Abstract
Background
Kawasaki disease is the most common cause of acquired heart disease in children in developed countries. The coronary arteries supplying the heart can be damaged in Kawasaki disease. The principal advantage of timely diagnosis is the potential to prevent this complication with early treatment. Salicylate (acetyl salicylate acid (ASA), aspirin) and intravenous immunoglobulin (IVIG) are widely used for this purpose. Salicylate is largely otherwise avoided in children because of concerns about serious side effects, particularly the risk of Reyes syndrome.
Objectives
The objective of this review was to evaluate the effectiveness of salicylate in treating and preventing cardiac consequences of Kawasaki disease in children.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group searched their trials register (last searched January 2009) and the Cochrane Central Register of Controlled Trials (CENTRAL) (last searched Issue 1, 2009). The authors searched MEDLINE (January 1966 to July 2006), EMBASE (January 1980 to July 2006), and CINAHL (1982 to July 2006), and reference list of articles. In addition, we contacted experts in the field.
Selection criteria
Randomised controlled trials (RCTs) of salicylate to treat Kawasaki disease in children were eligible for inclusion.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. Study authors were contacted for additional information.
Main results
We found one trial involving 102 children which was described as randomised, but it was not possible to confirm the method of treatment allocation. A second comparative study, possibly with a randomised treatment allocation, was also identified. The one randomised trial reported no association between the addition of ASA to IVIG treatment on the rate of coronary artery abnormalities at follow up, but with wide confidence limits. The second, possibly randomised trial did demonstrate a reduction in duration of fever with high dose ASA compared with low dose ASA, but was insufficiently powered to establish the effect on coronary artery abnormalities at follow up.
Authors' conclusions
Until good quality RCTs are carried out, there is insufficient evidence to indicate whether children with Kawasaki disease should continue to receive salicylate as part of their treatment regimen.
Plain language summary
Salicylate for treating Kawasaki disease in children and to prevent long‐term cardiac abnormalities
Kawasaki disease is an inflammation of the blood vessels (vasculitis) which predominantly affects young children, under the age of five years. It was first recognised in children in Japan and is the most common cause of acquired heart disease in children in developed countries. Kawasaki disease can be difficult to diagnose because it has similar symptoms to many common childhood infections. The most important complication of Kawasaki disease is caused by inflammation of the heart (coronary) arteries supplying blood to the heart muscle. This may lead to immediate heart problems and damage to the coronary arteries can also have long‐term effects. Salicylate (acetyl salicylate acid, aspirin) and intravenous immunoglobulin (IVIG) are widely used to treat Kawasaki disease, although salicylate is generally avoided in children because of concerns about serious side effects, particularly the risk of Reye's syndrome causing swelling of the brain and liver.
The review authors identified only one randomised controlled trial, from Japan, reported in 1991. A total of 102 children were randomised to receive IVIG with or without salicylate. There was no clear benefit of adding salicylate to immunoglobulin treatment on the rate of coronary artery abnormalities observed, up to 30 days. The spread of findings was wide and could include a beneficial effect of salicylate. There are theoretical grounds for using salicylate to prevent damage to the coronary arteries. However, there are concerns that aspirin use in children to treat fever can have adverse effects and children with Kawasaki disease who are treated with immunoglobulins have a very low rate of coronary artery abnormalities.
Background
Kawasaki disease, or mucocutaneous lymph node syndrome is a systemic vasculitis (inflammation of the blood vessels) which predominantly affects children under the age of five years (Royle 1998). It was first described by Dr Tomisaku Kawasaki in 1967 (Kawasaki 1967). Despite subsequent advances in treatment and research exploring a superantigen‐mediated role in the development of Kawasaki disease (Curtis 1995; Leung 1993; Leung 1995), the cause remains unknown. However, epidemiological studies support an infectious agent inducing the disease in a genetically susceptible minority (Harnden 2002). Reported incidence rates differ considerably throughout the developed world with rates in Japan 10 times those in the United States and 30 times those in the United Kingdom and Australia (Dhillon 1993; Royle 1998; Yanagawa 2001). Worldwide mortality rates also vary between 0.08% in Japan to 3.7% in the UK (Dhillon 1993). It is unclear whether mortality reflects intrinsic severity of disease, or early recognition and delivery of effective treatment.
The most important complication of Kawasaki disease, inflammation of the coronary arteries leading to formation of aneurysms, occurs in 20% to 30% of untreated patients (Kato 1995). Thrombosis within an aneurysm, myocardial infarction, and dysrhythmias (defective heart rhythms) may occur in the acute phase of the illness. Patients may also suffer long‐term illness or disease as a result of scarring of coronary arteries, thickening of the inner walls of the arteries, and accelerated atherosclerosis (progressive narrowing and hardening of the arteries).
There is no diagnostic test for the disease, and many cases are missed (Curtis 1995). Diagnosis is based on clinical criteria such as that summarised by the American Heart Association (AHA) in 1993 (Dajani 1993). These include fever for five or more days; a polymorphous exanthem (a rash of indefinite or variable appearance); non‐purulent conjunctivitis (inflammation of the membranes in the eye without pus); changes in the lips or within the mouth; redness and oedema with later desquamation (peeling of the skin) of the extremities; and at least one cervical lymph node that is >1.5 cm in diameter.
The diagnosis is made when the child has had five days of fever, four of the other five findings, and no evidence of another disease with similar clinical features. However, many common childhood infections have similar clinical features. Furthermore, the diagnostic features of Kawasaki disease may appear sequentially rather than simultaneously. The two features that doctors most often remember are desquamation of the rash and thrombocytosis (increased numbers of platelets in the bloodstream). Unfortunately, these features are the least useful in reaching an early diagnosis because they usually occur later in the disease (Curtis 1997). Moreover, the clinical diagnostic criteria do not identify every case; "incomplete" or "atypical" cases have come to light because coronary artery aneurysms have been found on echocardiography or at autopsy (Rowley 1987). One study has shown that more patients with partial findings developed coronary artery abnormalities than did those with a full clinical picture (Witt 1999). As a result of this, many feel justified in making a diagnosis of Kawasaki disease even when children do not meet all the AHA criteria.
The principal advantage of timely diagnosis of Kawasaki disease is the potential to prevent the complication of coronary artery abnormalities by early treatment. Salicylate (aspirin) is widely used for this purpose. Salicylate is now largely otherwise avoided in children because of concerns about serious side effects, particularly the risk of Reyes syndrome. The aims of this systematic review were (i) to identify all randomised controlled trials (RCTs) in which salicylate was used in the treatment of Kawasaki disease in children (ii) to assess the effectiveness and safety of salicylate.
One further Cochrane Systematic Review on Kawasaki disease has been completed: 'Intravenous immunoglobulin for the treatment of Kawasaki disease in children' (Oates‐Whitehead 2003), while another is still under way: 'Steroid hormone treatment for Kawasaki disease in children' (Liu 2001).
Objectives
The objective of this review was to evaluate the effectiveness of salicylate in treating, and preventing long‐term cardiac consequences of Kawasaki disease in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) examining the effectiveness of salicylate in the treatment of Kawasaki disease in children.
Types of participants
Children between the age of 0 to 18 years diagnosed with Kawasaki disease. As differing diagnostic criteria for the disease may have been used in studies, all studies were considered.
Types of interventions
Salicylate versus placebo or no treatment
Salicylate of differing doses
Salicylate and intravenous immunoglobulin versus intravenous immunoglobulin alone
Types of outcome measures
Primary outcomes
Death
Coronary artery aneurysms (diagnosed by echocardiography, coronary angiography or autopsy)
Myocardial function abnormalities (diagnosed by echocardiography, coronary angiography or autopsy)
Secondary outcomes
Duration of clinical symptoms
Adverse effects
Duration of hospital stay
All cardiac sequelae (all cardiac outcomes, however late they were reported including thrombosis within an aneurysm; myocardial infarction; dysrhythmias; long‐term morbidity as a result of scarring of coronary arteries; intimal thickening; and accelerated atherosclerosis)
Search methods for identification of studies
We searched for publications in the literature that described, or may have described the use of salicylate for the treatment of Kawasaki disease in children. No restrictions were made on language of publication.
Electronic searches
For the update the Cochrane Peripheral Vascular Diseases Group searched their Specialised Register (last searched January 2009) and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (last searched 2009, Issue 1) for publications describing randomised controlled trials of salicylate for the treatment of Kawasaki disease in children. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is constructed from electronic searches of MEDLINE (1950 to date), EMBASE (1980 to date), CINAHL (1982 to date) and AMED (1985 to date), and through handsearching relevant journals. The full list of journals that have been handsearched, as well as the search strategies used are described in the 'Search strategies for the identification of studies' section within the editorial information about the Cochrane PVD Group in The Cochrane Library,
http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/PVD/frame.html
For the original review the authors searched the following electronic databases using OVID software:
MEDLINE 1966 to July 2006; EMBASE 1980 to July 2006; CINAHL 1982 to July 2006.
For details of the search strategy used by the authors, seeAppendix 2.
For the original review the authors searched the following Trial Registers:
National Health and Medical Research Council (NHMRC) Clinical Trials Register; Meta‐Register.
Searching other resources
The authors also searched the citation lists of relevant publications, review articles, abstracts of scientific meetings and included studies for both published and unpublished works.
We also attempted to contact authors of all open and unpublished trials that we identified.
Data collection and analysis
Selection of trials
Two authors (SL, LH) selected studies after using the search strategy outlined above. Both authors independently assessed whether the studies met the inclusion criteria and any discrepancies were resolved by a third author (HB). We contacted the authors of articles if papers contained insufficient information to make a decision about eligibility.
Quality Assessment
Assessment of allocation concealment
Two authors (SL, LH) independently assessed the quality of all eligible studies. The quality of allocation concealment was graded as either adequate (A), unclear (B), or inadequate (C). If future trials are found, the same methodology will apply.
Assessment of methodological quality
Each item was rated as follows:
clearly yes ‐ rate A; not sure ‐ rate B (seek details from authors); clearly no ‐ rate C.
(i): Internal validity
Was the assigned treatment adequately concealed prior to allocation? Were the outcomes of patients who withdrew, or were excluded after allocation described and included in an "intention‐to‐treat" analysis? Were the outcome assessors blind to assignment status? Were the treatment and control groups comparable at entry? Were the participants blind to assignment status following allocation? Were the treatment providers blind to assignment status? Were the care programmes, other than the trial options, identical? Were the withdrawals < 10% of the study population?
(ii): External validity
Were the inclusion and exclusion criteria for entry clearly defined? Were the outcome measures used clearly defined? Were the accuracy, precision, and observer variation of the outcome measures adequate? Was the timing of the outcome measures appropriate? Were the outcome measures clearly reported?
Data collection
For each included trial, information was collected regarding location of the study, methods of the study (as per quality assessment checklist), participants' characteristics (age range, eligibility criteria), types of interventions and outcomes. We intended to seek missing data from the authors. For future updates of this review, should any trials be identified for inclusion, data extraction will be performed independently by at least two authors. Discrepancies will be resolved by discussion with a third author.
Analysis
One relevant study was found. In this trial (Furusho 1991), the method of randomisation could not be determined despite attempts to contact the authors. In a second study (Melish 1992) it was not possible to determine if the trial met the inclusion criteria, despite attempts to contact the first two authors. The latter trial is described, but the results are not presented graphically.
In the one trial that was definitely eligible for inclusion, relative risk (RR) was used as the measure of effect for each dichotomous outcome. Results for this study were expressed as relative risk with 95% confidence intervals and combined for meta‐analysis with RevMan software using the Peto‐modified Mantel‐Haenszel method (Mantel 1957). If there were sufficient data, a summary statistic for each outcome was to be calculated using a fixed‐effect model. Heterogeneity in the data was to be noted and cautiously explored using previously identified characteristics of the studies under the headings 'internal and external validity', particularly focusing on assessments of quality of allocation concealment. A random‐effects model was to be used where heterogeneity was detected. The authors planned to perform a priori sensitivity analyses on results to look at the possible contribution of differences in methodological quality; trials of high quality only compared with all trials to examine the stability of the results. Wherever possible, the outcomes were to be pooled statistically.
Continuous outcomes were to be shown as mean differences, and if pooling was appropriate and possible weighted mean differences (WMD) were to be used, with a fixed‐effects model.
For future updates of this review, if there are a sufficient number of trials of adequate size, it may be possible to conduct sub‐group analyses. Ability to conduct sub group analyses will also depend on whether or not the required information is recorded in the trial publications. Possible sub‐groups include:
Comparison of the effect of salicylate across differing diagnostic criteria
Timepoint in the disease process when salicylate is first administered
Duration of salicylate treatment
It is the intention of the review authors that a new search for randomised controlled trials will be performed yearly, and the review updated accordingly.
Results
Description of studies
Thirty‐two abstracts identified in the search initially appeared to fit the criteria for the review. After obtaining the full papers, the reference lists indicated another thirteen papers that were of interest. However, after reviewing all of the papers, we excluded forty three papers leaving two possible papers for inclusion. One of these papers (Furusho 1991) did state that the trial was randomised, however it failed to give a method of randomisation and the authors did not reply to requests for further information. The second of these papers (Melish 1992) did not indicate whether any randomisation was used. Again, the authors failed to reply to requests for further details. Both studies are reviewed, but in view of the uncertainties about method of treatment allocation, the Melish 1992 study was excluded, and no formal meta‐analysis of outcomes was undertaken. This decision will be reassessed if we receive clarification of the treatment allocation methods used.
The Furusho 1991 study compared ASA alone with IVIG and ASA treatment, and with IVIG alone. The dose of IVIG was 200 mg/kg daily for five days, and of ASA was 30 to 50 mg/kg/day in three divided doses until the fever had subsided, then 10 to 30 mg/kg/day once a day until "the acute reaction had also disappeared." Sixteen centres took part, and children were enrolled if they presented within seven days of the onset of symptoms. Children with recurrent Kawasaki disease, with coronary artery abnormalities at presentation, and with incomplete criteria were excluded. Two‐dimensional echocardiography was performed three times a week until 60 days after the onset of symptoms, and any abnormalities on echocardiography were followed by selective coronary angiography. The appearance of coronary artery abnormalities was presented both for lesions appearing before day 30 of the illness and those present on day 30. The results of the children receiving ASA alone are not reported here. Children in the two groups did not differ significantly in respect to age at presentation, sex, or time from onset of symptoms to trial entry.
The second study (Melish 1992) was described as a multicenter trial but without any mention of random treatment allocation. It compared low dose (3 to 8 mg/kg/day for three months, LDA) and high dose (100 mg/kg/day until illness day 14, then 3 to 8 mg/kg/day, HDA) ASA in 85 children with Kawasaki disease presenting in the first 10 days of illness. All children received 2 gm/kg of intravenous gamma globulin. Patients were hospitalised until afebrile for at least 24 hours. Children were stated not to differ in entry parameters: age, illness duration, race, white blood cell count (WBC), platelets, or acute phase reactants. The abstract reported the number of children "enrolled to date" suggesting that trial recruitment was not complete.
Risk of bias in included studies
The Furusho 1991 study stated that the method of treatment allocation was random, but did not describe the method of randomisation, and it was therefore not possible to determine the adequacy of concealment prior to allocation. Although the care given appeared otherwise to be equivalent, there appeared to be 12 withdrawals after randomisation, and neither participants, care providers nor outcome assessors were blind to treatment following allocation. The measures of external validity were satisfactory.
The Melish 1992 study was only reported in an abstract. It did not state whether the method of treatment allocation was random. The location and number of treatment centres was not described. The degree of allocation concealment, and assessments of internal and external validity were all unclear.
Effects of interventions
The Furusho 1991 study reported:
the incidence of new coronary artery lesions up to 30 days after the onset of symptoms (9 of 49 children receiving ASA and IVIG, 10 of 53 children receiving IVIG alone);
the prevalence of new coronary artery lesions at 30 days after the onset of symptoms (5 of 49 children receiving ASA and IVIG, 4 of 51 children receiving IVIG alone).
No significant difference was found in the incidence of CALs prior to 30 days from onset of symptoms (RR 0.97; 95% Confidence intervals (CI) 0.43 to 2.19) or at 30 days post onset of symptoms (RR 1.30; 95% CI 0.37 to 4.56).
The Melish 1992 study reported the following outcomes:
the existence of coronary artery abnormalities on admission (none of 45 children receiving LDA, 2 of 40 children receiving HDA);
the development of coronary artery abnormalities at follow up (1 of 45 children receiving LDA, 1 of 40 children receiving HDA);
the prevalence of fever (T ≥ 38oC) on day three (40% of 45 children receiving LDA, 18% of 40 children receiving HDA; absolute numbers not reported);
the prevalence of fever (T ≥ 38oC) on day six (9% of 45 children receiving LDA, 3% of 40 children receiving HDA; absolute numbers not reported);
the duration of hospitalisation (4.32 days in children receiving LDA, 3.32 days in children receiving HDA; standard deviations were not reported).
Salicylate levels were reported as being undetectable in children receiving LDA, whereas free (mean 2.6 mg/dl, range 1.6 to 11.5) and total (mean 10.8 mg/dl, range < 4 to 34) serum salicylate levels were detected in children receiving HDA. The authors stated that "bilirubin and ALT values showed no difference. After treatment, thromboxane and prostacyclin metabolites were suppressed in both HDA and LDA groups."
Discussion
The included RCT Furusho 1991 had no information regarding the method of randomisation and as the other study Melish 1992 did not state whether treatment was randomised, it was not included.
Results from three of the 43 excluded studies, including one non‐randomised study and two reviews, may be of interest to clinicians and fuel further research.
A non‐randomised study (Akagi 1991) was excluded as treatment was "assigned alternatively by age and sex". Reported outcomes were duration of fever and incidence of CALs in low and high dose ASA groups; no IVIG treatment was used in this trial. The high dose group were given 100 mg/kg/day of ASA for 14 days; the low dose group received 30 mg/kg/day for 14 days. It was not stated in the paper how long after the onset of symptoms and treatment outcomes were measured. The authors found no significant difference between incidence of CALs between the high dose and low dose groups (RR 0.71; 95% CI 0.25 to 2.00). There was however, a significant difference in favour of the high dose group in duration of fever (WMD ‐2.20; 95% CI ‐3.87 to ‐0.53) days. The results of this study cannot be extrapolated to children receiving IVIG treatment.
A 1997 meta‐analysis (Terai 1997), reviewed fifteen US and Japanese multicentre, randomised controlled studies regarding the effect of various doses of intravenous gamma globulin (IVIG) administered within the first seven days of illness on the prevalence of coronary artery abnormalities in Kawasaki disease. The authors state that the prevalence of coronary abnormalities was inversely related to the total dose of IVIG and was independent of the aspirin dose. However, none of the RCTs included actually directly compared ASA of different doses, ASA versus placebo or IVIG, or ASA versus IVIG alone. Therefore these results should be approached with caution.
A further meta‐analysis (Durongpisitkul 1995), reviewed 24 articles (28 studies). These included both retrospective and prospective studies, and the only included RCTs randomised IVIG, not ASA. This meta‐analysis pooled data from the sample populations from the various studies and analysed the resulting data set. Although the authors concluded that there was no statistically significant difference in the incidence of CALs both at 30 and 60 days between the high IVIG and low ASA versus high IVIG and high ASA groups, this was based on non‐random treatment allocation between high and low dose ASA.
The very limited evidence from the comparison studies above seem to concur that there was no demonstrable effect in reduction of CALs from salicylate treatment. However, the studies are underpowered, and important treatment effects are still possible. The non‐randomised study (Akagi 1991) and the possibly randomised comparison study (Melish 1992) did both find a relationship between increased ASA dosage and reduction in the duration of fever.
Authors' conclusions
Implications for practice.
We found one randomised controlled trial (RCT) examining the effect of ASA for the treatment of Kawasaki disease in children. This did not demonstrate any effect of salicylate in addition to IVIG on the rate of coronary artery lesions when compared with IVIG alone, but with very wide confidence limits. Although a non‐randomised study and a study where the method of treatment allocation could not be confirmed, reported no association between ASA in combination with IVIG or ASA alone, or low dose ASA compared with high dose ASA with effect on CALs, they did find a reduction in duration of fever in children treated with high dose ASA. Neither reported any deleterious effects from ASA administration. Thus, until good quality RCTs are carried out, the existing evidence from RCTs does not permit any recommendation for or against treatment of children with Kawasaki disease with salicylate in combination with IVIG.
Implications for research.
As there are theoretical grounds for considering that ASA, with its anti‐inflammatory and anti‐platelet properties, might help to prevent coronary artery disease, high quality randomised controlled trials on the efficacy of aspirin (or other similar drugs) used as an adjunct to high dose IVIG in the treatment of Kawasaki disease need to be performed, especially in the light of concerns that aspirin use in children to treat fever can have adverse effects. Given the very low rate of coronary artery abnormalities in children treated with IVIG, and the low rate of serious adverse effects from aspirin use in children, any trial would need to be extremely large in order to achieve adequate power.
What's new
| Date | Event | Description |
|---|---|---|
| 30 January 2009 | New search has been performed | Searches were re‐run and no new trials found. Search dates amended and minor changes made to the text. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 12 August 2008 | Amended | Converted to new review format. |
Acknowledgements
The review authors would like to thank Heather Maxwell for her searches of the Cochrane Peripheral Vascular Diseases Trials Register and the Consumer Network for their contribution to the plain language summary.
The authors would like to acknowledge the contribution made by Richmal Oates‐Whitehead and Kevin Roman to the protocol for this review.
Appendices
Appendix 1. Search strategy used to search Cochrane Central Register of Controlled Trials (CENTRAL)
| #1 | MeSH descriptor Mucocutaneous Lymph Node Syndrome explode all trees | 62 |
| #2 | mucocutan* near syndrome* | 82 |
| #3 | kawasaki* | 360 |
| #4 | (#1 OR #2 OR #3) | 368 |
| #5 | MeSH descriptor Anti‐Inflammatory Agents explode all trees | 25466 |
| #6 | MeSH descriptor Inflammation Mediators explode all trees | 8363 |
| #7 | aspirin* or salicyl* | 7619 |
| #8 | acetylsal* near acid* | 2032 |
| #9 | asa | 5383 |
| #10 | (anti‐inflam*) | 11684 |
| #11 | (antiinflam*) | 2922 |
| #12 | (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) | 44191 |
| #13 | (#4 AND #12) | 79 |
Appendix 2. Electronic databases search strategy
1. Kawasaki disease/ 2. Mucocutaneous Lymph Node Syndrome/ 3. 1 or 2 4. salicylate/ 5. salicylate$ 6. aspirin/ 7. asa 8. acetylsalicylic acid 9. 4 or 5 or 6 or 7 or 8 10. coronary aneurysm/ 11. coronary aneurysm$ 12. heart aneurysm/ 13. heart aneurysm$ 14. coronary vessel anomalies/ 15. 10 or 11 or 12 or 13 or 14 16. 3 and 9 17. 15 and 16
Data and analyses
Comparison 1. ASA + IVIG versus IVIG alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Coronary artery lesions within day 30 of illness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Coronary artery lesions at day 30 of illness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
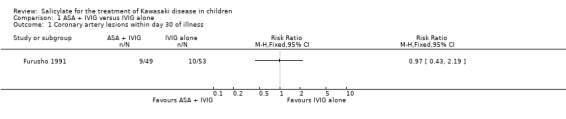
Comparison 1 ASA + IVIG versus IVIG alone, Outcome 1 Coronary artery lesions within day 30 of illness.
1.2. Analysis.
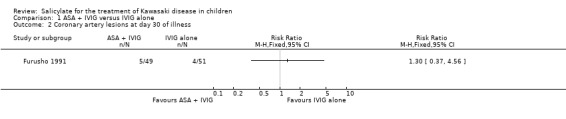
Comparison 1 ASA + IVIG versus IVIG alone, Outcome 2 Coronary artery lesions at day 30 of illness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Furusho 1991.
| Methods | Study design: multicentre randomised controlled trial. Method of randomisation: unclear. Number of centres: 16. Power calculations: no. Number of patients randomised: 163 (includes 3 arms ‐ only 102 children in 2 arms relevant). Number of patients analysed: 151. Number of patients excluded from analysis: 12. Reason for exclusions from analysis: not managed according to protocol. Intention‐to‐treat analysis: no. Source of funding: not stated. |
|
| Participants | Location: Japan, 16 institutes. Inclusion criteria: children with KD enrolled within 7 days of onset October 1985 to June 1986. Exclusion criteria: recurrent KD; coronary artery lesions on admission. |
|
| Interventions | Group 1: IVIG 200 mg/kg daily for 5 days + ASA 30 to 50 mg/kg/day in 3 divided doses until fever subsided, then 10 to 30 mg/kg daily once a day until "acute reaction had disappeared". Group 2: IVIG 200 mg/kg daily for 5 days without ASA. Group 3: ASA 30 to 50 mg/kg/day in 3 divided doses until fever subsided, then 10 to 30 mg/kg daily once a day until "acute reaction had disappeared". Only children in Groups 1 and 2 considered relevant for this review. |
|
| Outcomes | Coronary artery abnormalities before day 30 of illness; and at day 30 of illness. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
ASA: aspirin IVIG: intravenous imunoglobulin KD: Kawasaki disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akagi 1991 | QRCT |
| Barron 1990 | No comparison of interest. |
| Bavdekar 1998 | Case report |
| Bourillion 1989 | Review |
| Carriere 1998 | Not an RCT |
| Corregan 1986 | Not an RCT |
| Denning 1983 | Not an RCT |
| Durongpisitkul 1995 | Meta‐analysis |
| Falcini 2002 | Retrospective |
| Gersony 1998 | Not an RCT |
| Glode 1989 | No comparison of interest. |
| Harada 1991 | No comparison of interest. |
| Hicks 1979 | No comparison of interest. |
| Hsu 1993 | Retrospective |
| Hwang 1989 | Not an RCT |
| Hwang 1996 | Not an RCT |
| Ichida 1987 | Not an RCT |
| Jacobs 1978 | Not an RCT, but reports on ASA success. |
| Kato 1979 | This was a steroid comparison which is being dealt with by another review. |
| Kato 1987 | Guidelines |
| Koren 1985 | CCT |
| Koren 1986 | Review |
| Koren 1991 | Not an RCT |
| Koren 1998 | Not an RCT |
| Kusakawa 1987 | Not an RCT |
| Laupland 1999 | Review |
| Lee 1992 | Case report |
| Lux 1991 | Review |
| Mason 1999 | Review |
| Matsubara 1996 | Case reports |
| Melish 1982a | Review |
| Melish 1982b | Not an RCT |
| Melish 1992 | Abstract ‐ method of randomisation not stated. |
| Nakashima 1990 | Review |
| Newburger 1996 | No comparison of interest. |
| Okada 1991 | No comparison of interest. |
| Onouchi 1999 | Review |
| Pahl 1997 | Not an RCT |
| Rowley 1998 | Review |
| Saphyakhajon 1998 | Not an RCT |
| Sato 1999 | No comparison of interest. |
| Sundel 1997 | Not an RCT |
| Terai 1997 | Meta‐analysis |
| Tizard 1999 | Not an RCT |
ASA: aspirin CCT: controlled clinical trial QRCT: quasi randomised controlled trial RCT: randomised controlled trial
Contributions of authors
Samantha Love: Took the lead in writing the protocol and overall review, performed initial searches of databases for trials, was involved in determining if any trials were eligible for inclusion (with LH).
Harry Baumer: Jointly (with ROW) conceptualised the review, resolved discrepancies on trial selection, commented on and edited all sections of the protocol and review, led critical appraisal of the one included study, attempted to contact the author of one study potentially eligible for inclusion, and co‐ordinated responses to peer referee comments.
Linda Haines: Commented on and edited all sections of the protocol and review, was involved in determining whether any trials were eligible for inclusion.
Ian Maconochie: Commented on all sections of the review.
Amit Gupta: Commented on and edited all sections of the protocol and review.
Jaspal Dua: Commented on and edited all sections of the protocol and review.
Sources of support
Internal sources
Royal College of Paediatrics and Child Health, UK.
External sources
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Furusho 1991 {published data only}
- Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shuomiya K, Hayashidera T, et al. Intravenous gamma‐globulin for Kawasaki disease. Acta Paediatrica Japonica 1991;33(6):799‐804. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akagi 1991 {published data only}
- Akagi T, Kato H, Inoue O, Sato N. Salicylate treatment in Kawasaki disease: high dose or low dose?. European Journal of Pediatrics 1991;150(9):642‐6. [DOI] [PubMed] [Google Scholar]
Barron 1990 {published data only}
- Barron KS, Murphy DJ Jr, Silverman ED, Ruttenberg HD, Wright GB, Franklin W, et al. Treatment of Kawaski syndrome: a comparison of two dosage regimens of intravenously administered immune globulin. Journal of Pediatrics 1990;117(4):638‐44. [DOI] [PubMed] [Google Scholar]
Bavdekar 1998 {published data only}
- Bavdekar SB, Vaswani LK, Shalini H, Chandu KV, Gaikwad R, Kamat JR. Kawasaki disease. Journal of the Indian Medical Association 1998;96(8):257‐8. [PubMed] [Google Scholar]
Bourillion 1989 {published data only}
- Bourrillon A, Seban E, Vitoux‐Brot C. Kawasaki Syndrome [Le Syndrome de Kawasaki]. Presse Medicale 1989;18(18):933‐6. [PubMed] [Google Scholar]
Carriere 1998 {published data only}
- Carriere JP, Chaix Y, Grouteau E. Anti‐iinflammatory treatment in Kawasaki disease. Medecine et Maladies Infectieuses 1998;28(Spec. Issue. July):581‐5. [Google Scholar]
Corregan 1986 {published data only}
- Corregan JJ Jr. Kawasaki disease and the plight of the platelet. American Journal of Diseases in Childhood 1986;140(12):1223‐4. [DOI] [PubMed] [Google Scholar]
Denning 1983 {published data only}
- Denning DW. Kawasaki disease and aspirin [Letter]. Lancet 1983;2(8350):621. [DOI] [PubMed] [Google Scholar]
Durongpisitkul 1995 {published data only}
- Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta‐analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics 1995;96(6):1057‐61. [PubMed] [Google Scholar]
Falcini 2002 {published data only}
- Falcini F, Cimaz R, Calabri GB, Picco P, Martini G, Marazzi MG, et al. Kawasaki's disease in northern Italy: a multicentre retrospective study of 250 patients. Clinical & Experimental Rheumatology 2002;20(3):421‐6. [PubMed] [Google Scholar]
Gersony 1998 {published data only}
- Gersony WM. Predicting coronary aneurysms in Kawasaki disease [comment]. American Journal of Cardiology 1998;81(9):1162‐4. [DOI] [PubMed] [Google Scholar]
Glode 1989 {published data only}
- Glode MP, Joffe LS, Wiggins J Jr, Clarke SH, Hathaway WE. Effects of intravenous immune globulin on the coagulopathy of Kawasaki syndrome. Journal of Pediatrics 1989;115(3):469‐73. [DOI] [PubMed] [Google Scholar]
Harada 1991 {published data only}
- Harada K. Intravenous gamma‐globulin treatment in Kawasaki disease. Acta Paediatrica Japonica 1991;33(6):805‐10. [DOI] [PubMed] [Google Scholar]
Hicks 1979 {published data only}
- Hicks RV, Melish ME. Kawasaki syndrome: Rheumatic complaints and analysis of salicyate therapy. Arthritis & Rheumatism 1979;22(6):621. [Google Scholar]
Hsu 1993 {published data only}
- Hsu CH, Chan MR, Hwang FY, Kao HA, Hung HY, Hsu CH. Efficacy of plasmin‐treated intravenous gamma‐globulin for therapy of Kawasaki syndrome. Pediatric Infectious Disease Journal 1993;12(6):509‐12. [DOI] [PubMed] [Google Scholar]
Hwang 1989 {published data only}
- Hwang BT, Lin CY, Hsieh KS, Tsuei DH, Meng GC. High‐dose intravenous gamma‐globulin therapy in Kawasaki disease. Chung‐Hua Min Kuo Hsiao Erh Ko i Hseuh Hui Tsa Chih 1989;30(1):15‐22. [PubMed] [Google Scholar]
Hwang 1996 {published data only}
- Hwang KP, Wu JR, Huang LY, Liou CC, Huang TY. Clinical manifestations and effects of IVGG in patients with Kawasaki Disease. Kaoshiung Journal of Medical Sciences 1996;12(3):159‐66. [PubMed] [Google Scholar]
Ichida 1987 {published data only}
- Ichida F, Fatica NS, Engle MA, O'Loughlin JE, Klein AA, Synder MS, et al. Coronary artery involvement in Kawasaki syndrome in Manhattan, New York: risk factors and role of aspirin. Pediatrics 1987;80(6):828‐35. [PubMed] [Google Scholar]
Jacobs 1978 {published data only}
- Jacobs JC. Successful treatment of Kawasaki Disease with high dose aspirin. Pediatric Research 1978;12:494. [Google Scholar]
Kato 1979 {published data only}
- Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment of coronary artery involvement. Pediatrics 1979;63(2):175‐9. [PubMed] [Google Scholar]
Kato 1987 {published data only}
- Kato H, Koike S, Yokoyama T. Guideline for the treatment of cardiovascular sequelae in Kawasaki disease. Acta Paediatrica Japonica 1987;29(1):109‐14. [PubMed] [Google Scholar]
Koren 1985 {published data only}
- Koren G, Rose V, Lavi S, Rowe R. Probable efficacy of high‐dose salicylates in reducing coronary involvement in Kawasaki disease. JAMA 1985;254(6):767‐9. [PubMed] [Google Scholar]
Koren 1986 {published data only}
- Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of the risk factors for coronary aneurysms. Journal of Pediatrics 1986;108(3):388‐92. [DOI] [PubMed] [Google Scholar]
Koren 1991 {published data only}
- Koren G, Silverman E, Sundel R, Edney P, Newburger JW, Klein J, et al. Decreased protein binding of salicylates in Kawasaki disease. Journal of Pediatrics 1991;118(3):456‐9. [DOI] [PubMed] [Google Scholar]
Koren 1998 {published data only}
- Koren G, Schaffer F, Silverman E, Walker S, Duffy C, Stein L, et al. Determinants of low serum concentrations of salicylates in patients with kawasaki disease. Journal of Pediatrics 1998;112(4):663‐7. [DOI] [PubMed] [Google Scholar]
Kusakawa 1987 {published data only}
- Kusakawa S, Tatara K. Efficacies and risks of aspirin in the treatment of Kawasaki disease. Progress in Clinical & Biological Research 1987;250:401‐13. [PubMed] [Google Scholar]
Laupland 1999 {published data only}
- Laupland KB, Dele Davies H. Epidemiology, etiology and management of Kawasaki disease: state of the art. Pediatric Cardiology 1999;20(3):177‐83. [DOI] [PubMed] [Google Scholar]
Lee 1992 {published data only}
- Lee JH, Hung HY, Huang FY. Kawasaki disease with Reye syndrome: report of one case. Chung‐Hua Min Kuo Hsiao Erh Ko i Hseuh Hui Tsa Chih 1992;33(1):67‐71. [PubMed] [Google Scholar]
Lux 1991 {published data only}
- Lux KM. New hope for children with Kawasaki disease. Journal of Pediatric Nursing 1991;6(3):159‐65. [PubMed] [Google Scholar]
Mason 1999 {published data only}
- Mason WH, Takahashi M. Kawasaki syndrome. Clinical Infectious Diseases 1999;28(2):169‐85. [DOI] [PubMed] [Google Scholar]
Matsubara 1996 {published data only}
- Matsubara T, Mason W, Kashani IA, Kligerman M, Burns JC. Gastrointestinal hemorrhage complicating aspirin therapy in acute Kawasaki Disesae. Journal of Pediatrics 1996;128(5 Pt 1):701‐3. [DOI] [PubMed] [Google Scholar]
Melish 1982a {published data only}
- Melish ME. Kawasaki syndrome. Annual Review of Medicine 1982;33:569‐85. [DOI] [PubMed] [Google Scholar]
Melish 1982b {published data only}
- Melish ME. Kawasaki syndrome (the mucocutaneous lymph node syndrome). Pediatric Annals 1982;11(2):255‐68. [DOI] [PubMed] [Google Scholar]
Melish 1992 {published data only}
- Melish ME, Takahashi M, Shulman ST, Reddy DV, Mason WH, Elise Duffy C, et al. Comparison of low dose aspirin (LDA) vs. high dose aspirin (HDA) as an adjunct to intravenous gamma globulin (IVIG) in the treatment of Kawasaki disease. Pediatric Research 1992;31:170A. [Google Scholar]
Nakashima 1990 {published data only}
- Nakashima L, Edwards DL. Treatment of Kawasaki disease. Clinical Pharmacy 1990;9(10):755‐62. [PubMed] [Google Scholar]
Newburger 1996 {published data only}
- Newburger JW. Treatment of Kawasaki disease. Lancet 1996;347(9009):1128. [DOI] [PubMed] [Google Scholar]
Okada 1991 {published data only}
- Okada M, Satoh T, Hayashi T. Effect of intravenous gamma‐globulin on neutrophil function in Kawasaki disease. Acta Paediatrica Japonica 1991;33(6):785‐90. [DOI] [PubMed] [Google Scholar]
Onouchi 1999 {published data only}
- Onouchi Z, Kawasaki T. Overview of pharmocological treatment of Kawasaki disease. Drugs 1999;58(5):813‐22. [DOI] [PubMed] [Google Scholar]
Pahl 1997 {published data only}
- Pahl E. Cardiology for general pediatricians. Pediatric Annals 1997;26(2):129‐31. [Google Scholar]
Rowley 1998 {published data only}
- Rowley AH, Shulman ST. Kawasaki syndrome. Clinical Microbiology Review 1998;11(3):405‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saphyakhajon 1998 {published data only}
- Saphyakhajon P, Greene GR. Do we need high‐dose acetylsalicylic acid in Kawasaki disease?. Journal of Pediatrics 1998;133(1):167. [DOI] [PubMed] [Google Scholar]
Sato 1999 {published data only}
- Sato N, Sugimura T, Akagi T, Yamakawa R, Hashino K, Eto G, et al. Selective high dose gamma‐globulin treatment in Kawasaki disease: assessment of clinical aspects and cost effectiveness. Pediatrics International 1999;41(1):1‐7. [DOI] [PubMed] [Google Scholar]
Sundel 1997 {published data only}
- Sundel RP, Newburger JW. Management of acute Kawasaki disease. Progress in Pediatric Cardiology 1997;6(3):203‐9. [Google Scholar]
Terai 1997 {published data only}
- Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. Journal of Pediatrics 1997;131(6):888‐93. [DOI] [PubMed] [Google Scholar]
Tizard 1999 {published data only}
- Tizard EJ. Recognition and management of Kawasaki disease. Current Paediatrics 1999;9(2):97‐101. [Google Scholar]
Additional references
Curtis 1995
- Curtis N, Zheng R, Lamb JR. Evidence for a superantigen mediated process in Kawasaki disease. Archives of Disease in Childhood 1995;72(4):308‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 1997
- Curtis N. Kawasaki Disease. BMJ 1997;315(7104):322‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dajani 1993
- Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation 1993;87(5):1776‐80. [DOI] [PubMed] [Google Scholar]
Dhillon 1993
- Dhillon R, Newton L, Rudd PT, Hall SM. Management of Kawasaki disease in the British Isles. Archives of Diseases in Children 1993;69(6):631‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harnden 2002
- Harnden A, Alves B, Sheikh A. Rising incidence in Kawasaki disease in England: analysis of hospital admission data. BMJ 2002;324(7351):1424‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 1995
- Kato H, Akagi T, Sugimura T, Sato N, Kazue T, Hashino K, et al. Kawasaki disease. Coronary Artery Disease 1995;6(3):194‐206. [PubMed] [Google Scholar]
Kawasaki 1967
- Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involement with specific desquamation of the fingers and toes in children. Japanese Journal of Allergy 1967;16(3):178‐222. [PubMed] [Google Scholar]
Leung 1993
- Leung DY, Messmer HC, Fulton DR, Murray DL, Kotzin BL, Schlievert PM. Toxic shock syndrome toxin‐secreting Staphylococcus aureus in Kawasaki syndrome. Lancet 1993;342(8884):1385‐8. [DOI] [PubMed] [Google Scholar]
Leung 1995
- Leung DY, Giorno RC, Kazemi LV, Flynn PA, Busse JB. Evidence for superantigen involvement in cardiovascular injury due to Kawasaki syndrome. Journal of Immunology 1995;155(10):5018‐21. [PubMed] [Google Scholar]
Liu 2001
- Liu H, Zhou T, Wang X. Steroid hormone treatment for Kawasaki disease in children (Protocol for a Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 4. [Art. No.: CD003284. DOI: 10.1002/14651858.CD003284] [Google Scholar]
Mantel 1957
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
Oates‐Whitehead 2003
- Oates‐Whitehead RM, Baumer JH, Haines L, Love S, Maconochie IK, Gupta A, et al. Intravenous immunoglobulin for the treatment of Kawasaki disease in children (Cochrane Review). Cochrane Database of Systematic Reviews 2003, Issue 4. [Art. No.: CD004000. DOI: 10.1002/14651858.CD004000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowley 1987
- Rowley AH, Gonzalez ‐Crussi F, Gidding SS, Duffy CE, Shulman ST. Incomplete Kawasaki disease with coronary artery involvement. Journal of Pediatrics 1987;110(3):409‐13. [DOI] [PubMed] [Google Scholar]
Royle 1998
- Royle JA, Williams K, Elliot E, Sholler G, Nolan T, Allen R, et al. Kawasaki disease in Australia,1993‐1995. Archives of Disease in Childhood 1998;78(1):33‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witt 1999
- Witt MT, Minich LL, Bohnsack JF, Young PC. Kawasaki disease: more patients are being diagnosed who do not meet American Heart Association criteria. Pediatrics 1997;104(1):e10. [DOI] [PubMed] [Google Scholar]
Yanagawa 2001
- Yanagawa H, Nakamura Y, Yashiro M, Oki I, Hirata S, Zhang T, et al. Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics 2001;107(3):E33. [DOI] [PubMed] [Google Scholar]


