Abstract
Aim:
To mimic the ultrastructural morphology of the meniscus with nanofiber scaffolds coupled with controlled growth factor delivery to modulate cellular performance for tissue engineering of menisci.
Methods:
The authors functionalized collagen nanofibers by conjugating heparin to the following growth factors for sustained release: PDGF-BB, TGF-β1 and CTGF.
Results:
Incorporating growth factors increased human meniscal and synovial cell viability, proliferation and infiltration in vitro, ex vivo and in vivo; upregulated key genes involved in meniscal extracellular matrix synthesis and enhanced generation of meniscus-like tissue.
Conclusion:
The authors' results indicate that functionalizing collagen nanofibers can create a cell-favorable micro- and nanoenvironment and can serve as a system for sustained release of bioactive factors.
Keywords: : CTGF, heparin conjugation, meniscus, nanofibers, PDGF-BB, protein scaffolds, TGF-β1, tissue engineering
Lay abstract
Meniscal tears are a common injury to the part of the knee called the meniscus. Loss of meniscal tissue can lead to arthritis. In this study, the authors aimed to recreate the structure of the human meniscus using very thin (nanometers in diameter) fibers made of collagen. The authors also attached proteins called growth factors to the fibers. The addition of these proteins increased the growth rate of cells collected from human knee tissue. The levels of important genes involved in meniscal tissue formation were increased in these cells. These results show that adding proteins such as growth factors to collagen nanofibers can create an environment beneficial to growing meniscal tissue. Successful development of this technology could help in repairing meniscal damage in people.
Meniscal tears are among the most frequent orthopedic injuries leading to degeneration of knee articular cartilage [1]. Given the limited capacity for intrinsic regeneration in adult menisci, there is a significant unmet need for the repair and restoration of meniscal function after injury or degeneration [2]. Cell-based tissue engineering is an attractive approach for recapitulating the structure and function of the tissue, offering new treatment modalities for repair of meniscal tears and eventually enabling the replacement of lost meniscal tissue with a tissue-engineered construct [3,4].
Scaffolds with high surface area, porosity and interconnected 3D constructs are useful for facilitating cell attachment, enhancing the diffusing of nutrients and mimicking mechanical properties of the tissue. Electrospinning has been proposed for fabricating tissue with structural and mechanical properties mimicking native meniscal tissue [5,6]. The authors have previously electrospun PLA and collagen to replicate the nano- and microstructural organization and mechanical properties of the meniscus [5,7]. The authors found that scaffolds composed of electrospun collagen enhanced cell attachment, proliferation and tissue neogenesis relative to scaffolds composed of PLA.
Surface functionalization immobilizes biomolecules on the fiber surface via a chemical bond. This approach is predominantly used to control the release of bioactive proteins [8]. As one of many methods of surface functionalization, heparin is a naturally occurring highly sulfated glycosaminoglycan that can bind, release and protect multiple growth factors, including bFGF, TGF-β, VEGF, PDGF-BB and BMP-6 [9–12]. Heparin has therefore been widely investigated as a drug-delivery mechanism [13].
Bioactive molecules that can specifically stimulate cells to produce collagen are likely to be important with regard to generating neomeniscal tissue. Specifically, growth factors that enhance cell proliferation, differentiation and synthesis of extracellular matrix are valuable [14]. TGF-β is very effective at stimulating the production of glycosaminoglycans by meniscal cells in culture [15]. CTGF induces migration of endogenous mesenchymal stem cells, is profibrogenic and has been tested for meniscal healing in previous studies [16–18]. Another useful candidate is PDGF, which stimulates the proliferation of meniscal cells and is also known to play an important role in inducing angiogenesis [19]. The authors decided to study the effects of these growth factors because of their potential complementary advantages to meniscal tissue engineering. The objectives of the authors' study were to evaluate the effect of three growth factors (PDGF-BB, TGF-β1 and CTGF) on the upregulation of key genes involved in meniscal tissue synthesis, to document the release kinetics of growth factors coupled to heparin-conjugated collagen fibers and to analyze the effect of these heparin-conjugated growth factors on cell proliferation, matrix synthesis and potential for meniscal repair.
Methods
Fabrication of electrospun collagen type I scaffolds
Acid-soluble bovine collagen type I (Semed S; DSM Biomedical, PA, USA) at 16% (w/v) was dissolved in 20× phosphate-buffered saline (PBS) in absolute ethanol at a ratio of 1:1 v/v as described previously [5,6]. The collagen solution was placed in a syringe, which was controlled by a syringe pump (KDS200; KD Scientific Inc., MA, USA) operating at a feed rate of 0.1 ml h-1. A Teflon tube (Scientific Commodities, Inc., AZ, USA) connected the syringe to a 21-gauge needle. To spin the aligned fibers, a Ø90 × W200 portable DC90 stainless steel drum with a PTFE body (NanoNC, Seoul, South Korea) rotating at approximately 2400 r.p.m. was placed 12 cm from the needle tip, which was tilted at 45° to the drum. A portable NNC 30-kV, 2-mA, voltage-regulated power supply (NanoNC) generated a DC voltage of 18 kV between the syringe needle and rotating drum to eject the polymer jet. Electrospinning was performed in a fume hood under clean room conditions. Equipment surfaces were sterilized by 70% ethanol. The drum was covered with aluminum foil. Electrospun collagen nanofibers deposited on the aluminum foil were collected in the form of a cylindrical sheet, which was removed from the foil and cut into a flat sheet. Collagen scaffolds were crosslinked by soaking the sheets in 0.25% glutaraldehyde (Sigma-Aldrich, MO, USA) in absolute ethanol for 1 h. After fixation, the scaffolds were washed three times with absolute ethanol for 10 min per wash and stored at 4°C.
Heparin conjugation of electrospun scaffolds
An overview of heparin conjugation of growth factors to electrospun collagen fibers is illustrated in Supplementary Figure 1. In the first step of the process, the reactive amine groups (–NH2) on the collagen scaffolds are conjugated to the carboxyl groups (–COOH) on the heparin molecules to create an amide covalent bond. The negatively charged sulfonic groups (–SO3-) in the heparin molecules then bind growth factors via electrostatic interaction. Heparin conjugation of the discs of aligned electrospun collagen scaffolds was performed as follows. Collagen scaffolds (10 mm in diameter) were equilibrated with 0.05 M MES buffer (pH 5.6; Sigma-Aldrich) for 30 min. Heparin sodium salt was dissolved at a concentration of 1 mg/ml in 0.05 M MES buffer containing 25 mM EDC/10 mM NHS to activate the carboxyl groups of heparin. A total of 1 ml of this heparin solution was added to each sample, and the samples were incubated at room temperature for 4 h with gentle shaking for chemical conjugation between the carboxyl groups of heparin and the amine groups of collagen. After 4 h, the heparin-conjugated collagen scaffolds were washed with 1× PBS buffer three times. The amount of conjugated heparin was quantified using a toluidine blue assay. The collagen scaffolds (n = 3) were incubated with 1 ml of toluidine blue solution (0.4 mg/ml toluidine blue O, 2 mg/ml NaCl2 and 0.1 M HCl) for 2 h at room temperature with gentle agitation to create dye–heparin complexes. Scaffolds were rinsed with distilled water twice for 5 min, and the residual toluidine blue that was bound to the heparin was solubilized with a mixture of 0.1 M NaOH and absolute ethanol (1:4) and the absorbance of the resulting solution measured on a SpectraMax 384 plate reader (Molecular Devices, CA, USA) at 530 nm. The collagen scaffolds were incubated in 1× PBS (pH 7.0) with recombinant human PDGF-BB or TGF-β1 (R&D Systems, MN, USA) or CTGF (PeproTech, Inc., NJ, USA) at a concentration of 100 ng/ml for 12 h at 4°C. After each growth factor immobilization, collagen scaffolds were washed three times with 1× PBS and stored in 1× PBS at 4°C.
Release kinetics of PDGF-BB, TGF-β1 & CTGF from heparin-conjugated collagen scaffolds
To remove unbound growth factors, the scaffolds (n = 3 per condition) were washed three times with 1 ml release buffer composed of PBS with 0.1% (wt/v) bovine serum albumin. Growth factors in the wash supernatant were measured and used to calculate the amount of growth factor bound to the scaffolds (total amount of bound growth factor = original growth factor solution – quantity of growth factor in residual supernatant). To measure growth factor release rates, replicate growth factor-conjugated collagen scaffolds were placed in 1 ml release buffer in sterile 1.5-ml tubes at 37°C for up to 40 days without shaking or stirring. The supernatant was removed and replenished with fresh release buffer at predetermined time intervals. The harvested supernatants were centrifuged and stored at -80°C. Growth factor concentrations were analyzed by ELISA performed according to protocols provided by the manufacturers.
Tissue harvesting & cell isolation
Scripps Health Institutional Review Board approval (IRB-14-6320) was obtained to study deidentified human tissue samples provided by tissue banks. Normal human menisci and synovial tissues were harvested from six donors (mean age: 31 ± 8.9 years; age range: 17–39 years; sex: three females and four males). In a previous study, the authors did not find significant differences in the meniscogenic performance of cells harvested from vascular or avascular regions of the meniscus [5]. As previously described [20], the avascular meniscal region (the inner two-thirds of each meniscus) and synovial tissues were harvested, rinsed with sterilized 1× PBS, minced with a surgical blade and digested overnight at 37°C with collagenase 2 mg ml-1 (C5138; Sigma-Aldrich) in DMEM (Mediatech, Inc., VA, USA) and 1% penicillin–streptomycin–Fungizone (Life Technologies, CA, USA). Digested tissues were filtered through 100-μm cell strainers (BD Biosciences, CA, USA), and cells were seeded in monolayer culture in DMEM supplemented with 10% calf serum (Omega Scientific, Inc., CA, USA) and 1% penicillin–streptomycin–glutamine (Life Technologies). The isolated meniscus and synovial cells were cultured for three passages before seeding onto collagen scaffolds.
Characterization of scaffolds
Fourier transform IR spectroscopy (PerkinElmer, MA, USA) was carried out to document chemical changes after heparin conjugation (Supplementary Figure 1).
Cell seeding
Human meniscal and synovial cells were seeded at a nominal cell density of 0.5 × 106 per 10-mm diameter disc, averaging 2.27 mm in thickness, in the following types of collagen scaffolds: collagen scaffolds without heparin or any growth factors (COL), heparin-conjugated collagen scaffolds without growth factors (H–COL), PDGF-BB added to nonconjugated collagen scaffolds (P-COL), PDGF-BB added to heparin-conjugated collagen scaffolds (PH–COL), TGF-β1 added to nonconjugated collagen scaffolds (T–COL), TGF-β1 added to heparin-conjugated collagen scaffolds (TH–COL), CTGF added to nonconjugated collagen scaffolds (C-COL) and CTGF added to heparin-conjugated collagen scaffolds (CH–COL). Cell-seeded scaffolds were cultured as previously described [20]. Culture medium consisted of 3 ml of DMEM supplemented with 10% calf serum and 1% penicillin–streptomycin–glutamine for 1 day for initial cell attachment and scaffold colonization. The culture medium was then changed to serum-free ITS+ medium (Sigma-Aldrich) supplemented with the corresponding growth factor, with medium changes every 3 or 4 days for 2 weeks.
Cell viability
The viability of cells cultured on the scaffolds for 2 weeks was measured using calcein AM and ethidium homodimer-1 (LIVE/DEAD™ kit; Life Technologies) following the manufacturer's protocol [20]. Cells were imaged using an LSM 710 laser confocal scanning microscope (Zeiss, Oberkochen, Germany). For quantitative analysis (six donors, one image per donor), the fluorescence intensity of the live cells and dead cells was calculated with Image-Pro Premier 9.2 software (Media Cybernetics, Inc., MD, USA).
Gene expression
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and first-strand cDNA was made as per the manufacturer's protocol (High-Capacity cDNA Reverse Transcription Kits; Applied Biosystems, CA, USA) [20]. Quantitative reverse transcription PCR (n = 6) was performed using TaqMan® gene expression reagents (Applied Biosystems, CA, USA). COL1A1, COL2A1, ACAN, SOX9, COMP, THY-1, CHAD, PDGFRβ, CSPG4, ACTA2 and GAPDH were detected using Assays-on-Demand™ primer/probe sets (Applied Biosystems). Expression levels were normalized to GAPDH using the recommended ΔCt method, and fold change was calculated using the 2-ΔΔCT formula.
Ex vivo meniscal repair
The authors have previously reported on an ex vivo model to study repair of meniscal tears [21]. Meniscal tissue (n = 3) from the avascular zone was harvested from fresh young bovine knees, cut into rectangular sections (width: 12.25 ± 0.42 mm; length: 15.98 ± 1.45 mm; thickness: 2.37 ± 0.32 mm) and cultured in medium for 5 days in six-well plates with DMEM supplemented with 10% calf serum and 1% penicillin–streptomycin–glutamine. A longitudinal meniscal tear (nominally 10 mm in length) was created with a scalpel parallel to the circumferential direction of the collagen bundles. Scaffolds (COL, PH–COL, TH–COL or CH–COL) were freshly prepared (without seeding with any cells) and then inserted into the meniscal tears with the electrospun scaffold fibers aligned parallel to the circumferential fibers of the ex vivo meniscal tissue. The repaired meniscal explants were maintained in serum-free ITS+ medium supplemented without any growth factor at 8 ml well-1 for an additional 3 weeks with medium changes every 4–5 days.
In vivo subcutaneous implant model
All animal experiments were performed in compliance with protocols approved by the institutional animal care and use committee at The Scripps Research Institute (IACUC 18-0006). To evaluate the difference in in vivo response to growth factor-conjugated collagen scaffolds, four different scaffolds – COL, PH–COL, TH–COL and CH–COL (6-mm diameter discs each) – were implanted subcutaneously into wild-type mice (n = 6). Equal numbers of male and female mice weighing 25–30 g were used for the experiments. The scaffolds were washed thoroughly with PBS after staining with 0.20 mg/ml brilliant blue G dye to enable identification at the time of harvest. The animals were anesthetized with isoflurane. Four 0.5-cm incisions (one each on the cranial and caudal aspects on either side of the midline) were made on the dorsum of each animal and a subcutaneous pocket formed using blunt dissection. One randomized repair construct per group was placed in each pocket, and all incisions were closed by suturing with 6–0 Ethicon (8695G) and nonabsorbable Prolene (Ethicon, Inc., NJ, USA). The animals were killed by CO2 asphyxiation at 2 or 6 weeks.
Histology & immunohistochemistry
In vitro scaffolds, ex vivo meniscal explants and in vivo implanted scaffolds were fixed in Z-Fix (ANATECH LTD, MI, USA) for 2–3 days and decalcified in TBD-2 (Shandon, PA, USA). All samples were embedded in paraffin. Sections (5–7 μm thick) were stained with hematoxylin and eosin for histomorphometric analysis and with safranin O/fast green to assess glycosaminoglycan distribution. Additionally, paraffin sections from in vivo implants were stained with Masson's trichrome to determine the persistence of the collagen scaffolds. For detection of collagen type I and PDGFR-β by immunohistochemistry, paraffin sections were treated with hyaluronidase for 2 h and incubated overnight at 4°C with a primary antibody against collagen type I (ab34710, 1:200 dilution; Abcam, Cambridge, UK) and PDGFR-β (ab107169, 1:200 dilution; Abcam). ImmPRESS-AP anti-rabbit IgG (MP-5401; Vector Laboratories, CA, USA) for collagen type I antibody and ImmPRESS-HRP anti-rabbit IgG (MP-7401; Vector Laboratories) for PDGFR-β were used as secondary antibodies for 1 h and 30 min. An isotype control was used to monitor nonspecific staining. To quantify cell numbers for the ex vivo study and to determine the extent of cell migration into implanted collagen scaffolds, sections were stained with VECTASHIELD® mounting medium containing DAPI (Vector Laboratories).
Statistical analysis
Two-way analysis of variance and Mann–Whitney tests were used to detect statistically significant differences in gene expression between scaffold conditions and cell types. A p < 0.05 was considered statistically significant.
Results
Scaffold characterization
Fourier transform IR spectroscopy was performed on collagen scaffolds (Supplementary Figure 1). Heparin-conjugated collagen scaffolds displayed vibrations at 1236 cm-1 (SO2- asymmetric stretching). The characteristic absorption bands (S=O asymmetric stretch) between 1160 and 1260 cm-1 were attributed to the conjugated heparin. Moreover, the absorption of acetyl amine at 1650 cm-1 indicated stretching of C=O and bending of N–H. The spectrum of H–COL displayed higher absorption at 1650 cm-1 and 1597 cm-1 than the spectrum of COL, reflecting the formation of amide bonds between the amine groups of EDC and collagen and the carboxyl groups of heparin (Supplementary Figure 1A). Heparin concentration was threefold higher in H–COL scaffolds than COL scaffolds in the toluidine blue assay (Supplementary Figure 1C).
Immobilization of growth factors
The release of PDGF-BB, TGF-β1 and CTGF from heparin-conjugated collagen scaffolds was compared with the corresponding release from nonconjugated collagen scaffolds (Figure 1). H–COL scaffolds bound 76% of the original PDGF-BB in solution, whereas COL scaffolds bound 64%. H–COL scaffolds bound 78% of the original TGF-β1 in solution, whereas COL scaffolds bound 47%. H–COL scaffolds bound 45% of the original CTGF in solution, whereas COL scaffolds bound 40%. The nonconjugated collagen scaffolds exhibited a higher initial burst followed by a slower release, whereas the heparin-conjugated scaffolds exhibited a moderate initial burst release followed by a continuous release pattern over the same period. The cumulative release of all three heparin-conjugated growth factors was significantly higher than the release of the corresponding growth factors from nonconjugated scaffolds at each time point. Overall, the cumulative release of TGF-β1 was higher than either of the other growth factors. These results indicated that the high surface area of electrospun collagen resulted in absorption and subsequent rapid release of growth factors that were modulated by heparin conjugation.
Figure 1. . Growth factor release kinetics from collagen electrospun scaffolds.
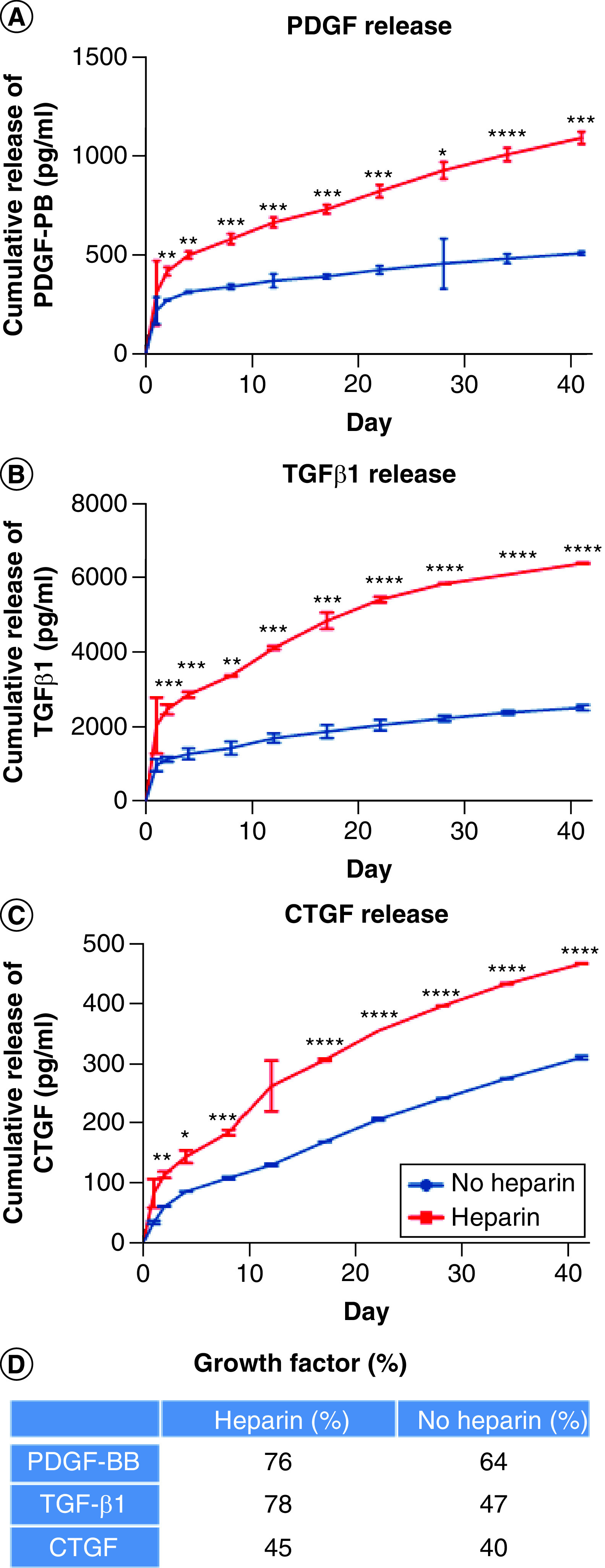
(A) PDGF-BB, (B) TGF-β1 and (C) CTGF were added to nonconjugated collagen electrospun scaffolds (no heparin) or to heparin-conjugated electrospun collagen scaffolds (Heparin) and cultured at 37°C for up to 40 days. Supernatants were collected at the indicated time points and analyzed for each growth factor by ELISA. Mann–Whitney test was used to determine significance (n = 3). (D) Growth factor loading efficiency (%).
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Cell seeding & viability
As demonstrated in confocal images, cell attachment, morphology and alignment provided evidence of high cell viability in heparin-conjugated collagen scaffolds (Figure 2) and nonconjugated collagen scaffolds (Supplementary Figure 2). In general, the viability of synovial cells was statistically equivalent to that of meniscal cells, with the exception of cells on H–COL and TGF-β1 bound to HEP-COL scaffolds (TH–COL). In addition, the viability of both meniscal and synovial cells on TH–COL scaffolds was higher than that observed with the other growth factors. Heparin conjugation alone (without growth factors) did not affect the viability of meniscal and synovial cells (Figure 2 & Supplementary Figure 2). The authors also found an interaction effect between heparin conjugation and growth factor type, with TGF-β1 enhancing viability of meniscal and synovial cells when conjugated to heparin (two-way analysis of variance: p < 0.001).
Figure 2. . Cell viability of human meniscal and synovial cells.
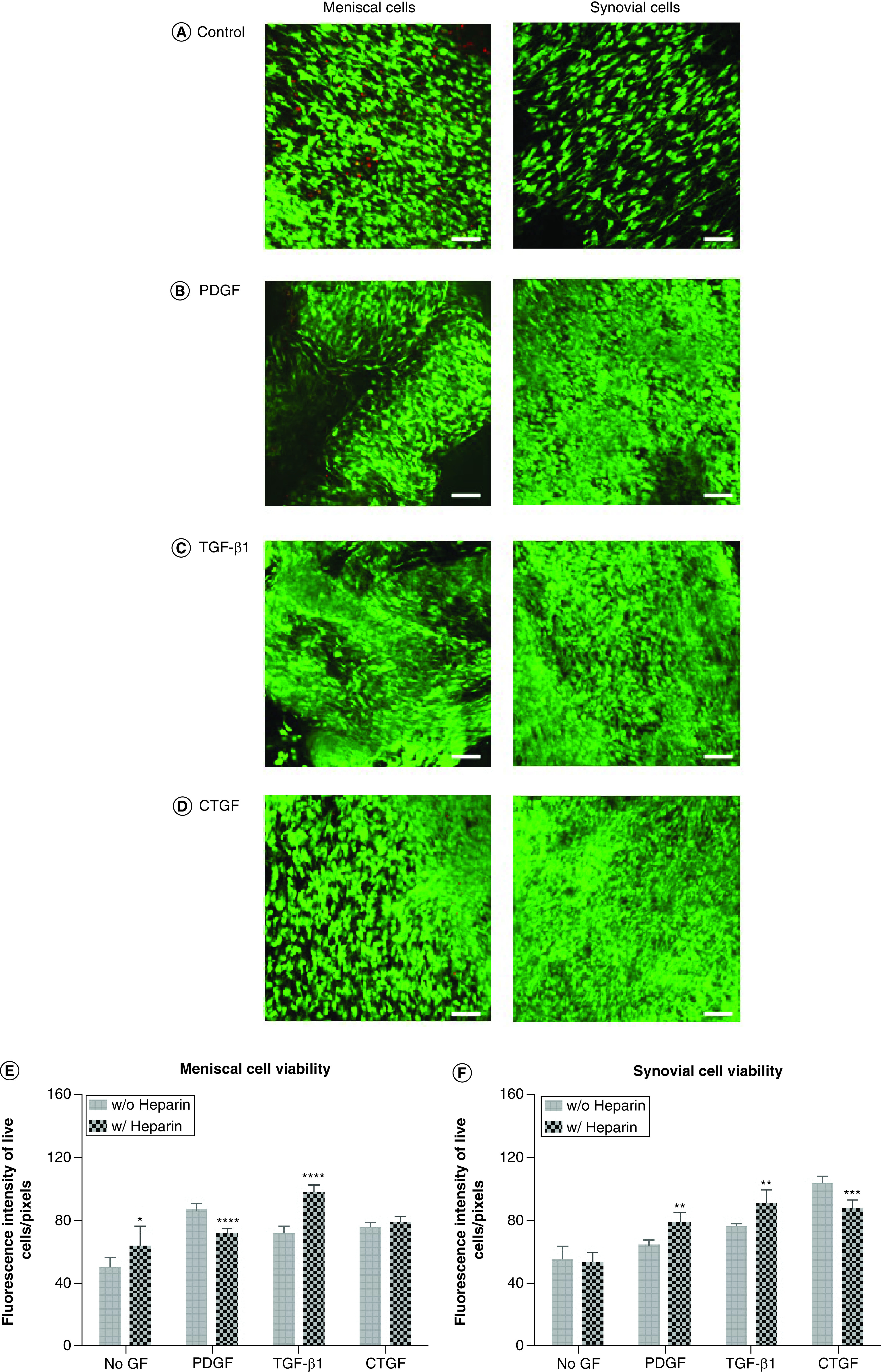
Viability of human meniscal avascular and synovial cells cultivated on (A) Control, (B) PDGF-BB, (C) TGF-β1 and (D) CTGF bound to heparin-conjugated collagen scaffolds. Magnification 10×. Scale bar = 200 μm. Quantitative analysis of fluorescence intensity of (E) live meniscal and (F) synovial cells in different growth factors on heparin-conjugated collagen scaffolds. Two-way ANOVA was used to determine significance (n = 6).
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
ANOVA: Analysis of variance.
Gene expression in response to growth factors
The authors previously documented changes in gene expression with dedifferentiation and redifferentiation of meniscal fibrochondrocytes [22]. Dedifferentiated cells in monolayer provide a useful reference for quantifying meniscogenic performance. In general, human meniscal and synovial cells cultured on different growth factors coated on all types of scaffolds expressed significantly higher levels of COL2A1, COL1A1, SOX9, THY-1, CHAD, PDGFRβ, CSPG4 and ACTA2 genes and significantly lower levels of ACAN relative to cells in monolayer culture (Figure 3 & Supplementary Figures 3 & 4). Heparin conjugation alone (without growth factor) resulted in significantly higher levels of SOX9, THY-1, CHAD, PDGFRβ and ACTA2 in synovial cells compared with meniscal cells (Supplementary Figure 3). Synovial cells expressed significantly higher levels of COL1A1, SOX9, THY-1, PDGFRβ and ACTA2 genes and significantly lower levels of ACAN relative to meniscal avascular cells. The TGF-β1-conjugated scaffolds generally induced significantly greater expression of COL1A1, ACAN, COMP, THY-1, CHAD, PDGFRβ and ACTA2 genes (Figure 3). In addition, compared with nonconjugated scaffolds, the presence of heparin generally induced significantly greater gene expression of PDGFRβ and ACTA2 – markers of perivascular cells (Figure 3 & Supplementary Figure 4) [23,24].
Figure 3. . Gene expression of human meniscal and synovial cells.
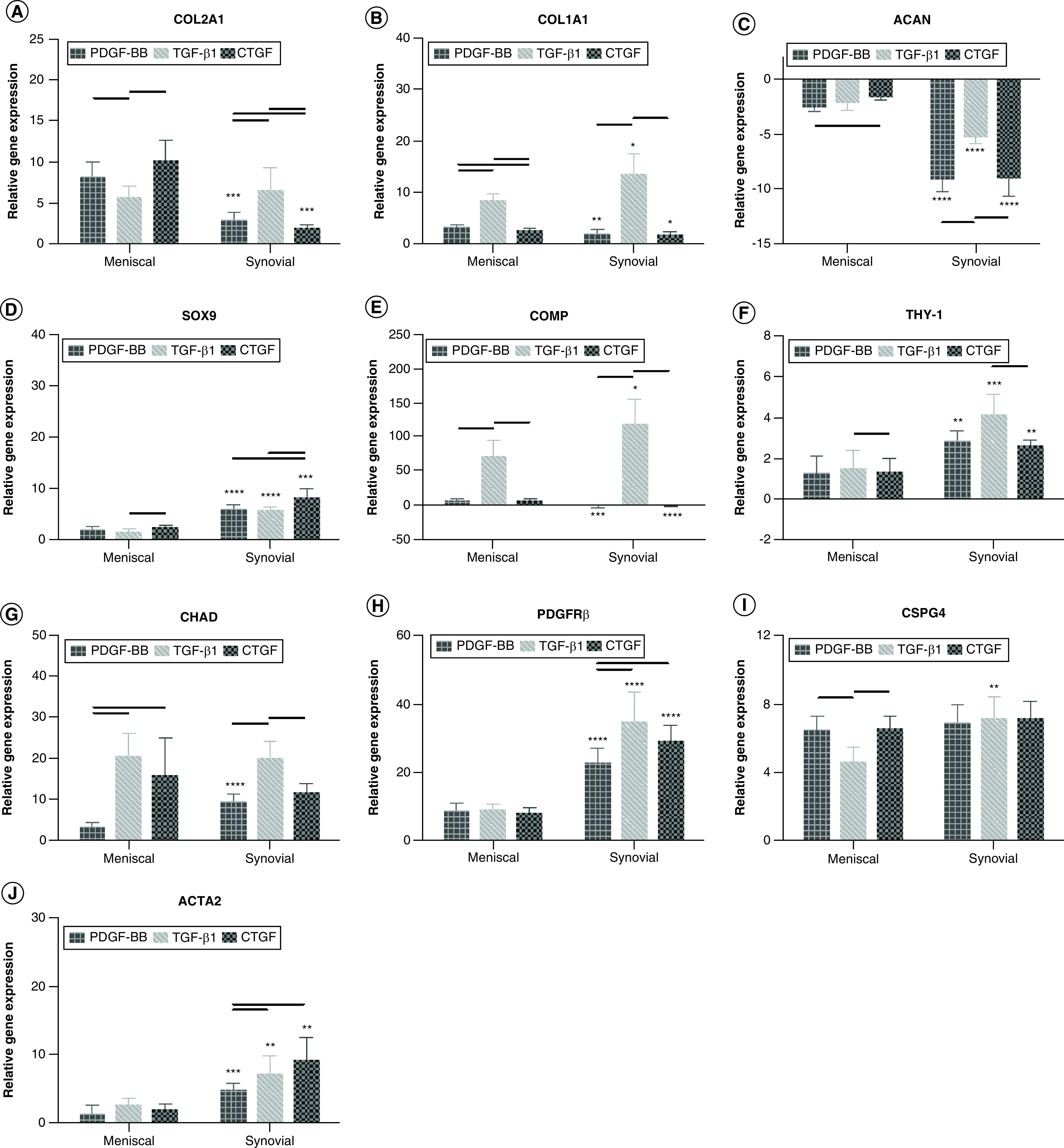
(A) COL2A1, (B) COL1A1, (C) ACAN, (D) SOX9, (E) COMP, (F) THY-1, (G) CHAD, (H) PDGFRβ, (I) CSPG4 and (J) ACTA2. Gene expression is relative to cells cultured under monolayer conditions. Two-way ANOVA and Mann–Whitney tests were used to determine significance (n = 6).
Horizontal line = p < 0.05 among growth factor groups.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 between cell types.
ANOVA: Analysis of variance.
Growth factors induce cell proliferation & migration & increase neotissue formation
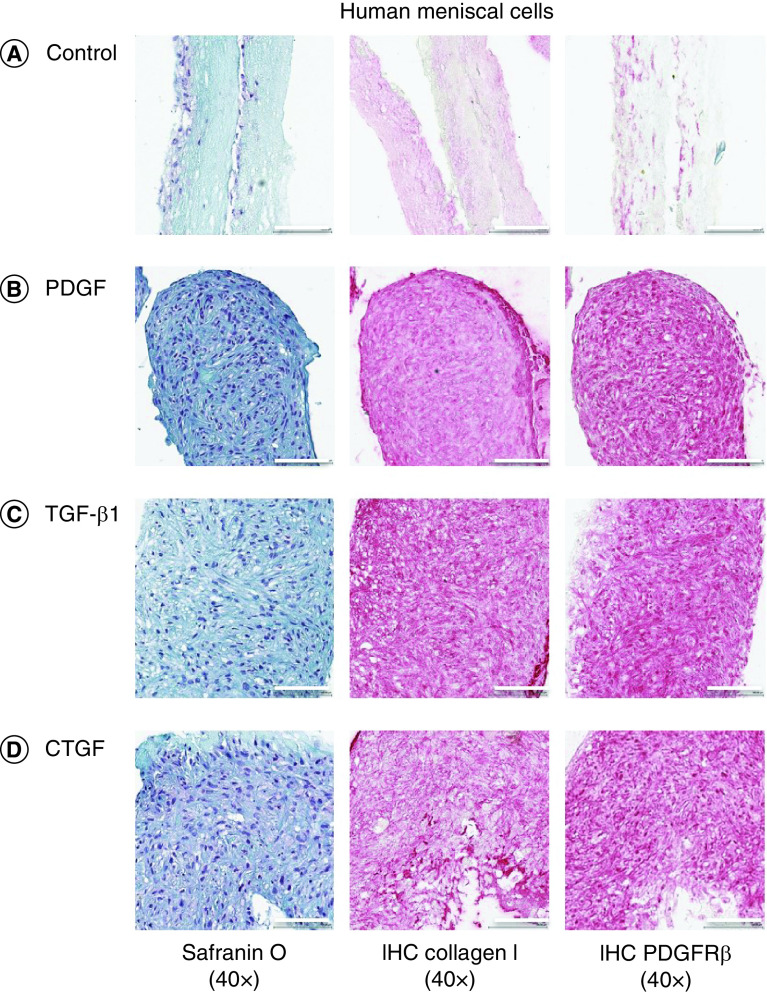
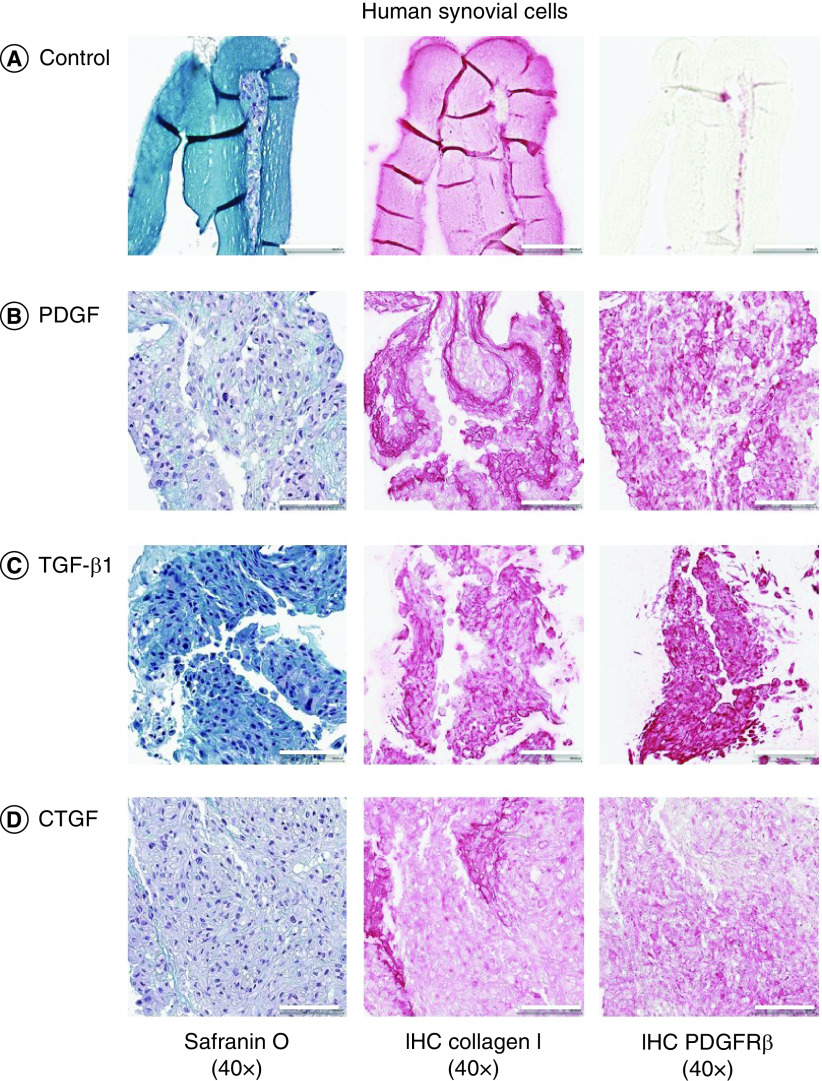
The authors assessed the efficacy of growth factors on cell migration and infiltration into growth factor-conjugated collagen and nonconjugated collagen scaffolds (Figures 4 & 5 & Supplementary Figures 5 & 6). Conjugating scaffolds with growth factors significantly increased the thickness of the tissues engineered from meniscal and synovial cells. After 2 weeks of culture, meniscal and synovial cells had migrated and infiltrated into scaffolds, as visualized by safranin O/fast green staining. By contrast, in the absence of growth factors, cells remained restricted to the surface of the scaffolds. On immunohistochemistry, significantly greater collagen type I deposition and PDGFRβ expression were noted in scaffolds that contained growth factors.
Figure 4. . Representative histological images of human meniscal cells.
Safranin O/fast green, collagen type I and PDGFRβ immunostaining of human meniscal avascular cells on different growth factors bound to heparin-conjugated collagen scaffolds. Magnification 40×. Scale bar = 100 μm.
Figure 5. . Representative histological images of human synovial cells.
Safranin O/fast green, collagen type I and PDGFRβ immunostaining of human synovial cells on different growth factors on heparin-conjugated collagen scaffolds. Magnification 40×. Scale bar = 100 μm.
Ex vivo response
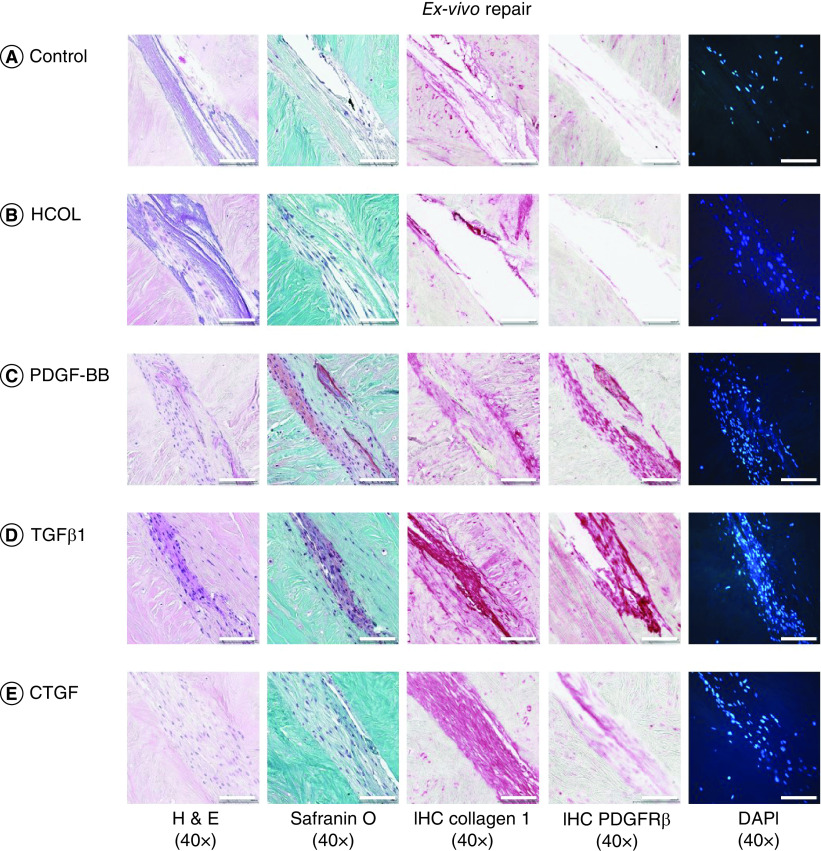
To evaluate the effect of growth factors on meniscal repair, collagen scaffolds conjugated to PDGF-BB, TGF-β1 and CTGF were implanted into an ex vivo model of bovine meniscal tear (Figure 6 & Supplementary Figure 7). After 3 weeks, implants containing any growth factor resulted in higher cell density in ex vivo host tissue within 100 μm of the graft–host interface – a significant increase compared with the nonconjugated collagen and heparin-conjugated collagen scaffolds without any growth factors. The addition of growth factors generated tissue that filled the tear and was histologically integrated into the host avascular tissue. On immunohistochemistry, significantly greater collagen type I staining and PDGFRβ expression were noted in cells migrating into scaffolds that were conjugated to TGF-β1 compared with other growth factors.
Figure 6. . Representative histological images of ex vivo meniscal repair.
Sections were stained with H&E, safranin O/fast green, collagen type I, PDGFRβ or DAPI. Surgically created tears were implanted with (A) nonconjugated collagen scaffolds, (B) heparin-conjugated collagen scaffolds, (C) PDGF-BB, (D) TGF-β1 or (E) CTGF bound to heparin-conjugated collagen scaffolds. Magnification 40×. Scale bar = 100 μm. Three replicates per bovine meniscus (n = 3). All constructs were cultured for 3 weeks and then processed for histology. The corresponding 10× magnification images are provided in Supplementary Figure 7.
H&E: Hematoxylin and eosin.
In vivo responses
To determine the potential for in vivo host cell migration, angiogenesis and implant integration, acellular growth factor-conjugated collagen and nonconjugated collagen scaffolds were implanted subcutaneously in mice. Within 2 weeks of implantation, a thin layer of tissue formed around the surface of the scaffolds (Supplementary Figure 8). These tissue layers on the scaffolds were rich in blood capillaries. More capillaries and cells invaded into the spaces of PDGF-BB- and TGF-β1-conjugated scaffolds compared with the nonconjugated collagen scaffolds and CTGF-conjugated scaffolds (Figure 7). Consistent with in vitro and ex vivo studies, significantly greater collagen type I matrix staining and PDGFRβ-positive cell expression were noted in cells migrating into scaffolds that contained TGF-β1 compared with other growth factors. At the end of 6 weeks, implanted scaffolds began to undergo remodeling and integration into host tissues, with loss of collagen I staining (Figure 8).
Figure 7. . Representative histological images of in vivo repair at 2 weeks.
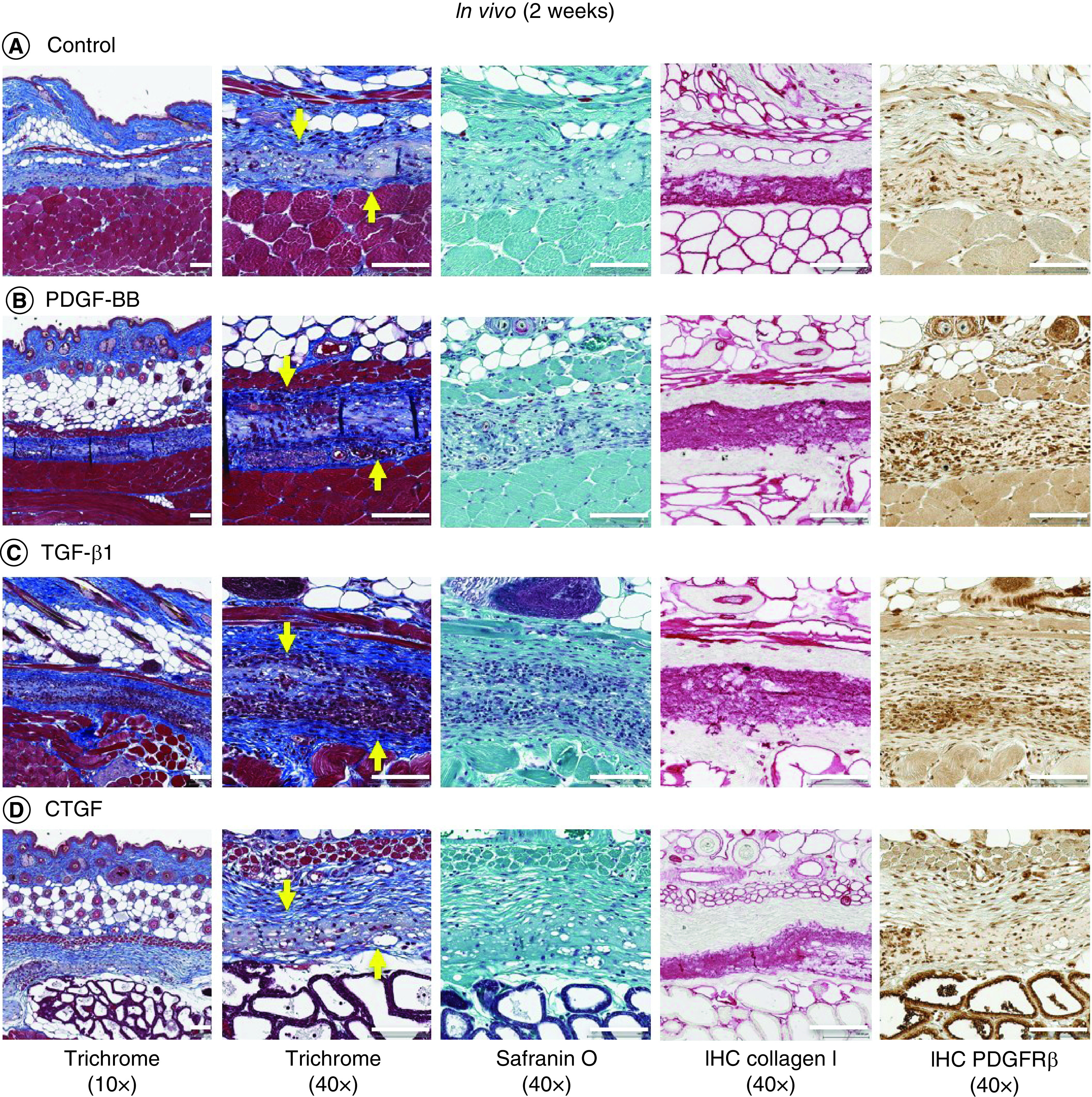
Trichrome (yellow arrow indicates scaffold implanted in subcutaneous tissue), safranin O/fast green, collagen type I and PDGFRβ immunostaining of (A) nonconjugated scaffolds, (B) PDGF, (C) TGF-β1 and (D) CTGF bound to heparin-conjugated collagen scaffolds after implantation in mice for 2 weeks. Low magnification 10×. Scale bar = 200 μm. High magnification 40×. Scale bar = 100 μm.
Figure 8. . Representative histological images of in vivo repair at 6 weeks.
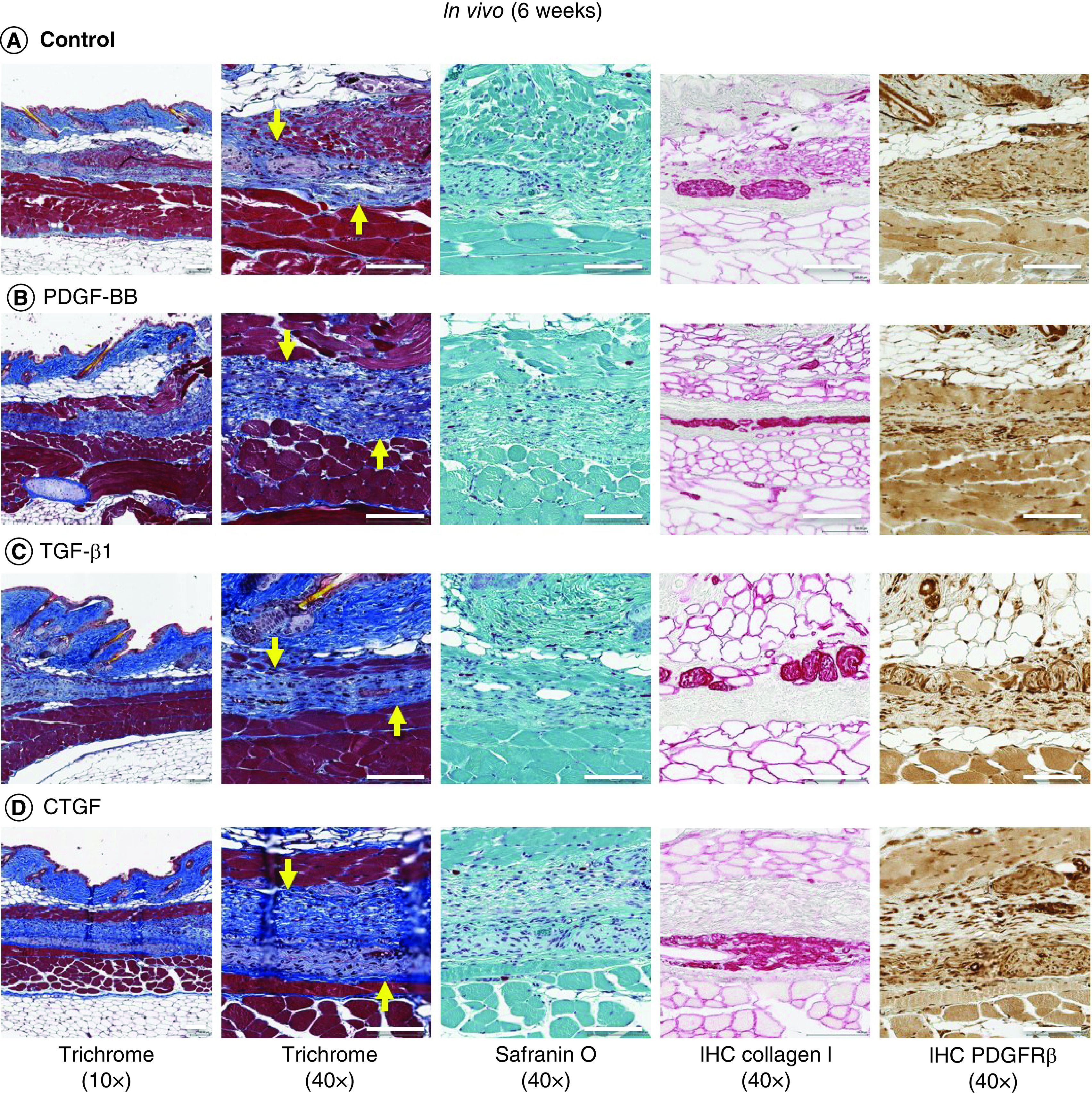
Trichrome (yellow arrow indicates scaffold implanted in subcutaneous tissue), safranin O/fast green, collagen type I and PDGFRβ immunostaining of (A) uncoated scaffolds, (B) PDGF, (C) TGF-β1 and (D) CTGF bound to heparin-conjugated collagen scaffolds after implantation in mice for 6 weeks. Low magnification 10×. Scale bar = 200 μm. High magnification 40×. Scale bar = 100 μm.
Discussion
Electrospun nanofibrous scaffolds are an attractive approach for meniscal tissue engineering. However, densely packed nanofibers can impede cell migration [25]. The authors used heparin conjugation to control the delivery of growth factors in electrospun collagen scaffolds to study the effect on cell migration and matrix synthesis. To the authors' knowledge, this is the first study to use electrospun collagen scaffolds conjugated to heparin and growth factors to show the potential for meniscal tissue engineering. As proof of concept for preclinical translation of delivery of meniscogenic bioactive molecules, the authors studied the ability of three growth factors (PDGF-BB, TGF-β1 and CTGF) to provide distinct biochemical cues to modulate cell survival, migration and proliferation and to induce differentiation of synovial cells. The authors used two cell types, meniscal cells from normal human meniscus and synovial cells, as a source of progenitor cells. The controlled local release of growth factors induced migration of human avascular meniscal cells and synovial cells throughout the scaffold and enhanced cell survival and proliferation and synthesis of meniscus-like neotissue.
The first objective of the present study was to devise a biocompatible scaffold that can be conjugated to growth factors with promise for meniscal applications. The authors had previously demonstrated that electrospinning can replicate the structural organization of meniscal tissue, with encouraging mechanical properties and cell compatibility, and found collagen scaffolds to be relatively more biocompatible than PLA, resulting in enhanced cell attachment and proliferation [5,7]. Collagen also provides a convenient method of heparin-conjugated delivery of growth factors. Heparin is a naturally occurring highly sulfated glycosaminoglycan with the ability to bind multiple growth factors, including bFGF, TGF-β, VEGF and PDGF-BB [9–12], and it therefore has been investigated as a drug-delivery mechanism [13]. The authors found that although growth factors could be passively adsorbed onto nonconjugated collagen scaffolds, this resulted in a high initial burst with a subsequent slower sustained release over 40 days, similar to that reported for PDGF-BB when added to PCL/gelatin scaffolds [26]. By contrast, heparin binding of growth factors moderated the initial burst release and resulted in higher levels of continuous release over the same period. The authors' results are consistent with reports of heparin conjugated to PCL scaffolds for drug delivery of bFGF and PDGF-BB [26–28].
The authors selected three different growth factors with differing actions for applications in meniscal tissue engineering. TGF-β is an important factor regulating meniscogenesis for tissue regeneration [5–7,21]. TGF-β1 is a more potent stimulator of protein and proteoglycan synthesis in meniscal explants than IGF-1 or bFGF [29]. CTGF has been implicated in cell migration, proliferation, angiogenesis and wound repair in general [30,31] as well as type I collagen synthesis in meniscal cells [32]. Sequential treatment with CTGF followed by TGF-β3 induces fibrochondrocytic differentiation of mesenchymal stem cells derived from bone marrow or synovium [17]. PDGF is a potent mitogen for cells of mesenchymal origin, such as progenitors, pericytes, fibroblasts and chondrocytes [33]. PDGF can significantly increase cell proliferation in meniscal explants [34] and cell migration of meniscal fibrochondrocytes [35]. The authors have previously shown that PDGF-BB induces migration of meniscal cells into the repair site of meniscal explants [6] and increases cell proliferation and infiltration in electrospun scaffolds seeded with human meniscal and synovial cells [20].
The authors compared these three growth factors for their ability to stimulate cell migration/distribution and differentiation, as measured by expression of meniscal ECM genes. Conjugating the scaffolds with three different growth factors induced higher levels of COL2A1, COL1A1, SOX9, THY-1, CHAD, PDGFRβ, CSPG4 and ACTA2 but lower levels of ACAN relative to cells in monolayer culture. ACAN and COL2A1 are typically coexpressed in hyaline cartilage [36]. It is possible that a collagen type I scaffold does not induce a hyaline-like cartilage response. TGF-β1 generally induced significantly greater gene expression in COL1A1, COMP, THY-1, CHAD, PDGFRβ and ACTA2 compared with PDGF-BB and CTGF. All three growth factors resulted in significant changes in gene expression and enhancement of cell proliferation, migration and synthesis of new tissue (relative to scaffolds without any growth factors). However, TGF-β1-bound scaffolds functioned the best over a variety of performance measures. TGF-β1 resulted in greater cell viability than CTGF or PDGF-BB. TGF-β1 also induced significantly greater gene expression of COL1A1, COMP, THY-1, CHAD, PDGFRβ and ACTA2 and significantly greater deposition of collagen type I. In vivo, TGF-β1 and PDGF-BB induced greater cell migration into scaffolds compared with PDGF-BB and scaffolds without any growth factors. Collectively, in vitro gene expression and ex vivo histology provided evidence of maintenance of phenotype in meniscal cells and efficacy of differentiation in synovial progenitors. An increase in cell migration, especially of PDGFRβ-positive cells, coupled with microvascular invasion is suggestive of a pericyte-like response with potential for in vivo repair.
The authors previously developed and characterized an ex vivo model to study the repair of meniscal tears and reported cell infiltration and cell migration into scaffolds as well as within the dense connective tissue of the meniscus [5–7,21]. In this model, the authors found that cell migration and infiltration into acellular scaffolds could be enhanced by growth factors conjugated to electrospun scaffolds and TGF-β1, resulting in greater collagen I deposition and enhanced integration into the host tissue compared with CTGF and PDGF-BB.
The authors also implanted acellular scaffolds subcutaneously in mice to assess the therapeutic potential of local growth factor delivery by enhancing host cell migration and angiogenesis in the short term and scaffold biodegradation and tissue remodeling in the long term. Overall, the authors found a common pattern of early angiogenesis at the surface of the implanted scaffold, accompanied by cell migration, followed later by scaffold degradation and absorption, eventually leading to remodeling and assimilation into host tissue. Scaffolds conjugated toTGF-β1 and PDGF-BB induced significantly greater cellular migration and angiogenesis, which supports potential use in tissue engineering for meniscal repair or replacement. The consistency of relevant findings across in vitro, ex vivo and in vivo models is encouraging and supports the ongoing use of the authors' models to screen multiple therapeutic approaches for enhancing meniscal repair.
Common cell sources studied for meniscal repair include meniscal fibrochondrocytes, articular chondrocytes and mesenchymal stem cells [1]. Although meniscal cells remain a standard for meniscogenesis, harvesting sufficient quantities of healthy autologous meniscal cells is clinically challenging. In the present study, the authors investigated the performance of cells from two sources: human meniscal fibrochondrocytes and synovial cells. Meniscal cells are among the most widely studied cell type for meniscal regeneration and tissue engineering. The authors have previously characterized the cell surface marker profiles of cells isolated from different regions of the meniscus [37]. The authors have also compared changes in gene expression associated with meniscal dedifferentiation and redifferentiation in monolayer and 3D culture, which indicated a potential use for meniscogenic tissue generation [22]. However, meniscal cells are not readily available for clinical applications. Multipotent progenitors are easily harvested from synovium and can be readily expanded in culture [38]. These cells have attractive differentiation potential relative to progenitors from muscle and bone marrow. Human synovium-derived progenitors are more chondrogenic than bone marrow-derived progenitors [39]. The authors previously documented the performance of cells from various tissue sources and found cells from synovium and infrapatellar fat pad to be the most meniscogenic [6]. Others have shown that intra-articular injection or implantation of aggregates of synovial cells promoted meniscal regeneration in rats, rabbits, pigs and primates [40–43]. In the present study, the cell viability and proliferation of synovial cells were at least equivalent to those of meniscal cells. In general, synovial cells expressed significantly higher levels of COL1A1, SOX9, THY-1, PDGFRβ and ACTA2 genes and significantly lower levels of ACAN relative to meniscal avascular cells in response to heparin-conjugated growth factors. These results support the potential of synovial cells for meniscal tissue engineering.
This study has several limitations. The authors' release rates in vitro may not reflect in vivo release rates, especially under mechanical loading. The authors studied the response of only two cell types to three growth factors. It is possible that other cell sources will perform differently and may respond differently to other growth factors. The in vivo subcutaneous implantation does not recapitulate the synovial joint in terms of biochemical milieu, type of host tissue, immune response and mechanical loading. Evaluation in a large animal model of meniscal regeneration is needed for clinical translation.
Conclusion
The authors fabricated a novel scaffold using electrospinning to generate collagen nanofibers conjugated to heparin and found that three factors (PDGF-BB, TGF-β1 and CTGF) bind with high efficiency, resulting in sustained release. PDGF-BB and TGF-β1 increased cell viability, proliferation and migration of host cells into scaffolds and upregulated key genes involved in meniscal tissue synthesis. The performance of human synovial cells was at least equivalent to that of avascular meniscal cells, which supports their potential for use in meniscal tissue engineering. Heparin-conjugated collagen nanofibrous scaffolds can create a cell-favorable nanoenvironment and can serve as a model system for studying the effects of sustained release of bioactive factors.
Summary points.
The objective of this study was to determine if heparin conjugation could control delivery of growth factors to enhance cellular performance for meniscal tissue engineering.
Heparin conjugation to electrospun collagen fibers increased binding and prolonged release of the following growth factors: PDGF-BB, TGF-β1 and CTGF.
Cell viability on scaffolds conjugated to TGF-β1 was higher than that observed on scaffolds conjugated to other growth factors.
All three growth factors increased expression of the following meniscogenic genes: COL2A1, COL1A1, SOX9, THY-1, CHAD, PDGFRβ, CSPG4 and ACTA2.
TGF-β1-conjugated scaffolds induced significantly greater gene expression of COL1A1, ACAN, COMP, THY-1, CHAD, PDGFRβ and ACTA2 than the other two growth factors.
Conjugating growth factors in general increased the thickness of tissues formed by meniscal and synovial cells.
Conjugating growth factors generated neotissue that filled a meniscal tear and integrated into host tissue in an ex vivo explant.
In vivo implantation of PDGF-BB- and TGF-β1-conjugated collagen scaffolds resulted in greater cellular migration of host cells onto the scaffold than that observed with nonconjugated scaffolds or scaffolds conjugated to CTGF.
Supplementary Material
Acknowledgements
The authors would like to thank Emily Lee and April Damon for technical assistance.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/nnm-2021-0313
Author contributions
J Baek, MK Lotz and DD D'Lima designed the study. J Baek generated the collagen electrospun scaffolds and conjugated them to heparin. J Baek conducted and interpreted the scanning electron microscopy and Fourier transform IR spectroscopy analyses. J Baek performed the cell culture studies and quantitative PCR analysis. J Baek and KI Lee conducted and interpreted the histological analysis. J Baek conducted the ex vivo repair model. J Baek and HJ Ra performed the in vivo subcutaneous implant model. J Baek, MK Lotz and DD D'Lima wrote the initial draft and revised the manuscript. All authors discussed the results and approved the final version of the manuscript.
Financial & competing interests disclosure
This work was supported by the National Institutes of Health (P01 AG007996. 2016), Shaffer Family Foundation and Donald and Darlene Shiley. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time, as they form part of an ongoing study.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Grogan SP, Baek J, D'Lima DD. Meniscal tissue repair with nanofibers: future perspectives. Nanomedicine (Lond.) 15(25), 2517–2538 (2020). [DOI] [PubMed] [Google Scholar]; • Useful review of the state of the art application of electrospinning in meniscal tissue engineering.
- 2.Whitehouse MR, Howells NR, Parry MC et al. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study. Stem Cells Transl. Med. 6(4), 1237–1248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Clinical study of mesenchymal stem cells in a collagen scaffold.
- 3.Morgan CD, Wojtys EM, Casscells CD, Casscells SW. Arthroscopic meniscal repair evaluated by second-look arthroscopy. Am. J. Sports Med. 19(6), 632–637 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Salata MJ, Gibbs AE, Sekiya JK. A systematic review of clinical outcomes in patients undergoing meniscectomy. Am. J. Sports Med. 38(9), 1907–1916 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Baek J, Sovani S, Glembotski NE et al. Repair of avascular meniscus tears with electrospun collagen scaffolds seeded with human cells. Tissue Eng. Part A 22(5–6), 436–448 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee KI, Olmer M, Baek J, D'Lima DD, Lotz MK. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater. 76, 126–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek J, Chen X, Sovani S, Jin S, Grogan SP, D'Lima DD. Meniscus tissue engineering using a novel combination of electrospun scaffolds and human meniscus cells embedded within an extracellular matrix hydrogel. J. Orthop. Res. 33(4), 572–583 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masters KS. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol. Biosci. 11(9), 1149–1163 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Guan R, Sun X-L, Hou S, Wu P, Chaikof EL. A glycopolymer chaperone for fibroblast growth factor-2. Bioconjug. Chem. 15(1), 145–151 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Sun B, Chen B, Zhao Y et al. Crosslinking heparin to collagen scaffolds for the delivery of human platelet-derived growth factor. J. Biomed. Mater. Res. B Appl. Biomater. 91B(1), 366–372 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Wu BM, Dunn JCY. The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin. Biomaterials 32(8), 2059–2069 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Wu BM, Dunn JCY. Enhancing angiogenesis alleviates hypoxia and improves engraftment of enteric cells in polycaprolactone scaffolds. J. Tissue Eng. Regen. Med. 7(12), 925–933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B, Lin Z, Mitsi M, Zhang Y, Vogel V. Heparin-induced conformational changes of fibronectin within the extracellular matrix promote hMSC osteogenic differentiation. Biomater. Sci. 3(1), 73–84 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J. Bone Joint Surg. Am. 70(8), 1209–1217 (1988). [PubMed] [Google Scholar]; • One of the earliest animal studies to show potential for meniscal repair.
- 15.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage 3(2), 127–138 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Tarafder S, Gulko J, Kim D et al. Effect of dose and release rate of CTGF and TGFβ3 on avascular meniscus healing. J. Orthop. Res. 37(7), 1555–1562 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CH, Rodeo SA, Fortier LA, Lu C, Erisken C, Mao JJ. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 6(266), 266ra171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Large animal study demonstrating effectiveness of spatiotemporal release of growth factors in regenerating meniscal tissue.
- 18.Tarafder S, Gulko J, Sim KH, Yang J, Cook JL, Lee CH. Engineered healing of avascular meniscus tears by stem cell recruitment. Sci. Rep. 8(1), 8150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evrova O, Buschmann J. In vitro and in vivo effects of PDGF-BB delivery strategies on tendon healing: a review. Eur. Cell Mater. 34, 15–39 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Baek J, Lee E, Lotz MK, D'Lima DD. Bioactive proteins delivery through core-shell nanofibers for meniscal tissue regeneration. Nanomedicine 23, 102090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek J, Lotz MK, D'Lima DD. Core–shell nanofibrous scaffolds for repair of meniscus tears. Tissue Eng. Part A 25(23–24), 1577–1590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grogan SP, Duffy SF, Pauli C, Lotz MK, D'Lima DD. Gene expression profiles of the meniscus avascular phenotype: a guide for meniscus tissue engineering. J. Orthop. Res. 36(7), 1947–1958 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry 99(1), 1–12 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Crisan M, Yap S, Casteilla L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3), 301–313 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Baker BM, Shah RP, Silverstein AM, Esterhai JL, Burdick JA, Mauck RL. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc. Natl Acad. Sci. USA 109(35), 14176–14181 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discusses incorporating water-soluble nanofiber-generated porosities to facilitate cell migration.
- 26.Lee J, Yoo JJ, Atala A, Lee SJ. The effect of controlled release of PDGF-BB from heparin-conjugated electrospun PCL/gelatin scaffolds on cellular bioactivity and infiltration. Biomaterials 33(28), 6709–6720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Yoo J, Atala A, Lee S. Controlled heparin conjugation on electrospun poly(ε-caprolactone)/gelatin fibers for morphology-dependent protein delivery and enhanced cellular affinity. Acta Biomater. 8(7), 2549–2558 (2012). [DOI] [PubMed] [Google Scholar]; • Demonstrates that modulation of growth factor release enhanced cellular performance on electrospun scaffolds.
- 28.Leong NL, Arshi A, Kabir N et al. In vitro and in vivo evaluation of heparin mediated growth factor release from tissue-engineered constructs for anterior cruciate ligament reconstruction. J. Orthop. Res. 33(2), 229–236 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage 12(9), 736–744 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell. Mol. Life Sci. 68(19), 3209–3217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramazani Y, Knops N, Elmonem MA et al. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 68–69, 44–66 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Furumatsu T, Kanazawa T, Miyake Y, Kubota S, Takigawa M, Ozaki T. Mechanical stretch increases Smad3-dependent CCN2 expression in inner meniscus cells. J. Orthop. Res. 30(11), 1738–1745 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22(10), 1276–1312 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB). J. Orthop. Res. 13(2), 201–207 (1995). [DOI] [PubMed] [Google Scholar]
- 35.Bhargava MM, Attia ET, Murrell GaC, Dolan MM, Warren RF, Hannafin JA. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am. J. Sports Med. 27(5), 636–643 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Felimban R, Ye K, Traianedes K et al. Differentiation of stem cells from human infrapatellar fat pad: characterization of cells undergoing chondrogenesis. Tissue Eng. Part A 20(15–16), 2213–2223 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Grogan SP, Pauli C, Lotz MK, D'Lima DD. Relevance of meniscal cell regional phenotype to tissue engineering. Connect. Tissue Res. 58(3–4), 259–270 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Futami I, Ishijima M, Kaneko H et al. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS ONE 7(9), e45517 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52(8), 2521–2529 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Katagiri H, Muneta T, Tsuji K et al. Transplantation of aggregates of synovial mesenchymal stem cells regenerates meniscus more effectively in a rat massive meniscal defect. Biochem. Biophys. Res. Commun. 435(4), 603–609 (2013). [DOI] [PubMed] [Google Scholar]; • Documents translational potential of synovium-derived stem cells.
- 41.Kondo S, Muneta T, Nakagawa Y et al. Transplantation of autologous synovial mesenchymal stem cells promotes meniscus regeneration in aged primates. J. Orthop. Res. 35(6), 1274–1282 (2017). [DOI] [PubMed] [Google Scholar]; • Unique translational study of meniscus regeneration in primates.
- 42.Nakagawa Y, Muneta T, Kondo S et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage 23(6), 1007–1017 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Hatsushika D, Muneta T, Horie M, Koga H, Tsuji K, Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. J. Orthop. Res. 31(9), 1354–1359 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.