Abstract
BACKGROUND:
Till now, no meta-analysis is available to address the clinical profile, risk factors, different interventions, and outcomes among COVID-19–associated rhino-orbito-cerebral mucormycosis (C-ROCM) cases.
MATERIALS AND METHODS:
Eight literature databases were screened using appropriate keywords from November 1, 2019, to June 30, 2021. The objectives were to analyze the clinical and microbiological profile, risk factor/comorbidity, intervention, and outcome. “R-metafor package” was used for analysis.
RESULTS:
A total of 23 studies were included. The mean age of presentation of C-ROCM was 54.6 years. The most common presentation was ptosis (72.7%), lid edema (60.6%), proptosis (60.6%), ophthalmoplegia (57.3%), loss of vision (53.7%), facial edema (34.7%), and nasal-blockage (11.8%). Evidence of intracranial spread was seen in 42.8% of cases. Rhizopus was the most common fungus (57.1%) isolated in fungal culture. Among C-ROCM patients, diabetes was the commonest comorbid condition, and the use of corticosteroids related to COVID-19 treatment was the most common risk factor (85.75%). Compared to controlled diabetics, C-ROCM was significantly higher among uncontrolled diabetics (odds ratio [OR] 0.15, 95% confidence interval [C.I.] 0.041–0.544, P = 0.0010). However, no significant association was seen between C-ROCM and COVID-19 severity (OR 0.930, 95% C.I. 0.212–4.087, P = 0.923). For treatment, amphotericin-B was the most common antifungal drug used which was followed by surgical options. However, mortality was high (prevalence 0.344, 95% C.I. 0.205–0.403) despite treatment.
CONCLUSION:
Although local rhino-orbito symptoms were the first to appear, rapid intracranial extension was seen in a significant number of C-ROCM cases. Uncontrolled diabetes and excessive use of corticosteroid were the most common risk factors present among the C-ROCM cases. High index clinical suspicion is imperative (specifically among COVID-19 patients with diabetes), and routine screening may be helpful.
Keywords: COVID-19–associated mucormycosis, cerebral, COVID-19, mucormycosis, orbital, rhino, rhino-orbito-cerebral mucormycosis, SARS CoV-2
Introduction
Fungal infections are being recognized as important secondary infections among patients with COVID-19.[1,2] Pre-existing comorbidities, injudicious use of corticosteroids and antimicrobials, and lapses in infection control practices contributed to opportunistic fungal infection in COVID-19 cases.[3] Common systemic fungal infections reported among COVID-19 population are candidemia, invasive aspergillosis, and mucormycosis.[4,5] COVID-19–associated mucormycosis (CAM) is gaining significant attention across the globe.[6,7] The general population incidence of mucormycosis is low (0.005–1.7/million populations); however, a surge of increases in the number of cases has been reported in recent COVID-19 pandemic.[8]
In immunocompetent patients, the incidence of opportunistic infections is low in the maxillofacial region, which may be attributed to its high vascularity. However, mucormycosis is especially important in this region as they colonize in the nasal mucosa and invade the local defense mechanisms in this area and can lead to invasive infections.[9] Rhino-orbito-cerebral mucormycosis (ROCM) represents the most common form of mucormycosis involving the maxillofacial and orbital region, which may further spread intracranially in its advanced stage and is reported among COVID-19 patients.[10] Critically ill patients on oxygen therapy, mechanical ventilation, and prolonged intensive care unit stay are known risk factors of mucormycosis, especially C-ROCM patients,[10] and high rate of mortality was seen among those with intra-cranial extension.
Although ROCM is very much relevant in the context of COVID-19, and few systematic reviews have addressed this issue, till now, no meta-analysis is available to address the demographic and clinical profile, risk factors, impact of different interventions, and outcomes among C-ROCM cases. In this context, the current systematic review and meta-analysis was undertaken to address these knowledge gaps.
Materials and Methods
This meta-analysis was undertaken and reported in accordance with “Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines”[11] (PRISMA) (PROSPERO registration I.D. CRD42021262943).
Objectives
Primary objectives
Clinical and demographic profile of C-ROCM patients
Microbiological profile of C-ROCM cases
Association between presence of risk factor/comorbidity and occurrence of C-ROCM
Evaluation of different intervention used in ROCM and their outcome (treatment success, treatment failure, and death).
Secondary objectives
Association between C-ROCM and diabetes (prevalence of diabetes among C-ROCM; both controlled and uncontrolled diabetes mellitus [DM])
Association of mortality and DM in C-ROCM
Association between C-ROCM and COVID-19 severity.
Inclusion criteria
Studies including diagnosed/confirmed mucormycosis patients, as defined by “Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium”[12] of all age groups associated with concurrent or post-COVID-19 infection
All types of observational study (prospective, retrospective, cohort, etc.) and case series reporting C-ROCM were included in our meta-analysis.
Exclusion criteria
Case reports, guidelines, consensus, editorials, and review articles were excluded from our meta-analysis.
Study population
Confirmed ROCM patients[12] including all age groups concurrent or post-COVID-19 C-ROCM.
Definitions
COVID-19 severity: COVID-19 severity definitions were adopted according to the World Health Organization “Therapeutics and COVID-19: living Guideline”[13] and patients were categorized into critical, severe, and nonsevere COVID-19 categories.[13]
Confirmed ROCM: Confirmed C-ROCM cases was defined according to the guidelines set by “Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium”[12]
Treatment success: Defined as response to treatment, i.e., stabilization of disease and disease-free patient[14]
Treatment failure: Death/mortality or progression of disease (showing radiological or clinical progression).[14]
Literature search and screening of articles
Eight literature databases were searched, i.e., PubMed, EMBASE, Google Scholar, Web of Science, Scopus, MedRxiv, BioRxiv, and SSRN from November 1, 2019, to June 30, 2021. Keywords used for data search were “Mucormycosis,” rhino, orbital, cerebral, ROCM, CAM, COVID-19, SARS CoV-2 without language restriction [detailed search strategy is given in Supplementary Table 1]. With completion of detailed search, duplicates were removed following which two authors (AB and PS) screened the articles/studies using the title and the abstracts of the study as predefined by the inclusion and exclusion criteria, following which full text of the relevant article was further evaluated for inclusion of our meta-analysis by using predefined inclusion and exclusion criteria. Any discrepancy among authors was resolved by consulting with BM.
Supplementary Table 1.
Detailed search strategy
| Key word Search: |
| 1# ((((((((“mucormycosis”[All Fields]) OR (“mucor”) OR OR (“mycoses”) [All Fields]) (“zygomycosis” [All Fields])) OR (“phycomycosis” [All Fields]) OR (“rhizopus”) [All Fields]) [All Fields]) OR (“absidia”) [All Fields]) OR (“cunninghamella”) [All Fields]) OR (“fungal infection”) [All Fields]) OR (“apophysomyces”) [All Fields] OR (“lichtheimia”) [All Fields] OR (“saksenaea”) [All Fields] OR (“rhizomucor”) 2#(“rhino”[All Fields]) OR (“nose”) OR (“ENT”) OR (“Nasal”) 3#(“orbital”[All Fields]) OR (“orbito”[All Fields]) OR (“orbit”[All Fields]) OR (“Ocular”) OR (“Eye”) OR (“Ophthal*”) 4#(“cerebral”[All Fields]) OR (“Brain”) OR (“Cranial”) OR (“Cranium”) 5# 2# OR 3# OR 4# 6# (“COVID-19”[All Fields]) OR (“sars cov 2”[All Fields]) 7# #1 AND #5 AND #6 |
ENT=Ear nose and throat
Data extraction
PS and AB independently extracted the data directly from full-length articles into structured tables. Collected data consisted of study country of origin, study type, risk factor or comorbidity, laboratory parameters, anatomic location of infection, diagnostic method, clinical manifestation, details of therapy used (antibiotic, steroid, antifungal use, surgery, etc.), and outcome of treatment (clinical and radiological response, laboratory parameters, hospital stay, treatment success and mortality etc.). In case of any study where our studied outcome parameters were not reported, corresponding authors were contacted for data via e-mail.
Quality assessment
The “methodological quality assessment” of the observational studies was assessed using “Newcastle–Ottawa Scale,”[15] and “risk of bias (ROB)” was assessed under three domains, i.e., selection, comparability, and outcome.[16] For assessing the quality of case series, scale mentioned by Murad et al.[17] was used (ROB domain evaluated: selection, ascertainment, causality, and reporting). Two investigators PS and AB independently evaluated the ROB of all the included studies. BM was consulted to solve the issue in case any discrepancy. Only high-quality studies were included in the meta-analysis.
Publication bias
”Publication bias” was evaluated by constructing a funnel plot and “Egger's regression test.”[18]
Statistical analysis
For single-group dichotomous data, pooled proportion/prevalence with 95% confidence interval (C.I.) was calculated. In case of dichotomous data having two groups, log odds ratio (OR) with 95% C.I. was used as a measure of association. In case of continuous data, for single-group analysis, pooled mean with 95% C.I. was calculated, and for two groups, mean difference with 95% C.I. was calculated. I2 and Chi2 statistics were used to assess heterogeneity.[19] In case of high heterogeneity (>50%), “random-effect model” was used for the analysis, else a “fixed-effect model” was used.[20] For the meta-analysis, “R software with Metafor Package”, R Foundation for Statistical Computing, Vienna, Austria” was used. Data conversion to different formats was done as per Wan et al., 2014.[21] Proportion was obtained as a ratio of total sample size. For prevalence, it was multiplied by 100.
Exploration of heterogeneity
For significantly high heterogeneity (>50%), etiology of high heterogeneity was investigated using “subgroup and meta-regression analysis.” Meta regression was conducted in case where the confounding variable was reported in 10 or more studies.[22]
Subgroup analysis
”Subgroup analysis” was done on the basis of study design (i.e., prospective vs. retrospective) and on the basis of studies including only severe and critical category COVID-19 population versus studies including COVID-19 population of all severity category.
Meta regression
Many factors (e.g., age, sex, comorbid condition, and therapy) may influence the mortality associated with C-ROCM.[23] In this context, “a meta-regression analysis” was done to observe the effect of these confounders on the final result, especially in the outcome of the disease (mortality).
Results
Details of included studies
After searching eight literature databases, we identified 827 articles. After removing duplicates, 615 studies were obtained, which were carried forward for further screening using title and abstract. Full-text screening was done for 67 relevant articles, out of which 23 studies fulfilling “predefined inclusion/exclusion criteria” were finally included in the systematic review and meta-analysis. The majority of included studies were from India (14 studies[6,7,24,25,26,27,28,29,30,31,32,33,34] ) followed by 4 studies from Egypt,[35,36,37,38] 1 from Iran,[39] 1 from Turkey,[14] 1 from Chile,[40] 1 from French[41] and 1 from the Netherlands.[42] Among all these included articles, 4 were preprint[31,33,34,41] [detailed characteristics of included studies showed in Supplementary Table 2 and Supplementary Figure 1 (733.4KB, tif) shows PRISMA flow diagram].
Supplementary Table 2.
Details of included studies
| Author, year/country/study design time/duration of study | Study population/sample size (n) | Age group (years) | COVID-19 category/T | Presenting clinical feature/COVID-19 marker at presentation |
|---|---|---|---|---|
| Deepti Satish[1] 2021/India Retrospective study March-December 2020 were included |
Patients with invasive fungal infection n=25 COVID-19 (+) =11 |
30-74 | Asymptomatic: 2 Mild: 2 Moderate: 3 Severe: 4 Concurrent=8 Post-COVID-19=3 |
Unilateral facial swelling, retro-orbital pain, ptosis, and headache |
| Mishra et al.[2] 2021/India Retrospective study August-December 2020 |
COVID-19-associated ROCM patient n=10 |
37-78 | Mild=3 Moderate=6 Severe=1 |
Ocular pain=1 Loss of vision=2 Chemosis=2 Epistaxis=1 Facial pain=1 Headache=1 Ocular swelling=1 Nasal block=2 Nasal crusting=1 |
| Sen et al.[3] 2021/India Retrospective study August-December 2020 |
COVID-19-associated ROCM cases n=6 |
60.5 (range 46.2-73.9) | Moderate-to-severe COVID-19=5 Post-COVID-19 cases=5 Concurrent cases=1 T=30-42 days |
Initial presentation: pain, redness, periocular swelling=6 Developed ptosis, ophthalmoplegia, painful loss of vision average 2 days from day of presentation |
| Sarkar et al.[4] 2021/India Case series October-November 2020 |
A cluster of clinically diagnosed orbital mucormycosis with concurrent COVID-19 illness over 2 months n=10 | 45.5 | Mild-to-moderate: 1 Severe: 9 |
CRAO=6 Loss of vision=4 DKA=4 |
| Moorthy et al.[5] 2021/India Retrospective observational multicenter study May 2020-December 2020 |
Patients with aggressive maxillofacial and ROCM cases concurrent or post COVID-19 positive n=18 |
54.6 (35-73) | No detail Concurrent case=4 Post COVID-19=14 |
Loss of vision=12/18 maxillary sinusitis=18/18 Maxillary necrosis=14/18 Intracranial extension=9/18 |
| Sharma et al.[6] 2021/India Prospective observational study August-December 2020 |
Mucormycosis of PNS with history of COVID-19 n=23 |
NA | NA Concurrent COVID-19 infection: 4 Post COVID-19 mucormycosis: 14 |
Intraorbital extension=43.47% Intracranial extension=8.69% Involvement of ethmoid sinus=100% Classical black escher on hard palate=9/23 |
| Nehara et al.[7] 2021/India Case series November-December 2020 |
COVID-19-associated ROM patient n=5 |
62.2 | Severe to critical=2 Mild-to-moderate=3 Concurrent COVID-19=3 Post COVID-19=2 |
Loss of vision=3 Headache=3 Ptosis=2 Proptosis=4 Palate inv=3 Complete ophthalmoplegia=2 Epistaxis=3 |
| Ravani et al.[8] 2021/India Retrospective study September 2020-mid-March 2021 |
All biopsy-proven mucormycosis patient Total n=31 COVID-19=19 All are post-COVID, no case of concurrent COVID-19 |
56.3 | NA T=average 2 months No concurrent COVID-19 case |
Commonest presentation: Diminution of vision (<6/60 in 80.64%), ophthalmoplegia (77.4%) |
| Diwakar et al., 2021[9]/India Case series Preprint |
CAM patient of pediatric age group with type 1 DM n=2 |
Case 1=11 Case 2=13 |
Asymptomatic COVID-19 infection ROCM developed during course of DKA T=3 days (case 1) T=20 (case 2) |
Case 1: Weight loss, unilateral pain and swelling of eye, high-grade fever, complete ophthalmoplegia Case 2: Unilateral pain, swelling and diplopia, complete ophthalmoplegia, nonreactive pupil |
| Gangneux et al., 2021[10]/French National multicenter observational study 18 French ICU Preprint February 29 and July 9, 2020 |
Severe COVID-19 population with IFI≥18 years age group n=57 |
59.4±12.5 | Severe COVID-19=57 | Mucormycosis prevalence 1.2% |
| Buil et al., 2021[11]/Netherlands Case series |
Secondary fungal infections caused by Mucorales species in COVID-19 patients n=4 |
>50 | 3 CAM cases occurred in ICU one outside ICU | Pulmonary, rhino-orbital cerebral and disseminated infection |
| Fouad et al., 2021[12]/Egypt Retrospective observational study March 25, 2020-September 25 |
ROCM patients n=12 COVID-19 positive=6 Concurrent case=5 Post-COVID-19 positive=1 |
51.2 (16.7) | Moderate-to-severe=5 | Orbital invasion=all Cerebral invasion=8 Lid edema(50%), conjunctival chemosis (50%) diminution of vision (41.7%), proptosis (33.3%), facial edema (25%), nasal Crusts (25%), total ophthalmoplegia (16.7%) Paralytic esotropia (8.3%) |
| El-Kholy et al., 2021[13]/Egypt/longitudinal prospective study August 2020-December 2020 |
COVID-19-associated acute invasive fungal rhino sinusitis n=36 |
52.92±11.30 | Mild=11 Moderate=13 Severe=12 MV needed=3 |
Headache and facial pain (75%) Facial numbness (66.7%) Ophthalmoplegia, and visual Loss (63.9%) Proptosis=19 Diplopia=6 Palatal involvement=11.1% Necrosis of cheek=1 Orbital apex syndrome=3 |
| Wael F. Ismaiel et al., 2021[14]/Egypt/retrospective study January 2017-December 2020 |
Diagnosed AIFRS n=56 Total post COVID-19 cases=18 |
58.38±12.2 | ARDS=3 | - |
| Manar M. Ashour, et al., 2021[15] Case series May 2020-February 2021 |
Confirmed COVID-19 AIFS n=7 |
- | T=12-35 days | Right upper eyelid edema=4 Total ophthalmoplegia=5 No PL=5, black nasal crusts=6 Rapidly deteriorating visual acuity=1 Conjunctival chemosis=1 Proptosis=1 Paralytic esotropia=1 Bilateral oroantral fistulae=1 Eyelid edema=1 Reduced visual acquity=1 Facial edema=2 Skin discoloration=1 bilateral Panophthalmitis=1, necrosed nasal septum=1Nacrosed hard palate=1 Painful orbital proptosis=1 Drop of vision=1 Panophthalmitis=1 Septal ulceration=1 Panophthalmitis=4 Orbital compartment syndrome=1 Optic nerve ischemia/inflammation=2 Bilateral orbital panophthalmitis=2 Intracranial complications were: Perineural spread=6 Cavernous sinus involvement=6 Meningeal/epidural infiltration=3 ICA vasculitis/thrombosis=4 ICA mycotic aneurysm=intracerebral Abscess=2 and cerebral infarctions=3 |
| Ricardo Rabagliati et al.[16]/2021/Santiago, Chile Retrospective case series During May 18-July 18, 2020 |
Nonimmunocompromised adult CAIMI cases with severe COVID-19 cases admitted in ICU Total severe to critical COVID-19 cases=146 n=16 |
Median age65 (30-89) | All severe to critical COVID-19 cases T=18.5 (range 1-47) days 14.5 days (range 0-28) After ICU admission 12.5 days (range 0-28) after IMV |
- |
| Bayram et al., 2021[17]/prospective observational study March 2020-December 2020 |
COVID-19-associated ROM cases in severe COVID-19 patients n=11 |
- | All severe COVID-19 cases T=5.1±1.8 days (2-8 days) Increased D-dimer=all patient Low molecular weight heparin received=all mean D-dimer level=1362.4±468.9 μg/L |
Proptosis (100%) Ophthalmoplegia (63.6%), orbital pain (81.8%), conjunctival Hyperemia/chemosis (81.8%), ptosis (63.6%), fixed and dilated pupil (63.6%), vision loss (63.6%), endophthalmitis (54.5%), decreased vision (27.3%), orbital apex syndrome 7 (63.3%) syndrome, orbital cellulitis (36.4%) |
| Joshi et al., 2021[18]/India/retrospective study March 1, 2021-April 15, 2021 | COVID-19 and invasive ROCM cases | 55.2±13, range 34-76 | Severe to critical COVID-19 | - |
| Atul Patel et al., 2021[19]/India/multicenter retrospective study in 16 healthcare centers across India September-December 2020 |
Confirmed mucormycosis cases with and without COVID-19 Total mucormycosis patient=287 CAM=187 (65.2%) |
53.4 (SD 17.1) | T=median time 18 days (IQR 11-27) | Commonest is rhino-orbital mucormycosis (58.2%), followed by ROCM, pulmonary and other sites |
| Muley et al., 2021[20]/India/observational study April 15, 2021, and May 15, 2021 Preprint |
Probable RMMn=30 | - | 23 (76.6%) gave a history of hospitalization due to COVID-19 17 (23.3%) had no history of hospitalization |
6.6% of cases were in Grade 1, while 53.5% were in Grade 2, and 40% were in Grade 3 |
| Farzad Pakdel et al., 2021[21]/Iran/cross-sectional descriptive multicenter study in five COVID-19 hospitalized centers in Tehran, Iran April-September 2020 |
Biopsy-proven ROCM patients with COVID-19 n=15 |
52 (range 14-71) | Mild=3 Moderate=5 Severe=7 T: 1-37 days |
Unilateral periorbital pain and edema (73%), ptosis (73%), acute vision loss (73%), proptosis (67%), unilateral facial edema (60%), cranial nerve palsy (60%), headache (33%), fever (27%), nasal blockage (13%) and ear pain (7%) |
| Rajalakshmi Arjun et al., 2021[22]/India/case series | COVID-19-associated rhino-orbital mucormycosis n=10 |
53.0±12.1 | All cases had past H/O COVID-19 T=17.0±3.6 days |
Headache=6 Facial swelling=3 Facial numbness=1 Lid swelling=4 Black discoloration of palate=1 Ocular pain=3 Earache=1 Diplopia=1 Sphenoid=8 Intracranial spread=3 Optic nerve involvement=2 |
| Sebastian et al., 2021[23]/India/case series | CAIFS n=3 |
61±2.64 | Severe to critical=3 Bilateral COVID-19 pneumonia with ARDS=3 Altered sensorium=1 RF and shock=1 |
Nasal blockage=1 facial swelling=1 Periorbital swelling=2 Blackening of middle turbinate=2 Blackening of the lateral wall of nose with crusts and discharge=1 Thick dirty nasal discharge=1 Eye pain=1 Restricted eye movements=1 Diminution of vision=1 Cavernous sinus thrombosis=1 Proptosis=1 Periorbital bluish discoloration=1 |
|
| ||||
| Author, year/country/study design time/duration of study | Co-morbidities/risk factor | Hyperglycemia at presentation | Treatment | Comment |
|
| ||||
| Deepti Satish[1] 2021/India Retrospective study March-December 2020 were included |
Immunocompromised-23 Immunocompetent=2 Majority of the patients with DM and HTN with underlying IHD and CKD Leukemia=1 |
CAM cases had HbA1c level 7-15 mg/dl (majority >10 mg/dl) COVID-19 negative Mucor patients had HbA1c level 6-13 mg/dl (majority <10 mg/dl) |
Empirical IV Amph B in severe CAM patients and debridement done once stable COVID-negative cases: Debridement followed by IV AmphB IV AmphB dose: 50 mg/kg/day with cumulative dose of 1.5-2 g Postoperatively local diluted AmphB douching all cases Debridement: 20 |
Details of COVID-19 patient profile is not available Redebridement done in 1 patient after 1 month due to vision loss Significant delay in the surgical management of moderate-to-severe COVID-19 patients were there due to lack of fitness for GA |
| Mishra et al.[2] 2021/India Retrospective study August-December 2020 |
DM=8 HTN=3 CKD=2 CLD=1 IHD=1 Hypothyroidism=2 |
No detail available | Steroid=6 Amph B=10 Posaconazole=1 RDV=6 Tocilizumab=1 |
4/10 patients were diagnosed and managed for COVID-19 6/10 were treated for COVID-19 outside Lost to follow-up=1 |
| Sen et al.[3] 2021/India Retrospective study August-December 2020 |
DM (Type 2): 100%2/6 were diagnosed of DM with the onset of COVID-19 | Average FBS=222.5±144.4 (86-404) mg/dL at presentation Mean duration of DM=5.9±4.9 (median 6.5) years Uncontrolled DM=5 DKA=3 Case 2: Moderate NPDR with DME |
Antifungals started after microbiological confirmation LipAmpB B (5 mg/kg/day, up to a maximum of 10 mg/kg/day I.V. for CNS infections) Oral posaconazole (loading dose 300 mg BD day 1 followed by maintenance dose 300 mg OD) Orbital-exenteration: In suboptimal response to systemic antifungals within 72 h |
Male cases 100% All were referred cases |
| Sarkar et al.[4] 2021/India Case seriesOctober-November 2020 |
DM: 10/10 (100%) Steroid: 10/10 (100%) |
DKA at admission: 4/10 DKA during COVID-19 therapy: 5/10 |
All patient received dexamethasone as per NIH guideline LipAmpB: All cases RDV=4/10 Ventilatory support=9/10 |
Detail dose not given |
| Moorthy et al.[5] 2021/India Retrospective observational multicenter study May 2020-December 2020 |
DM: 16/18 | All poorly controlled DM | Mucormycosis: LipAmpB at 3-5 mg/kg to a cumulative dose of 3-5 g Aspergillosis: Oral voriconazole (6 mg/kg IV 12 hourly Day 1/4 mg/kg IV 12 hourly subsequently) and posaconazole (200 mg) Orbital exenteration: 7 Maxillectomy: 11 Endoscopic sinus surgery: 17 |
Maxillary necrosis=14/18, which is statistically significant (P=0.03) Lost to follow up=1 |
| Sharma et al.[6] 2021/India Prospective observational study August-December 2020 |
DM: 21 HTN: 14 Renal failure: 1 Steroid user=100% |
DM: 21 Uncontrolled DM: 12 (HbA1c>6.5%) |
Intra-orbital AmpB: 2 | None gave consent for orbital exenteration Mortality: 0% |
| Nehara et al.[7] 2021/India Case series November-December 2020 |
DM: 5 HTN: 2 |
Uncontrolled DM=3 DKA=1 Ketosis without acidosis=1 |
LipAmpB=all Posaconazole=1 MV=1 Broad-spectrum antibiotics=all Ionotrops=2 |
- |
| Ravani et al.[8] 2021/India Retrospective study September 2020-mid-March 2021 |
COVID-19 positivity: 61.2% Uncontrolled DM: 96.7% Steroid user: 61.2% HTN=54.8% IHD=3.22% Kidney disease=6.45% |
Uncontrolled type 2 DM=29 (93.54%) Uncontrolled type I DM=1 Newly diagnosed DM=6 Average duration of DM=4.4 years Average HBA1C=7.57 mmol/mol Duration of steroid=7-14 days |
IV LipAmpB B: 31 (100%) after microbiological confirmation Sinus debridement: 31 (100%) Exenteration: 4 (12.9%) IV LipAmpB dose=3-5 mg/kg body weight/day Oral posaconazole: Add-on therapy at time of resolution (5 mg/kg body weight/day) |
Cerebral involvement and HbA1c ≥8 found to be significant in prediction of 75-day mortality (P≤0.05) |
| Diwakar et al., 2021[9]/India Case seriesPreprint |
Type 1 DMNO H/O Steroid exposure |
Both are cases pf type 1 DM | I.V. LipAmpB=2 Retro orbital AmpB=2 Craniotomy (for brain abscess)=2 Orbital exenteration with resection of the involved sinuses. =2 I.V LipAmpB dose (10 mg/kg/day) in 5% dextrose over 1 h for 6 weeks |
Case 1: hypokalemia (serum K+=2.8 mmol/L), hyperglycemia 96 (RBS=329 mg/dL Case 2: LFT: Hypoalbuminaemia 130 (3 g/dL) and low albumin/globulin ratio (1.00) |
| Gangneux et al., 2021[10]/French National multicenter observational study 18 French ICU Preprint February 29 and July 9, 2020 |
DM: 32·9% HTN: 50·1% Lymphopenia at presentation: 64·2% Mean duration of MV: 27·1±19·8 days |
- | Detail for mucormycosis patients not available | Detail profile of for mucormycosis patients not available |
| Buil et al., 2021[11]/Netherlands Case series |
DM=2 Steroid=4 MV=4 CLL=1 Obesity=1 |
DM=2 Uncontrolled DM=1 |
Tocilizumab=1 LAmpB=4 Prosaconazole=1 Voriconazole=1 Isavuconazole=2 INF Gama=1 |
Microscopy showed nonseptate hyphae in the patient’s urine in 1 case died 3 out of 4 cases |
| Fouad et al., 2021[12]/Egypt Retrospective observational study March 25, 2020-September 25 |
DM=10 Hematological malignancy=2 received chemotherapy CKD=3 IHD=1 |
DM=10 Uncontrolled DM=10/12 cases (83.3%) Occurrence of DM at time of ROCM diagnosis=9 Median HbA1c=9.7% DKA=1 |
Debridement done=7 (58.3%) | - |
| El-Kholy et al., 2021[13]/Egypt/longitudinal prospective study August 2020-December 2020 |
DM (27.8%) HTN (16.67%) Associated malignancy, uncontrolled leukemia, and pancreatic cancer=5.56% |
DM=10 (27.8%) 3/10 COVID-19-associated due to corticosteroid therapy |
Antifungal therapy and surgery=34 Only antifungal therapy=2 LipAmpB=26 Voriconazole=8 Both=2 Posaconazole=3 |
Individual data for COVID-19-associated mucormycosis not given Early cases (n=7) with limited sinonasal involvement showed better results |
| Wael F. Ismaiel et al., 2021[14]/Egypt/retrospective study January 2017-December 2020 |
DM=44.4% of post-COVID-19 AIFRS Immunosuppressive=16.7% HTN=10 obesity=8 Smoking=12 Allergic rhinitis=14 Asthma=4 COBD and Cardiac diseases=5 Otitis media=6 Renal dysfunction=3 Liver dysfunction=2 Thrombocytopenia and leucopenia=4 Immunosuppressive drugs=3 Antibiotic or antiviral therapy=12 Steroid use=7 Chemotherapy=1 |
DM=44.4% of post-COVID-19 AIFRS | - | Incidence of AIFRS is more prominent in post-COVID-19 patients than in non-COVID-19 especially in immunocompromised patients, diabetic, renal and liver dysfunction patients and patients with risk factors for rhino sinusitis |
| Manar M. Ashour, et al., 2021[15] Case series May 2020-February 2021 |
DM (n=6), HTN (n=2), end stage renal disease (n=2), hyperlipidemia (n=2), ischemic heart disease (n=1), previous cerebral stroke (n=1) | - | AmpB=7 Itraconazole=1 Ambisome=1 Surgical debridement=7 |
Patients had radiologic features of aggressive late-stage forms of the disease process with a consequent long-term morbidity rate of 100% and a high mortality rate of 37.5% |
| Ricardo Rabagliati et al.[16]/2021/Santiago, Chile Retrospective case series During May 18-July 18, 2020 |
HTN=9 (56.3%) Asthma/COPD=4 (25%) DM=4 (25%) Obesity=3 (18.8%) Median worst PaO2/FiO2 for each patient was 124 (range 57-476) None were immunocompromised |
- | Antifungal therapy=13 (81.3%) Voriconazole=10 (76.9%) LipAmpB=2 (15.4%) Isavuconazole=1 (7.7%) Therapeutic drug monitoring received with patients under voriconazole therapy=9 patients receiving (median 3.9 mg/L; range 0.1-7.2 mg/L) |
Severe hypoxia, broad-spectrum Antibiotics, high corticosteroid doses, prolonged ICU stay, long intubation period, and airway/lung damage and infarction areas are predisposing condition for critically ill patients |
| Bayram et al., 2021[17]/prospective observational study March 2020-December 2020 |
COVID-19-associated ARDS=11 Corticosteroid=11 DM=8 (72.7%) CRF=3 ARF=2 Hematological malignancy=1 under immunosuppressive |
Type 2 DM=8 (72.7%), all uncontrolled Mean duration of diagnosis=12.1±4.4 years (range: 7-19 years) |
IV and retro bulbar LipAmpB=all cases Intravitreal LipAmpB in patients with endophthalmitis IV dose=1.0 mg/kg/day, increasing a total dose of 2.5-3 g Retro bulbar dose=1 ml of 3.5 mg/ml Intravitreal dose=5 μg/0.1 ml Second surgical procedure=8 (72.7%) Mean number of retrobulbar and intravitreal AmpB injections=2.2±0.6 and 2.3±0.5 Interval between repeat injections varied from 2-8 days |
Diagnosis of mucormycosis was made in all patients during the COVID-19 treatment The mean MIC of amphotericin B was 2.5±1.0 μg/ml (range: 1.5-4 μg/ml) The MIC of voriconazole was >32 μg/ml in all patients All endophthalmitis cases showed posterior scleritis, two developed retinochoroiditis followed by retinoschisis, and one had corneal edema |
| Joshi et al., 2021[18]/India/retrospective study March 1, 2021-April 15, 2021 |
DM=22 Uncontrolled DM=13 HIV=2 MV received=2 Corticosteroids=2 (duration, 10-14 days) |
- | Amphotericin B=all Surgical debridement=10 Orbital exenteration=10 s |
- |
| Atul Patel et al., 2021[19]/India/multicenter retrospective study in 16 healthcare centers across India September-December 2020 |
COVID-19 only 61 (32.6%) Glucocorticoids 48/61 (78.7) DM=113 (60.4%) Posttrauma=3 (1.6%) Hematological malignancy=2 (1.1%) Renal transplantation=3 (1.6%) Median cumulative Dexamethasone-equivalent dose=84 mg (range 18-1,343 mg) Tocilizumab=5 (2.7%) Hypoxemia due to COVID-19 and inappropriate glucocorticoid administration were associated with development of late CAM |
Uncontrolled DM=62.7% DKA=early (28%), late CAM (5%) Newly diagnosed DM=39/187 (20.9%) COVID-19 was the only underlying disease in 61/187 (32.6%) |
LipAmp B=136 (72.7%) AmpD deoxycholate=31 (16.6%) Posaconazole=73 (39.0%) Isavuconazole=19 (10.2%) Single antifungal drug=95 (50.8%) Concurrent=13 (7.0%) Sequential=79 (42.5%) Combined medical and surgical therapy=131 (70.1%) LipAmpB dose=5 mg/kg 1×/d for 4-6 weeks AmpB deoxycholate=1 mg/kg 1×/d for 6-8 week Sequential antifungal drug treatment improved mucormycosis survival |
CAM prevalence=0.27% among hospitalized COVID-19 patients Newly diagnosed DM in CAM patients were significantly higher (P=0.02) DKA seen more in non-CAM patients (16/187 [8.6%]) than in CAM patients (27/100 [27%]; P=0.0001) COVID-19-related hypoxemia and improper glucocorticoid use independently were associated with CAM |
| Muley et al., 2021[20]/India/observational study April 15, 2021, and May 15, 2021 Preprint |
76.66% had a history of COVID-19, and 66.66% had hospitalization and steroid administration history | 84% patients had diabetes mellitus | 36% were operated on by OMFS, and 6.6% were referred to an ENT specialist | - |
| Farzad Pakdel et al., 2021[21]/Iran/cross-sectional descriptive multicenter study in five COVID-19 hospitalized centers in Tehran, Iran April-September 2020 |
DM=13 (86%) HTN=7 (46%) Hematologic malignancies=2 (13%), Asthma 2 (13%) Cardiovascular disease 2 (13%), hepatic cirrhosis 1 (6%), hypothyroidism 1 (6%), TB 1 (6%), immunosuppressive therapy 7 (46%), chemotherapy 2 (13%), neutropenia 3 (20%), ketoacidosis 1 (6%) |
46.6% patients previously received intravenous corticosteroid therapy | IV AmpB: All cases Oral posaconazole: 4 (27%) Combined anti-fungal therapy: 6 (40%) IV AmpB (5 mg/kg daily for 4-6 weeks) Orbital exenteration=5 Palatal debridement=2 |
Anti-fungal combination therapy was significantly associated with Better outcome (P=0.003) |
| Rajalakshmi Arjun et al., 2021[22]/India/case series | DM=all HTN=2 Hypothyroidism=1 CAD=3 CKD=1 RA=1 Supplemental O2 required=8 Steroid therapy=8 Broad-spectrum IV antibiotics=9 Steam inhalation: 9 |
DM=all cases Mean HbA1c: 10.2±2.0 |
Endoscopic sinus surgery and debridement was done in all patients within 24 h Antifungals used included liposomal amphotericin B Amphotericin B deoxycholate and isavuconazole |
- |
| Sebastian et al., 2021[23]/India/case series | DM=1 CABG=1 CKD=1 HTN=1 MV=2 (Case 1=on mechanical ventilation for 7 days) Peptic ulcer=1 BSA=3 Steroid=3 Renal replacement therapy=2 |
All three are diabetic controlled | Case 1=liposomal amphotericin B was given (total dose of 3050 mg). Subsequently he was continued on voriconazole Surgical debridement was done Case 2=total dose of 850 mg of liposomal amphotericin B |
Case 1: Although clinical resolution was seen, during the post-COVID recovery phase patient developed myocarditis with cardiac arrhythmia and expired Case 2=surgical debridement could not be carried Case 3=developed massive peptic ulcer bleed and went into irreversible shock and died |
MV=Mechanical ventilation, IMV=Invasive mechanical ventilation, ARDS=Acute respiratory distress syndrome, ROCM=Rhino-orbito-cerebral mucormycosis, CAM=COVID-19 associated mucormycosis, T=Time to develop mucormycosis from COVID-19 diagnosis, IHD=Ischemic heart disease, CKD=Chronic kidney disease, RDV=Remdesivir, FBS=Fasting blood sugar level, DKA=Diabetic ketoacidosis, NPDR=Nonproliferative diabetes mellitus, DME=Diabetic macular edema, CRAO=Central retinal arterial occlusion, PNS=Paranasal sinus, BSA=Broad spectrum IV antibiotics, AIFRS=Acute invasive fungal rhino sinusitis, RMM=Rhinomaxillary mucormycosis, ICU=Intensive care unit, OMFS=Oral and maxillofacial surgeons, PL=Perception of light, IFI=Invasive fungal infections, SD=Standard deviation, IQR=Interquartile range, NA=Not available, DM=Diabetes mellitus, HTN=Hypertension, ARF=Acute renal failure, COPD=Chronic obstructive pulmonary disease, CRF=Chronic respiratory failure, CABG=Coronary artery bypass grafting, HbA1c=Hemoglobin A1c, RBC=Red blood cell, MIC= Minimal inhibitory concentration, LFT= Liver function test, NIH=National institute of health, ENT= Ear nose and throat, ICA= Internal carotid artery RF=Respiratory failure, TB= Tuberculosis, RA= Rheumatoid arthritis, GA= General anaesthesia, ROM=Rhino orbital mucormycosis, AIFS= Acute invasive fungal sinusitis, CAIMI=Covid-19 associated invasive mucorales infection
Publication bias
The Egger's test shows no evidence of publication bias with P = 0.633. [Supplementary Figure 2 (218.8KB, tif) ].
Primary objective
Demographic profile of patients with COVID-19–associated rhino-orbito-cerebral mucormycosis
Mean age at presentation
A total of 17 studies[6,7,14,25,26,27,29,31,32,34,35,37,39,40,41,42,43] reported mean age of presentation of C-ROCM. The mean age of presentation of C-ROCM was 54.6 years (95% C.I. 44–65, I2 = 99%, random-effect model) [Figure 1]. In the pooled data, it was seen that Bayram et al.[14] and Diwakar et al.[31] reported extremes of age group (mean age 73 years and 12 years, respectively) as compared to other studies. Hence, a sensitivity analysis was conducted by excluding these two studies; however, the mean age of presentation remained similar (56.8 years [95% C.I. 54–58]) and heterogeneity reduced to 50% (I2 = 50%, fixed-effect model) [Supplementary Figure 3 (323.5KB, tif) ].
Figure 1.

Mean age at presentation in C-ROCM patients
Sex ratio
Males were the more frequently affected gender (17 studies,[6,7,14,24,25,26,27,28,29,31,33,34,35,37,39,40,42,43] prevalence 0.784, 95% C.I. 0.721–0.846, I2 = 28.96%, fixed-effect model).
Clinical presentation of COVID-19–associated rhino-orbito-cerebral mucormycosis population
Mean time period of presentation of C-ROCM from the time of COVID-19 diagnosis was 20 days (10 studies,[7,14,14,26,29,34,35,40,42,43] 95% C.I. 15–24 days) [Supplementary Figure 4 (238.1KB, tif) ]. There was no significant difference in odds of occurrence of ROCM among patients with concurrent COVID-19 infection versus post-COVID-19 infection (7 studies,[6,26,28,29,30,37,42] OR [log scale] 0.306, 95% C.I. 0.042–2.246, P = 0.244, I2 = 79.46%, random-effect model) [Supplementary Figure 5 (178.2KB, tif) ].
Regarding ocular presentation, unilateral involvement was commonest, while bilateral ocular involvement was reported only in small number of patients (2/12, i.e., 16.7%, single study[37] ). The most common ocular presentations were lid edema (9 studies,[25,26,29,31,34,35,37,39,43] prevalence 0.606, 95% C.I. 0.385–0.826), proptosis (8 studies,[14,26,29,35,37,38,39,43] prevalence 0.606, 95% C.I. 0.385–0.826), ophthalmoplegia (8 studies,[14,29,30,31,35,37,38,43] prevalence 0.573, 95% C.I. 0.395–0.751), loss of vision (10 studies,[6,14,25,26,27,28,29,35,38,39] prevalence 0.537, 95% C.I. 0.282–0.793), facial edema (6 studies,[29,34,35,37,39,43] prevalence 0.347, 95% C.I. 0.216–0.477), and ptosis (4 studies,[14,26,29,39] prevalence 0.727, 95% C.I. 0.534–0.920). Pupil involvement was reported by 3 studies[14,26,31] (prevalence 0.581, 95% C.I. 0.362–0.801), while optic nerve involvement was reported by 2 studies[34,35] (prevalence 0.230, 95% C.I. 0.031–0.430). Rare but vision-threatening presentations included panophthalmitis (single study,[35] n = 7, unilateral in 4/7 and bilateral in 1/7), endophthalmitis (single study,[14] 6/11, 54.5%), orbital compartment syndrome (single study,[35] 1 out of 7, 14.2%), and central retinal artery occlusion (single study,[27] 6 out of 10, 60%).
Regarding rhino/nasal presentation the most common presenting features reported were nasal blockage (2 studies,[29,39] n = 25, overall 3 out of 25, prevalence 0.118, 95% C.I. −0.008–0.244), black nasal discharge (single study,[29] n = 5, 2 out of 5), and epistaxis (2 studies,[25,29] n = 15, 3 out of 15).
Palatal involvement was also reported by few studies (3 studies,[26,28,29] n = 21, overall 13 out of 21, prevalence 0.575, 95% C. I. 0.162–0.989). “Classic black Eschar” has been reported in small number of patients (single study,[28] n = 23, 9 out of 23), and bleeding gum (single study,[25] n = 10, 1 out of 10) has also been reported as infrequent presentation [detail clinical presentation and their prevalence in C-ROCM patients is summarized in Table 1].
Table 1.
Pooled prevalence of proportion of various clinical presentations described among COVID-19-associated rhino-orbito-cerebral mucormycosis patient population
| Clinical feature | Number of studies/reference | Event/total | Pooled data |
SE | Over all P | Heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|---|
| Pooled proportion | 95% CI | I2 (%) | Tau 2/Q/DF | Heterogeneity P | |||||
| Clinical feature | |||||||||
| Loss of vision | 10[6,14,25,26,27,29,35,38,39,44] | 71/141 | 0.537 | 0.282-0.793 | 0.130 | <0.001 | 93 | 0.151/147.530/9 | <0.001 |
| Lid edema | 9[25,26,29,31,34,35,37,39,43] | 35/70 | 0.515 | 0.278-0.751 | 0.121 | <0.001 | 84 | 0.105/52.026/8 | <0.001 |
| Ophthalmoplegia | 8[14,29,30,31,35,37,38,43] | 66/107 | 0.573 | 0.395-0.751 | 0.091 | <0.001 | 72 | 0.042/25.310/7 | <0.001 |
| Proptosis | 8[14,26,29,35,37,38,39,43] | 56/95 | 0.606 | 0.385-0.826 | 0.112 | <0.001 | 87 | 0.082/56.483/7 | <0.001 |
| Facial edema | 6[29,34,35,37,39,43] | 19/52 | 0.347 | 0.216-0.477 | 0.067 | <0.001 | 9 | 0.003/5.514/5 | <0.001 |
| Palatal involvement | 3[26,29,44] | 13/21 | 0.575 | 0.162-0.989 | 0.211 | 0.006 | 79 | 0.104/9.717/2 | 0.008 |
| Ptosis | 4[14,26,29,39] | 26/37 | 0.727 | 0.534-0.920 | 0.099 | <0.001 | 53 | 0.020/6.458/3 | 0.091 |
| Pupil involvement | 3[14,26,31] | 11/19 | 0.581 | 0.362-0.801 | 0.112 | <0.001 | 0 | 0.000/0.356/2 | 0.837 |
| Palatal Eschar | 6[26,29,34,35,38,44] | 20/74 | 0.356 | 0.058-0.654 | 0.152 | 0.019 | 91 | 0.120/59.771/5 | <0.001 |
| Optic nerve involvement | 2[34,35] | 4/17 | 0.230 | 0.031-0.430 | 0.102 | 0.023 | 0 | 0.000/0.163/1 | 0.687 |
| Nasal blockage | 2[29,39] | 3/25 | 0.118 | −0.008-0.244 | 0.064 | 0.067 | 0 | 0.000/0.067/1 | 0.796 |
| Radiological feature | |||||||||
| Sinonasal involvement | 4[24,38,39,43] | 47/79 | 0.549 | −0.013-1.11 | 0.287 | 0.056 | 98 | 0.319/235.393/3 | <0.001 |
| Rhino-orbital involvement | 8[7,14,26,31,37,39,43,44] | 165/259 | 0.708 | 0.539-0.877 | 0.086 | <0.001 | 87 | 0.044/57.998/7 | <0.001 |
| Intracranial spread | 13[6,7,14,24,25,26,27,29,32,35,37,39,44] | 103/333 | 0.428 | 0.297-0.560 | 0.067 | <0.001 | 82 | 0.042/69.656 | <0.001 |
| Involvement of orbital apex | 2[14,34] | 8/21 | 0.257 | −168-0.882 | 0.268 | 0.183 | 89 | 0.129/9.578/1 | 0.002 |
| Involvement of cavernous sinus | 8[26,29,30,31,32,37,38,39] | 36/132 | 0.403 | 0.22-0.595 | 0.098 | <0.001 | 88 | 0.059/60.787/7 | <0.001 |
CI=Confidence interval, SE=Standard error
Radiological involvement
Radiological investigation, especially computed tomography (CT) scan and magnetic resonane imaging (MRI), has been used as a diagnostic tool to evaluate the extent of involvement of mucormycosis infection almost all the authors. Our meta-analysis showed the most common involvement to be rhino-orbital involvement (8 studies,[7,14,26,28,31,37,39,43] prevalence 0.708, 95% C.I. 0.539–0.877), followed by sinonasal involvement (4 studies,[24,38,39,43] prevalence 0.549, 95% C.I. −0.013–1.11) and intracranial involvement (13 studies,[6,7,14,24,25,26,27,28,29,32,35,37,39] prevalence 0.428, 95% C.I. 0.297–0.560). Involvement of orbital apex (2 studies,[14,34] prevalence 0.257, 95% C.I. −168–0.882) and cavernous sinus was seen in small number of patients (8 studies,[26,29,30,31,32,37,38,39] prevalence 0.403, 95% C.I. 0.22–0.595).
Regarding paranasal sinus involvement, “ethmoid sinus” was the most common paranasal sinus involved which was reported to be 80% (n = 10, 8 out of 10) by Arjun et al.,[34] 90.9% (n = 11, 10 out of 11) by Bayram et al.,[14] and 100% (n = 23, 23 out of 23) by Sharma et al.[28] Pansinusitis was seen in 60% (n = 5, 3 out of 5) by Nehara et al.,[29] 77.4% (n = 31, 24 out of 31) by Ravani et al.,[30] 90.9% (n = 11, 10 out of 11) by Bayram et al.,[14] and 100% (n = 6, 6 out of 6) patients by Sen et al.[26] Pakdel et al.[39] reported that the most common sinus involvement was pansinusitis in their study population.
Regarding orbital involvement, isolated orbital involvement was seen in 13% of cases (n = 15, 2 out of 15) by Pakdel et al.[39] Extraocular muscle involvement was seen in 19.35% of cases (n = 31, 6 out of 31) and orbital cellulitis in 61.29% (n = 31, 19 out of 31) by Ravani et al.[30] Subperiosteal abscess was seen in 5.6% of patients (n = 36, 2 out of 36) by El-Kholy et al.[38]
Regarding intracranial involvement, transverse and sigmoid sinus thrombosis was seen in 2.7% (n = 36, 1 out of 36), temporal lobe abscess in 2.7% (n = 36, 1 out of 36), encephalitis in 5.4% (n = 36, 2 out of 36), and central retinal artery thrombosis in 5.4% (n = 36, 2 out of 36) by El-Kholy et al.[38] Cavernous sinus thrombosis was seen in 60% (n = 5, 3 out of 5) patients by Nehara et al.[29] and 3.22% (n = 31, 3 out of 31) by Ravani et al.[30] Internal carotid artery thrombosis was seen in 6.45% (n = 31, 2 out of 31) and 16.7% (n = 25, 4 out of 25) by Ravani et al.[30] and Fouad et al.,[37] respectively. Brain infarct was seen in 20% (n = 5, 1 out of 5) patients by Nehara et al.[29] while cerebral abscess was seen in 16.7% (n = 25, 4 out of 25) population by Fouad et al.;[37] meningitis was seen in 8% (n = 25, 2 out of 25) by Joshi et al.;[32] 5th nerve involvement in 4% (n = 25, 1 out of 25) patients; and black turbinate sign in 32% cases (n = 25, 8 out of 25) in a single study.[32]
Very small number of studies reported pulmonary involvement and disseminated form of mucormycosis in C-ROCM patients. Buil et al.[42] reported bilateral consolidations in 25% patients (n = 4, 1 out of 4) and pulmonary cavities and a reversed halo-sign in 25% patients (n = 4, 1 out of 4). Rabagliati et al.[40] reported pulmonary cavitations in 6.25% (n = 16, 1 out of 16), pulmonary nodules in 6.25% (n = 16, 1 out of 16), both cavitations and nodule in 6.25% (n = 16, 1 out of 16), pleural effusion in 12.5% (n = 16, 2 out of 16), and pneumothorax and bullas in 12.5% patients (n = 16, 2 out of 16). Patel et al.[7] reported pulmonary involvement in 8.6% (n = 187, 16 out of 187) of C-ROCM patients. Sebastian et al.[43] reported bilateral nonlobar ground-glass opacities with septal thickening in 66.6% (N = 3, 2 out of 3); fibroparenchymal scarring in 33.3% (N = 3, 2 out of 3); subpleural fibrotic bands in the left lower lobe in 33.3% (N = 3, 2 out of 3), and pericardial effusion in 33.3% patients (N = 3, 2 out of 3). Dissemination of mucormycosis has been reported in 25% by Buil et al.[42] (N = 4, 1 out of 4) and 2.1% by Patel et al.[7] (n = 187, 4 out of 187) in their study population [Table 1].
Details of COVID-19 laboratory parameter
Very few studies reported laboratory parameters in the C-ROCM cases. Gangneux et al.[41] in their study population of invasive fungal infection reported that 64.2% (n = 57) patients presented lymphopenia at the time of first presentation. Bayram et al.[14] in their study population of C-ROCM patients reported that there was increased level of D-dimer (mean 1362.4 ± 468.9 μg/L, n = 11).
Microbiological profile of COVID-19–associated rhino-orbito-cerebral mucormycosis
Rhizopus species were the most common fungal species identified among positive fungal culture (7 studies,[27,29,31,36,40,42,43] prevalence 0.571, 95% C.I. 0.3–0.84). Mixed fungal growth was seen in 8.1% growth (6 studies,[6,7,26,38,42,43] prevalence 0.081, 95% C.I. −0.007–0.169). Bacterial coinfection has been reported by 2 studies[26,40] (prevalence 0.316, 95% C.I. 0.053–0.580).
Association of risk factor/co-morbidity for the occurrence of COVID-19–associated rhino-orbito-cerebral mucormycosis
Comorbidity
Diabetes mellitus
Diabetes is one of the most important comorbidities among C-ROCM patients. Pooled proportion of diabetes among C-ROCM patients were 0.79 (19 studies,[6,7,14,25,26,27,28,29,31,32,33,34,35,37,39,40,41,42,43] 95% C.I. 0.686–0.895). Mean duration of diabetes at the time of diagnosis of C-ROCM was 5.9 year as reported by Sen et al.[26] (n = 6) while 4.4 years by Ravani et al.[30] (n = 31) and 12.1 years by Bayram et al.[14] n = 11). Mean HBA1C at time of presentation was varied as reported by different authors (7–15 mg/dl by Satish et al.,[24] n = 15; 6.5 mg/dl by Sharma et al.,[28] n = 25; 7.57 mg/dl by Ravani et al.,[30] n = 31; 9.7 mg/dl by Fouad et al.,[37] n = 12; and 10.2 gm/dl by Arjun et al.,[34] n = 12). Average fasting blood sugar at presentation is reported by single study[26] as 222.5 mg/dl (n = 6).
Other comorbidities
Apart from DM, other comorbidities that were prevalent among C-ROCM population were hypertension (12 studies,[14,25,26,28,29,34,35,39,40,41,43] prevalence 0.435, 95% C.I. 0.340–0.530), chronic kidney disease (9 studies,[7,14,25,28,30,34,35,37,43] prevalence 0.114, 95% C.I. 0.032–0.196), hematological malignancy (5 studies,[7,14,37,39,42] prevalence 0.066, 95% C.I. −0.012–0.144), coronary artery disease (3 studies,[14,26,43] prevalence 0.235, 95% C.I. 0.052–0.419), acute renal failure (3 studies,[14,28,43] prevalence 0.058, 95% C.I. −0.016–0.132) and ischemic heart disease (3 studies,[25,35,37] prevalence 0.125, 95% C.I. −0.01–0.26).
Other rare comorbidities reported were atrial fibrillation (single study,[14] prevalence 9%, total population [n = 11]), chronic renal failure 36% (single study,[14] n = 11), hypothyroidism 10% (single study,[25] n = 10), hyperthyroidism 9% (single study,[14] n = 11), chronic liver disease 10% (single study,[25] n = 10), chronic obstructive pulmonary disease 9% (single study,[14] n = 11), and obesity 25% (single study,[42] n = 4).
Risk factors of C-ROCM
A significant proportion of C-ROCM patients in our study population received corticosteroid therapy as part of COVID-19 treatment protocol (proportion 0.857, 14 studies,[6,7,14,25,26,27,28,29,32,33,34,39,42,43] 95% C.I. 0.784–0.930). Mean duration of steroid use related to COVID-19 was reported by single study[14] (n = 11) as 8 days while another study[32] reported to be average 10–14 days (n = 25). Total corticosteroid dose equivalent received due to COVID-19 was reported to be 84 mg (cumulative dose of dexamethasone equivalent = 84 mg [18–1343 mg]) by single study[7] (n = 187).
Few studies reported use of mechanical ventilation as another risk factor. Pooled data showed that proportion of patients received mechanical ventilation as part of COVID-19 treatment protocol to be 0.454 (4 studies,[27,29,32,43] 95% C.I. −0.048–0.956). Similarly, use of supplemental oxygen has been reported as another risk factor by Mishra et al.[25] (40% patients, n = 10) and Arjun et al.[34] (80% patients, n = 10). Median worst PaO2/FiO2 for each patient was reported to be 124 (range 57–476) in the population as reported by Rabagliati et al. in their study population (N = 16, single study[40] ).
Arjun et al.[34] also reported use of broad-spectrum antibiotic as risk factor of occurrence of C-ROCM in their study population (90%, n = 10). No data were available regarding use of iron chelating in any of the included studies.
Intervention and outcomes analysis
Antifungal therapy
Amphotericin B was the mainstay of systematic as well as local antifungal therapy used in all studies. Pooled result showed that the proportion of C-ROCM patients receiving amphotericin B was 0.930 (14 studies,[6,7,14,25,26,27,28,29,30,31,32,34,35,39,42,43] 95% C.I. 0.873–0.987) among which proportion of liposomal amphotericin B given was 0.902 (12 studies,[6,7,14,25,26,27,28,29,30,31,34,42,43] 95% C.I. 0.825–0.978). Apart from intravenous form, proportion of patients receiving intraorbital amphotericin B was 0.506 (4 studies,[14,26,28,31] 95% C.I. −0.048–1.062), by intravitreal route in 54.5% of patients (in patients with with C-ROCM associated panophthalmitis, single study[14] ). Voriconazole (2 studies,[26,43] proportion 0.206, 95% C.I. −0.054–0.467) and posaconazole were another antifungals used in the management of ROCM cases although very few studies reported.
Other concomitant therapeutic treatments given were broad-spectrum antibiotics (100% patients single study,[43] n = 3), tocilizumab (10% cases, 1 study,[25] n = 10), vasopressor (100% patients, single study,[43] n = 3) and intravenous (I.V.) dexamethasone (2 studies[14,27] 100% patients, n = 21).
Surgical treatment
In C-ROCM cases, 70.9% cases were required mechanical debridement of para nasal sinus (13 studies,[14,24,25,26,27,28,29,30,32,34,35,37,43] proportion 0.709, 95% C.I. 0.587–0.832) while “functional endoscopic sinus surgery” was possible in 71.2% cases (4 studies,[6,25,26,27] proportion 0.712, 95% C.I. 0.416–1.007). Orbital decompression was done in 10% cases[25] (single study, n = 10) while exenteration was done in 21.2% patients (10 studies,[6,24,25,26,27,28,29,31,32,37] proportion 0.212, 95% C.I. 0.092–0.333). Other surgeries performed were maxillectomy in 34.6% patients (3 studies,[6,25,27] n = 37, 95% C.I. 0.004–0.688), ethmoidectomy in 10% (single study,[25] n = 10), and craniotomy in 10% patients (single study,[25] n = 10).
Outcome analysis
Overall treatment success was seen in 60.8% patients [15 studies,[6,7,14,24,25,27,28,29,34,35,37,39,42,43] 95% C.I. 0.470–0.745, Figure 2a], while continued intracranial spread was seen despite treatment in 42.8% ROCM patients (13 studies,[6,7,14,24,25,27,28,29,32,35,37,39] 95% C.I. 0.297–0.560). Treatment failure/mortality was seen in 34.4% of cases [15 studies,[6,7,14,24,25,26,27,28,29,34,35,37,39,42,43] 95% C.I. 0.205–0.483, Figure 3a], with mean time to death found to be 19 days (3 studies,[29,35,42] n = 16, 95% C.I. 3–35 days).
Figure 2.
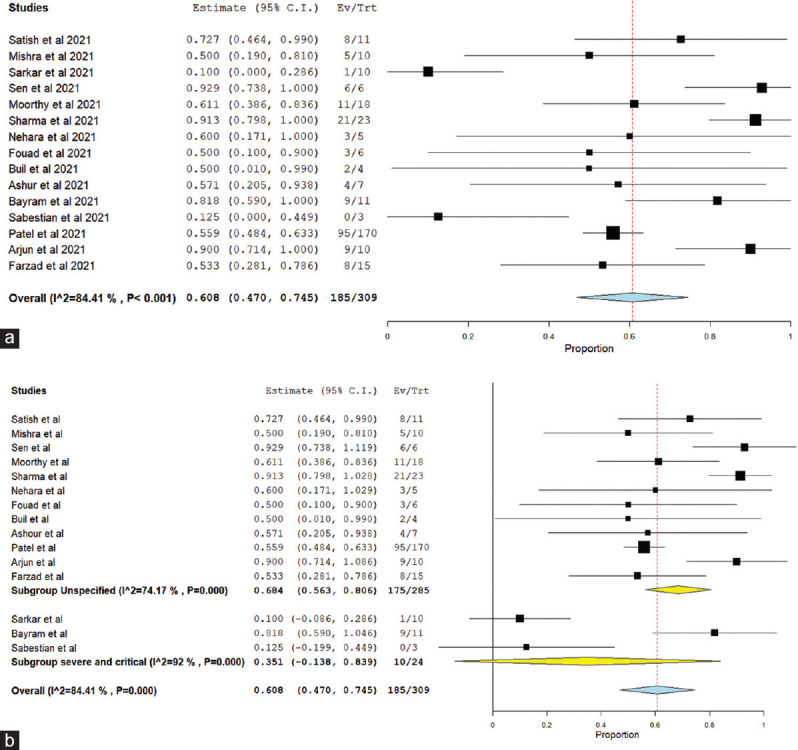
(a) Treatment success in C-ROCM population, (b) subgroup analysis of treatment success in C-ROCM patients based on severity of COVID-19
Figure 3.
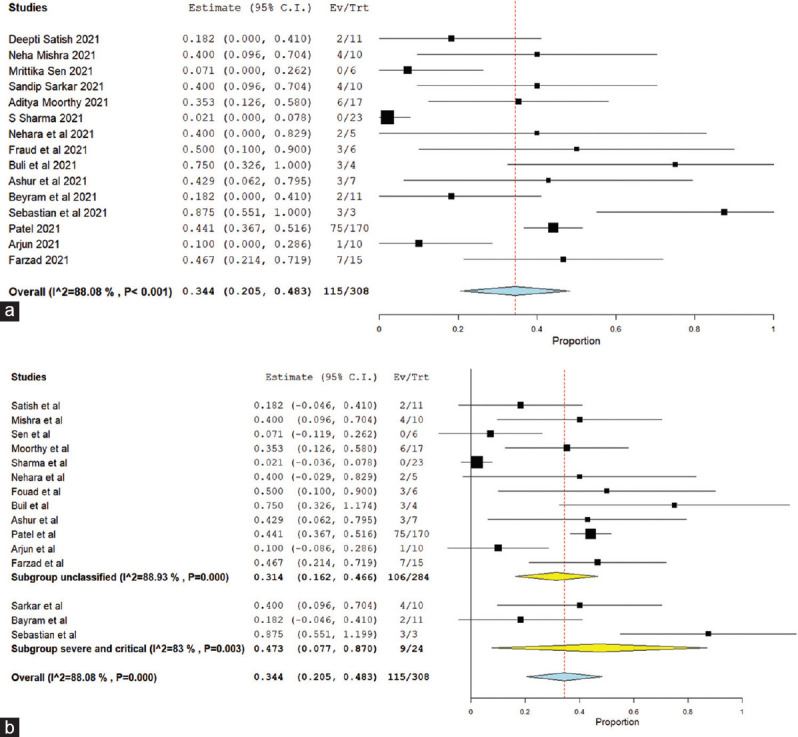
(a) Treatment failure/mortality in C-ROCM population, (b) subgroup analysis treatment failure/mortality based on severity of COVID-19
In the pooled result, high heterogeneity was seen while analyzing “treatment success” and “treatment failure” as indicated by I2 84% and 88% respectively [Figure 2a and Figure 3a]. To explore the etiology of “high heterogeneity,” we conducted “subgroup analysis” on the basis of “studies including “only severe and critical COVID-19 population” versus studies including “patients of all severity category of COVID-19” and study design (prospective vs. retrospective study). The high heterogeneity was not explained by subgroup analysis on the basis of COVID-19 severity [Figures 2b and 3b]. However, while doing subgroup analysis based on study design (prospective vs. retrospective study), very less heterogeneity was seen among prospective studies as compared to retrospective studies both in mortality and treatment success [Supplementary Figures 6 (307.5KB, tif) and 7 (308.9KB, tif) ].
Association between occurrence of C-ROCM and diabetic control
Prevalence of diabetes among C-ROCM cases was found to be 23.1% in controlled versus 68.5% in uncontrolled DM patients (11 studies,[6,14,26,26,27,28,29,31,32,37,42,43] OR 0.150, 95% C.I. 0.041–0.544, I2 = 64.5%, random-effect model, P = 0.001), indicating significant association between uncontrolled DM with C-ROCM patients [Figure 4].
Figure 4.

Association of controlled versus uncontrolled DM for occurrence of C-ROCM
Association between C-ROCM and COVID-19 severity
No significant difference was found with regard to occurrence of C-ROCM and COVID-19 severity (nonsevere vs. severe-to-critical COVID-19, 8 studies,[24,25,27,28,29,38,39,42] OR 0.930, 95% C.I. 0.212–4.087, I2 = 74%, random-effect model, P = 0.923) [Supplementary Figure 8 (189.3KB, tif) ].
Meta regression
We evaluated the effect of different covariates on the incidence of occurrence of C-ROCM associated mortality in “univariate meta regression model.” Covariates that were reported in 10 or more studies were evaluated. The covariates evaluated were diabetes, age, male sex, liposomal amphotericin B treatment, and mechanical debridement. Among all these, only mechanical debridement has significant impact on mortality (i.e., protective effect as indicated by negative correlation, −0.004 P = 0.002) [Supplementary Table 3 and Supplementary Figure 9 (127.1KB, tif) ]. All other covariates did not show significant correlation with ROCM-associated mortality.
Supplementary Table 3.
Univariate meta regression to evaluate the association between mortality and different covariates
| Parameter | Number of studies | Intercept parameters | Covariate parameters | Overall model omnibus P | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Coefficient | P | Coefficient | Confidence interval | SE | P | ||||
|
| |||||||||
| Lower | Upper | ||||||||
| Age | 13[2,3,4,5,7,11,12,15,17,19,21,22,23] | 0.593 | 0.301 | −0.005 | −0.024 | 0.015 | 0.010 | 0.629 | 0.629 |
| Male sex | 13[2,3,4,5,6,7,11,12,15,17,19,21,22] | 0.251 | 0.363 | 0.001 | −0.006 | 0.008 | 0.003 | 0.800 | 0.800 |
| DM | 14[2,3,4,5,6,7,11,12,15,17,19,22,23] | 0.068 | 0.858 | 0.003 | −0.006 | 0.011 | 0.004 | 0.557 | 0.557 |
| Liposomal amphotericin B therapy | 10[2,3,4,5,6,5,7,11,17,19,22,23] | 0.727 | 0.144 | −0.005 | −0.016 | 0.006 | 0.005 | 0.368 | 0.368 |
| Debridement | 10[1,2,3,4,6,7,12,15,17,22,23] | 0.548 | <0.001 | −0.004 | −0.007 | −0.002 | 0.001 | 0.002 | 0.002** |
*Statistically significant. SE=Standard error
Discussion
This systematic review and meta-analysis provides the global scenario of C-ROCM, its epidemiology, clinical presentation, and microbiological spectrum, which became a matter of concern due to sudden rise of mucormycosis/black fungus cases in the whole world parallel to ongoing COVID-19 pandemic.
Our meta-analysis showed that occurrence C-ROCM was more prevalent in elderly population with a mean age of presentation of 54.6 years and male gender being commonly affected. The lag period between COVID-19 diagnosis and diagnosis of ROCM was found to be average of 20 days (95% C.I. 15–24 days).
The most common presenting feature in C-ROCM was proptosis (60.6%), lid edema (60.7%), ophthalmoplegia (57.3%), loss of vision (53.7%), facial edema (34.7%), and ptosis (4 studies, 72.7%). Involvement of palate was not uncommon (35.6%). Rare occurrence of panophthalmitis (n = 4/7) and endophthalmitis[14,35] (6/11) is also being reported as sight-threatening complication though in very few studies. Although intracranial dissemination was frequently seen owing to the contiguous spread, pulmonary involvement[7,40,42,43] (21/210) or dissemination to other organs was very rare[7,42] (5/191).
CT scan and MRI remain the most common radiological tool for diagnosis, evaluation of the extent of disease, and monitoring of response to treatment.[6,26,28] Rhino-orbital involvement was the most common (70.8%) presentation followed by sinonasal (54.9%) and cerebral involvement (42.8%). Cavernous sinus involvement was seen in 40.7% patients. Ethmoid sinus was the single most common paranasal sinus involved[14,28,30,34] (80% by Arjun et al.[34] [n = 10], 90.9% by Bayram et al.[14] [n = 11], and 100% involvement reported by Sharma et al.[28] [n = 23]).
Rhizopus species were the most common species identified (57.1%) in total ROCM patients in our meta-analysis.
Diabetes was the most common comorbid condition in C-ROCM patients (79%). After diabetes, the most common associated comorbidities found among C-ROCM patients were hypertension (43.5%), coronary artery disease (23.5%), ischemic heart disease (12.5%), chronic kidney disease (11.4%), and hematological malignancy (6.6%).
Among other risk factors, 85.7% C-ROCM cases received corticosteroid; 12/20, i.e., 61%, received supplemental oxygen therapy;[25,34] and 14/43, i.e., 45.4%, also received mechanical ventilation as part of COVID-19 therapy. One hypothesis says, increased use of corticosteroid as a part of COVID-19 treatment protocol served as exaggerating factor for glucose homeostasis which act as predisposing condition for the patients to opportunistic fungal infection.[6,44,45,46] However, the dose of corticosteroid used as part of COVID-19 treatment protocol is not reported in maximum of the study. Use of broad-spectrum antibiotic as the risk factor is also reported by single study.[34]
In our meta-analysis, significant association (P = 0.001) was found between uncontrolled DM (68.5%) with C-ROCM patients as compared to controlled diabetes (23.1%). However, no association was seen between occurrence of COVID-19 severity and C-ROCM. Similarly, no significant difference was seen with respect to occurrence of C-ROCM among concurrent COVID-19 population versus post-COVID-19 population (OR 0.306, 95% C.I. 0.042–2.246, P = 0.244).
Alteration of iron metabolism occurs in severe COVID-19[44] and iron overload (excessive free iron seen in acidosis) are other established risk factors for opportunistic fungal infection.[47,48] However, none of the included in our meta-analysis has reported or gave specific consideration in this aspect.
Antifungal therapy was the mainstay of treatment both as presumptive and postdiagnosis therapy, and I.V. amphotericin B (93%) has been started as definitive treatment in all of the included studies in our meta-analysis, while liposomal amphotericin B was given in 90% of patients. Amphotericin B was given by intraorbital route in case of orbital involvement by few authors (16/42, 50.5%) and intravitreal route in case of panophthalmitis (6/11, 54.5%, single study[14] ); however, details of the antifungal susceptibility are not reported in maximum of the studies. The impact of liposomal amphotericin B needs further evaluation and newer antifungal option has to be taken into consideration.[49,50,51,52,53,54,55,56,57,58,59,60]
Mechanical debridement was the commonly performed surgical procedure (70.9%) followed by maxillectomy (15/37, 34.6%), while exenteration was required in 21.2% patients. Less frequently performed surgeries were ethmoidectomy (1/10, i.e., 10%, single study[25] ), orbital decompression (1/10, i.e., 10%, single study[25] ), and craniotomy (1/10, i.e., 10%, single study[25] ). Although complete removal of infected tissue by surgical debridement at the earliest in addition to antifungal therapy is recommended as definitive treatment modality in patients with C-ROCM, it is not possible in all the cases, especially in patients with advanced disease at presentation or due to fitness issue for general anesthesia in maximum of the studies, leading to higher mortality rate.
Regarding the treatment outcome, treatment success was seen in 59.8% patients while continued intracranial spread was seen in 30.9% and treatment failure/mortality was seen in as high as 37.3% of cases despite treatment. While exploring the source of high heterogeneity, in treatment success and treatment failure, no difference was seen while doing subgroup analysis based on studies including only severe and critical versus studies including all category COVID-19 population. On the other hand, study design (prospective vs. retrospective study) seems to explain high heterogeneity in our meta-analysis. On univariate analysis for possible correlation of comorbidity and risk factors, mechanical debridement shows significant impact on mortality in C-ROCM patients (i.e., protective effect as indicated by coefficient − 0.004, P = 0.002). Again requirement of only debridement indirectly indicates a less severe form of disease without intracranial extension and without requiring other more invasive therapy. The protective effect of debridement may thus be explained in a dual manner.
Strength and weakness
This current meta-analysis provides the most recent and largest overview of the C-ROCM worldwide, which is the first meta-analysis published in this regard also. However, limited data regarding laboratory parameter of C-ROCM patients are available.
Conclusion
Our meta-analysis showed that occurrence C-ROCM was more prevalent in elderly population and among males with the most common presenting feature being loss of vision, proptosis, lid edema, ophthalmoplegia, facial edema, and ptosis. Although intracranial dissemination was frequently seen, pulmonary involvement or dissemination to other organs was very rare. CT scan and MRI remain the most common radiological tool for diagnosis and for evaluation of the extent of disease. KOH positivity for Mucor species was seen in 62% patients while 59.3% culture was positive for Mucor, Rhizopus species was the most common species to be identified (44.8%). 67.7% of the population had concomitant diabetes and 85.7% cases received corticosteroid as part of COVID-19 therapy. Compared to controlled DM, the incidence of ROCM was higher among patients with uncontrolled DM. However, no association was seen between COVID-19 severity and occurrence of ROCM. Amphotericin B, more specifically liposomal amphotericin B, was a mainstay of therapy along with other surgical management options. However, despite treatment, mortality/treatment failure was seen in 37.3% of cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Preferred Reporting Items for Systematic Review and Meta-Analysis flow diagram of included studies
Publication bias Funnel plot showing publication bias in case of mortality associated with COVID-19associated rhino-orbital-cerebral mucormycosis
Sensitivity analysis in terms of mean age of presentation by excluding Bayram et al. and Diwakar et al.
Mean duration between COVID-19 diagnosis and rhino-orbital-cerebral mucormycosis diagnosis
Comparison of incidence of C-ROCM between concurrent vs. post-COVID 19 patients
Mortality subgroup analysis prospective versus retrospective study design
Treatment success subgroup analysis prospective vs. retrospective study design
Occurrence of C-ROCM between non-severe vs. severe to critical COVID-19 population
Meta regression plot showing correlation of mechanical debridement with mortality due to C-ROCM
References
- 1.Baiou A, Elbuzidi AA, Bakdach D, Zaqout A, Alarbi KM, Bintaher AA, et al. Clinical characteristics and risk factors for the isolation of multi-drug-resistant Gram-negative bacteria from critically ill patients with COVID-19. J Hosp Infect. 2021;110:165–71. doi: 10.1016/j.jhin.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ripa M, Galli L, Poli A, Oltolini C, Spagnuolo V, Mastrangelo A, et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin Microbiol Infect. 2021;27:451–7. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaton RA, Gibbons CL, Cooper L, Malcolm W, McKinney R, Dundas S, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect. 2020;81:952–60. doi: 10.1016/j.jinf.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nucci M, Barreiros G, Guimarães LF, Deriquehem VA, Castiñeiras AC, Nouér SA. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64:152–6. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Arkel AL, Rijpstra TA, Belderbos HN, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–5. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids – An unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021;20:418–25. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–59. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Tawfiq JA, Alhumaid S, Alshukairi AN, Temsah MH, Barry M, Al Mutair A, et al. COVID-19 and mucormycosis superinfection: The perfect storm. Infection. 2021;49:833–53. doi: 10.1007/s15010-021-01670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PRISMA. [Last accessed on 2021 Aug 29]. Available from: http://prisma-statement.org/PRISMAStatement/
- 12.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–76. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therapeutics and COVID-19: Living Guideline. [Last accessed on 2021 Jul 26]. Available from: https://app.magicapp.org/#/guideline/nBkO1E .
- 14.Bayram N, Ozsaygılı C, Sav H, Tekin Y, Gundogan M, Pangal E, et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65:515–25. doi: 10.1007/s10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: A systematic review and meta-analysis. PLoS One. 2016;11:e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–3. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochrane Handbook for Systematic Reviews of Interventions. [Last accessed on 2018 Dec 07]. Available from: http://handbook .
- 20.Sarma P, Kaur H, Kumar H, Mahendru D, Avti P, Bhattacharyya A, et al. Virological and clinical cure in covid-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis. J Med Virol. 2020;92:776–85. doi: 10.1002/jmv.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons; 2019. [Last accessed on 2021 Aug 30]. Available from: https://training.cochrane.org/handbook . [Google Scholar]
- 23.British Society for Haematology. Cullis JO, Fitzsimons EJ, Griffiths WJ, Tsochatzis E, Thomas DW. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331–40. doi: 10.1111/bjh.15166. [DOI] [PubMed] [Google Scholar]
- 24.Satish D, Joy D, Ross A, Balasubramanya Mucormycosis coinfection associated with global COVID-19: A case series from India. Int J Otorhinolaryngol Head Neck Surg. 2021;7:815–20. [Google Scholar]
- 25.Mishra N, Mutya VS, Thomas A, Rai G, Reddy B, Mohanan AA, et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7:867–70. [Google Scholar]
- 26.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: A tale of two pathogens. Indian J Ophthalmol. 2021;69:244–52. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–4. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135:442–7. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nehara HR, Puri I, Singhal V, Ih S, Bishnoi BR, Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J Med Microbiol. 2021;39:380–3. doi: 10.1016/j.ijmmb.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–8. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diwakar J, Samaddar A, Konar SK, Bhat MD, Manuel E, Hb V, et al. First Report of COVID-19-Associated Rhino-Orbito-Cerebral Mucormycosis in Pediatric Patients with Type 1 Diabetes Mellitus. Rochester, NY: Social Science Research Network; [Last accessed on 2021 Jul 24]. Available from: https://papers.ssrn.com/abstract=3863080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi AR, Muthe MM, Patankar SH, Athawale A, Achhapalia Y. CT and MRI findings of invasive mucormycosis in the setting of COVID-19: Experience from a single center in India. AJR Am J Roentgenol. 2021;217:1431–2. doi: 10.2214/AJR.21.26205. [DOI] [PubMed] [Google Scholar]
- 33.Muley P, Chitguppi R, Jambure R. Proposal for a Novel Grading System for Rhino-Maxillary Mucormycosis Based on the Analysis of 30 Cases. Rochester, NY: Social Science Research Network; 2021. [Last accessed on 2021 Jul 24]. Available from: https://papers.ssrn.com/abstract=3854282 . [Google Scholar]
- 34.Arjun R, Felix V, Niyas VKM, Kumar MAS, Krishnan RB, Mohan V, et al. COVID-19 associated Rhino-orbital Mucormycosis: a Single Centre Experience of Ten Cases. QJM. 2021 Jun 28;:hcab176. doi: 10.1093/qjmed/hcab176. doi: 10.1093/qjmed/hcab176. Epub ahead of print. PMID: 34181023. [DOI] [PubMed] [Google Scholar]
- 35.Ashour MM, Abdelaziz TT, Ashour DM, Askoura A, Saleh MI, Mahmoud MS. Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: A case series and a review of literature. J Neuroradiol. 2021;48:319–24. doi: 10.1016/j.neurad.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismaiel WF, Abdelazim MH, Eldsoky I, Ibrahim AA, Alsobky ME, Zafan E, et al. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. Am J Otolaryngol. 2021;42:103080. doi: 10.1016/j.amjoto.2021.103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fouad YA, Abdelaziz TT, Askoura A, Saleh MI, Mahmoud MS, Ashour DM, et al. Spike in rhino-orbital-cerebral mucormycosis cases presenting to a tertiary care center during the COVID-19 pandemic. Front Med (Lausanne) 2021;8:645270. doi: 10.3389/fmed.2021.645270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Kholy NA, El-Fattah AM, Khafagy YW. Invasive fungal sinusitis in post COVID-19 patients: A new clinical entity. Laryngoscope. 2021;131:2652–8. doi: 10.1002/lary.29632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pakdel F, Ahmadikia K, Salehi M, Tabari A, Jafari R, Mehrparvar G, et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses. 2021;64:1238–52. doi: 10.1111/myc.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, Garcia P. COVID-19-associated mold infection in critically Ill patients, Chile. Emerg Infect Dis. 2021;27:1454–6. doi: 10.3201/eid2705.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gangneux JP, Dannaoui E, Fekkar A, Luyt CE, Botterel F, de Prost N, et al. High Prevalence of Fungal Infections in Mechanically Ventilated COVID-19 Patients in the ICU: The French Multicenter MYCOVID Study. Rochester, NY: Social Science Research Network; 2021. [Last accessed on 2021 Jul 24]. Available from: https://papers.ssrn.com/abstract=3858565 . [Google Scholar]
- 42.Buil JB, van Zanten AR, Bentvelsen RG, Rijpstra TA, Goorhuis B, van der Voort S, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Euro Surveill. 2021;26:2100510. doi: 10.2807/1560-7917.ES.2021.26.23.2100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sebastian SK, Kumar VB, Gupta M, Sharma Y. Covid Assossiated Invasive Fungal Sinusitis. Indian Journal of Otolaryngology and Head and Neck Surgery. 2021 Feb;:1–4. doi: 10.1007/s12070-021-02471-6. DOI: 10.1007/s12070-021-02471-6. PMID: 33649716; PMCID: PMC7905418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: The perfect storm for mucormycosis. J Fungi (Basel) 2021;7:298. doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarma P, Bhattacharyya A, Kaur H, Prajapat M, Prakash A, Kumar S, et al. Efficacy and safety of steroid therapy in COVID-19: A rapid systematic review and Meta-analysis. Indian J Pharmacol. 2020;52:535–50. doi: 10.4103/ijp.ijp_1146_20. doi: 10.4103/ijp.ijp_1146_20. PMID: 33666200; PMCID: PMC8092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattacharyya A, Sarma P, Sharma DJ, Das KK, Kaur H, Prajapat M, et al. Rhino-orbital-cerebral-mucormycosis in COVID-19: A systematic review. Indian J Pharmacol. 2021;53:317–27. doi: 10.4103/ijp.ijp_419_21. doi: 10.4103/ijp.ijp_419_21. PMID: 34414911; PMCID: PMC8411962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edeas M, Saleh J, Peyssonnaux C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–5. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaushal K, Kaur H, Sarma P, Bhattacharyya A, Sharma DJ, Prajapat M, et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care. 2022;67:172–81. doi: 10.1016/j.jcrc.2021.09.023. doi: 10.1016/j.jcrc.2021.09.023. Epub 2021 Nov 20. PMID: 34808527; PMCID: PMC8604557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prajapat M, Sarma P, Shekhar N, Prakash A, Avti P, Bhattacharyya A, et al. Update on the target structures of SARS-CoV-2: A systematic review. Indian J Pharmacol. 2020;52:142–9. doi: 10.4103/ijp.IJP_338_20. doi: 10.4103/ijp.IJP_338_20. Epub 2020 Jun 3. PMID: 32565603; PMCID: PMC7282679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarma P, Bhattacharyya A, Prakash A, Kaur H, Prajapat M, Borah M, et al. Yogic Neti-Kriya Using Povidone Iodine: Can it have a Preventive Role Against SARS-CoV-2 Infection Gateway? Indian J Otolaryngol Head Neck Surg. 2021:1–7. doi: 10.1007/s12070-021-02885-2. doi: 10.1007/s12070-021-02885-2. Epub ahead of print. PMID: 34692450; PMCID: PMC8520578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prajapat M, Shekhar N, Sarma P, Avti P, Singh S, Kaur H, et al. Virtual screening and molecular dynamics study of approved drugs as inhibitors of spike protein S1 domain and ACE2 interaction in SARS-CoV-2. J Mol Graph Model. 2020;101:107716. doi: 10.1016/j.jmgm.2020.107716. doi: 10.1016/j.jmgm.2020.107716. Epub 2020 Aug 21. PMID: 32866780; PMCID: PMC7442136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarma P, Shekhar N, Prajapat M, Avti P, Kaur H, Kumar S, et al. In-silico homology assisted identification of inhibitor of RNA binding against 2019-nCoV N-protein (N terminal domain) J Biomol Struct Dyn. 2021;39:2724–32. doi: 10.1080/07391102.2020.1753580. doi: 10.1080/07391102.2020.1753580. Epub 2020 May 18. PMID: 32266867; PMCID: PMC7256351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shekhar N, Sarma P, Prajapat M, Avti P, Kaur H, Raja A, et al. In Silico Structure-Based Repositioning of Approved Drugs for Spike Glycoprotein S2 Domain Fusion Peptide of SARS-CoV-2: Rationale from Molecular Dynamics and Binding Free Energy Calculations. mSystems. 2020;5:e00382–20. doi: 10.1128/mSystems.00382-20. doi: 10.1128/mSystems.00382-20. PMID: 32963099; PMCID: PMC7511214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avti P, Chauhan A, Shekhar N, Prajapat M, Sarma P, Kaur H, et al. Computational basis of SARS-CoV 2 main protease inhibition: an insight from molecular dynamics simulation based findings. J Biomol Struct Dyn. 2021:1–11. doi: 10.1080/07391102.2021.1922310. doi: 10.1080/07391102.2021.1922310. Epub ahead of print. PMID: 33998950; PMCID: PMC8127165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chauhan A, Avti PK, Shekhar N, Prajapat M, Sarma P, Sangwan N, et al. An insight into the simulation directed understanding of the mechanism in SARS CoV-2 N-CTD, dimer integrity, and RNA-binding: Identifying potential antiviral inhibitors. J Biomol Struct Dyn. 2021:1–13. doi: 10.1080/07391102.2021.1996463. doi: 10.1080/07391102.2021.1996463. Epub ahead of print. PMID: 34751101. [DOI] [PubMed] [Google Scholar]
- 56.Kaur H, Shekhar N, Sharma S, Sarma P, Prakash A, Medhi B. Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes. Pharmacol Rep. 2021;73:736–49. doi: 10.1007/s43440-020-00195-y. doi: 10.1007/s43440-020-00195-y. Epub 2021 Jan 3. PMID: 33389725; PMCID: PMC7778723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarma P, Prajapat M, Avti P, Kaur H, Kumar S, Medhi B. Therapeutic options for the treatment of 2019-novel coronavirus: An evidence-based approach. Indian J Pharmacol. 2020;52:1–5. doi: 10.4103/ijp.IJP_119_20. doi: 10.4103/ijp.IJP_119_20. Epub 2020 Mar 11. PMID: 32201439; PMCID: PMC7074432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaur H, Kaur M, Bhattacharyya A, Prajapat M, Thota P, Sarma P, et al. Indian contribution toward biomedical research and development in COVID-19: A systematic review. Indian J Pharmacol. 2021;53:63–72. doi: 10.4103/ijp.ijp_168_21. doi: 10.4103/ijp.ijp_168_21. PMID: 33976001; PMCID: PMC8216129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhattacharyya A, Kumar S, Sarma P, Kaur H, Prajapat M, Shekhar N, et al. Safety and efficacy of lopinavir/ritonavir combination in COVID-19: A systematic review, meta-analysis, and meta-regression analysis. Indian J Pharmacol. 2020;52:313–23. doi: 10.4103/ijp.IJP_627_20. doi: 10.4103/ijp.IJP_627_20. PMID: 33078733; PMCID: PMC7722914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prakash A, Kaur S, Kaur C, Prabha PK, Bhatacharya A, Sarma P, et al. Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: A systematic review. Indian J Pharmacol. 2021;53:236–43. doi: 10.4103/ijp.ijp_382_21. doi: 10.4103/ijp.ijp_382_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- 1.Satish D, Joy D, Ross A, Balasubramanya Mucormycosis coinfection associated with global COVID-19: A case series from India. Int J Otorhinolaryngol Head Neck Surg. 2021;7:815–20. [Google Scholar]
- 2.Mishra N, Mutya VS, Thomas A, Girish Rai G, Reddy B, Mohanan AA, et al. A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7:867–70. [Google Scholar]
- 3.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: A tale of two pathogens. Indian J Ophthalmol. 2021;69:244–52. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002–4. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids – An unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021;20:1–8. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135:442–7. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nehara HR, Puri I, Singhal V, Ih S, Bishnoi BR, Sirohi P. Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the north-western part of India. Indian J Med Microbiol. 2021;39:380–3. doi: 10.1016/j.ijmmb.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69:1563–8. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diwakar J, Samaddar A, Konar SK, Dattatraya M, Emma M, Veenakumari BN, et al. First Report of COVID-19-Associated Rhino-Orbito-Cerebral Mucormycosis in Pediatric Patients with Type 1 Diabetes Mellitus. Rochester, NY: Social Science Research Network; 2021. [Last accessed on 2021 Jul 24]. Available from: https://papers.ssrn.com/abstract=3863080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangneux JP, Dannaoui E, Fekkar A, Edouard LC, Francoise B, Nicolas P, et al. High Prevalence of Fungal Infections in Mechanically Ventilated COVID-19 Patients in the ICU: The French Multicenter MYCOVID Study. Rochester, NY: Social Science Research Network; 2021. [Last accessed on 2021 Jul 24]. Available from: https://papers.ssrn.com/abstract=3858565 . [Google Scholar]
- 11.Buil JB, van Zanten AR, Bentvelsen RG, Rijpstra TA, Goorhuis B, van der Voort S, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Euro Surveill. 2021;26:2100510. doi: 10.2807/1560-7917.ES.2021.26.23.2100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouad YA, Abdelaziz TT, Askoura A, Saleh MI, Mahmoud MS, Ashour DM, et al. Spike in rhino-orbital-cerebral mucormycosis cases presenting to a tertiary care center during the COVID-19 pandemic. Front Med (Lausanne) 2021;8:645270. doi: 10.3389/fmed.2021.645270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Kholy NA, El-Fattah AM, Khafagy YW. Invasive fungal sinusitis in post COVID-19 patients: A new clinical entity. Laryngoscope. 2021;131:2652–8. doi: 10.1002/lary.29632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismaiel WF, Abdelazim MH, Eldsoky I, Ibrahim AA, Alsobky ME, Zafan E, et al. The impact of COVID-19 outbreak on the incidence of acute invasive fungal rhinosinusitis. Am J Otolaryngol. 2021;42:103080. doi: 10.1016/j.amjoto.2021.103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashour MM, Abdelaziz TT, Ashour DM, Askoura A, Saleh MI, Mahmoud MS. Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: A case series and a review of literature. J Neuroradiol. 2021;48:319–24. doi: 10.1016/j.neurad.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, Garcia P. COVID-19-associated mold infection in critically Ill patients, Chile. Emerg Infect Dis. 2021;27:1454–6. doi: 10.3201/eid2705.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayram N, Ozsaygılı C, Sav H, Tekin Y, Gundogan M, Pangal E, et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65:515–25. doi: 10.1007/s10384-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi AR, Muthe MM, Patankar SH, Athawale A, Achhapalia Y. CT and MRI findings of invasive mucormycosis in the setting of COVID-19: Experience from a single center in India. AJR Am J Roentgenol. 2021;217:1431–2. doi: 10.2214/AJR.21.26205. [DOI] [PubMed] [Google Scholar]
- 19.Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–59. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muley P, Chitguppi R, Jambure R. Proposal for a Novel Grading System for Rhino-Maxillary Mucormycosis Based on the Analysis of 30 Cases. Rochester, NY: Social Science Research Network; 2021. [Last accessed on 2021 Jul 24]. Available from: https://papers.ssrn.com/abstract=3854282 . [Google Scholar]
- 21.Pakdel F, Ahmadikia K, Salehi M, Tabari A, Jafari R, Mehrparvar G, et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses. 2021;64:1238–52. doi: 10.1111/myc.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arjun R, Felix V, Niyas VKM, Kumar MAS, Krishnan RB, Mohan V, Ansar A, Gautaam S, Lalitha S, et al. COVID-19 associated Rhino-orbital Mucormycosis: a Single Centre Experience of Ten Cases. QJM. 2021 Jun 28;:hcab176. doi: 10.1093/qjmed/hcab176. doi: 10.1093/qjmed/hcab176. Epub ahead of print. PMID: 34181023. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian SK, Kumar VB, Gupta M, Sharma Y. Covid Assossiated Invasive Fungal Sinusitis. Indian Journal of Otolaryngology and Head and Neck Surgery. 2021 Feb;:1–4. doi: 10.1007/s12070-021-02471-6. DOI: 10.1007/s12070-021-02471-6. PMID: 33649716; PMCID: PMC7905418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Review and Meta-Analysis flow diagram of included studies
Publication bias Funnel plot showing publication bias in case of mortality associated with COVID-19associated rhino-orbital-cerebral mucormycosis
Sensitivity analysis in terms of mean age of presentation by excluding Bayram et al. and Diwakar et al.
Mean duration between COVID-19 diagnosis and rhino-orbital-cerebral mucormycosis diagnosis
Comparison of incidence of C-ROCM between concurrent vs. post-COVID 19 patients
Mortality subgroup analysis prospective versus retrospective study design
Treatment success subgroup analysis prospective vs. retrospective study design
Occurrence of C-ROCM between non-severe vs. severe to critical COVID-19 population
Meta regression plot showing correlation of mechanical debridement with mortality due to C-ROCM


