Abstract
Background/Aim: The effect of sarcopenia on patients with severe Covid-19 disease is unknown. We aimed to assess the influence of baseline computed tomography (CT)-based body composition parameters (pectoralis muscle area, pectoralis muscle index, skeletal muscle gauge) on clinical variables in patients with severe Covid-19 disease. Patients and Methods: Chest CT scans of adult patients with confirmed Covid-19 who were hospitalized from March 2020 to May 2021 at a level-one medical center in Germany were retrospectively analyzed. Pectoralis muscle area, pectoralis muscle index and skeletal muscle gauge were measured on the first CT scan after admission. Body composition parameters were assessed for association with clinical variables and 30-day mortality. Results: A total of 46 patients were included. None of the body composition parameters was a predictor for 30-day mortality, duration of hospital stay, duration of intensive care unit treatment, or duration of invasive mechanical ventilation. Conclusion: Pectoralis muscle composition parameters in CT chest scans did not predict outcomes in adult patients with severe Covid-19 infection.
Keywords: Covid-19, body composition, computed tomography, pectoralis muscle, mortality
Sarcopenia is an abnormal body composition defined as the loss of muscle mass, low muscle strength, and impaired muscle quality (1). Screening measures include clinical parameters as well as image-based techniques (1,2). Sarcopenia is common in patients aged 65 years and older and has been related to worse clinical outcome, disability, and mortality (3-7). Chest scans with either computed tomography (CT) or magnetic resonance imaging are considered the gold-standard for quantitative evaluation of body composition (1). Measurements such as skeletal muscle mass or density can be used as a surrogate marker for sarcopenia and muscle quality (8-10).
The etiology of sarcopenia is manifold and includes nutritional, environmental, behavioral, and medical factors. Sarcopenia is associated with malnutrition, metabolic dysregulation and chronic inflammation, increasing vulnerability of affected adults to various diseases (9,11). Suboptimal protein intake has been associated with sarcopenia, but the diagnosis does not depend on body weight and body mass index (12).
The pectoralis muscle index (PMI) and the pectoralis muscle area (PMA) have been shown to be indicators of sarcopenia and predictors of clinical variables such as length of hospital stay and mortality for multiple diseases (7,9,13-17). In addition, measurements of muscle density on CT scans are regarded as an indicator of muscle quality, reflecting lipid content (10). The skeletal muscle gauge (SMG) integrates both the PMI and muscle density and has been shown to be associated with outcomes in patients with cancer (18,19).
The effect of sarcopenia on patients suffering from Coronavirus 2019 disease (Covid-19) caused by severe acute respiratory syndrome coronavirus 2 is yet unknown. The disease affects mostly elderly people at highest risk for sarcopenia, with age being an independent risk factor for worse outcome (20,21).
Some authors have described that certain body composition parameters can identify unfavorable outcome in patients with Covid-19. For example, a higher level of adipose tissue has been associated with higher rates of hospitalization and mechanical ventilation (22-24). In another study, higher visceral fat area and higher abdominal circumference measured at the lumbar level were correlated with higher rates of transfer to the Intensive Care Unit (ICU) and mechanical ventilation, but mortality was not investigated (25).
Regarding measurement of skeletal mass, however, the literature results are controversial. For example, in one study, PMI and PMA were associated with length of hospital stay and death in Covid-19 patients (26). However, no strong association between skeletal muscle index (SMI) and clinical variables or mortality was found in another study (27). Density of the pectoralis muscle has been associated with the risk of death in symptomatic patients with Covid-19 (28). Yet no effect of the paraspinal muscle index at the T12 level with clinical outcomes was found in a Chinese cohort of patients hospitalized with Covid-19 disease (29). The literature is still preliminary and shows some variation regarding the muscle region measured and the level where muscle mass is assessed (9,30). However, if an association between muscle mass and clinical outcomes of patients with Covid-19 existed, it might be an important means of triage at hospital admission.
The purpose of the present study was to analyze the effect of pectoralis muscle composition, as measured by PMA, PMI and SMG on CT chest scans, on clinical variables. Length of hospital stay, length of ICU stay, length of invasive mechanical ventilation, and mortality at 30 days were assessed in patients hospitalized with severe Covid-19 infection.
Patients and Methods
Study population. We retrospectively analyzed a sample of patients admitted to a level-one medical center in Germany with polymerase chain reaction-confirmed symptomatic Covid-19 infection between March 2020 and May 2021. Patients that had undergone a CT chest scan after admission to our clinic were included. Patients were followed-up until discharge or death. The patient cohort included both primary admissions and referrals from other hospitals to the ICU unit. Scans with strong motion artifacts were excluded. For all patients, the length of overall hospital stay, length of ICU stay, length of invasive mechanical ventilation, and mortality at 30 days were noted. Data on clinical variables and mortality were obtained from the hospital system.
Image analysis. All CT scans were obtained on a multidetector CT scanner (Siemens Somatom Definition AS+; Siemens Healthcare, Germany). During the Covid-19 pandemic the scanner was set aside for suspected or confirmed cases. Patients were positioned in supine position. The CT protocol was as follows: Acquisition slice thickness 1 mm with 5 mm reconstructions, tube voltage 120 kV, automatic tube current modulation, pitch factor 1.2, and collimation 0.6 mm.
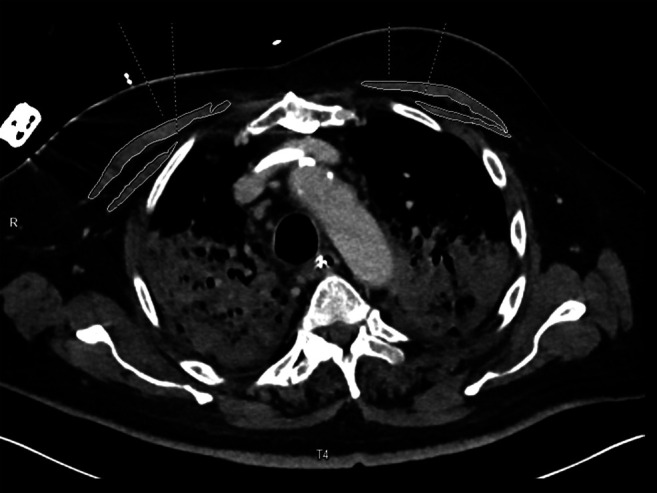
We used the first CT scan of patients after hospital admission. All images were assessed in consensus by two experienced radiologists (HK and AS) who were blinded to the clinical course of the patients. Measurements were performed on axial images at the T4 level in the soft tissue window (window of 45 to 250 HU) on a dedicated workstation (Infinitt PACS, Version 3.0; Infinitt Healthcare, Seoul, Republic of Korea). A line was drawn along the contours of the pectoralis major and minor muscles on both sides and the bilateral areas as determined by the software were added to obtain the PMA (Figure 1). Muscle density was measured for each side on all contrast scans and the mean was calculated. The PMI was calculated by dividing the PMA by the patient’s height. SMG was calculated multiplying the PMI by the mean muscle density as reported previously (18). SMG units are cm2 HU/m2 but are reported as arbitrary units (AU) for simplicity. A further variable was calculated dividing the mean density by the PMA.
Figure 1. Computed tomography of the thorax of a 67-year-old male patient after contrast injection. The identified muscle parameters were: pectoralis muscle area 18.0 cm2, pectoralis muscle index 5.8 cm2/m2, muscle density 22.5 HU, skeletal muscle gauge 132.3 AU. The patient died after 13 days on Intensive Care Unit.
Statistical analysis. Mean and standard deviation as well as median and interquartile range (IQR) were calculated for continuous variables. To assess the impact of pectoralis muscle composition on clinical variables and mortality, we used a multivariable logistic regression model. Odds ratios are presented together with 95% confidence intervals (95% CI). The resulting p-values were interpreted in an exploratory sense.
Results
Included patients and muscle mass analysis. Of the 74 patients screened, 46 underwent a chest CT scan at admission and were included in the analysis. There were 19 female and 27 male patients. The median age was 64.5 years and median body mass index was 27.3 kg/m2. Baseline characteristics of patients, PMA, PMI, SMG and density/PMA are summarized in Table I.
Table I. Patient characteristics (n=46).
BMI: Body mass index; IMV: invasive mechanical ventilation; PMA: pectoralis muscle area; PMI: pectoralis muscle index; SMG: skeletal muscle gauge.
Association between muscle parameters and clinical outcomes (Table II). Nineteen patients died within 30 days after admission (41.3%). Neither PMI nor PMA were strongly associated with 30-day mortality. Likewise, SMG, muscle density and density divided by PMA showed no significant association with mortality. Moreover, neither age nor sex were predictors of death at 30 days in our cohort.
Table II. Association of pectoralis muscle composition parameters with 30-day mortality.
BMI: Body mass index; CI: confidence interval; IMV: invasive mechanical ventilation; OR: odds ratio; PMA: pectoralis muscle area; PMI: pectoralis muscle index; SMG: skeletal muscle gauge.
The median length of hospital stay was 19.0 days, with 37 patients (80.4%) being admitted to the ICU for a median of 11.5 days. A total of 33 patients (71.7%) received invasive mechanical ventilation for a median duration of 228.5 hours. No association was found between PMA, PMI, SMG or density/PMI for length of hospital stay nor length of ICU stay. No association was found between either variable and length of invasive mechanical ventilation (Table III).
Table III. Association of pectoralis muscle composition parameters with clinical variables (univariable model).
BMI: Body mass index; CI: confidence interval; ICU: Intensive Care Unit; IMV: invasive mechanical ventilation; PMA: pectoralis muscle area; PMI: pectoralis muscle index; SMG: skeletal muscle gauge.
Discussion
We aimed to evaluate whether muscle-based body composition parameters as measured on chest CT scans were prognostic factors for mortality and clinical variables such as length of hospital stay and length of invasive mechanical ventilation in hospitalized patients with severe Covid-19 disease. Our study is a comprehensive analysis, associating multiple sarcopenia measures including PMA, PMI, muscle density, and SMG with clinical variables for patients hospitalized with Covid-19. We were not able to find an association between any variable and clinical outcomes nor 30-day mortality in our cohort.
It has been shown that sarcopenia can serve as a predictor of length of hospital stay and mortality in critically ill and trauma patients (16,31-34). In patients with Covid-19, the results are less clear. Muscle area measurements and skeletal mass indices as an indicator for sarcopenia have been conducted at different muscle levels, both at the thoracic and the pelvic level, with conflicting results. In a study by Schiaffino et al., lower cross-sectional area of paravertebral muscles were positively associated with ICU admission and mortality (30). Skeletal muscle indices were not calculated. In a cohort with 116 patients by Feng et al., no association between paraspinal muscle index at the T12 level and clinical outcomes was found (29). Higher muscle attenuation was associated with reduced critical illness or death, yet only for female patients. Kim et al. calculated the SMI at the T12 level and found that sarcopenia was associated with prolonged hospital stay but not mortality in patients with Covid-19 (35). In their sample, the ICU admission rate was only 8.3% and mortality was 5.8%. Hocaoglu et al. showed that higher muscle density as measured in non-contrast CT was inversely associated with death in patients with Covid-19 (28). However, over 40% of the cohort of 217 patients were outpatients and PMI was not measured. Furthermore, only major pectoralis muscle was measured. No association with mortality was found for SMI measured below the lung base in a study by Moctezuma-Velázquez et al. (27). Ufuk et al. presented an association between PMA and PMI on negative clinical outcomes such as intubation, length of hospital stay, and mortality (26).
Our cohort differs strongly from those analyzed previously and our patients were more severely affected than in other samples. The median age for our cohort was 65 years, with no patient being younger than 41 years, which is older than the cohort analyzed by Ufuk et al. (median age of 48 years) (26). This may have introduced selection bias, explaining why neither age nor sex were a significant predictor of mortality in our cohort. Overall 30-day mortality was 41%, while it ranged between 5.8% and 31.8% in other studies (26,28,35). This is mirrored in the high proportion of our patients treated in the ICU (80.4%) and requiring mechanical ventilation (71.7%). In the sample by Kim et al., the ICU admission rate was only 8.3% (35). The percentage of patients receiving mechanical ventilation were 6.6%, 11.5% and 21.4% in the study by Kim et al., Ufuk et al., and Besutti et al., respectively (22,26,35). The study by Moctezuma-Velázquez had a patient cohort with characteristics closer to ours, with an ICU admission rate of 40% and a mortality of 25%, revealing no association with clinical outcomes (27). A large proportion of our patients (73.9%) had a hospital stay of more than 10 days, with a median of 19 days, compared with 18.5% and 7 days for the sample by Ufuk et al. (26). Density values in our sample were measured in contrast CT scans, with other studies using non-contrast images (28,29). Hocaoglu et al. measured the pectoralis muscle density in non-contrast scans, finding cut-off values of 34.1 HU for men and 15.9 HU for women for association with increased mortality (28). Muscle density values are typically higher when measured in the venous phase (36). Nevertheless, our mean density was lower than the cut-off determined by Hocaoglu et al. for the male group, indicating a more critical disease stage.
Sarcopenia may potentially play a role in clinical outcome of patients with Covid-19. Yet we were not able to find such association in patients with severe disease. A possible explanation for this may be found in the nature of the disease, which causes multisystem inflammation and organ failure and subsequent mortality (27). In a cohort already severely affected by the disease in a progressed state, muscle mass at admission may no longer be a good predictor for outcome, as muscle loss is already advanced (37,38).
Our study has several limitations. Firstly, it was a single-center study with a small cohort. Not all symptomatic patients with Covid-19 at our center underwent a chest CT scan at admission. Only those with respiratory distress or infiltrates on chest X-ray were further evaluated at the discretion of the attending physician. Thus, patients with symptomatic but mild disease were not analyzed. In the follow-up, only mortality at 30 days was noted. As we evaluated pectoralis muscle composition as a proxy indicator for sarcopenia, the effect of muscle mass at other levels remains unknown. We did not associate our muscle indices with comorbidities.
In conclusion, in patients severely affected by Covid-19, pectoralis muscle composition parameters were not associated with length of hospital or ICU stay, length of mechanical invasive ventilation, or 30-day mortality. Further research is warranted to determine whether muscle indices predict clinical outcome in patients with a less advanced stage of disease.
Conflicts of Interest
None declared.
Authors’ Contributions
HK: Data analysis, validation and writing original draft. MT: Supervision, validation, investigation, data analysis and writing original draft. CB: Review and editing. JO: validation, review and editing. AW: statistical analysis, review and editing. MP: supervision and writing original draft. AS: supervision, data analysis, review and editing.
References
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) , Extended Group for EWGSOP2 Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liguori I, Russo G, Aran L, Bulli G, Curcio F, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin Interv Aging. 2018;13:913–927. doi: 10.2147/CIA.S149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57(12):M772–M777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 4.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Rossi S, Di Noia V, Tonetti L, Strippoli A, Basso M, Schinzari G, Cassano A, Leone A, Barone C, D’Argento E. Does sarcopenia affect outcome in patients with non-small-cell lung cancer harboring EGFR mutations. Future Oncol. 2018;14(10):919–926. doi: 10.2217/fon-2017-0499. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54(1):56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 7.Surov A, Wienke A. Sarcopenia predicts overall survival in patients with malignant hematological diseases: A meta-analysis. Clin Nutr. 2021;40(3):1155–1160. doi: 10.1016/j.clnu.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Hemke R, Buckless C, Torriani M. Quantitative imaging of body composition. Semin Musculoskelet Radiol. 2020;24(4):375–385. doi: 10.1055/s-0040-1708824. [DOI] [PubMed] [Google Scholar]
- 9.Ali AM, Kunugi H. Screening for sarcopenia (physical frailty) in the COVID-19 era. Int J Endocrinol. 2021;2021:5563960. doi: 10.1155/2021/5563960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bak SH, Kwon SO, Han SS, Kim WJ. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: a case control study. Respir Res. 2019;20(1):226. doi: 10.1186/s12931-019-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch AA, Hayhoe RPG, Cameron D. The relationships between sarcopenic skeletal muscle loss during ageing and macronutrient metabolism, obesity and onset of diabetes. Proc Nutr Soc. 2020;79(1):158–169. doi: 10.1017/S0029665119001150. [DOI] [PubMed] [Google Scholar]
- 12.Wannamethee SG, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc. 2015;74(4):405–412. doi: 10.1017/S002966511500169X. [DOI] [PubMed] [Google Scholar]
- 13.Diaz AA, Martinez CH, Harmouche R, Young TP, McDonald ML, Ross JC, Han ML, Bowler R, Make B, Regan EA, Silverman EK, Crapo J, Boriek AM, Kinney GL, Hokanson JE, Estepar RSJ, Washko GR. Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir Res. 2018;19(1):62. doi: 10.1186/s12931-018-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinsey CM, San José Estépar R, van der Velden J, Cole BF, Christiani DC, Washko GR. Lower pectoralis muscle area is associated with a worse overall survival in non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(1):38–43. doi: 10.1158/1055-9965.EPI-15-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh J, Song IK, Nam JS, Lee SW, Lee EH, Choi IC. Sarcopenia as a prognostic factor for outcomes after isolated tricuspid valve surgery. J Cardiol. 2020;76(6):585–592. doi: 10.1016/j.jjcc.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 16.DeAndrade J, Pedersen M, Garcia L, Nau P. Sarcopenia is a risk factor for complications and an independent predictor of hospital length of stay in trauma patients. J Surg Res. 2018;221:161–166. doi: 10.1016/j.jss.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Surov A, Wienke A. Low skeletal muscle mass predicts relevant clinical outcomes in head and neck squamous cell carcinoma. A meta analysis. Ther Adv Med Oncol. 2021;13:17588359211008844. doi: 10.1177/17588359211008844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, Nyrop KA, Alston SM, Williams GR. Beyond sarcopenia: Characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018;24(3):278–284. doi: 10.1111/tbj.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, Benbow JM, Muss HB. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res. 2017;23(3):658–665. doi: 10.1158/1078-0432.CCR-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risk for COVID-19 infection, hospitalization, and death by age group | CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalizationdeath-by-age.html. [Last accessed on June 14th, 2021]
- 22.Besutti G, Pellegrini M, Ottone M, Cantini M, Milic J, Bonelli E, Dolci G, Cassone G, Ligabue G, Spaggiari L, Pattacini P, Fasano T, Canovi S, Massari M, Salvarani C, Guaraldi G, Rossi PG, Reggio Emilia COVID-19 Working Group The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLoS One. 2021;16(5):e0251768. doi: 10.1371/journal.pone.0251768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viddeleer AR, Raaphorst J, Min M, Beenen LFM, Scheerder MJ, Vlaar APJ, Amsterdam UMC COVID-19 Biobank , Beudel M, Hemke R. Intramuscular adipose tissue at level Th12 is associated with survival in COVID-19. J Cachexia Sarcopenia Muscle. 2021;12(3):823–827. doi: 10.1002/jcsm.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe M, Caruso D, Tuccinardi D, Risi R, Zerunian M, Polici M, Pucciarelli F, Tarallo M, Strigari L, Manfrini S, Mariani S, Basciani S, Lubrano C, Laghi A, Gnessi L. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319. doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen A, Bressem K, Albrecht J, Thieß HM, Vahldiek J, Hamm B, Makowski MR, Niehues A, Niehues SM, Adams LC. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ufuk F, Demirci M, Sagtas E, Akbudak IH, Ugurlu E, Sari T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur J Radiol. 2020;131:109271. doi: 10.1016/j.ejrad.2020.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moctezuma-Velázquez P, Miranda-Zazueta G, Ortiz-Brizuela E, González-Lara MF, Tamez-Torres KM, Román-Montes CM, Díaz-Mejía BA, Pérez-García E, Villanueva-Reza M, Tovar-Méndez VH, Medrano-Borromeo C, Martínez-Valenzuela A, Jandete-Medina MÁ, Martínez-Guerra BA, Uscanga-Domínguez L, Sifuentes-Osornio J, Ponce-de-León A, Olivas-Martinez A, Moctezuma-Velázquez C. Low thoracic skeletal muscle area is not associated with negative outcomes in patients with COVID-19. Am J Phys Med Rehabil. 2021;100(5):413–418. doi: 10.1097/PHM.0000000000001716. [DOI] [PubMed] [Google Scholar]
- 28.Hocaoglu E, Ors S, Yildiz O, Inci E. Correlation of pectoralis muscle volume and density with severity of COVID-19 pneumonia in adults. Acad Radiol. 2021;28(2):166–172. doi: 10.1016/j.acra.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Z, Zhao H, Kang W, Liu Q, Wu J, Bragazzi NL, Ma X, Wang W, Rong P. Association of paraspinal muscle measurements on chest computed tomography with clinical outcomes in patients with severe Coronavirus disease 2019. J Gerontol A Biol Sci Med Sci. 2021;76(3):e78–e84. doi: 10.1093/gerona/glaa317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiaffino S, Albano D, Cozzi A, Messina C, Arioli R, Bnà C, Bruno A, Carbonaro LA, Carriero A, Carriero S, Danna PSC, D’Ascoli E, De Berardinis C, Della Pepa G, Falaschi Z, Gitto S, Malavazos AE, Mauri G, Monfardini L, Paschè A, Rizzati R, Secchi F, Vanzulli A, Tombini V, Vicentin I, Zagaria D, Sardanelli F, Sconfienza LM. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology. 2021;300(2):E328–E336. doi: 10.1148/radiol.2021204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji Y, Cheng B, Xu Z, Ye H, Lu W, Luo X, Fu S, Fang X. Impact of sarcopenic obesity on 30-day mortality in critically ill patients with intra-abdominal sepsis. J Crit Care. 2018;46:50–54. doi: 10.1016/j.jcrc.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Joyce PR, O’Dempsey R, Kirby G, Anstey C. A retrospective observational study of sarcopenia and outcomes in critically ill patients. Anaesth Intensive Care. 2020;48(3):229–235. doi: 10.1177/0310057X20922234. [DOI] [PubMed] [Google Scholar]
- 33.Kou HW, Yeh CH, Tsai HI, Hsu CC, Hsieh YC, Chen WT, Cheng HT, Yu MC, Lee CW. Sarcopenia is an effective predictor of difficult-to-wean and mortality among critically ill surgical patients. PLoS One. 2019;14(8):e0220699. doi: 10.1371/journal.pone.0220699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng CC, Lee ZY, Chan WY, Jamaluddin MF, Tan LJ, Sitaram PN, Ruslan SR, Hasan MS. Low muscularity as assessed by abdominal computed tomography on intensive care unit admission is associated with mortality in a critically ill Asian population. JPEN J Parenter Enteral Nutr. 2020;44(3):425–433. doi: 10.1002/jpen.1666. [DOI] [PubMed] [Google Scholar]
- 35.Kim JW, Yoon JS, Kim EJ, Hong HL, Kwon HH, Jung CY, Kim KC, Sung YS, Park SH, Kim SK, Choe JY. Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with Coronavirus disease 2019. J Gerontol A Biol Sci Med Sci. 2021;76(8):e110–e116. doi: 10.1093/gerona/glab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Vugt JLA, Coebergh van den Braak RRJ, Schippers HJW, Veen KM, Levolger S, de Bruin RWF, Koek M, Niessen WJ, IJzermans JNM, Willemsen FEJA. Contrast-enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin Nutr. 2018;37(5):1707–1714. doi: 10.1016/j.clnu.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Baggerman MR, van Dijk DPJ, Winkens B, van Gassel RJJ, Bol ME, Schnabel RM, Bakers FC, Olde Damink SWM, van de Poll MCG. Muscle wasting associated co-morbidities, rather than sarcopenia are risk factors for hospital mortality in critical illness. J Crit Care. 2020;56:31–36. doi: 10.1016/j.jcrc.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Ju S, Choi SM, Park YS, Lee CH, Lee SM, Yoo CG, Kim YW, Han SK, Lee J. Rapid muscle loss negatively impacts survival in critically ill patients with cirrhosis. J Intensive Care Med. 2020;35(7):663–671. doi: 10.1177/0885066618775706. [DOI] [PubMed] [Google Scholar]