Abstract
There is a significant research-to-practice gap with respect to reaching underserved populations with evidence-based tobacco cessation treatments. Increasing enrollment in evidence-based treatments is necessary to reduce tobacco use and tobacco-related health inequities. The purpose of the current study was to evaluate whether Motivation And Problem Solving (MAPS), a flexible, holistic counseling/navigation approach delivered via phone, and proactive provision of Nicotine Replacement Therapy (NRT) would improve Quitline enrollment among a sample of low SES smokers who were not motivated to quit. In a 3×2 factorial design, cigarette smokers (N = 603) were randomized to one of six treatment conditions (Standard Treatment, MAPS-6, or MAPS-12 by NRT or no NRT). Results indicated that both MAPS-6 and MAPS-12 increased Quitline enrollment compared to Standard Treatment (ps < .03). There were no differences between MAPS conditions. NRT did not increase Quitline enrollment. MAPS is an effective intervention with the potential to be disseminated and implemented in healthcare and community settings to increase the reach of evidence-based interventions for tobacco cessation.
Keywords: Tobacco cessation, Evidence-based interventions, Treatment engagement, Smoking
Motivation and Problem Solving (MAPS) is an acceptable, scalable, and efficacious intervention to increase enrollment in an evidence-based intervention for smoking cessation among those with low socioeconomic status.
Implications.
Practice Providing MAPS via phone counseling has the ability to reach large numbers of individuals not motivated to quit smoking and has the potential to increase enrollment into evidence-based smoking cessation interventions.
Policy MAPS is a cost-effective intervention aimed at increasing enrollment in smoking cessation treatment and policies aimed at incorporating such interventions into existing infrastructure should be considered.
Research Future research should focus on the implementation and dissemination of MAPS, given its high potential for scalability and broad impact.
Smoking remains the leading preventable cause of morbidity and mortality, accounts for about 30% of all cancer deaths, and is a leading cause of health inequities [1–8]. Fortunately, quitting smoking improves health and greatly reduces the risk of developing cancer—former smokers have an almost 40% lower risk of developing lung cancer within five years of quitting compared to individuals who continue to smoke [9].
A significant advance in cancer prevention and control has been the development of evidence-based interventions (EBIs) for tobacco cessation, including those delivered via Quitlines [10]. Quitlines typically provide free EBIs in several different formats (e.g., phone counseling, web-based programs, text messaging, pharmacotherapy) to help tobacco users quit and have consistently been found to be efficacious and cost-effective [11,12]. Quitlines are available in all 50 states, Puerto Rico, and Guam, and increasing their reach has the potential to greatly impact tobacco use at the population level [13,14]. In particular, increasing Quitline reach among underserved populations could reduce tobacco-related health inequities [15–17].
Unfortunately, individuals of lower SES are less likely to be successful in quitting smoking compared to those of higher SES, in part because of lack of access to, and awareness of, EBIs such as Quitlines [3,6,7,13,14]. In addition, the vast majority of Quitlines (81%) only enroll smokers who are motivated to quit [18], and only about 10–20% of smokers are motivated to quit at any particular moment in time [19]. Moreover, differences regarding intention to quit are related to SES, such that individuals with lower income or education are less likely to intend to quit both within the next 6 months and long term [20]. Consequently, despite immense potential, Quitlines are underutilized, reaching less than 1% of smokers as of 2017 [21].
The lack of enrollment of tobacco users in EBIs for cessation, such as Quitlines, is a major breakdown in the translation of research-to-practice [22–24]. Although the vast majority of smokers, and particularly low SES smokers, might be categorized as “unmotivated” to quit, motivation can change over relatively short periods of time [25–27]. As such, interventions that influence or leverage motivation changes could be effective at increasing treatment enrollment and the reach of EBIs, particularly for populations that bear a disproportionate burden of smoking and face unique barriers to cessation.
Motivation And Problem Solving (MAPS) is a flexible, holistic intervention approach that utilizes an overarching motivational enhancement framework combined with practical problem solving strategies for enhancing behavior change [28]. MAPS is appropriate for individuals regardless of their motivation to change and previous research has demonstrated the efficacy of MAPS and its precursors in improving smoking treatment enrollment, cessation rates, relapse prevention, and in reducing alcohol use [29–32].
Proactively providing nicotine replacement therapy (NRT), often in conjunction with brief advice to quit, is another strategy designed to promote cessation among unmotivated smokers by increasing motivation to quit and moving people towards making an active quit attempt. Numerous studies have found that the provision of NRT in this manner is related to decreased cigarette consumption and an increased likelihood of quitting [33–41]. For example, Wennike et al. found that among those not interested in quitting, individuals randomized to receive nicotine gum (vs placebo gum) were more successful at sustaining smoking reduction and had increased smoking abstinence at 12 and 24 month follow-ups [38]. More recently, Dahne et al. found that providing two weeks of NRT may have more impact on cessation-related outcomes among lower than higher SES smokers [41]. Thus, provision of proactive NRT may not only promote an active quit attempt, but also encourage smokers to enroll in EBIs.
This randomized clinical trial tested the efficacy of both MAPS and the provision of proactive NRT with respect to increasing enrollment in EBIs delivered via a Quitline among a sample of low SES, unmotivated to quit, cigarette smokers. Participants were randomized to one of the following in a 3×2 design: (a) Standard Treatment (ST), (b) ST with the proactive provision of NRT (ST+NRT), (c) MAPS-6, (d) MAPS-6 with the proactive provision of NRT (MAPS-6+NRT), (e) MAPS-12, or (f) MAPS-12 with the proactive provision of NRT (MAPS-12+NRT). We hypothesized that participants receiving MAPS and proactive NRT would be more likely to engage in a Quitline-delivered EBI (i.e., the Quitline) than the comparison conditions. Tobacco abstinence was also assessed. It should be noted that MAPS and proactive NRT were designed to increase treatment enrollment (rather than cessation) and that actual cessation treatment was provided by the Quitline independent of the current study.
METHOD
Participants
From June 2011 to January 2013, 603 low-income, unmotivated smokers were enrolled. Participants were recruited in Houston, TX from the Harris County Hospital District, radio and newspaper ads, and via community organizations. Inclusion criteria included the following: ≥18 years of age, current smoker of at least one cigarette per day for the past year, not ready to quit in the next 30 days, household income less than 200% of the federal poverty level, valid home address, functioning telephone number, and the ability to speak, read, and understand English. Exclusion criteria included: carbon monoxide (CO) breath sample lower than 5 parts-per-million (ppm), less than a 6th grade literacy level, enrolled in any other smoking study in the past 90 days, used tobacco products other than cigarettes regularly, reported abuse or dependence on addictive substances other than nicotine, were pregnant or lactating, or had another household member enrolled in the study.
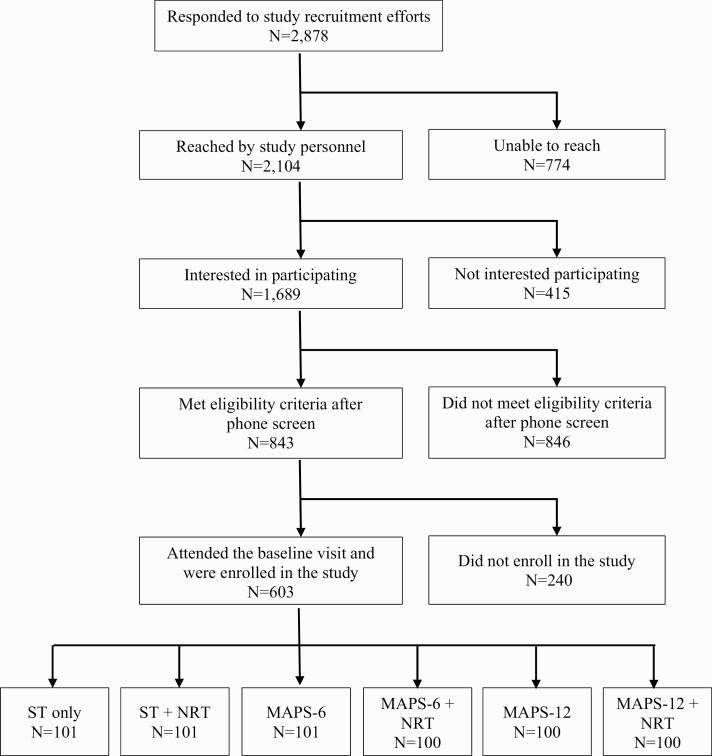
Figure 1 shows the consort diagram. Of the 2,878 who responded to recruitment efforts, 1,689 completed the initial phone screen, and 843 were eligible. Of those 843 smokers, 603 met all eligibility criteria, attended a baseline visit, and were consented by study staff. Written informed consent was obtained from all participants. All study procedures were approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (MD Anderson); clinicaltrials.gov registration #NCT00984724.
Fig 1.
| Consort diagram.
Procedures
This study used a form of adaptive randomization, called minimization, which allows treatment groups to remain balanced regarding participant characteristics that may be related to time of accrual and was based on gender, race/ethnicity, age, income, and cigarettes smoked per day. Participants were randomized to treatment group via computer program: (a) ST, (b) ST+NRT, (c) MAPS-6, (d) MAPS-6+NRT, (e) MAPS-12, or (f) MAPS-12+NRT. All participants received ST—a letter referring smokers to the Quitline and standard self-help materials. Participants were followed for 2 years and attended five in-person visits (Baseline, Months 6, 12, 18, and 24), during which they completed computer-administered self-report questionnaires. Participants were provided with a $50 gift card for each completed visit.
MAPS intervention.
In addition to the Quitline referral letter and self-help materials, participants in MAPS-6 and MAPS-12 received up to 6 or 12 proactive counseling sessions via the phone, respectively, over a period of up to 24 months. MAPS is a holistic, dynamic approach that integrates two empirically validated treatment approaches—motivational enhancement and practical problem solving—into a coordinated and comprehensive treatment that addresses not only motivation to quit, but also a variety of concerns and barriers for low income smokers, many of which reduce motivation and ability to quit smoking [28]. MAPS is strongly grounded in motivational enhancement approaches such as Motivational Interviewing (MI)[42–43] and was developed for anyone regardless of readiness to change/quit smoking. Key clinical principles derived from MI included in MAPS are expressing empathy, managing discord, developing discrepancy, and supporting self-efficacy. MAPS utilizes a “wellness program” approach that in addition to focusing on smoking, also addresses life events, stressors, and concerns that impact the lives of low income individuals (e.g., stressors, family issues, financial hardships). By addressing the larger context in which smoking occurs, many of the barriers and factors that impact engaging in a quit attempt can be addressed.
In order to fully address changes in motivation, MAPS allows the counselor to shift therapeutic strategies within a single session. That is, MAPS requires the counselor to carefully assess and attend to changes in motivation so that they can be addressed in the moment as they emerge, which is different from other stage-based conceptualizations of behavior change. MAPS assumes that motivation is a fluid and dynamic construct that can fluctuate on a day-to-day or even moment-to-moment basis depending upon the intra- and interpersonal context. Although MAPS is a manualized treatment, counselors in this study were not provided with a specific agenda for each session. Counselors were trained to listen for specific language related to changes in motivation to quit, such that they shift between motivational enhancement and skills-based talk. This approach allowed the counselor to dynamically address changes in motivation and to comprehensively address various topics most relevant to the participant. In the current study, participants were not provided with cessation treatment to quit smoking during sessions, given the target goal of enhancing enrollment in EBIs for cessation. If participants indicated an interest in cessation treatment, the counselor referred them to the Quitline; they were able to continue sessions with a MAPS counselor after the referral was made. Additional details about MAPS can be found elsewhere [28]. MAPS-6 and MAPS-12 groups differed only in the potential number of calls. Calls typically lasted 20–30 minutes and timing of the calls was completely flexible based on the needs of the individual. As such, participants might not complete all calls designed for their assigned condition.
Proactive NRT.
Participants assigned to groups with proactive provision of NRT received 300 pieces of nicotine gum at their baseline visit. They were not given specific instructions to cut down their smoking or to quit. Rather, they were told that they could use the gum in any way that was helpful. For example, they could use the gum to reduce their smoking if they wanted too, in settings where smoking was not allowed, to reduce cravings, or in any other situation that they thought the gum could be useful.
NRT during a quit attempt.
If participants indicated that they were ready to initiate a quit attempt, all participants, regardless of intervention condition, could request and receive up to 600 pieces of nicotine gum. Participants received gum in-person and were provided with instructions on use at that time. For participants in the NRT conditions, this means that they could receive up to 900 pieces of gum.
Treatment delivery and integrity.
Study staff who conducted assessments were blinded to treatment condition; participants were not blinded. All counseling sessions were conducted by counselors (master’s level with 1+ years of counseling experience) trained in MAPS. Participants in MAPS-6 completed an average of 4.06 counseling sessions and 58.2% completed all sessions. Participants in MAPS-12 completed an average of 6.69 sessions and 37.5% completed all sessions. All MAPS sessions were recorded and a subset (5.4%) were randomly selected and coded for treatment fidelity with a modified Motivational Interviewing Treatment Integrity Manual (MITI) [44]. Average global ratings (scored on a 5-point Likert scale from 1 = low to 5 = high) were high: evocation (M = 4.01), collaboration (M = 4.12), autonomy/support (M = 4.29), empathy (M = 4.41), direction (M = 4.47), and desirable shifting (M = 4.16). The desirable shifting subscale is unique to MAPS and captures appropriate transitions between problem solving and motivational enhancement techniques. Ratio of reflections to questions was good (2:1), as was the % of open ended questions (72.67%), % MI adherence (100%), and % complex reflections (72.77%).
Measures
Questionnaires assessing demographic information and tobacco history were administered at baseline. Quitline use and smoking abstinence were measured at each follow-up visit.
Demographics questionnaire and tobacco use.
Demographic information (e.g., age, race/ethnicity) and cigarettes smoked per day were collected.
Quitline enrollment.
The primary outcome of treatment enrollment was assessed at each visit with two questions, “Are you currently receiving services from the Quitline?” and “Did you receive services from the Quitline?” If a participant responded in the affirmative to either question, his/her Quitline enrollment status was coded as “Yes” for that visit. Once a visit was coded “Yes,” that status was carried forward to all subsequent visits.
Tobacco abstinence.
Tobacco abstinence (7-day and 30-day point prevalence), based on recommendations from the Society for Research on Nicotine and Tobacco for cessation induction trials (i.e., among smokers not ready to quit) [45], was assessed at the Months 6, 12, 18, and 24 visits. Abstinence at all follow-ups was biochemically verified using a CO level of <6 ppm.
Analytic plan
Generalized estimating equations (GEE) with binomial probit link function were used to model changes in Quitline enrollment and smoking abstinence with respect to MAPS and proactive NRT. GEE is an appropriate analytic strategy for analyzing binomial outcomes and nested data obtained from repeated longitudinal observations [46]. MAPS (ST, MAPS-6, MAPS-12), NRT (NRT, no NRT), and Time (follow-up time points at months 6, 12, 18, and 24) were entered as independent variables (IVs) in a main effects model, followed by examining a series of interaction models in which the three independent variables and their second-order interaction (MAPS × NRT, MAPS × Time, and NRT × time) were entered as IVs, followed by a model with both the second- and third-order (MAPS × NRT × time) interaction terms. We used a compound symmetry, or exchangeable working correlation matrix.
Missing data were handled with multiple imputation procedures to minimize bias and maximize power in estimation of treatment effects. First, missing observations were imputed with the multivariate imputation by chained equations (MICE) algorithm under SAS PROC MI, and 40 imputed data sets were generated. Second, the GEE models were estimated separately on each imputed data set. Third, the GEE parameter estimates and their standard errors were pooled using Rubin’s rules [47]. To maximize the plausibility of the missing at random (MAR) assumption, multiple imputation models included outcome (e.g., Quitline enrollment), predictor (e.g., treatment conditions), and covariates (gender, age, partner status, ethnicity, education, employment status, and income), as well as auxiliary variables (cigarettes smoked per day and alcohol use). Auxiliary variables are considered associated with missing data mechanisms (i.e., probability of missingness) [48] but are not part of the analytic models. Sensitivity analyses were performed, comparing the results from the imputed models with models that excluded missing data (completers only). No differences in results were found. Hence, only the findings from the imputed models are reported. A few participants (6.3%) reported previous use of the quitline at baseline. When this variable was included in the models, results did not change.
RESULTS
Participant demographic data (N = 603) and percent missing data are reported in Table 1. No significant differences were found among demographic variables across the 6 groups.
Table 1.
| Participants characteristics
| Variables | Mean (SD), % | ||||||
|---|---|---|---|---|---|---|---|
| ST | ST+NRT | MAPS-6 | MAPS-6+NRT | MAPS-12 | MAPS-12 +NRT |
p | |
| N | 101 | 101 | 100 | 101 | 100 | 100 | |
| Demographics | |||||||
| Age (years) | 47.5 (10.0) | 46.5 (10.8) | 46.8 (10.7) | 46.8 (11.2) | 47.2 (10.7) | 46.9 (10.3) | .99 |
| Female | 53% | 54% | 54% | 55% | 54% | 54% | .99 |
| Married/living with Partner | 27% | 17% | 22% | 19% | 20% | 17% | .50 |
| Race/ethnicity | |||||||
| White, non-Hispanic | 18% | 18% | 17% | 18% | 19% | 21% | .99 |
| Black, non-Hispanic | 64% | 61% | 64% | 63% | 64% | 61% | |
| Hispanic/Latino | 7% | 10% | 9% | 7% | 7% | 6% | |
| Other | 11% | 11% | 10% | 12% | 10% | 13% | |
| Socioeconomics | |||||||
| Education (years) | 11.9 (1.9) | 12.1 (1.7) | 12.1 (2.0) | 12.0 (1.7) | 11.9 (1.7) | 12.0 (1.8) | .96 |
| Annual income ≤ $10,830 | 51% | 52% | 58% | 50% | 57% | 55% | .97 |
| Employed | 20% | 28% | 29% | 25% | 16% | 21% | .34 |
| No health insurance | 63% | 56% | 63% | 64% | 56% | 59% | .45 |
| Smoking characteristics | |||||||
| Cigarettes per day | 17.1 (8.6) | 17.8 (10.6) | 15.4 (8.8) | 16.5 (8.5) | 16.7 (10.1) | 15.1 (9.0) | .33 |
| Years smoked | 25.3 (11.8) | 25.9 (13.7) | 24.0 (10.7) | 25.3 (12.2) | 23.7 (12.1) | 23.3 (12.5) | .59 |
| Smoke menthol cigarettes | 65% | 69% | 79% | 65% | 69% | 72% | .19 |
| Smoke within 5 minutes of waking | 43% | 46% | 39% | 45% | 54% | 45% | .38 |
| Missing data (%) | |||||||
| 6 months | 22% | 27% | 23% | 36% | 23% | 39% | |
| 12 months | 31% | 26% | 30% | 37% | 30% | 36% | |
| 18 months | 29% | 30% | 30% | 32% | 25% | 39% | |
| 24 months | 28% | 31% | 35% | 37% | 30% | 43% |
MAPS motivation and problem solving; NRT nicotine replacement therapy; SD standard deviation; ST standard treatment.
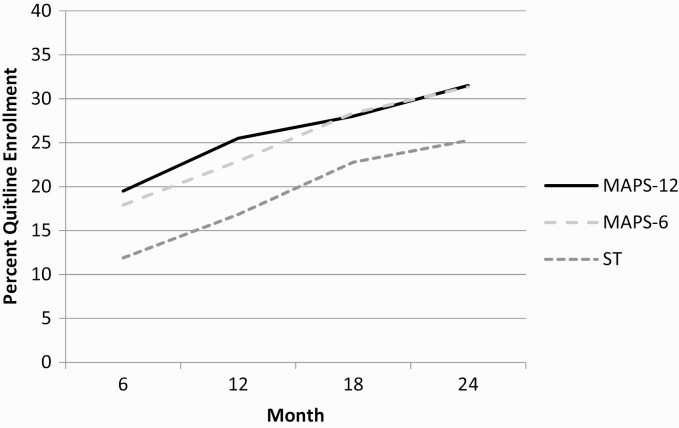
A simple main effects model regressed Quitline enrollment on MAPS, NRT, and Time. There was a significant main effect of both MAPS-6 (t = 2.30, p = .02; OR = 1.60, 95% CI = 1.07–2.39) and MAPS-12 (t = 2.45, p = .01; OR = 1.68, 95% CI = 1.11–2.54), with participants in both groups significantly more likely to report enrolling in Quitline services across the follow-up time points than participants who were assigned to ST (Fig. 2). MAPS-6 and MAPS-12 did not differ significantly from one another in their effects on Quitline enrollment (t = 0.22, p = .83, OR = 1.05, 95% CI = 0.70–1.57). No significant effects were found for proactive NRT, and for the second and third order interaction terms in subsequent models that added them. Finally, we added covariates (gender, age, marital status, ethnicity, education, and number of cigarettes smoked per day) to the simple main effects model and found that the significant effect of both MAPS conditions remained unchanged (MAPS-6 vs. ST, t = 2.40, p = .02, OR = 1.65, 95% CI = 1.10–2.47; MAPS-12 vs. ST, t = 2.54, p = .01, OR = 1.71, 95% CI = 1.13–2.59).
Fig 2.
| Quitline enrollment for ST, MAPS-6, and MAPS-12. MAPS motivation and problem solving.
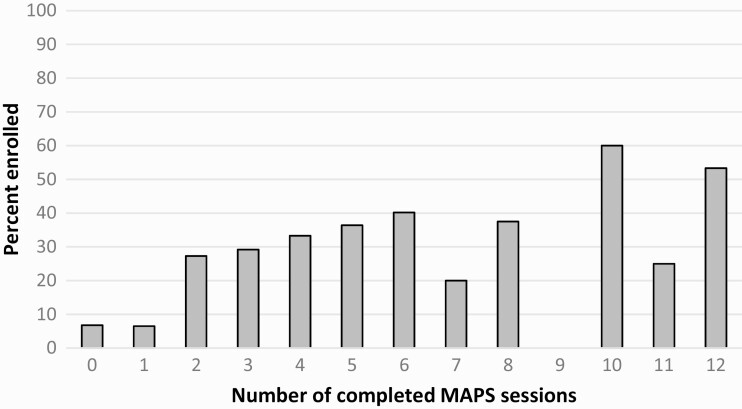
Given the overall effect of MAPS on Quitline enrollment, we conducted a separate logistic regression to examine the incremental effect of MAPS sessions on participants’ enrollment status at 24 months. There was a significant dose response effect (χ 2 = 3.79, p = .05), such that each additional MAPS session increased participants’ odds of enrolling in the Quitline by 7% (OR = 1.07, 95% CI = 1.00–1.14). Adding covariates to the model did not change the results (OR = 1.08, 95% CI = 1.01–1.17). Figure 3 shows the dose–response relationship of calls for all four MAPS groups combined over time with Quitline enrollment.
Fig 3.
| Dose response effect of completed MAPS sessions for all four MAPS conditions on Quitline enrollment. MAPS motivation and problem solving.
Given recent research showing that the provision of NRT was more strongly associated with quitting in low income smokers as compared to high income smokers [41], we examined the interaction of demographics (education, income, gender, age, race/ethnicity, partner status) and treatment conditions on Quitline enrollment and abstinence. Results indicated no significant interactions.
No significant main or interaction effects of MAPS or proactive NRT were found on either 7-day or 30-day point prevalence. Results remained generally unchanged (i.e., no significant main or interaction effects) when we only used data from those participants who engaged in Quitline treatment.
Discussion
Proactive phone counseling using MAPS increased the likelihood of enrolling in Quitline treatment among low SES, unmotivated smokers. There were no differences between MAPS-6 and MAPS-12, but there was a dose response effect such that each additional call increased the likelihood of Quitline enrollment by 7%. MAPS calls were well received by unmotivated smokers, as over half of participants in each group completed at least six calls (MAPS-6 = 58%; MAPS-12 = 53.5%). The provision of proactive NRT did not increase enrollment in the Quitline. There were no differences on abstinence outcomes, which is not surprising given that the current study was designed to test the effect of MAPS in increasing enrollment in the Quitline, as opposed to increasing abstinence.
Low SES populations experience higher levels of chronic stressors and negative life events, which influence motivation to quit smoking and long-term cessation outcomes [7,49–52]. MAPS may be particularly useful for this population given it is designed as a dynamic, flexible treatment approach that addresses a variety of concerns that may impact quitting smoking (e.g., family and job stressors, financial strain). In addition to the flexibility in topic and timing of sessions, MAPS is highly scalable. Previous research has demonstrated that Quitline counselors can be effectively trained in MAPS [53], and state Quitlines could consider incorporating counseling/navigation for those not ready to quit smoking, which is generally counter to current practice [18]. Although the “conversion rate” of Quitline callers to enrollment in treatment is lower among less motivated smokers [54], low SES populations are less motivated to quit and our approaches need to be broadened to more effectively reduce health inequities [20]. Moreover, MAPS could be adopted and implemented in numerous healthcare and community settings through the use of patient navigators, community health workers, and other lay health workers [55]. Current research is evaluating the efficacy of MAPS in engaging underserved tobacco users in EBIs through Federally Qualified Health Centers in a stepped care approach [56]. Future research should broadly explore dissemination of MAPS through existing infrastructures (e.g., state Quitlines, healthcare systems).
Despite previous studies showing that proactive NRT given to unmotivated smokers impacts EBI enrollment and quit rates [33–39,41], provision of NRT did not affect either in this study. Furthermore, unlike Dahne et al. [41], the lack of effect was not moderated by income. However, 20% of participants had incomes ≥$50,000 in Dahne et al., whereas our extremely low income sample severely restricted our ability to examine the moderating effect of income on NRT. Nevertheless, given that the effect of NRT on making cessation attempts and outcomes were stronger among low income smokers in Dahne et al., one might have expected a significant effect in the current study. Unlike prior research, the current study outcome was EBI enrollment, whereas prior studies focused on quit attempts and actual cessation. Thus, several possibilities might account to the lack of effect of proactive NRT. First, prior research often provided only a “sampling” of NRT to smokers [41], whereas participants in the current study received an ample supply (~25 days or more), which might have led them to believe that they had the tools to quit on their own without additional help, and thus they did not initiate contact with the Quitline. Or it may be the case that many smokers contact the Quitline simply to get NRT and that incentive was removed in the current study. Additional research is needed to better understand the use and role of proactive NRT in studies attempting to increase enrollment in Quitline services. With regard to abstinence, participants in the current study were provided scant instruction on how to use the product (to mimic what would occur when accessing NRT over-the-counter). Prior studies have often provided NRT with additional instruction by counselors or clinic staff.
Limitations include that only the nicotine gum was provided (vs. patch, etc.) and that we relied on self-report of EBI enrollment in the Quitline (vs. receiving data from the Quitline on services provided). This study did not include non-daily/occasional smokers and thus it is unclear whether this subgroup would benefit from MAPS. Finally, we do not have data on NRT use at follow-up, and we do not know the impact of NRT use on study outcomes. Furthermore, this study only provided nicotine gum (as opposed to other forms of NRT, such as the nicotine patch), so form of NRT may have impacted usage.
Findings suggest that MAPS is an acceptable, scalable, and efficacious intervention to increase enrollment in an EBI for smoking cessation among low SES individuals. MAPS has the potential to reach large numbers of individuals and is easily disseminated via phone counseling. As such, MAPS has the potential for high impact if disseminated widely.
Contributor Information
Christine Vinci, Moffitt Cancer Center, Tampa, FL, USA; University of South Florida, Tampa, FL, USA.
Cho Lam, University of Utah and the Huntsman Cancer Institute, Salt Lake City, UT, USA.
Chelsey R Schlechter, University of Utah and the Huntsman Cancer Institute, Salt Lake City, UT, USA.
Yusuke Shono, University of Utah and the Huntsman Cancer Institute, Salt Lake City, UT, USA.
Jennifer I Vidrine, Moffitt Cancer Center, Tampa, FL, USA; University of South Florida, Tampa, FL, USA.
David W Wetter, University of Utah and the Huntsman Cancer Institute, Salt Lake City, UT, USA.
Funding
This work was supported by awards from the National Cancer Institute (R01CA141613 and P30CA042014); the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR002538); and the Huntsman Cancer Foundation.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: The authors have no conflicts of interest to declare.
Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
TRANSPARENCY STATEMENTS
• This study was preregistered at: https://clinicaltrials.gov/ct2/show/NCT00984724. This analysis plan was not formally preregistered.
• Deidentified data from this study are not available in a public archive. Deidentified data from this study will be made available (as allowable according to institutional IRB standards) by emailing Dr. David Wetter.
• Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing Dr. David Wetter.
• Materials used to conduct the study are not publicly available.
References
- 1. American Cancer Society. Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2. Barbeau EM, Krieger N, Soobader MJ. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94(2):269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolen JC, Rhodes L, Powell-Griner EE, Bland SD, Holtzman D. State-specific prevalence of selected health behaviors, by race and ethnicity—Behavioral Risk Factor Surveillance System, 1997. MMWR CDC Surveillance Summaries. 2000;49(2):1–60. [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Current cigarette smoking among adults - United States, 2005–2012. Morbidity and Mortality Weekly Report. 2015;63(02):29–34. [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 6. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 7. Vinci C, Guo L, Spears CA, et al. Socioeconomic indicators as predictors of smoking cessation among Spanish-Speaking Mexican Americans. Ethn Health. 2019;24(7):841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wetter DW, Cofta-Gunn L, Fouladi RT, et al. Understanding the associations among education, employment characteristics, and smoking. Addict Behav. 2005;30(5):905–914. [DOI] [PubMed] [Google Scholar]
- 9. Tindle HA, Stevenson Duncan M, Greevy RA, et al. Lifetime smoking history and risk of lung cancer: results from the Framingham heart study. J Natl Cancer Inst. 2018;110(11):1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. North American Quitline Consortium. 2016. Promoting evidence based quitlines services across diverse communities in North America. Available at http://www.naquitline.org/. Accessed 1 January 2021.
- 11. Fiore MC. A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service report. JAMA. 2000;283(24):3244–3254. [PubMed] [Google Scholar]
- 12. Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Syst Rev. 2013;( 8):CD002850. [DOI] [PubMed] [Google Scholar]
- 13. Abrams DB, Graham AL, Levy DT, Mabry PL, Orleans CT. Boosting population quits through evidence-based cessation treatment and policy. Am J Prev Med. 2010;38(3 Suppl):S351–S363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glasgow RE, Klesges LM, Dzewaltowski DA, Bull SS, Estabrooks P. The future of health behavior change research: what is needed to improve translation of research into health promotion practice? Ann Behav Med. 2004;27(1):3–12. [DOI] [PubMed] [Google Scholar]
- 15. Bock BC, Papandonatos GD, de Dios MA, et al. Tobacco cessation among low-income smokers: motivational enhancement and nicotine patch treatment. Nicotine Tob Res. 2014;16(4):413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christiansen BA, Reeder KM, TerBeek EG, Fiore MC, Baker TB. Motivating low socioeconomic status smokers to accept evidence-based smoking cessation treatment: a brief intervention for the community agency setting. Nicotine Tob Res. 2015;17(8):1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill S, Amos A, Clifford D, Platt S. Impact of tobacco control interventions on socioeconomic inequalities in smoking: review of the evidence. Tob Control. 2014;23(e2):e89–e97. [DOI] [PubMed] [Google Scholar]
- 18. Cummins SE, Bailey L, Campbell S, Koon-Kirby C, Zhu SH. Tobacco cessation quitlines in North America: a descriptive study. Tob Control. 2007;16Suppl 1:i9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: current population survey results. Prev Med. 2003;36(6):710–720. [DOI] [PubMed] [Google Scholar]
- 20. Reid JL, Hammond D, Boudreau C, Fong GT, Siahpush M; ITC Collaboration . Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: findings from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2010;12 Suppl:S20–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. North American Quitline Consortium. 2017. Results from the 2017 NAQC Annual Survey of Quitlines. Available at http://www.naquitline.org/?page=2017Survey
- 22. Balas EA, Boren SA. Managing clinical knowledge for health care improvement. Yearbook of Medical Informatics. 2000;9(01): 65–70. [PubMed] [Google Scholar]
- 23. Grant J, Green L, Mason B. Basic research and health: a reassessment of the scientific basis for the support of biomedical science. Research Evaluation. 2003;12(3):217–224. [Google Scholar]
- 24. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes JR, Keely JP, Fagerstrom KO, Callas PW. Intentions to quit smoking change over short periods of time. Addict Behav. 2005;30(4):653–662. [DOI] [PubMed] [Google Scholar]
- 26. Hughes JR, Solomon LJ, Fingar JR, Naud S, Helzer JE, Callas PW. The natural history of efforts to stop smoking: a prospective cohort study. Drug Alcohol Depend. 2013;128(1-2):171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peters EN, Hughes JR. The day-to-day process of stopping or reducing smoking: a prospective study of self-changers. Nicotine Tob Res. 2009;11(9):1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vidrine JI, Reitzel LR, Figueroa PY, et al. Motivation and Problem Solving (MAPS): motivationally based skills training for treating substance use. Cogn Behav Pract. 2013;20(4):501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Correa-Fernández V, Díaz-Toro EC, Reitzel LR, et al. Combined treatment for at-risk drinking and smoking cessation among Puerto Ricans: a randomized clinical trial. Addict Behav. 2017;65:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McClure JB, Westbrook E, Curry SJ, Wetter DW. Proactive, motivationally enhanced smoking cessation counseling among women with elevated cervical cancer risk. Nicotine Tob Res. 2005;7(6):881–889. [DOI] [PubMed] [Google Scholar]
- 31. Reitzel LR, Vidrine JI, Businelle MS, et al. Preventing postpartum smoking relapse among diverse low-income women: a randomized clinical trial. Nicotine Tob Res. 2010;12(4):326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wetter DW, Mazas C, Daza P, et al. Reaching and treating Spanish-speaking smokers through the National Cancer Institute’s Cancer Information Service. A randomized controlled trial. Cancer. 2007;109(2 Suppl):406–413. [DOI] [PubMed] [Google Scholar]
- 33. Bolliger CT, Zellweger JP, Danielsson T, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. Bmj. 2000;321(7257):329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72(3):371–381. [DOI] [PubMed] [Google Scholar]
- 35. Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam TH. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction. 2011;106(6):1155–1163. [DOI] [PubMed] [Google Scholar]
- 36. Fagerström KO. Can reduced smoking be a way for smokers not interested in quitting to actually quit? Respiration. 2005;72(2):216–220. [DOI] [PubMed] [Google Scholar]
- 37. Fagerström KO, Tejding R, Westin A, Lunell E. Aiding reduction of smoking with nicotine replacement medications: hope for the recalcitrant smoker? Tob Control. 1997;6(4):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98(10):1395–1402. [DOI] [PubMed] [Google Scholar]
- 39. Jardin BF, Cropsey KL, Wahlquist AE, et al. Evaluating the effect of access to free medication to quit smoking: a clinical trial testing the role of motivation. Nicotine Tob Res. 2014;16(7):992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carpenter MJ, Wahlquist AE, Dahne J, et al. Nicotine replacement therapy sampling for smoking cessation within primary care: results from a pragmatic cluster randomized clinical trial. Addiction. 2020;115(7):1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dahne J, Wahlquist AE, Smith TT, Carpenter MJ. The differential impact of nicotine replacement therapy sampling on cessation outcomes across established tobacco disparities groups. Prev Med. 2020;136:106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guildford Press; 1991. [Google Scholar]
- 43. Miller WR, Rollnick S. Motivational Interviewing (2nd Edition). New York, NY: Guilford Press; 2002. [Google Scholar]
- 44. Moyers T, Martin T, Manuel J, Miller W, Ernst D. Revised global scales: Motivational interviewing treatment integrity 3.1. 1 (MITI 3.1. 1). Unpublished manuscript,University of New Mexico, Albuquerque, NM. 2010. [Google Scholar]
- 45. Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. [DOI] [PubMed] [Google Scholar]
- 46. Lee JH, Herzog TA, Meade CD, Webb MS, Brandon TH. The use of GEE for analyzing longitudinal binomial data: a primer using data from a tobacco intervention. Addict Behav. 2007;32(1):187–193. [DOI] [PubMed] [Google Scholar]
- 47. Rubin DB. Multiple Imputation for Survey Nonresponse. New York: Wiley; 1987. [Google Scholar]
- 48. Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 49. Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896(1):3–15. [DOI] [PubMed] [Google Scholar]
- 50. Businelle MS, Kendzor DE, Reitzel LR, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol. 2010;29(3):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gallo LC, de Los Monteros KE, Shivpuri S. Socioeconomic status and health: what is the role of reserve capacity? Curr Dir Psychol Sci. 2009;18(5):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cambron C, Haslam AK, Baucom BRW, et al. Momentary precipitants connecting stress and smoking lapse during a quit attempt. Health Psychol. 2019;38(12):1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas JL, Luo X, Bengtson J, et al. Enhancing Quit & Win contests to improve cessation among college smokers: a randomized clinical trial. Addiction. 2016;111(2):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Warlick C, Richter KP, Mussulman LM, Nazir N, Patel V. Prevalence and predictors of quitline enrollment following hospital referral in real-world clinical practice. J Subst Abuse Treat. 2019;101:25–28. [DOI] [PubMed] [Google Scholar]
- 55. Roland KB, Milliken EL, Rohan EA, et al. Use of community health workers and patient navigators to improve cancer outcomes among patients served by federally qualified health centers: a systematic literature review. Health Equity. 2017;1(1):61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fernandez ME, Schlechter CR, Del Fiol G, et al. QuitSMART Utah: an implementation study protocol for a cluster-randomized, multi-level sequential multiple assignment randomized trial to increase reach and impact of tobacco cessation treatment in Community Health Centers. Implement Sci. 2020;15(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]