Smoking and rural residence are independently correlated with the development of presumed ocular histoplasmosis syndrome, both with and without choroidal neovascularization. Diabetes may also be correlated with ocular histoplasmosis without choroidal neovascularization.
Key words: case-control study, diabetes, epidemiology, presumed ocular histoplasmosis syndrome, rural residence, smoking, urbanicity
Abstract
Purpose:
To investigate the relationship of smoking, urbanicity, and diabetes to presumed ocular histoplasmosis syndrome (POHS) and associated choroidal neovascularization (CNV).
Methods:
Medical records of 751 adult patients with POHS were reviewed, including 603 patients without CNV and 148 patients with CNV. Age-matched and gender-matched controls were randomly selected from the same practice for comparison. Statistical comparisons of smoking history, urbanicity, and diabetic history were performed using chi-square and conditional logistic regression analyses.
Results:
Increased rates of current or former smoking, rural residence, and diabetes were found in patients with POHS compared with controls. POHS patients with CNV had increased rates of current or former smoking and rural residence as compared with controls.
Conclusion:
A history of current or past smoking is associated with an increased risk of developing both POHS alone and POHS with CNV. We did not find a significant additional risk of smoking on the development of CNV in patients with POHS. Patients living in rural locations are more likely than those in urban locations to develop both POHS and POHS with CNV. Diabetics may be more likely to develop POHS than nondiabetics.
Presumed ocular histoplasmosis syndrome (POHS) is characterized by distinctive fundus findings in the absence of ocular inflammation and is a major cause of vision loss in young patients in endemic areas. The most probable etiology of POHS is pulmonary infection with Histoplasma capsulatum, with resultant dissemination to the choroid and subsequent inflammatory reaction and chorioretinal scarring.1,2 POHS patients are at risk for significant loss of vision from central chorioretinal scarring and/or choroidal neovascularization (CNV).
A retrospective case–control study by Chheda et al3 suggested an increased risk of CNV in patients with POHS who were cigarette smokers. However, their study compared neovascular POHS patients with normal control patients (without POHS). Because of this, they were unable to determine whether the effect of smoking was on the development of the neovascular component of POHS or on the development of POHS itself. Their conclusion of increased risk of CNV among patients with POHS who smoked was supported by an incidental finding in a study designed to investigate genetic risk factors for CNV in patients with POHS.4 That study showed an unusually high prevalence of smoking in patients with POHS, both with (62.9%) and without (48.3%) CNV.
There are reasons to suspect that cigarette smoking may affect the development of POHS itself rather than just the occurrence of neovascularization in these patients. In the United States, POHS is most commonly found in the Ohio and Mississippi valley regions,1 which is also the region of the United States with the highest per capita rate of cigarette smoking (Figure 1).5 Cigarette smoking has been shown to increase the risk of pulmonary infections such as influenza, pneumonia, and tuberculosis, probably because of structural changes in the respiratory tract and a decrease in immune response.6 In addition, cigarette smokers are more likely to develop a chronic disseminated form of pulmonary histoplasmosis,2 suggesting that smokers may have increased dissemination or more end-organ susceptibility to infectious foci.
Fig. 1.
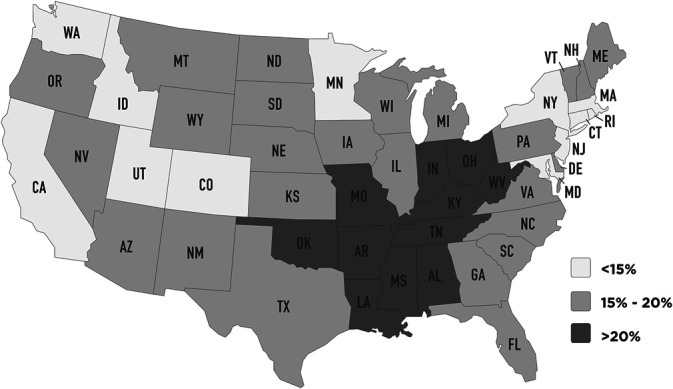
Percentage of adults in each state who were current smokers in 2017. Each state is designated with its standard United States postoffice abbreviation. (Public domain data from Centers for Disease Control and Prevention, Behavioral Risk Factor Surveillance System, [2017]).
We undertook this study to evaluate the risk of smoking on the development of POHS with and without CNV. As secondary measures, we also evaluated the effect of diabetes and the effect of rural versus urban residence on POHS.
Methods
This study was designed as a retrospective pair-matched case–control study with 1:3 cases:controls. We reviewed electronic patient records from an integrated multicenter optometry and ophthalmology practice with offices throughout northern Indiana. The institutional ethics committee prospectively ruled that IRB approval was not required. The rationale for IRB exemption was that the study posed minimal risk to patients and complied with federal guidelines for IRB exemption (Title 45 CFR 46.104 paragraph d 4 ii) in that the data had been previously recorded in the patient charts, all extracted patient information was deidentified before analysis, and the investigators did not contact the subjects or reidentify the subjects. The study was conducted in compliance with the ethical guidelines of the Declaration of Helsinki and in compliance with all federal and state laws, including the Health Insurance Portability and Accountability Act.
All patients aged 18 and older seen in the 7-year period between January 2013 and December 2019 for whom a diagnostic code of histoplasmosis (B39.9) and/or posterior chorioretinal scar (H31-011-H31.013) had been recorded were eligible for consideration in the study. We reviewed the records for each patient, along with any fundus photographs, fluorescein angiograms, and correspondence attached to the record, looking for documentation of the classic triad of POHS, including 1) typical peripapillary atrophy or pigment changes, 2) “punched-out” chorioretinal scars in the macula or periphery, and 3) CNV. For the purposes of this study, CNV was presumed to be present if that diagnosis had been documented by a retinal specialist or if the patient had a history of intravitreal injections or laser treatment for that diagnosis. Patients were included in the study groups if at least two of the three classic findings of POHS were documented in the chart or visible on fundus photographs of either eye at any time during the study period. Patients were excluded from the study group if they had documented evidence of uveitis at any time during the study period or if no smoking history had been recorded in the chart.
To obtain control groups for comparison, we accessed the same electronic medical record database from which our study patients were selected (Compulink EyeMD). The electronic medical record was used to generate a report of all patients aged 18 and older seen in the practice during the last year of the study period, including medical record number, age, and gender. Patients were removed from consideration for the control group if they had either diagnostic code used to create the list of potential study patients, leaving 159,787 patients as a pool of potential controls. SAS 9.4 statistical software (MatchCC macro) was then used to randomly select three controls from those in the pool who had both age and gender matching those of each study patient.
Data extracted from the record for both study patients and controls included age, gender, zip code of home address, history of diabetes, and smoking history. The smoking history in the medical record was categorized as nonsmoker (never smoked), former smoker, and current smoker. To reduce the risk of bias, investigators did not view the smoking history or diabetes history until after the decision of inclusion or exclusion in the study had been made.
We used the residential zip code to categorize each patient and control into either an urban or rural place of residence. Our system for using zip codes to determine urbanicity was based on recommendations from Hall et al.7 We designated a zip code as urban if the population density was greater than 1,000 people per square mile (ppsm), but adjustments in the category for each zip code were made for several conditions. Unusually small zip code areas encompassing only a small town were categorized as rural despite density greater than 1,000 ppsm. Zip codes consisting only of post office boxes were categorized with the same designation as the zip code within which they exist geographically. Unusually large zip code areas containing an urbanized area of at least 30,000 people were categorized as urban despite a density lower than 1,000 ppsm. Zip codes that were part of an “urban cluster” surrounding a metropolitan area were categorized as urban even if their density was less than 1,000 ppsm, on the assumption that most people live within the urbanized portion of the zip code and/or commute into the city for work. In general, rural was the default if there was insufficient evidence to support an urban classification.
Statistical comparison of the groups (POHS with CNV, POHS without CNV, and controls) was performed using chi-square analysis to univariably compare the smoking, diabetes, and urbanicity status of the patients with POHS with their matched controls. Multivariable conditional logistic regression analysis was then used to independently evaluate smoking, diabetes, and urbanicity as predictors of POHS with and without CNV. Statistical significance was defined as a P-value < 0.05. For the purposes of the logistic regression analysis, smokers and former smokers were grouped together and compared with nonsmokers. Statistical analyses were performed with SAS 9.4 statistical software.
Results
Our records search yielded 2,283 patients of age 18 or older with a diagnosis of histoplasmosis and/or chorioretinal scar. We excluded 1,526 patients from the study due to not meeting our diagnostic criteria for POHS. Many of these were patients with chorioretinal scars from causes other than POHS. Others were patients who were diagnosed or suspected clinically to have POHS but for whom we could not document the full study criteria based on information and images in the patient record. Of the remaining 757 patients meeting the criteria for POHS, we excluded three because of a history of uveitis, two because of the smoking history not documented in the chart, and one due to CNV felt to be caused by macular degeneration rather than ocular histoplasmosis, which left 751 ocular histoplasmosis patients in our study group. Of these, 603 patients had POHS without CNV (POHS group) and 148 had POHS with CNV (POHS/CNV group). An urbanicity designation could not be determined for 1 patient due to an unknown local zip code, and this patient was excluded from the statistical evaluation of urbanicity. The demographics and results for the study and control groups are presented in Table 1.
Table 1.
Demographic and Results Data for Study Groups and Their Matched Control Groups
| POHS | POHS Controls | POHS/CNV | POHS/CNV Controls | |
| Number of patients | 603 | 1809 | 148 | 444 |
| Average age in years | 61.1 | 61.1 | 54.3 | 54.3 |
| Female | 380 (63.0%) | 1,140 (63.0%) | 83 (56.1%) | 259 (56.1%) |
| Male | 223 (37.0%) | 669 (37.0%) | 65 (43.9%) | 185 (43.9%) |
| Rural | 340 (56.5%) | 834 (46.1%) | 90 (60.8%) | 185 (41.7%) |
| Urban | 262 (43.5%) | 975 (53.9%) | 58 (39.2%) | 259 (58.3%) |
| Current smoker | 153 (25.4%) | 286 (15.8%) | 43 (29.1%) | 71 (16.0%) |
| Former smoker | 148 (24.5%) | 378 (20.9%) | 33 (22.3%) | 73 (16.4%) |
| Ever smoker (current + former) | 301 (49.9%) | 664 (36.7%) | 76 (51.4%) | 144 (32.4%) |
| Nonsmoker | 302 (50.1%) | 1,145 (63.3%) | 72 (48.6%) | 300 (67.6%) |
| Diabetic | 151 (25.0%) | 363 (20.1%) | 16 (10.8%) | 58 (13.1%) |
The univariable comparison between the POHS group and their gender-matched and age-matched controls showed that 49.9% of patients with POHS were current or former smokers compared with 36.7% of controls (P < 0.001). In addition, 56.5% of the patients with POHS were found to live in zip codes designated as primarily rural, as compared with 46.1% of controls (P < 0.001). Diabetes was also associated with POHS; 25.0% of patients with POHS were diabetic compared with 20.1% of controls (P = 0.010). The conditional logistic regression comparison between the POHS group and their matched controls showed significantly increased risk for current or former smoking history (OR = 1.696, CI = 1.402–2.051, P < 0.001), history of diabetes (OR = 1.309, CI = 1.045–1.640, P = 0.019), and rural location (OR = 1.516, CI = 1.254–1.834, P < 0.001). The logistic regression results are presented in Tables 2 and 3.
Table 2.
Logistic Regression Results Using Smoking, Diabetes, and Urbanicity to Predict the Presumed Ocular Histoplasmosis Cases*
| P | Odds Ratio Estimate | Odds Ratio 95% Confidence Interval | ||
| Smoking (vs. nonsmoking) | <0.001 | 1.696 | 1.402 | 2.051 |
| Diabetic (vs. nondiabetic) | 0.019 | 1.309 | 1.045 | 1.640 |
| Rural (vs. urban) | <0.001 | 1.516 | 1.254 | 1.834 |
The regression analysis involves a total of 603 cases and 1809 matched controls.
Table 3.
Logistic Regression Results Using Smoking, Diabetes, and Urbanicity to Predict the Presumed Ocular Histoplasmosis With Choroidal Neovascularization Cases*
| P | Odds Ratio Estimate | Odds Ratio 95% Confidence Interval | ||
| Smoking (vs. nonsmoking) | <0.001 | 2.188 | 1.474 | 3.248 |
| Diabetic (vs. nondiabetic) | 0.718 | 0.894 | 0.485 | 1.645 |
| Rural (vs. urban) | <0.001 | 2.205 | 1.481 | 3.283 |
The regression analysis involves a total of 148 cases and 444 matched controls.
For the POHS/CNV group, univariable comparison with their matched controls shows that 51.4% of patients with POHS/CNV were current or former smokers compared with 32.4% of controls (P < 0.001). Patients with POHS/CNV were more likely to live in rural locations, with 60.8% of patients versus 41.7% of their controls living in zip codes designated as rural (P < 0.001). Diabetes was not found to be associated with POHS/CNV; 10.8% of patients were diabetic, whereas 13.1% of controls were diabetic (P = 0.473). The conditional logistic regression comparison between the POHS/CNV group and their matched controls showed significant increased risk for current or former smoking history (OR = 2.188, CI = 1.474–3.248, P < 0.001) and rural location (OR = 2.205, CI = 1.481–3.283, P < 0.001). There was no association of diabetes with the POHS/CNV group (OR = 0.894, CI = 0.485–1.645, P = 0.718).
To estimate if smoking confers an additional risk of CNV development in a patient who has POHS, we compared the rate of past or current smoking for the two groups (49.9% of the POHS patients vs. 51.4% of the POHS/CNV patients). Chi-square analysis demonstrated that this difference in smoking rates for the two groups was not statistically significant (P = 0.755).
Discussion
Our study demonstrates that a history of current or former smoking is significantly correlated with the development of POHS, both with and without CNV. This correlation between smoking and POHS itself (without CNV) may have implications concerning the pathogenesis of POHS. We postulate that cigarette smoking may actually increase the risk of chorioretinal scarring in an individual exposed to the Histoplasma capsulatum fungus. The etiology of this increased risk is unknown but could be due to pulmonary damage caused by smoking that gives the organism increased access to the vascular system to aid in dissemination, to an immunosuppressive effect of smoking,8 or to an ocular effect such as choroidal thinning9,10 that improves access of the organism to the choroid and RPE.
Our study did not show a significantly higher rate of smoking in patients with POHS with CNV as compared with patients with POHS without CNV, implying that smoking may not significantly increase the risk of developing CNV in patients with POHS. Our findings support those of Benedict et al11 in a recent epidemiologic study using insurance claim data in which they did not find an association between smoking and CNV in patients with POHS.
Chheda et al used the patient's zip code as a surrogate for educational level and income level. We chose instead to use zip codes as an indicator of urbanicity, and demonstrated an association between rural residence and POHS, both with and without CNV. Smoking is known to be more common among rural residents,12 but even when smoking was controlled for in our study, a significant association between rural residence and POHS was found. Histoplasmosis has historically been associated with rural areas, although more recent large well-publicized outbreaks have been documented in urban areas, usually related to demolition of structures in areas of high population density.13 The principal transmission of histoplasmosis is by inhalation of spores aerosolized by soil disruption, such as by plowing fields, digging holes, or during construction.14 It is often associated with bird droppings; so those with exposure to chicken coops, bats, and starlings in rural areas would be presumably more likely to come in contact with the fungus.13
We also found a history of diabetes to be associated with POHS but not with neovascular POHS. This association was statistically significant but was weaker than the associations with smoking and rural location. The reason for this association of diabetes with POHS is unclear, but there are several possible explanations. Diabetics have an increased risk of infection due to hyperglycemia, decreased function of T cells and neutrophils, decreased secretion of inflammatory cytokines, local factors such as angiopathy and neuropathy, and increased susceptibility to oxidative stress.15 Several studies have linked diabetes to an increased risk of common and opportunistic infections, including respiratory tract infections and fungal infections.16–18 A case report by Niknam et al19 suggests that uncontrolled diabetes may be a risk factor for severe or disseminated histoplasmosis in immunocompetent patients. However, the prevalence of diabetes in our patients in all groups was higher than that of the general Indiana population. Because diabetics are strongly encouraged to receive yearly dilated eye examinations, they are probably overrepresented in eye care practices generally. In addition, screening for diabetic retinopathy may increase the likelihood that asymptomatic histoplasmosis lesions are found and/or documented, which could explain the association with POHS but not with neovascular POHS. To the best of the authors' knowledge, an association between POHS and diabetes has not been previously investigated or reported, making additional studies on the subject advisable for clarification of this association.
This study is retrospective and can demonstrate association but not causation. It is possible that these associations of smoking and rural residence with POHS may be due to additional unmeasured differences between the groups such as behaviors or socioeconomic factors that could put them at higher risk for POHS. Another limitation of the study is that patients were characterized as living in urban or rural areas based on their current zip code, although the Histoplasma exposure presumably occurred in the past when the patient may have lived in a different zip code area.
Our study also relied on patient reporting of their smoking status, which may not always be accurate. We grouped smokers and former smokers together for the statistical analysis because of a tendency for patient self-reporting to switch between these two groups when the medical history was obtained at different encounters over time. Grouping former and current smokers together for analysis is also reasonable because any pertinent effect of smoking on the development of POHS would presumably need to have been in effect at the time of exposure to the fungus.
Because of the large number of potential control patients available in our database, we were able to select all of our matched controls from the final year of the study period. There is some risk of bias if the controls are not distributed throughout the study period, especially if the rate of smoking changes markedly during the study. However, although adult cigarette smoking rates are gradually decreasing in the United States, the rates in Indiana have remained relatively stable during the study period (21.9% in 2013–21.1% in 2018).5 In addition, because we evaluated current and former smokers together, any bias because of change in smoking rates should be minimized.
Our study implies that a history of smoking may play a role in the pathogenesis of POHS but does not support the conclusion that smoking increases the risk of CNV in patients with POHS. Because POHS is often found in young adults, smoking cessation or avoidance in young people may decrease the risk of POHS chorioretinal scarring and its associated CNV and vision loss later in life. More studies are warranted to increase our understanding of the pathogenesis of POHS to help promote primary prevention of the disease.
Footnotes
None of the authors has any financial/conflicting interests to disclose.
This study was performed at The Retinal Institute, Fort Wayne, Indiana.
The authors would like to acknowledge the assistance of Frederick L. Ferris III, MD in critically reviewing our manuscript.
Contributor Information
Brad F. Richey, Email: bradrichey3@yahoo.com.
Rachel S. Obrock, Email: rachel.obrock@gmail.com.
Zachary M. Gee, Email: zachary.gee03@gmail.com.
David Y. Lu, Email: dlu2@hfhs.org.
Gordon Jacobsen, Email: GJACOBS2@hfhs.org.
References
- 1.Diaz RI, Sigler EJ, Rafieetary MR, Calzada JI. Ocular histoplasmosis syndrome. Surv Ophthalmol 2015;60:279–295. [DOI] [PubMed] [Google Scholar]
- 2.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007;20:115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chheda LV, Ferketich AK, Carroll CP, et al. Smoking as a risk factor for choroidal neovascularization secondary to presumed ocular histoplasmosis syndrome. Ophthalmology 2012;119:333–338. [DOI] [PubMed] [Google Scholar]
- 4.Wilkes MF, Miller DM, Mitchell MD, et al. Investigation of choroidal neovascularization risk alleles in ocular histoplasmosis. Ophthalmology 2014;121:1487–1488.e1. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. State tobacco activities tracking & evaluation (STATE) system. Available at: https://www.cdc.gov/statesystem/cigaretteuseadult.html 2017. Accessed June 19, 2020.
- 6.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med 2004;164:2206–2216. [DOI] [PubMed] [Google Scholar]
- 7.Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U.S. epidemiologic studies. J Urban Health 2006;83:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu F, Liang CL, Liu H, et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget 2017;8:268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigler EJ, Randolph JC, Calzada JI, Charles S. Smoking and choroidal thickness in patients over 65 with early-atrophic age-related macular degeneration and normals. Eye (Lond) 2014;28:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin W, Sumit K, Jianbin D, et al. Choroidal structural changes in smokers measured using choroidal vascularity index. Invest Ophthalmol Vis Sci 2019;60:1316–1320. [DOI] [PubMed] [Google Scholar]
- 11.Benedict K, Shantha JG, Yeh S, et al. Presumed ocular histoplasmosis syndrome in a commercially insured population, United States. PLoS One 2020;15:e0230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buettner-Schmidt K, Miller DR, Maack B. Disparities in rural tobacco use, smoke-free policies, and tobacco taxes. West J Nurs Res 2019;41:1184–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deepe GS. Outbreaks of histoplasmosis: the spores set sail. Plos Pathog 2018;14:e1007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz JH. Environmental and wilderness-related risk factors for histoplasmosis: more than bats in caves. Wilderness Environ Med 2018;29:531–540. [DOI] [PubMed] [Google Scholar]
- 15.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab 2012;16(Suppl 1):S27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis 2005;41:281–288. [DOI] [PubMed] [Google Scholar]
- 17.Carey IM, Critchley JA, DeWilde S, et al. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 2018;41:513–521. [DOI] [PubMed] [Google Scholar]
- 18.Poradzka A, Jasik M, Karnafel W, Fiedor P. Clinical aspects of fungal infections in diabetes. Acta Pol Pharm 2013;70:587–596. [PubMed] [Google Scholar]
- 19.Niknam N, Malhotra P, Kim A, Koenig S. Disseminated histoplasmosis presenting as diabetic keto-acidosis in an immunocompetent patient. BMJ Case Rep 2017;2017:bcr2016217915. [DOI] [PMC free article] [PubMed] [Google Scholar]


