Abstract
OBJECTIVE:
Stress exacerbates the pathophysiology of major neurodegenerative disorders. In this study, the zebrafish (Danio rerio), the frequently used model for experimental studies of stress and other central nervous system disorders, was used to evaluate the anxiolytic potential of flavonoids, namely silibinin and naringenin on alleviating acute stress-induced anxiety.
MATERIALS AND METHODS:
A molecular docking study with Molegro Virtual Docker software was done to assess the binding potential of flavonoids on serotonin and dopamine receptors. To determine the bioactivity and investigate the toxicity of the flavonoids, silibinin, and naringenin, brine shrimp lethality assay, and an acute toxicity study was conducted according to Organization for Economic Co-operation and Development (OECD) Test Guideline 203. The effect of silibinin and naringenin was assessed using behavioral tasks such as the novel tank assay and the light-dark test on the zebrafish model of acute stress.
RESULTS:
Molecular docking studies showed a higher affinity of silibinin and naringenin for the serotonin and dopamine receptors. In comparison to the LC50 value, 13.15 μg/ml of the reference standard potassium dichromate, silibinin, and naringenin yielded higher LC50 values, 34.10 μg/ml and 91.33 μg/ml, respectively. The LC50 value of silibinin and naringenin was observed to be >100 mg/l from the acute toxicity study on adult zebrafish. After transferring to a novel tank, silibinin and naringenin-treated zebrafish groups were found to explore the upper level of the tank, similar to standard drugs, and spent a long time in the upper level of the tank compared to the control group (p < 0.01). Both silibinin and naringenin treatment group spent increased amounts of time in the tank's illuminated part in contrast to that of the dark side as evidenced by the number of zebrafish entering or remaining in the illuminated part of the tank through the light-dark test. Silibinin and naringenin treated groups were found to spend increased time in the light side significantly on the day 15th of evaluation as compared to the control group (p < 0.05 and p < 0.001, respectively).
CONCLUSION:
The flavonoids, silibinin, and naringenin were found to mitigate acute stress-induced anxiety, owing to their anxiolytic properties in the zebrafish model and may be explored as the potential therapeutic agents for treating anxiety.
Keywords: Anxiety, behaviour, flavonoids, stress, zebrafish
Introduction
The environment exposes all humans to a variety of stress triggers in their daily lives that may have both positive and negative effects on the physiology of the body, thus affect health in general. The coping mechanisms to combat stress may lead to improved memory, alertness, energy, and focus. However, mood, productivity, and quality of life are affected by chronic stress which ultimately leads to anxiety, depression, and other disorders.[1] In the recent past, owing to fast-paced lifestyles, chronic stress has been shown to cause an increase in the incidence of depression, anxiety, and other mood disorders.[2]
Only a few of the many pharmacologically active constituents found in nature are neuroactive. Flavonoids, the polyphenolic compounds are found abundantly in plants, cereals, teas, fruits, vegetables, and wines, making them essential components of a natural human diet. Flavonoids have a variety of pharmacological properties such as neuroprotection through the modulation of neurotransmitter release and stimulation of hippocampal neurogenesis.[3]
Furthermore, flavonoids may promote memory and learning by modulating certain signaling pathways and through blockade of neuroinflammation, thus preventing us from developing brain disorders such as Alzheimer's disease and Parkinsonism.[4]
Recent trend increases toward investigating the role of phytocomponents in improving memory, learning, and cognitive functions. Antioxidant properties of flavonoids' may be attributed to their neuroprotective properties. The neuroprotective action of flavonoids appears to be influenced by their bioavailability, particularly their presence in the brain in vivo.[5]
Silybum marianum (L.) Gaertn., with the common name Milk thistle belonging to the family Asteraceae, is a recognized traditional herb with a wide range of biological properties and has traditionally been used for detoxification and the treatment of certain liver diseases. Silymarin is the primary extract, containing silibinin (synonymous with silybin), the flavonolignan which is a mix of two diastereomers (A and B). Isosilibinin, dehydro silibinin, silidianin, silicristin, taxifolin, and quercetin are some of the other components of silymarin.[6] The emerging evidence from modern medicine has shown that silymarin and silibinin may be helpful in the treatment of hepatic disorders, hyperglycemia, mushroom poisoning, certain types of nephrotoxicity, neurodegenerative diseases, and a variety of cancers.[7]
Naringenin (4-oxo, 5,7-trihydroxy flavanone), a dietary flavonoid found in citrus fruit peels, has been shown to have several biological properties, including neuroprotective activity[8] and monoamine oxidase inhibitory activity.[9] Naringenin has already been evaluated pharmacologically as a potential antioxidant (acting directly or indirectly) through the modulation of oxidative cascades as well as neurodegenerative processes.[10]
Screening of potential drug candidates by using rodent experimental models for various central nervous system (CNS) diseases is cumbersome and expensive. The zebrafish (Danio rerio, small cyprinid fishes) has gained popularity in recent decades as an alternative model for screening novel compounds with potential for treating a variety of diseases due to their low maintenance costs, ease of handling, and rapid reproducing ability. Furthermore, zebrafish is found to have similar landmarks of neuroanatomy and neurotransmitter systems with mammals,[11,12] and they react predictably to anxiolytic and anxiogenic drugs while screening behavioral changes.[13,14,15]
To support the major objectives of the current work, we probed to investigate the potential neuropharmacological and toxicological effects of silibinin and naringenin on stress-induced anxiety in the zebrafish model and to determine their binding interactions by using docking analysis on the proposed targets (dopamine and serotonin receptors).
Materials and Methods
Brine shrimp lethality assay
The preliminary toxicological evaluation of silibinin and naringenin was carried out using the brine shrimp lethality assay, which was modified slightly from the previous method.[16]
Artemia salina (commonly known as Brine shrimp) is zooplankton that belongs to the genus Artemia of aquatic crustaceans and is used to feed larval fishes. In a conical vessel (separating funnel) containing seawater, cysts of A. salina (150 mg) were incubated to hatch. After 24 h, a 0.06% yeast solution was added to feed the larvae, which were then filled with seawater, and aeration was maintained continuously for 48 h. Active nauplii released from eggshells were collected from the hatching chamber after 48 h and used in the subsequent assay.
Using a Pasteur pipette, 10–15 nauplii were collected from the hatching chamber and placed in the 24 wellplates. Each well was filled with 0.5 ml of seawater with or without increasing concentrations of the flavonoids silibinin and naringenin ranging from 0.1 to 1000 μg/ml with the same range of concentrations of potassium dichromate that served as a positive control, were added and incubated for 24 h at RT. The number of surviving nauplii in each well was counted after 24 h. The best-fit line plot was used to calculate LC50 values from a graph of concentration versus percentage lethality. The following formula was applied to calculate the nauplii death percentage:
% Mortality = [Number of non-surviving nauplii/ (Number of non-surviving nauplii + Number of surviving nauplii)] X 100
Animals
Wild-type zebrafish (D. rerio) adults of both sexes were procured and acclimatized to laboratory conditions for 15 days in glass tanks with filters. The photoperiod was set for 12–16 h, and the temperature was kept between 21 and 25°C with constant aeration. Dry pellets of commercial fish food and active brine shrimp were provided in sufficient amounts twice daily. The proposal has been approved by the Institutional Animal Ethics Committee, and the approval number has been assigned as IAEC/XLIII/SRU424/2015.
Acute toxicity test (OECD test guideline-203)
Acute toxicity indicates the adverse effects of a substance produced by single or multiple exposures in a short period. Fish were fed twice a day until 24 h before the test began. At 24-h intervals, dissolved oxygen, pH value, and temperature were measured. A limit test at 100 mg of active ingredient per liter was carried out to arrive at a median lethal concentration of >100 mg/l. For the acute toxicity study, 10 fish per test concentration (100 mg/l) of silibinin and naringenin and a control group were used. The static method was used, in which the test system remained unchanged throughout the observation period (4 days). Fish were inspected at regular intervals at 24, 48, 72, and 96 h, and the zebrafish were considered dead when no visible movement was observed. During the observation period, visible abnormalities such as loss of equilibrium, respiratory function, pigmentation, swimming behavior, and other clinical signs were recorded to examine the signs of toxicity.
Neuropharmacological evaluation
Zebrafish were randomly divided into groups of ten fish per concentration. The effect of flavonoids on anxiety was investigated at concentrations of 0.1, 1, and 10 μM by immersion in a 3-liter dosing tank for 30 min and evaluated using a novel tank method and light-dark test after 2-min acute stress exposure using a fishnet to chase the fish. Fluoxetine and diazepam were used as reference standards and were dosed at 0.1, 1, and 10 μM. To reduce the stress of handling zebrafish, a semi-static-renewal procedure lasting 24 h was used. The control group was treated using similar procedures as adopted for the test system. Zebrafish was subjected to the drugs for 15 days, with major evaluations of behavioral parameters performed on days 7, 8, 14, and 15, that was manually analyzed from video footage recorded for 5 min by the observers who were blinded to the treatment.[17]
Novel tank test
The term “novelty” refers to a novel or unacquainted experience. The brain interprets novel conditions as stressful, eliciting anxiety-like behaviors due to a lack of previous experience. Novel tank diving assay (or open tank model) is a novel behavioral task used in basic neuroscience research to assess stress responses associated with novelty. Primarily, the test involves observing the fish's initial tendency of diving to the bottom of the new tank and gradually swimming to its upper level, thereby evaluating vertical exploratory behavior in a novel setting.[18]
This procedure was adapted from previously published reports that employed similar testing procedures.[13,17,18,19] After drug exposure, behavioral analysis was performed by transferring the fish from the housing tank to a novel environment, devoid of the drug(s). The apparatus consisted of a narrow, transparent tank that allowed for minimal, lateral movement while allowing for easy vertical and horizontal movement. The tank was separated virtually into nearly three equal horizontal portions by a dividing line on the outer walls of the tank (e.g., with a permanent marker). To enable observation and to minimize the impact of the surrounding area, the lateral and posterior sides were visually blocked using a self-adhesive film opaque in nature. For 3 min, a video was recorded and analyzed later manually.
The following endpoints were recorded: the time required to reach the tank's top portion (sec), the number of transitions or entries to the top portion of the tank (sec), the time consumed in tank's upper level (sec), the duration of freezing (sec), and the number of erratic movements.
Light-dark test
The scototaxis (dark/light preference) test has been put forward as another method for assessing anxiety type of behavior in various species of teleost. In comparison to the novel tank diving test, where the novelty of the environment serves as the primary aversive stimulus, the light-dark test is primarily driven by motivational conflict between approach and avoidance. It is a deceptively simple technique, parallel to the light-dark box of murine model, establishing preference by relying on the exploratory behavior of the fish in a black and white compartment of the tank.[20,21]
To conduct the light-dark test, the test tank was divided into two equally sized dark and white compartments, with the dark compartment externally covered with a black chart. The tank water level was kept at 4 cm (3 l) to allow zebrafish to freely swim between the two compartments.
In silico docking analysis
The docking software Molegro Virtual Docker was used for in silico docking analysis. Silibinin and naringenin were extracted from the PubChem compound database and stored in the “. mol” format for further analysis.
The receptor for dopamine D3 type with PDB ID as 3PBL comprises of two chains with 432 and 423 amino acid residues in the A and B chains, respectively,[22] and the serotonin receptor, as wild-type human serotonin transporter in complex with paroxetine and 8B6 Fab in cryo-EM structure (6VRH) was obtained in “.pdb” format from the Protein Data Bank database.
Preparation of ligand
The standard drugs fluoxetine, diazepam, rivastigmine, and scopoleptin were obtained and saved in “. mol” format from the database Drug Bank.
Libraries containing phytocompounds and other standard ligands were loaded into the Molegro virtual docking toolbox and further analyzed using the Molegro virtual docker. Several drug docking patterns and test scores were compared to the docking patterns of the reference standard and the control group.
Statistical analysis
The values obtained from all the tests were expressed as mean ± Standard deviation (SD) The effects of drugs on behavioral parameters were studied using the one-way analysis of variance, followed by a post hoc test using Dunnett's method, where appropriate. Using GraphPad Prism 8.0 statistical analysis was performed and statistical significance was considered when the difference in mean ± SD values between different groups was shown with p < 0.05.
Results
In the brine shrimp lethality assay, almost all shrimps survived throughout the observation period in the control sets that were left blank (24 h). In comparison to the cytotoxic component potassium dichromate (positive control), where complete mortality was observed with an LC50 value of 13.15 μg/ml, shrimps began dying only after 12 h, and considerable lethality was observed after 21 h at the highest treated concentration of 1000 μg/ml for the flavonoids, silibinin, and naringenin treated brine shrimps.
After 24 h, the flavonoids, silibinin, and naringenin showed significantly lower toxicity having LC50 values 34.10 μg/ml and 91.33 μg/ml, respectively, as compared to the standard cytotoxic agent, potassium dichromate with LC50 value 13.15 μg/ml [Figure 1].
Figure 1.
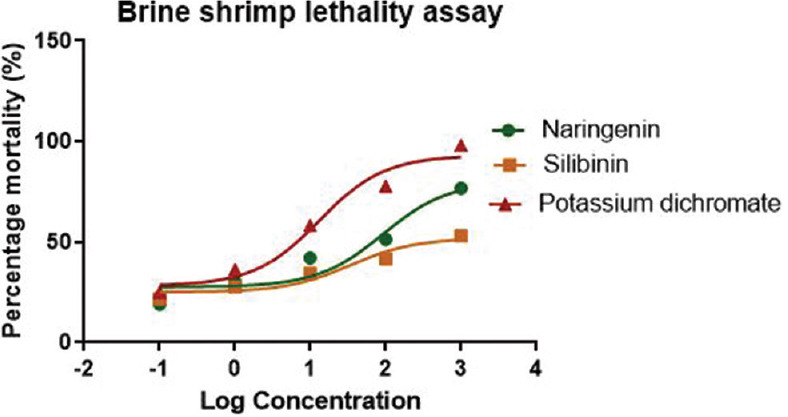
Effect of flavonoids on brine shrimp lethality. Potassium dichromate served as positive control. Results were stated as percentage mortality (mean ± standard error of the mean) of n = 3 observations
Acute toxicity test
During the observation period (i.e., at 24, 48, 72, and 96 h) of the test, no visible abnormalities such as loss of equilibrium, respiratory function, pigmentation, swimming behavior, and other clinical signs, as well as no mortality, were observed in the silibinin and naringenin exposed zebrafish groups and in the control group (data not shown).
Zebrafish exposed to 100 mg/l flavonoids exhibited normal behavior and swimming patterns with no mortality. After 72 h, a change in the color of the naringenin solution was observed. The exact cause for these discolorations remains to be conclusively identified. However, no mortality was recorded in the naringenin as well as the silibinin-treated zebrafish groups.
Neuropharmacological evaluation in zebrafish
During the 15-day treatment period, behavioral parameters were assessed using a novel tank test on days 7 and 14 and a scototaxis test was performed on days 8 and 15. These two tests were carried out on different days to avoid handling stress to zebrafish due to experimental procedures. Similar care was undertaken to avoid stress and a 7-day interval was allowed between each testing.
Novel tank test
The time consumed in the upper level of the test tank by the zebrafish was greater in the silibinin and naringenin treated groups as compared to the reference standard treated groups. This effect in the treatment groups and the reference standard treated group was concentration-dependent and the time consumed in the tank's upper level was maximal at the highest concentration (10 μM) as compared to the untreated control group which showed a decrease in time spent in the upper part and the zebrafish from this group preferred to be in the bottom and middle level of the tank, as evidenced by the video recordings [Figure 2].
Figure 2.
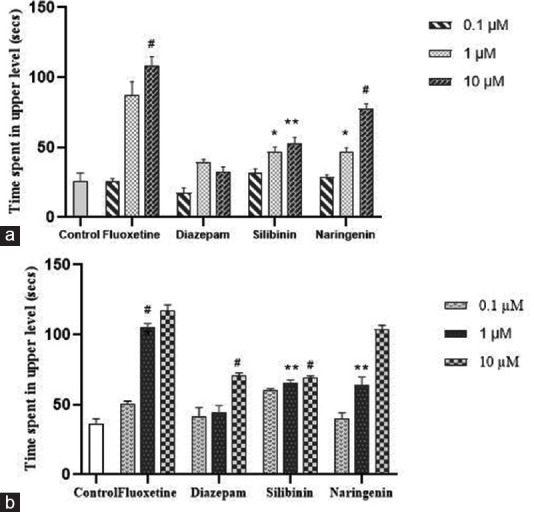
Effect of treatment on time spent in upper level of test tank in novel tank teston day 7 (a) and on day 14 (b). Data were presented as mean ± standard deviation (n = 10), *p < 0.05, **p< 0.01 and #p< 0.001 versus control, analyzed by means of one-way analysis of variance with Dunnet's post hoc test
Effects on swimming pattern
Chronic fluoxetine exposure had a significant effect on zebrafish behavior, where a reduction in the inactivity to enter the tank's upper level, and an increase in the entries to the top was observed. Similarly, treatment with flavonoids reduced the number of erratic movements was observed in contrast to the control group, which exhibited erratic movements when transferred to a novel tank during the observation period.
Effects on shoal cohesion
Due to the exposure to acute stress, the control groups showed increased shoal cohesion or crowding behavior, whereas the silibinin and naringenin treated groups showed decreased shoal cohesion during the observation period, similar to the fluoxetine and diazepam treated groups, which exhibited normal distance and shoaling behavior.
Light-dark test
On day 8 of the evaluation, silibinin and naringenin showed an extended time spent in the light compartment for 146 s and 171 s, respectively, similar to fluoxetine and diazepam treated groups, which showed a latency of 158 s and 140 s, respectively, at the highest exposure concentration (10 μM), whereas the control group of fish spent <150 s in the illuminated side of the tank [Figure 3].
Figure 3.
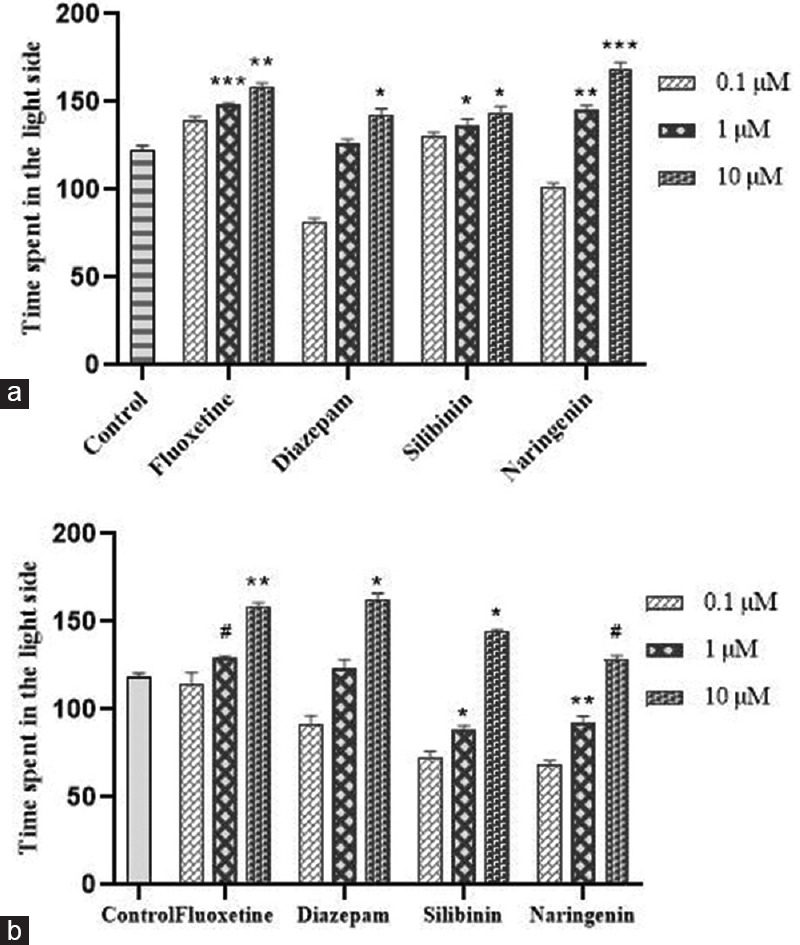
Effect of treatment on time spent in the light side of the tank in light/dark test on day 8 (a) and on day 15 (b). Data were presented as mean ± standard deviation (n = 10), *p < 0.05, **p < 0.01 and #p< 0.001 versus control, analyzed by the one-way analysis of variance with Dunnet's post hoc test
Although acute fluoxetine treatment did not affect light avoidance in zebrafish, it led to an increase in locomotion at the highest exposure concentration. With chronic exposure, fluoxetine exhibited an increase in the time consumed in the illuminated part of the tank at the higher concentration (10 μM) whereas both silibinin and naringenin treatment has increased the time spent in the light side of the tank significantly.
Effect on the number of fish and number of entries to the light side
The effect on the number of zebrafish entering and the number of entries by each zebrafish into the light compartment was found to be concentration-dependent, with the highest concentration (10 μM) causing a rise in the number of fish remaining in the tank's light side and the number of entries into the light compartment in all drug-treated groups. The control group exhibited rapid movement and a decline in the number of fish remaining in the illuminated part of the tank [Figure 4].
Figure 4.
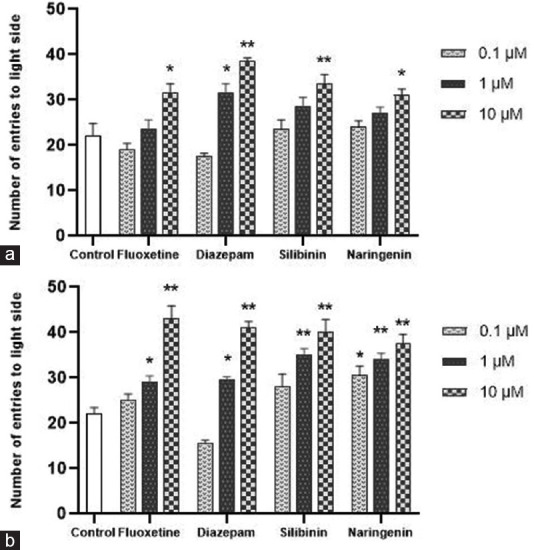
Effect of treatment on the number of entries to light side of the tank in light/dark test on day 8 (a) and on day 15 (b). Data were presented as mean ± standard deviation (n = 10), *p < 0.05, **p < 0.01 versus control, analyzed by the one-way analysis of variance with Dunnet's post hoc test
In silico docking analysis
The molecular docking study revealed that flavonoids, silibinin, and naringenin have the potential to interact with dopamine and serotonin receptors, as evidenced by their affinity for the target receptors used in the study. Silibinin was found to interact with the target molecules such as Lys 221, Arg 219, Val 214, Val 133, Gln 217, Arg 218, Arg 220, Val 320, Trp 1158, Gly 319, Val 213, Lys 325, Val 132, Leu 215, Arg 210, Tyr 1161, Asp 1159 on the dopamine receptor (PDB ID: 3PBL) and the interaction sites on the serotonin receptor (6VRH) were identified as Asp 328, Glu 494, Gly 324, Gly 498, Gly 578, Gly 582, Ile 327, Leu 557, Met 558, Phe 556, Pro 499, Pro 560, Pro 561, Ser 555, Ser 559, Thr 323, Tyr 495, Tyr 579. Similarly, Naringenin was found to interact with Arg 149, Asp 127, Gly 141, Ile 126, His 137, Met 134, Met 153, Thr 63, Thr 130, Thr 142, Tyr 138, Arg 100, Asn 173, Gly 171, Gly 176, Phe 170, Thr 174, Thr 175 on dopamine receptor with PDB ID as 3PBL and the serotonin receptor (6VRH) the interaction sites were identified as Asn 112, Asn 316, Asp 393, Gln 111, Gly 113, Gly 114, Lys 314, Lys 319, Phe 311, Tyr 110, Asp 21, Gln 47, Ile 22, Ile 114, Pro 115, Ser 113, Tyr 112 [Tables 1 and 2, Figures 5 and 6].
Table 1.
Docking study of ligands on dopamine receptor (PDB ID: 3PBL) based on MolDock score
| Flavanoid | Ligands | MolDock score | Rerank score | HBond |
|---|---|---|---|---|
| [01]Silibinin | Silibinin | −110.178 | −73.0613 | −8.07463 |
| [00]Silibinin | Silibinin | −109.083 | −98.2515 | −9.40337 |
| [02]Silibinin | Silibinin | −103.773 | −97.2328 | −6.74556 |
| [00]Naringenin | Naringenin | −89.8094 | −82.648 | −5 |
| [01]Naringenin | Naringenin | −83.2909 | −74.6334 | −2.99362 |
| [02]Naringenin | Naringenin | −80.1884 | −57.1281 | −3.30482 |
Table 2.
Docking analysis of ligands on the serotonin receptor (6VRH) based on MolDock score
| Flavanoid | Ligands | MolDock score | Rerank score | HBond |
|---|---|---|---|---|
| [00]Silibinin | Silibinin | −115.908 | −99.6174 | −3.33175 |
| [02]Silibinin | Silibinin | −112.233 | −97.3305 | −5.44722 |
| [01]Silibinin | Silibinin | −110.62 | −92.9124 | −10.5742 |
| [00]Naringenin | Naringenin | −84.1574 | −75.4056 | −8.91331 |
| [01]Naringenin | Naringenin | −82.3335 | −71.2042 | −9.51531 |
| [02]Naringenin | Naringenin | −76.3737 | −7.71734 | −0.660189 |
Figure 5.
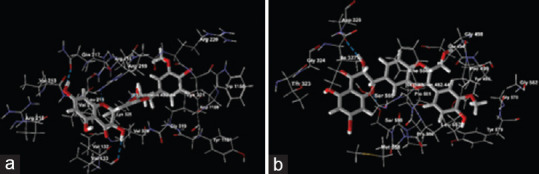
Docked View of silibinin on (a) dopamine receptor (PDB ID: 3PBL) and (b) serotonin receptor (PDB ID: 6VRH)
Figure 6.
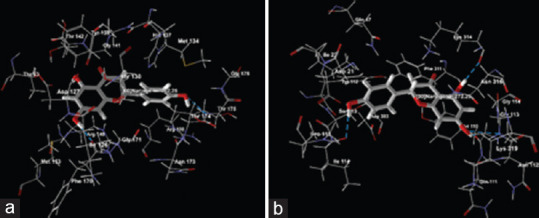
Docked View of naringenin on (a) dopamine receptor (PDB ID: 3PBL) and (b) serotonin receptor (PDB ID: 6VRH)
Discussion
A. salina L.(Artemiidae), an invertebrate species commonly known as brine shrimp that belongs to the fauna of saline aquatic networks, is employed in bioassays to test toxicity by estimating the median lethal concentration (LC50), which has been reported for many toxins and plant extracts.[23] Several studies have shown that a brine shrimp lethality assay is a convenient method for toxicity investigations preliminarily, screening of medicinal plants, and monitoring the isolation of various bioactive compounds with LC50 values of <1000 μg/ml. The flavonoids, silibinin, and naringenin were found to have LC50 values of >1000 μg/ml in this study, implying greater safety margins as compared to the reference standards.[24] Further studies are warranted in other animal models, including rodents to confirm the anxiolytic effects of the flavonoids, silibinin, and naringenin. In the field of aquatic toxicology, acute toxicity evaluation in zebrafish according to OECD TG 203, is widely accepted.[25] In this study, the OECD Test Guideline 203 limit test was done at 100 mg/L of silibinin and naringenin to establish that the LC50 is greater than the prescribed limits.
In the novel tank protocol, the silibinin and naringenin treated groups were found exploring the tank's upper part after transferring to a new tank similar to the standard drugs. Zebrafish prefer to swim in groups, and this behavior is referred to as shoal cohesion. This behavioral strategy is believed to be active against predators in several species of fishes.[12]
In the novel tank test method, zebrafish exhibit vigorous behavioral responses to novelty-evoked anxiety. It is based on the animals' instinct of seeking protection by diving, freezing, and decreasing their exploration in an unfamiliar environment. When the fish begins to gradually acclimatize to the new setting, the exploring activity increases usually (e.g., increase in locomotion, increase in the number of entries to the upper level of the tank, and decreased freezing). The novelty-based tank paradigm has developed into a helpful tool to screen drugs, as anxiogenic and anxiolytic agents can alter anxiety type of behavior. For example, ethanol, nicotine, morphine, amphetamine, benzodiazepines, and cocaine have all been studied in zebrafish.[13] Following acute stress exposure, flavonoids had an anxiolytic-like effect similar to fluoxetine or diazepam, reversing the behavioral and locomotor changes induced by the acute stress procedure, in contrast to the control group, which exhibited an increase in time consumed in the tank's lower part and no sedation or relevant motor side-effects.
However, when compared to the novel tank test at the final stage of evaluation on day 15, Fluoxetine and Naringenin showed a reduction in the magnitude of effect on time spent on the light-dark test, whereas Diazepam showed remarkable effects on a light-dark test, highlighting the sensitivity of these tasks to evaluate serotonergic agents where zebrafish behavior may be regulated.[15] The previous reports also support the neuroprotective potential of silibinin and naringenin on different experimental models of CNS disorders like depression, cerebral ischemia, and Alzheimer's disease, and so on revealing their neurotoxicity ameliorative effects. Aβ induced toxicity on cognitive impairment was inhibited by Silibinin treatment.[26] Another study on chronically stressed mice with behavioral despair paradigm revealed that silibinin exerted beneficial effects, specifically by modulating inflammation and oxidative stress.[27] Oral administration of naringenin was shown to ameliorate the memory deficit in the Aβ-induced mouse model of AD.[28] There are several studies available to reveal the strong anti-oxidant and anti-inflammatory properties of naringenin demonstrating its protective role against various neurological disorders and showing biological values in the conditions of epilepsy, depression, Parkinson's disease, dementia, and ischemic stroke.[29] Taken together, the present study might be proving helpful to extend the investigations on the molecular mechanisms and safety of the neuroprotective flavonoids studied to broaden the clinical applications in the management or mitigation of the complications of neurodegenerative diseases.
Conclusion
The geotaxis and the scototaxis model have demonstrated an increase in the response of adult zebrafish in their differential susceptibility to manipulations by the serotonergic system or other variables remains collectively shown in the tests (e.g., response to novelty vs. warfare or fear vs. anxiety). The results from our studies indicate that flavonoids, silibinin, and naringenin have an anxiolytic effect in the zebrafish model of acute stress-induced anxiety. Further research on the safety and toxicity of flavonoids must be carried out for evaluating their potential clinical applications in the treatment of anxiety disorders and neurodegenerative conditions.
Financial support and sponsorship
This research project was funded by the “GATE-Young Faculty Research Grant” of Sri Ramachandra Institute of Higher Education & Research (DU), Chennai, Tamil Nadu, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Esch T, Stefano GB, Fricchione GL, Benson H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23:199–208. [PubMed] [Google Scholar]
- 2.Chakravarty S, Reddy BR, Sudhakar SR, Saxena S, Das T, Meghah V, et al. Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: Altered brain proteome profile implicates mitochondrial dysfunction. PLoS One. 2013;8:e63302. doi: 10.1371/journal.pone.0063302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vafeiadou K, Vauzour D, Lee HY, Rodriguez-Mateos A, Williams RJ, Spencer JP. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch Biochem Biophys. 2009;484:100–9. doi: 10.1016/j.abb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Vafeiadou K, Vauzour D, Spencer JP. Neuroinflammation and its modulation by flavonoids. Endocr Metab Immune Disord Drug Targets. 2007;7:211–24. doi: 10.2174/187153007781662521. [DOI] [PubMed] [Google Scholar]
- 5.Dajas F, Rivera-Megret F, Blasina F, Arredondo F, Abin-Carriquiry JA, Costa G, et al. Neuroprotection by flavonoids. Braz J Med Biol Res. 2003;36:1613–20. doi: 10.1590/s0100-879x2003001200002. [DOI] [PubMed] [Google Scholar]
- 6.Gazák R, Walterová D, Kren V. Silybin and silymarin – New and emerging applications in medicine. Curr Med Chem. 2007;14:315–38. doi: 10.2174/092986707779941159. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin – A promising new treatment for cancer. Anticancer Agents Med Chem. 2010;10:186–95. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- 8.Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res. 2005;39:1119–25. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- 9.Olsen HT, Stafford GI, van Staden J, Christensen SB, Jäger AK. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J Ethnopharmacol. 2008;117:500–2. doi: 10.1016/j.jep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 10.El Madani MA, ELSalam RM, Attia AS, El-Shenawy SM, Arbid MS. Neuropharmacological effects of naringenin, harmine and adenosine on parkinsonism induced in rats. Pharm Lett. 2016;8:45–57. [Google Scholar]
- 11.Panula P, Chen YC, Priyadarshini M, Kudo H, Semenova S, Sundvik M, et al. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Maximino C, Puty B, Benzecry R, Araújo J, Lima MG, de Jesus Oliveira Batista E, et al. Role of serotonin in zebrafish (Danio rerio) anxiety: Relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology. 2013;71:83–97. doi: 10.1016/j.neuropharm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Cachat JM, Canavello PR, Elegante MF, Bartels BK, Elkhayat SI, Hart PC, et al. InZebrafish models in neurobehavioral research. Totowa, NJ: Humana Press; 2011. Modeling stress and anxiety in zebrafish; pp. 73–88. [Google Scholar]
- 14.Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maximino C, da Silva AW, Gouveia A, Jr, Herculano AM. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:624–31. doi: 10.1016/j.pnpbp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Manfra L, Savorelli F, Pisapia M, Magaletti E, Cicero AM. Long-term lethal toxicity test with the crustacean Artemia franciscana. JoVE (Journal of Visualized Experiments) 2012 Apr 14;62:e3790. doi: 10.3791/3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giacomini AC, Abreu MS, Giacomini LV, Siebel AM, Zimerman FF, Rambo CL, et al. Fluoxetine and diazepam acutely modulate stress induced-behavior. Behav Brain Res. 2016;296:301–10. doi: 10.1016/j.bbr.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton TJ, Morrill A, Lucas K, Gallup J, Harris M, Healey M, et al. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci Rep. 2017;7:15081. doi: 10.1038/s41598-017-15374-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni P, Chaudhari GH, Sripuram V, Banote RK, Kirla KT, Sultana R, et al. Oral dosing in adult zebrafish: Proof-of-concept using pharmacokinetics and pharmacological evaluation of carbamazepine. Pharmacol Rep. 2014;66:179–83. doi: 10.1016/j.pharep.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Maximino C, Marques de Brito T, Dias CA, Gouveia A, Jr, Morato S. Scototaxis as anxiety-like behavior in fish. Nat Protoc. 2010;5:209–16. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- 21.Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014;37:264–78. doi: 10.1016/j.tins.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330:1091–5. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DJ, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–4. [PubMed] [Google Scholar]
- 24.Bastos ML, Lima MR, Conserva LM, Andrade VS, Rocha EM, Lemos RP. Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents. Ann Clin Microbiol Antimicrob. 2009;8:16. doi: 10.1186/1476-0711-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.OECD. Test No. 203: Fish, Acute Toxicity Test, OECD Guidelines for the Testing of Chemicals. Sec. 2. Paris: OECD Publishing; 2019. [Google Scholar]
- 26.Sciacca MF, Romanucci V, Zarrelli A, Monaco I, Lolicato F, Spinella N, et al. Inhibition of Aβ amyloid growth and toxicity by silybins: The crucial role of stereochemistry. ACS Chem Neurosci. 2017;8:1767–78. doi: 10.1021/acschemneuro.7b00110. [DOI] [PubMed] [Google Scholar]
- 27.Garikapati DR, Shaik PB, Penchalaiah H. Evaluate neuroprotective effect of silibinin using chronic unpredictable stress (cus) model. Int J Physiol Pathophysiol Pharmacol. 2018;10:184–91. [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Kuboyama T, Tohda C. A systematic strategy for discovering a therapeutic drug for Alzheimer's disease and its target molecule. Front Pharmacol. 2017;8:340. doi: 10.3389/fphar.2017.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin I, Majid S, Farooq A, Wani HA, Noor F, Khan R, et al. Naringenin (4, 5, 7-trihydroxyflavanone) as a potent neuroprotective agent: From chemistry to medicine. Stud Natl Prod Chem. 2020;65:271–300. [Google Scholar]


