Supplemental Digital Content is available in the text.
Keywords: endocarditis, heart diseases, humans, mortality, pulmonary valve
Abstract
Background:
The Melody valve was developed to extend the useful life of previously implanted right ventricular outflow tract (RVOT) conduits or bioprosthetic pulmonary valves, while preserving RV function and reducing the lifetime burden of surgery for patients with complex congenital heart disease.
Methods:
Enrollment for the US Investigational Device Exemption study of the Melody valve began in 2007. Extended follow-up was completed in 2020. The primary outcome was freedom from transcatheter pulmonary valve (TPV) dysfunction (freedom from reoperation, reintervention, moderate or severe pulmonary regurgitation, and/or mean RVOT gradient >40 mm Hg). Secondary end points included stent fracture, catheter reintervention, surgical conduit replacement, and death.
Results:
One hundred seventy-one subjects with RVOT conduit or bioprosthetic pulmonary valve dysfunction were enrolled. One hundred fifty underwent Melody TPV replacement. Median age was 19 years (Q1–Q3: 15–26). Median discharge mean RVOT Doppler gradient was 17 mm Hg (Q1–Q3: 12–22). The 149 patients implanted >24 hours were followed for a median of 8.4 years (Q1–Q3: 5.4–10.1). At 10 years, estimated freedom from mortality was 90%, from reoperation 79%, and from any reintervention 60%. Ten-year freedom from TPV dysfunction was 53% and was significantly shorter in children than in adults. Estimated freedom from TPV-related endocarditis was 81% at 10 years (95% CI, 69%–89%), with an annualized rate of 2.0% per patient-year.
Conclusions:
Ten-year outcomes from the Melody Investigational Device Exemption trial affirm the benefits of Melody TPV replacement in the lifetime management of patients with RVOT conduits and bioprosthetic pulmonary valves by providing sustained symptomatic and hemodynamic improvement in the majority of patients.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00740870.
What Is Known
The Melody transcatheter pulmonary valve delays reoperation in patients who otherwise may undergo repeat open-heart surgery for right ventricular outflow tract conduit or pulmonary valve replacement.
The Melody IDE trial (Post-Approval Study of the Investigational Device Exemption Cohort) is the largest trial initiated in the United States for a transcatheter pulmonary valve replacement device.
Numerous important procedural and mid-term clinical insights related to transcatheter pulmonary valve replacement have been reported, but longer-term follow-up has been awaited.
What the Study Adds
The Melody IDE trial demonstrates a decade of follow-up, which affirms the important contribution of transcatheter pulmonary valve replacement to the lifetime care of patients with repaired complex congenital heart disease and a dysfunctional right ventricular outflow tract conduit or bioprosthetic pulmonary valves.
The first transcatheter pulmonary valve replacement (TPVR) reported 2 decades ago by Bonhoeffer et al1 marked the advent of a therapy that altered the landscape for managing right ventricular outflow tract (RVOT) disease in patients with congenital and acquired heart disease. Indeed, as the first-in-human transcatheter heart valve, the Melody transcatheter pulmonary valve (TPV) ushered in a revolution in cardiac care, the less invasive treatment of failing heart valves for a wide variety of conditions and patient ages.
Designed to restore hemodynamic function in failing RVOT conduits or bioprosthetic pulmonary valves (BPV) without open surgery, the Melody TPV delays reoperation in a population of patients who might otherwise undergo repeat open-heart surgery for RVOT conduit or pulmonary valve replacement. The Post-Approval Study of the Investigational Device Exemption Cohort (Melody IDE trial), which began in 2007, remains the largest trial initiated in the United States for a TPVR device (NCT00740870). Since Zahn et al2 reported the 6-month follow-up of the first 34 IDE patients in 2009, analyses of Melody IDE trial data alone and in combination with 2 other prospective Melody valve trials have led to numerous important procedural and clinical insights.3–11
As more patients undergo TPVR with the Melody valve, prospective, long-term data will be essential to informing the lifetime management of patients with an RVOT conduit or BPV. Few studies have included post-TPVR follow-up beyond 5 years. Long-term outcomes remain an important gap in our understanding of this therapy.7,12–18 The original Melody IDE trial design included 5-year prospective follow-up. However, based on favorable intermediate outcomes and the need to establish data on longer-term valve performance, it was extended through 10 years. The trial is now complete and culminates with this 10-year follow-up analysis of patient outcomes and valve performance.
Methods
Patients
The eligible study population included patients ≥5 years of age and ≥30 kg in weight who had a dysfunctional RVOT conduit ≥16 mm in diameter or a stented BPV with internal diameter ≥18 mm and ≤22 mm at time of implant. Detailed inclusion criteria, reported previously, are summarized in Table S1.2,3,7 All patients provided written, informed consent, and participating centers maintained Institutional Review Board approval throughout the study. Medtronic (Minneapolis, MN) sponsored the trial. T.K. Jones had access to all trial data. S. Weng was responsible for data integrity and data analyses. The data, analytic methods, and study materials are owned by the sponsor and will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Outcomes
The primary outcome measure was freedom from TPV dysfunction, defined as freedom from RVOT reoperation, catheter reintervention on the TPV, or hemodynamic dysfunction of the TPV (moderate or greater pulmonary regurgitation [PR] by Doppler echocardiography and/or a mean Doppler RVOT gradient >40 mm Hg). Additional time-dependent outcomes included stent fracture, catheter reintervention, surgical RVOT reoperation, and death. Serial assessments of functional status (New York Heart Association [NYHA] class), hemodynamic status of the TPV (RVOT gradient and PR severity), and exercise cardiopulmonary function are also reported, along with 6-month postimplant assessments that were not reported for the entire cohort in prior reports, including cardiac magnetic resonance imaging. For serial echocardiographic results, patients who were explanted or underwent a valve-in-valve replacement were excluded from the analysis after that event. We also performed a subgroup analysis of patients who underwent TPVR into a protected environment (protected conduit), defined as a stented BPV (including Hancock conduits) or an RVOT conduit with at least one new prestent. Echocardiographic, cardiac magnetic resonance, and cardiopulmonary exercise test data for this study were reported by the individual sites.
Follow-Up
Patients were initially enrolled for a 5-year follow-up period or until the TPV was explanted. At 5 years, 102/122 (84%) patients had completed their follow-up visit (Figure S1). Two years after the final implant, the study protocol was modified, and all active patients were approached to consent for an additional 5 years (10 total years) of follow-up. Patients were followed until the earliest date of TPV explant, death, or end of study participation. Patients who did not provide consent to extend follow-up from 5 to 10 years were considered to have completed the study. This resulted in fewer patients completing follow-up beyond 5 years, with natural attrition due to explantation, death, and withdrawals. However, at 10 years, 94% of those who remained (58/62) had completed follow-up (Figure S1). Patients who notified the investigator that follow-up would no longer be maintained according to the protocol were considered to withdraw from the study. Patients were categorized as lost to follow-up if follow-up was discontinued without known exit and 3 attempts to make contact were unsuccessful. Echocardiographic and cardiopulmonary exercise test data were collected preimplant, at discharge, 3 months, 6 months, 1 year, and annually thereafter. Cardiac magnetic resonance data were collected before implant and at 6 months.
Statistical Analysis
Continuous data are presented as median (quartile 1–quartile 3) and categorical data are presented as number and percent of total. Kaplan-Meier estimates are presented as mean with 95% CI. Competing risk analysis was used to estimate the cumulative incidence function for any reintervention, death without any reintervention, explant, and death without explant, separately. Multivariable Cox regression with stepwise selection was used to assess the impact of prespecified factors on the time-to-any reintervention outcome. Protected conduit status, as defined above, was forced in the model. Other factors considered in the selection process included sex, age, NYHA class, primary implant indication, number of previous open-heart surgeries, preimplant and postimplant PR severity, and peak RVOT gradient (assessed by catheterization). In the stepwise selection process, the significance levels for entering and removing a factor into or from the model were 0.2 and 0.1, respectively. Analyses were performed using SAS version 9.4.
Results
Patients
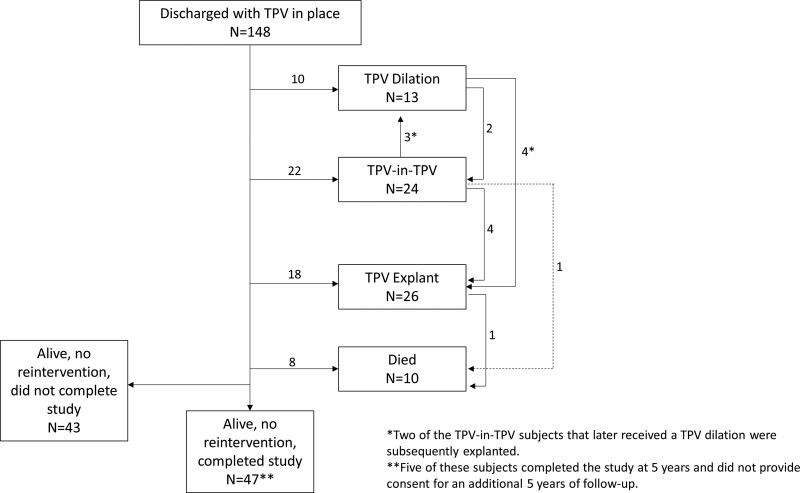
A total of 171 patients were enrolled in the trial at 5 centers between January 2007 and January 2010, and 150 had a Melody valve implanted. Patient characteristics are reported in Table S2. Briefly, patients were predominantly male (64%), median age of 19 years, and commonly presented with a homograft (73%). Preexisting stents in the RVOT were uncommon (25% present). In one patient, emergent surgery was performed due to conduit rupture and the Melody valve was explanted. The remaining 149 patients were followed for a median of 8.4 years (5.4–10.1). Of those, 58 patients completed the 10-year follow-up assessment. Reasons for and timing of study exit are detailed in Figure S1, and patient flow according to post-TPVR outcome is presented in Figure 1.
Figure 1.
Patient flow. Depicts patient status following discharge, showing the numbers of patients who underwent transcatheter pulmonary valve (TPV) dilation, TPV-in-TPV, or explant.
Long-Term Survival and TPV Function
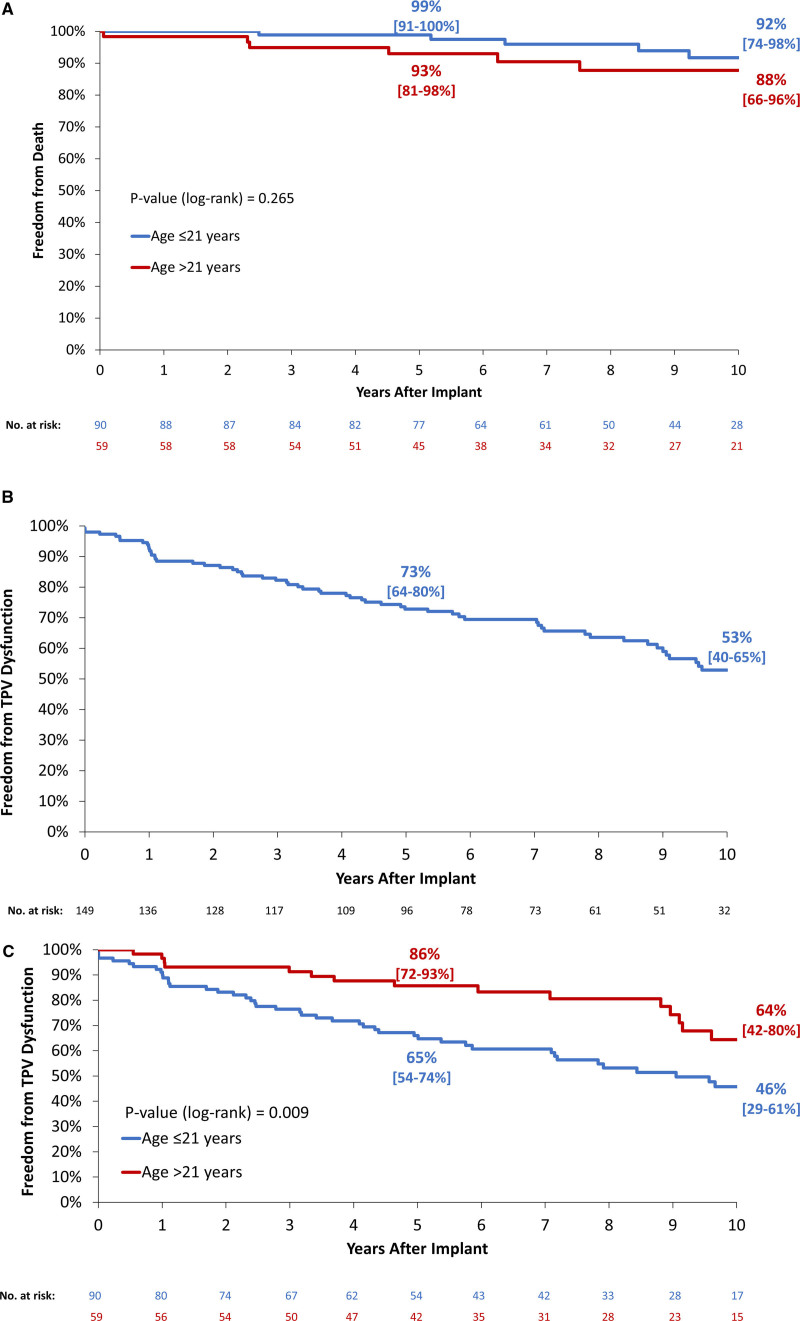
Estimated freedom from mortality at 10 years was 90% (95% CI, 79%–96%) and did not differ by age group (Figure 2A). Eleven patients died over the course of the trial. Five deaths were related to endocarditis and one each from respiratory failure, acute hydrocephalus, hypernatremia and cerebral edema, presumed arrhythmia, septic shock unrelated to endocarditis, and an unknown cause. Estimated freedom from TPV dysfunction was 53% at 10 years (40%–65%; Figure 2B) and was significantly shorter in patients ≤21 years of age at implant (Figure 2C).
Figure 2.
Survival and transcatheter pulmonary valve (TPV) dysfunction. Kaplan-Meier curves depict (A) estimated freedom from all-cause death by age (≤21 vs >21 y), and freedom from TPV dysfunction, (B) overall, and (C) by age (≤21 vs >21 y). Estimates displayed with (95% CI). End point evaluated in patients implanted >24 h.
TPV Reintervention
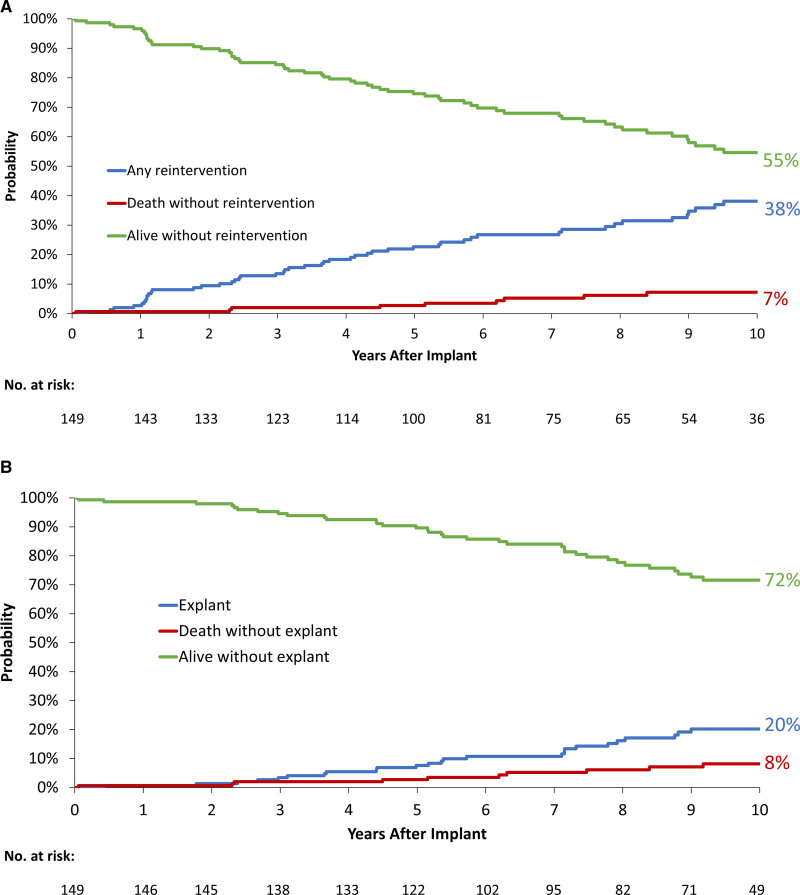
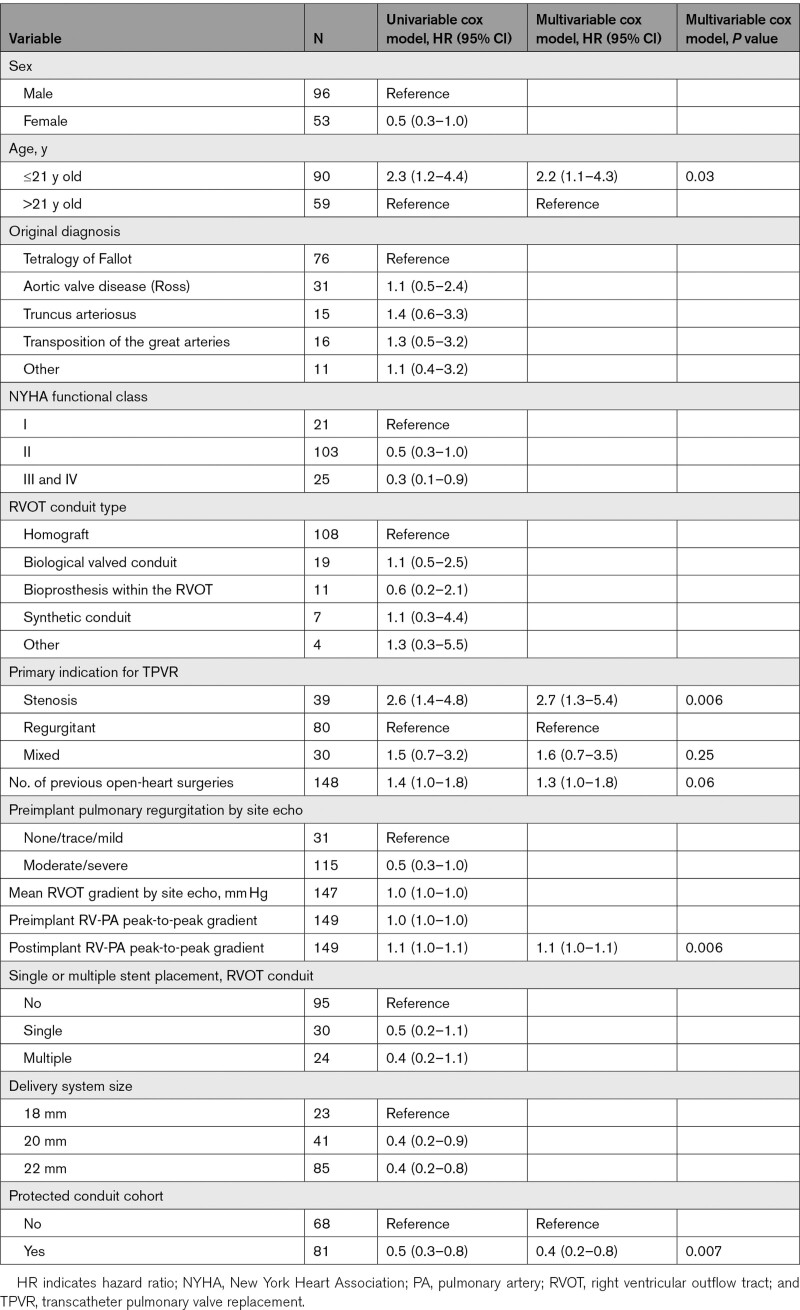
At 10-year post-TPVR, estimated reintervention-free survival was 55% (45%–63%; Figure 3A), estimated freedom from any TPV reintervention was 60% (47%–71%) and estimated freedom from RVOT reoperation was 79% (67%–87%; Figure S2). Estimated freedom from reintervention was significantly shorter in patients ≤21 years old at implant. Estimated freedom from reintervention was longer in the protected conduit cohort, while freedom from explant was similar (Figure S2). On multivariable Cox regression analysis, there was lower risk of reintervention in patients with than in patients without a protected conduit (hazard ratio [HR], 0.4 [95% CI, 0.2–0.8], P=0.007). There was a higher risk of reintervention in patients ≤21 years of age at implant compared with those >21 years old (HR, 2.2 [95% CI, 1.1–4.3], P=0.03) and in patients with a primary indication of stenosis as compared to regurgitation (HR, 2.7 [95% CI, 1.3–5.4], P=0.006). More prior open-heart surgeries was also associated with higher risk of reintervention (HR, 1.3 [95% CI, 1.0–1.8], P=0.06), as was higher postimplant RV-pulmonary artery peak-to-peak gradient (HR, 1.1 [95% CI, 1.0–1.1], P=0.006; Table). Competing outcome curves depicting the cumulative incidences of reintervention and death or explant and death are shown in Figure 3.
Figure 3.
Competing outcomes of reintervention, explant, and death. Competing outcome curves show the cumulative incidences of (A) reintervention and death or (B) explant and death. Estimated cumulative incidences for each outcome and Kaplan-Meier estimates at 10 y are indicated. End point evaluated in patients implanted >24 h.
Table.
Univariable and Multivariable Cox Regression Analysis for Time-to-Any Reintervention Among Patients Implanted >24 Hours
Long-Term Melody TPV Hemodynamics and Functional Status
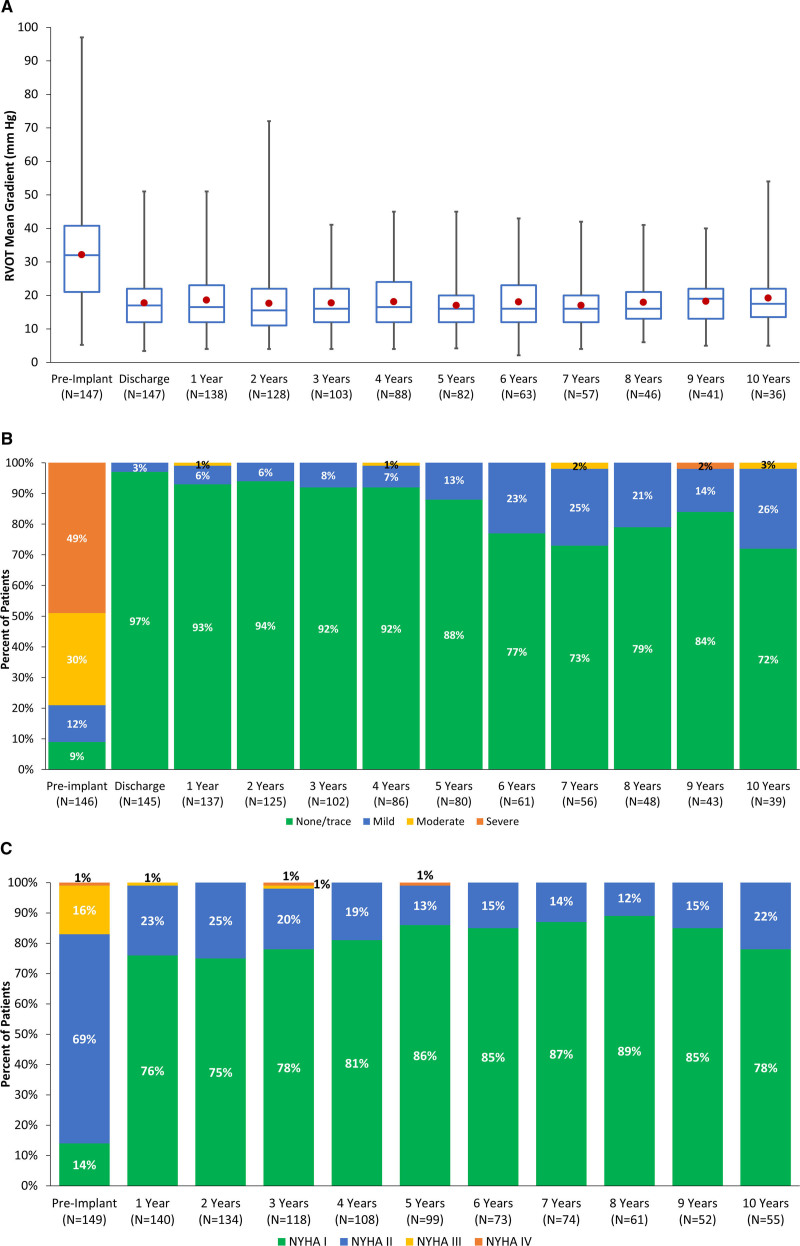
Mean gradient across the RVOT assessed by echocardiogram at each study time point are summarized in Figure 4A and demonstrate consistently lower gradients through 10 years compared with preimplant. At 10 years, 72% of patients still enrolled had trace or no PR, 26% had mild PR, and 3% had moderate PR (Figure 4B). At 10 years, most patients (78%) were in NYHA functional class I and the remaining patients reported NYHA class II symptoms (Figure 4C).
Figure 4.
Echocardiographic and New York Heart Association (NYHA) functional class. In A, Mean right ventricular outflow tract (RVOT) gradients before and after implant and at annual study visits are depicted by box plots. Center line represents the median, and the marker the mean; the limits of the box are the 25th and 75th percentiles, and the whiskers indicate minimum–maximum. B, Pulmonary regurgitation severity before and after implant and at each annual follow-up visit. Percentages are rounded to the nearest whole number. For RVOT gradient and pulmonary regurgitation data, end points are assessed in patients implanted >24 h. Data were collected until patients received surgical or catheter-based reintervention, which rendered the original Melody valve nonfunctional. C, NYHA classification over time. Data represent outcomes in the group of patients with follow-up evaluation performed at each study visit.
Other Device-Related Outcomes
A total of 28 patients were diagnosed with endocarditis during follow-up, with a relatively steady incidence rate over the study period. Estimated freedom from TPV-related endocarditis was 81% at 10 years (69%–89%), with an annualized rate of 2.0% (1.3%–3.0%) per patient-year. Estimated freedom from any endocarditis was 76% (63%–85%) at 10 years, with an annualized incidence of 3.0% per patient-year (Figure S3A). A major stent fracture was diagnosed in 23 patients, 20 who had no prestent placed at the time of TPVR while 3 had 1 or more new prestent. Freedom from major stent fracture was 84% at 10 years (70%–92%), similar to the rate at 5 years (85% [78%–91%]), as there were few new fractures diagnosed beyond 4-year postimplant (Figure S3B).
Short- and Mid-Term Outcomes Not Previously Reported
Previously unreported 5-year cardiopulmonary exercise test results according to baseline NYHA status are summarized in Table S3, and magnetic resonance imaging outcomes according to primary indication for implant are summarized in Table S4. In both PR and mixed disease cohorts, indexed RV end-diastolic volume on cardiac magnetic resonance decreased significantly relative to pre-TPVR, and indexed LV end-diastolic volume increased.
Discussion
This report of 10-year outcomes in the original Melody TPVR IDE cohort is the longest follow-up obtained in any prospective transcatheter heart valve study. It is among very few long-term prospective studies of cohorts after heart valve replacement regardless of the valve studied or means of implantation. Although many patients did not consent to 5 additional years of late follow-up, compliance of those still in the study at year 10 was excellent, with 94% of evaluable subjects (58/62) completing the 10-year follow-up visit and a median follow-up of 8.4 years (5.4–10.1) in the entire cohort. A number of significant insights regarding the role of this therapy in the lifetime management of patients with RVOT conduit and BPV disease emerged from this study.
Long-Term Survival
Estimated survival 10 years after TPVR for the entire cohort was 90%. Endocarditis was the most common cause of death and contributed to 5 of the 11 deaths, while a variety of noncardiac causes prevailed in the other 6 patients, consistent with the overall health status of adolescent and adult survivors of repaired complex congenital heart disease.19,20 When compared with similar patient populations undergoing surgical treatment of RVOT disease, mortality rates were nearly identical.21,22 This would suggest that long-term survival following transcatheter treatment of RVOT conduit or BPV dysfunction is not inferior to traditional surgical RVOT conduit or pulmonary valve replacement.23,24
Not surprisingly, freedom from valve dysfunction in this study and similar surgical series was associated with patient age. Patients treated at a younger age and size require a smaller initial RVOT conduit or BPV. This, in turn, creates a limitation in the caliber of the implanted Melody valve. These patients will outgrow the implant faster than patients who are fully grown at the time of TPVR. Conceptually, the reduced long-term function of the Melody valve after implant in younger patients is consistent with well-established contemporary surgical series and should prompt providers following these patients to be mindful of this time- and growth-dependent risk.
Functional Status and Clinical Outcomes
This study with extended follow-up demonstrated Melody valve durability in patients that did not require reintervention for as long as 10 years, with no significant evolving stenosis or regurgitation (Figure 4). Consistent with this experience, most patients reported no or only mildly limited exercise intolerance over the duration of the study. Furthermore, results of serial cardiopulmonary exercise test evaluation summarized for the full study cohort in this report for the first time correlated with reported and sustained improvement in exercise capacity across NYHA functional classes. Thus, the development of new cardiac symptoms in a previously well patient after Melody TPVR should prompt further investigation of acute changes possibly related to rare, rapid deterioration of valve function as seen in some cases of endocarditis.10
Endocarditis
The annualized incidence rate of TPV-related endocarditis over the duration of the study was 2.0% (1.3%–3.0%) per patient-year. This time-dependent outcome remained relatively stable over the duration of the study. Of concern, endocarditis was the leading cause of mortality in the trial, accounting for 5 of the 11 deaths. The small number of patients in this trial make ascertainment of risk factors for endocarditis or death from endocarditis problematic. Much larger, multicenter data registries of patients who have undergone TPVR and followed over many years are needed to address this question in a statistically valid way. Such clarity will be a welcome addition to our understanding of the lifetime role of TPVR therapy and how best to avoid or at least minimize this serious and time-dependent outcome.
TPV Reintervention
Patients who underwent TPVR at a younger age and within a smaller RVOT conduit or bioprosthetic valve were subject to shorter freedom from reintervention. The association between younger implant age and shorter duration to valve dysfunction and reintervention was previously reported by Armstrong et al,11 who analyzed outcomes from the 3 multicenter regulatory studies of the Melody valve. This independent association holds true through the full 10-year follow-up of the Melody IDE cohort and should serve as an important reminder that younger patients, in particular, require close monitoring to provide timely reintervention in an effort to preserve their lifetime cardiovascular health.
Major stent fracture, defined as Melody valve stent fracture leading to hemodynamically defined valve dysfunction, was largely identified in the first several years following valve implantation. In the initial version of the IDE protocol prestenting of the RVOT conduit was precluded. Indeed, the tendency of the Melody stent frame to fracture and the value of prestenting was first defined and subsequently validated in the early years of this trial. Once prestenting was introduced into the trial, it provided the opportunity to explore the role of prestenting in prolonging freedom from reintervention. The benefit of creating a protected conduit by placing one or more bare or covered stents before TPV implant, or implanting into a rigid, stented BPV, has been reported in previous analyses of this and other regulatory studies of the Melody valve.4,9 In this long-term analysis, patients who underwent TPVR into a protected conduit were found to have a sustained and stable hemodynamic and clinical benefit of this adjunctive therapy over 10 years. Similarly, as also observed in prior studies, there is a long-term outcome advantage to performing TPVR with the lowest possible residual gradient, which is achieved with a combination of conduit preparation and postdilation of the Melody valve if necessary.
Stenosis as the original implant indication and higher residual postimplant RVOT gradient increased the risk of receiving reintervention. The anatomic milieu in which the Melody valve was designed to function included a majority of patients with obstructed RVOT conduits and BPV in whom complete relief of RVOT obstruction could be a significant procedural challenge. Smaller existing RVOT conduits or changes in conduit caliber due to healing response, calcification, or immune-mediated conduit shrinkage created technical challenges especially given that large diameter covered stents were largely unavailable in the United States when the valves were implanted in this cohort.25 It is not surprising that reintervention would occur sooner in these patients, again emphasizing the importance of close surveillance when a higher initial or postimplant gradient is observed. These findings support the strategy of (1) optimizing RVOT conduit preparation with thorough predilation and multiple prestents, as required, to eliminate recoil, and (2) intentional BPV frame fracture with high-pressure balloons, all in an effort to provide the largest possible landing zone for the Melody valve.26
When considering the lifetime management of patients with a surgically placed RVOT conduit or BPV, it can be useful to view this in the context of competing outcomes. Reintervention-free survival 10-year post-TPVR was 55% (45%–63%), even accounting for the higher reintervention rate in patients implanted before prestenting was permitted. Currently, this number lacks comparative context with other forms of treatment. However, going forward, this methodology may be a useful way to evaluate the late outcomes of this historically important study with other surgical or transcatheter therapies developed in the future for this patient population.
Limitations
The total number of patients followed to 10 years was limited due to attrition over the duration of the study. Also, patients initially consented for 5 years and not all opted to continue for 10-year follow-up. Modifications to inclusion criteria and procedural options during the study may have introduced minor confounding with respect to analyzed factors associated with time-related outcomes. Imaging and cardiopulmonary core laboratory data were not available for long-term follow-up in this trial. Additionally, the study was not randomized and direct comparisons between the Melody TPV and surgery or other transcatheter devices were not studied.
Conclusions
The 10-year Post-Approval Study of the Investigational Device Exemption Cohort (Melody valve IDE trial) represents a landmark achievement in affirming the important contribution of TPVR to the lifetime care of patients with repaired complex congenital heart disease and a dysfunctional RVOT conduit or BPV. At 10 years, 53% of subjects were free of TPV dysfunction and, importantly, 79% of subjects were free of reoperation, while no new unanticipated safety risks were identified in late follow-up. The Melody valve, therefore, fulfills its original design intent to prolong the useful life of existing RVOT conduits or BPV while providing relief of pulmonary valve dysfunction in the majority of subjects. Awareness of the risk factors for earlier TPV dysfunction and reintervention identified in this study should inform physicians caring for these patients about optimal goals for valve implantation and how best to maintain vigilance in long-term care.
Article Information
Acknowledgments
We acknowledge the considerable work done by the study personnel at each of the pivotal study institutions over the 10 years of this study. We also acknowledge Jessica Dries-Devlin, PhD, CMPP, for editorial support, Megan Mueller for study management support, both Medtronic employees, and Kristin Boulware, formerly of Medtronic, for earlier study management support.
Sources of Funding
This work was supported by Medtronic (Mounds View, MN).
Disclosures
Dr Jones reports significant: investigator, proctor, and consultant for Medtronic. Dr McElhinney reports significant: investigator, proctor, and consultant for Medtronic. Dr Vincent reports modest: consultant and proctor for Medtronic. Dr Hellenbrand reports significant: former investigator, consultant, and proctor for Medtronic. Dr Cheatham reports significant: consultant, proctor, principal investigator for Medtronic; Consultant for NuMED. Dr Berman reports modest: consultant for Medtronic. Dr Zahn reports significant: former investigator, current proctor, and current consultant for Medtronic. S. Weng reports significant: employee and shareholder of Medtronic. The other authors report no conflicts.
Supplemental Material
Figures S1–S3
Tables S1–S4
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BPV
- bioprosthetic pulmonary valve
- HR
- hazard ratio
- IDE
- investigational device exemption
- NYHA
- New York Heart Association
- PR
- pulmonary regurgitation
- RVOT
- right ventricular outflow tract
- TPV
- transcatheter pulmonary valve
- TPVR
- transcatheter pulmonary valve replacement
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.121.010852.
For Sources of Funding and Disclosures, see page 32.
Contributor Information
Doff B. McElhinney, Email: doff@stanford.edu.
Julie A. Vincent, Email: jav2136@cumc.columbia.edu.
William E. Hellenbrand, Email: william.hellenbrand@yale.edu.
John P. Cheatham, Email: john.cheatham@nationwidechildrens.org.
Darren P. Berman, Email: dberman@chla.usc.edu.
Evan M. Zahn, Email: evan.zahn@cshs.org.
Danyal M. Khan, Email: danyal.khan@nicklauschildrens.org.
John F. Rhodes, Jr, Email: rhodesjf@musc.edu.
Shicheng Weng, Email: shicheng.weng@medtronic.com.
Lisa J. Bergersen, Email: lisa.bergersen@cardio.chboston.org.
References
- 1.Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0 [DOI] [PubMed] [Google Scholar]
- 2.Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the Melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit: early results from the U.S. Clinical trial. J Am Coll Cardiol. 2009;54:1722–1729. doi: 10.1016/j.jacc.2009.06.034 [DOI] [PubMed] [Google Scholar]
- 3.McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, Vincent JA. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US Melody valve trial. Circulation. 2010;122:507–516. doi: 10.1161/CIRCULATIONAHA.109.921692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElhinney DB, Cheatham JP, Jones TK, Lock JE, Vincent JA, Zahn EM, Hellenbrand WE. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circ Cardiovasc Interv. 2011;4:602–614. doi: 10.1161/CIRCINTERVENTIONS.111.965616 [DOI] [PubMed] [Google Scholar]
- 5.Batra AS, McElhinney DB, Wang W, Zakheim R, Garofano RP, Daniels C, Yung D, Cooper DM, Rhodes J. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. Am Heart J. 2012;163:280–287. doi: 10.1016/j.ahj.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 6.Priromprintr B, Rhodes J, Silka MJ, Batra AS. Prevalence of arrhythmias during exercise stress testing in patients with congenital heart disease and severe right ventricular conduit dysfunction. Am J Cardiol. 2014;114:468–472. doi: 10.1016/j.amjcard.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 7.Cheatham JP, Hellenbrand WE, Zahn EM, Jones TK, Berman DP, Vincent JA, McElhinney DB. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US Melody valve investigational device exemption trial. Circulation. 2015;131:1960–1970. doi: 10.1161/CIRCULATIONAHA.114.013588 [DOI] [PubMed] [Google Scholar]
- 8.Jones TK, Rome JJ, Armstrong AK, Berger F, Hellenbrand WE, Cabalka AK, Benson LN, Balzer DT, Cheatham JP, Eicken A, et al. Transcatheter pulmonary valve replacement reduces tricuspid regurgitation in patients with right ventricular volume/pressure overload. J Am Coll Cardiol. 2016;68:1525–1535. doi: 10.1016/j.jacc.2016.07.734 [DOI] [PubMed] [Google Scholar]
- 9.Cabalka AK, Hellenbrand WE, Eicken A, Kreutzer J, Gray RG, Bergersen L, Berger F, Armstrong AK, Cheatham JP, Zahn EM, et al. Relationships among conduit type, pre-stenting, and outcomes in patients undergoing transcatheter pulmonary valve replacement in the prospective North American and European Melody valve trials. JACC Cardiovasc Interv. 2017;10:1746–1759. doi: 10.1016/j.jcin.2017.05.022 [DOI] [PubMed] [Google Scholar]
- 10.McElhinney DB, Sondergaard L, Armstrong AK, Bergersen L, Padera RF, Balzer DT, Lung TH, Berger F, Zahn EM, Gray RG, et al. Endocarditis after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2018;72:2717–2728. doi: 10.1016/j.jacc.2018.09.039 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AK, Berger F, Jones TK, Moore JW, Benson LN, Cheatham JP, Turner DR, Rhodes JF, Vincent JA, Zellers T, et al. Association between patient age at implant and outcomes after transcatheter pulmonary valve replacement in the multicenter Melody valve trials. Catheter Cardiovasc Interv. 2019;94:607–617. doi: 10.1002/ccd.28454 [DOI] [PubMed] [Google Scholar]
- 12.Fraisse A, Aldebert P, Malekzadeh-Milani S, Thambo JB, Piéchaud JF, Aucoururier P, Chatelier G, Bonnet D, Iserin L, Bonello B, et al. Melody ® transcatheter pulmonary valve implantation: results from a French registry. Arch Cardiovasc Dis. 2014;107:607–614. doi: 10.1016/j.acvd.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Fiszer R, Dryżek P, Szkutnik M, Góreczny S, Krawczuk A, Moll J, Moszura T, Pawlak S, Białkowski J. Immediate and long-term outcomes of percutaneous transcatheter pulmonary valve implantation. Cardiol J. 2017;24:604–611. doi: 10.5603/CJ.a2017.0023 [DOI] [PubMed] [Google Scholar]
- 14.Cools B, Brown S, Budts W, Heying R, Troost E, Boshoff D, Eyskens B, Gewillig M. Up to 11 years of experience with the Melody valved stent in the right ventricular outflow tract. EuroIntervention. 2018;14:e988–e994. doi: 10.4244/EIJ-D-18-00054 [DOI] [PubMed] [Google Scholar]
- 15.Borik S, Crean A, Horlick E, Osten M, Lee KJ, Chaturvedi R, Friedberg MK, McCrindle BW, Manlhiot C, Benson L. Percutaneous pulmonary valve implantation: 5 years of follow-up: does age influence outcomes? Circ Cardiovasc Interv. 2015;8:e001745. doi: 10.1161/CIRCINTERVENTIONS.114.001745 [DOI] [PubMed] [Google Scholar]
- 16.Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. doi: 10.1161/CIRCULATIONAHA.107.735779 [DOI] [PubMed] [Google Scholar]
- 17.Nordmeyer J, Ewert P, Gewillig M, AlJufan M, Carminati M, Kretschmar O, Uebing A, Dähnert I, Röhle R, Schneider H, et al. Acute and midterm outcomes of the post-approval MELODY Registry: a multicentre registry of transcatheter pulmonary valve implantation. Eur Heart J. 2019;40:2255–2264. doi: 10.1093/eurheartj/ehz201 [DOI] [PubMed] [Google Scholar]
- 18.Georgiev S, Ewert P, Eicken A, Hager A, Hörer J, Cleuziou J, Meierhofer C, Tanase D. Munich comparative study: prospective long-term outcome of the transcatheter Melody valve versus surgical pulmonary bioprosthesis with up to 12 years of follow-up. Circ Cardiovasc Interv. 2020;13:e008963. doi: 10.1161/CIRCINTERVENTIONS.119.008963 [DOI] [PubMed] [Google Scholar]
- 19.Diller GP, Kempny A, Alonso-Gonzalez R, Swan L, Uebing A, Li W, Babu-Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation. 2015;132:2118–2125. doi: 10.1161/CIRCULATIONAHA.115.017202 [DOI] [PubMed] [Google Scholar]
- 20.Montanaro C, Merola A, Kempny A, Alvarez-Alvarez B, Alonso-Gonzalez R, Swan L, Uebing A, Li W, Babu-Narayan SV, Gatzoulis MA, et al. The outcome of adults born with pulmonary atresia: high morbidity and mortality irrespective of repair. Int J Cardiol. 2019;280:61–66. doi: 10.1016/j.ijcard.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 21.Buber J, Assenza GE, Huang A, Valente AM, Emani SM, Gauvreau K, Marshal AC, McElhinney DB, Landzberg MJ. Durability of large diameter right ventricular outflow tract conduits in adults with congenital heart disease. Int J Cardiol. 2014;175:455–463. doi: 10.1016/j.ijcard.2014.06.023 [DOI] [PubMed] [Google Scholar]
- 22.Brown JW, Ruzmetov M, Rodefeld MD, Eltayeb O, Yurdakok O, Turrentine MW. Contegra versus pulmonary homografts for right ventricular outflow tract reconstruction: a ten-year single-institution comparison. World J Pediatr Congenit Heart Surg. 2011;2:541–549. doi: 10.1177/2150135111415711 [DOI] [PubMed] [Google Scholar]
- 23.Batlivala SP, Emani S, Mayer JE, McElhinney DB. Pulmonary valve replacement function in adolescents: a comparison of bioprosthetic valves and homograft conduits. Ann Thorac Surg. 2012;93:2007–2016. doi: 10.1016/j.athoracsur.2012.02.039 [DOI] [PubMed] [Google Scholar]
- 24.Tweddell JS, Pelech AN, Frommelt PC, Mussatto KA, Wyman JD, Fedderly RT, Berger S, Frommelt MA, Lewis DA, Friedberg DZ, et al. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation. 2000;102(19 suppl 3):III130–III135. doi: 10.1161/01.cir.102.suppl_3.iii-130 [DOI] [PubMed] [Google Scholar]
- 25.Bishnoi RN, Jones TK, Kreutzer J, Ringel RE. NuMED Covered Cheatham-Platinum Stent™ for the treatment or prevention of right ventricular outflow tract conduit disruption during transcatheter pulmonary valve replacement. Catheter Cardiovasc Interv. 2015;85:421–427. doi: 10.1002/ccd.25682 [DOI] [PubMed] [Google Scholar]
- 26.Shahanavaz S, Asnes JD, Grohmann J, Qureshi AM, Rome JJ, Tanase D, Crystal MA, Latson LA, Morray BH, Hellenbrand W, et al. Intentional fracture of bioprosthetic valve frames in patients undergoing valve-in-valve transcatheter pulmonary valve replacement. Circ Cardiovasc Interv. 2018;11:e006453. doi: 10.1161/CIRCINTERVENTIONS.118.006453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.