ABSTRACT
We recently reported the successful treatment of a case of periprosthetic joint infection (PJI) with phage. Phage activity against bacteria causing PJI has not been systematically evaluated. Here, we examined the in vitro activity of seven phages against 122 clinical isolates of Staphylococcus aureus recovered between April 1999 and February 2018 from subjects with PJI. Phages were assessed against planktonic and biofilm phenotypes. Activity of individual phages was demonstrated against up to 73% of bacterial isolates in the planktonic state and up to 100% of biofilms formed by isolates that were planktonically phage susceptible. Susceptibility to phage was not correlated with small-colony-variant phenotype for planktonic or biofilm bacteria; correlation between antibiotic susceptibility and planktonic phage susceptibility and between biofilm phage susceptibility and strength of biofilm formation were noted under select conditions. These results demonstrate that phages can infect S. aureus causing PJI in both planktonic and biofilm phenotypes, and thus are worthy of investigation as an alternative or addition to antibiotics in this setting.
KEYWORDS: antibiotic resistance, biofilm, phage, periprosthetic joint infection
INTRODUCTION
Phages are bacteriotropic viruses that target bacterial hosts with high specificity. Since their discovery at the turn of the 20th century, they have been used for a variety of applications, including biotechnology, medical diagnostics, and treatment of bacterial infections, also known as “phage therapy.” Human use of phage therapy has occasionally been reported in the form of clinical trials but has predominantly occurred as case reports (1). Phage therapy is the subject of current therapeutic interest in the wake of mounting antimicrobial resistance.
Biologically distinct from small-molecule antibiotics, phages have several theoretical advantages as therapeutics, including their environmental abundance, their minimal off-target effects owing to host specificity, and ability to replicate through the attainment of bacterial clearance (2, 3). As strict obligate intracellular parasites of bacteria, phages must evolve in parallel with their hosts, a phenomenon referred to as a “coevolutionary arms race” which renders phages an appealing treatment strategy for bacteria with acquired or biofilm-associated antimicrobial resistance. The anti-biofilm potential of phages was first recognized in the mid-1990s in a demonstration by Doolittle et al. of T4 phage infection of Escherichia coli biofilms (4). Anti-biofilm activity has since been described in the literature for a variety of applications, including for Staphylococcus aureus infection (see [5–9] for examples). The mechanism underlying this activity may be exacted through depolymerase (so called “ectolysins”) enzymes which bind polysaccharide in capsule, lipopolysaccharide, or extrapolymeric substance, or through endolysins which bind peptidoglycan (10–13). Additional mechanisms have been proposed, including endolysin-mediated transcriptional downregulation of bacterial autolysin (14), quorum sensing inhibition (15), and stress response activation (16). Importantly, there is debate as to whether phages may augment biofilm formation in certain settings, such as through lysis-mediated release of extracellular DNA, a component of the EPS (16–18).
In medicine, bacterial biofilms can cause chronic, recalcitrant infections. PJI is one example of a biofilm-associated infection that may benefit from therapeutic phage (19–22). PJI is a rare but serious complication of joint arthroplasty, oftentimes leading to chronic infection, diminished function of the affected limb, and occasionally amputation, among other adverse outcomes (23). Though PJI occurs in only approximately 1–3% of joint arthroplasty cases, increasing numbers of individuals will be affected because the number of arthroplasty surgeries is increasing over time (23). Fifty to 60 percent of PJI cases are caused by members of the bacterial genus Staphylococcus, with 27% of cases caused by S. aureus (23). Current management strategies for PJI may insufficiently eradicate infection, due in part to drug-resistant organisms and/or the presence of bacterial biofilms, which exhibit intrinsic resistance to the immune system and traditional antibiotic interventions.
Phage therapy has been considered for treatment of foreign body associated infections in only a few in vitro and in vivo studies. Barros et al. demonstrated reductions in bacterial density of drug-resistant S. aureus, Enterococcus faecalis, and E. coli PJI isolates in vitro when treated planktonically with phage at a multiplicity of infection (MOI) of 10 (24). Kaur et al. used hydrogel-embedded MR-5 phage, linezolid, or a combination thereof to determine which treatment resulted in the greatest inhibition of methicillin-resistant S. aureus biofilm formation on orthopedic grade K-wires in vitro. Combination therapy prevented bacterial colonization and was associated with lower rates of phage and linezolid resistance-associated mutations versus subjects in either single therapy group (25). In an in vivo study by Cobb et al., treatment of an experimental S. aureus infected rat bicortical femur defect with phage-loaded hydrogel and fosfomycin resulted in lower bone bacterial densities compared with mono-treated and control groups (26). Similarly, in work by Morris et al. on rats with methicillin-susceptible S. aureus-infected total knee arthroplasties treated intraperitoneally with vancomycin, StaPhage cocktail, or vancomycin plus phage, the only difference in bacterial loads compared with untreated controls was in combination-treated animals (27). There have been seven reported compassionate uses of phage in human case reports for the treatment of PJI, all with apparently successful outcomes (28–34), alongside largely successful case series and case reports of osteoarticular indication (35–39). Finally, four of seven case reports of phage therapy in non-PJI device-related infections have been documented as successful (40–44).
Prior studies have considered the effect of single phages or cocktails against a small number of laboratory strains or non-PJI clinical isolates of S. aureus, and therefore, the ability to extrapolate the therapeutic potential of phage for PJI is limited (45). The objective of this study was to systematically examine the effect of phages against a large collection of clinical S. aureus isolates with a view toward better understanding their potential impact in the setting of PJI. This is the largest to date evaluating phage against clinical PJI isolates.
RESULTS
Planktonic susceptibility testing.
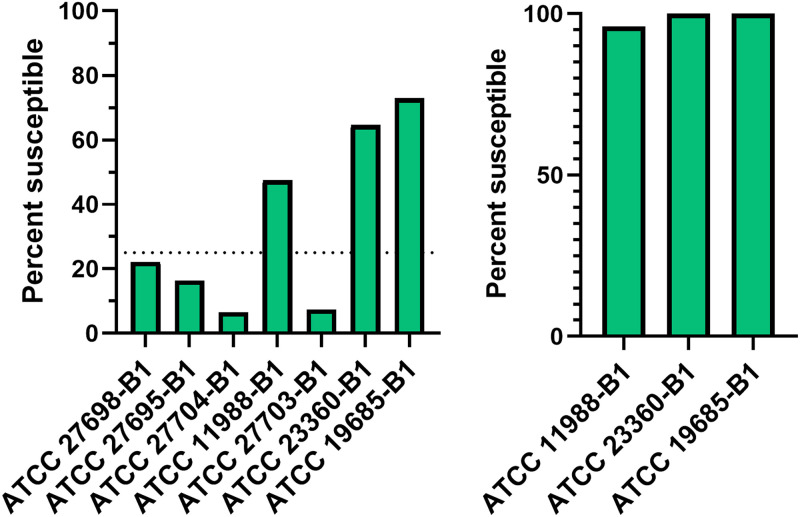
Testing seven phages from the American Type Culture Collection (ATCC) against each of 122 isolates (Table 1) planktonically revealed phage susceptibility ranging between 6 and 73% across the collection (Figure 1a). Twenty-three isolates were not susceptible to any phage in the collection (Table S1). Three (ATCC 11988-B1, ATCC 19685-B1 and ATCC 23360-B1) of the seven phages demonstrated activity against at least 25% of the collection and were therefore advanced to subsequent biofilm testing (Figure 1b). No associations were found between planktonic phage susceptibility and small versus normal colony variant phenotypes (Table 2). Generally, no correlation between planktonic phage susceptibility and antibiotic susceptibility was demonstrated, excepting a positive correlation found between oxacillin susceptibility and ATCC 11988-B1 susceptibility (Table 3).
TABLE 1.
Select clinical characteristics of PJI Staphylococcus aureus isolates
| Isolate feature (n = 122) | No. (%) |
|---|---|
| Methicillin susceptibility | |
| Susceptible | 51 (42) |
| Resistant | 36 (29) |
| Unknown | 35 (29) |
| Source | |
| Knee | 64 (52) |
| Hip | 41 (34) |
| Shoulder | 7 (6) |
| Elbow | 7 (6) |
| Othera | 3 (2) |
| Small-colony variant | |
| No | 118 (97) |
| Yes | 4 (3) |
Tibial component, ankle and finger joint.
FIG 1.
Phage activity against PJI Staphylococcus aureus isolates. Seven phages were tested against 122 S. aureus isolates. (a) Results of planktonic susceptibility testing using all seven phages. (b) The three phages that yielded plaques or zones of clearance for ≥25% of the bacterial collection in planktonic experiments were evaluated for anti-biofilm activity isolates for which planktonic activity had been observed. This included phage ATCC 11988-B1 (58 bacterial isolates); phage ATCC 19685-B1 (89 bacterial isolates); and phage ATCC 23360-B1 (79 bacterial isolates). The percentage of biofilms susceptible to each phage is shown.
TABLE 2.
Correlational analysis of planktonic phage susceptibility and small-colony-variant statusa
| No. of small-colony variants | No. of normal colony variants |
P value |
||||||
|---|---|---|---|---|---|---|---|---|
| ATCC 27698-B1 | ATCC 27695-B1 | ATCC 27704-B1 | ATCC 11988-B1 | ATCC 27703-B1 | ATCC 23360-B1 | ATCC 19685-B1 | ||
| 2 | 119 | 0.3951 | 1.000 | 1.000 | 1.000 | 1.000 | 0.5398 | 1.000 |
The Fisher’s exact test was used to interrogate the relationship between planktonic phage susceptibility and small-colony variant or normal colony phenotype, α = 0.05.
TABLE 3.
Correlational analysis of planktonic phage susceptibility and phage susceptibilitya
| Drug | No. of drug-susceptible isolates | No. of drug intermediate isolates | No. of drug-resistant isolates | Phage |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 27698-B1 |
ATCC 27695-B1 |
ATCC 27704-B1 |
ATCC 11988-B1 |
ATCC 27703-B1 |
ATCC 23360-B1 |
ATCC 19685-B1 |
||||||||||||||||||
| S | R | P | S | R | P | S | R | P | S | R | P | S | R | P | S | R | P | S | R | P | ||||
| Oxacillin | 75 | NA | 38 | 25 | 88 | 1.000 | 19 | 94 | 0.289 | 7 | 106 | 1.000 | 54 | 59 | 0.017 | 9 | 104 | 0.268 | 73 | 40 | 1.000 | 82 | 31 | 0.657 |
| Clindamycin | 77 | NA | 35 | 24 | 88 | 0.466 | 19 | 93 | 0.417 | 7 | 105 | 1.000 | 53 | 59 | 0.315 | 9 | 103 | 0.718 | 72 | 40 | 1.000 | 81 | 31 | 0.649 |
| Rifampin | 111 | NA | 2 | 25 | 88 | 0.395 | 19 | 94 | 1.000 | 7 | 106 | 1.000 | 54 | 59 | 0.497 | 9 | 104 | 1.000 | 73 | 40 | 1.000 | 82 | 31 | 1.000 |
| Daptomycin | 56 | NA | 0 | 15 | 41 | 1.000 | 10 | 46 | 1.000 | 5 | 51 | 1.000 | 25 | 31 | 1.000 | 3 | 53 | 1.000 | 34 | 22 | 1.000 | 38 | 18 | 1.000 |
| Levofloxacin | 46 | 33 | 34 | 25 | 88 | 0.522 | 19 | 94 | 0.412 | 7 | 106 | 1.000 | 54 | 59 | 0.426 | 9 | 104 | 0.342 | 73 | 40 | 0.527 | 82 | 31 | 0.803 |
| Linezolid | 34 | NA | 0 | 7 | 27 | 1.000 | 4 | 30 | 1.000 | 3 | 31 | 1.000 | 23 | 11 | 1.000 | 4 | 30 | 1.000 | 26 | 8 | 1.000 | 28 | 6 | 1.000 |
The Fisher’s exact test was used to interrogate the relationship between planktonic phage susceptibility and susceptibility to antibiotics shown, α = 0.05. NA = not applicable, S = susceptible, R = resistant.
Biofilm susceptibility testing.
Only isolates susceptible to each of the following three phages planktonically were included in phage anti-biofilm experiments: ATCC 11988-B1 was tested against 58 isolates; ATCC 19685-B1 was tested against 89 isolates; and ATCC 23360-B1 was tested against 79 isolates. Of the subset with consistent susceptibility across technical and biological duplicates in addition to a standard deviation of the average percent reduction across biological duplicates less than or equal to 5% of the value of average reduction, statistically significant reductions between control and phage-treated biofilms were found in 63 (100%) isolates treated with ATCC 23360-B1; 81 (100%) isolates treated with ATCC 19685-B1; and 26 (96%) isolates treated with ATCC 11988-B1 (Figure 1b).
Strength of biofilm formation was assessed for association with biofilm phage susceptibility (Table 4). No associations were observed between strength of biofilm formation and percent optical density (OD492) reduction, except between nonadherent or weak biofilm formers and moderate/strong or strong biofilm formers treated with ATCC 11988-B1 and between weak/moderate or moderate and moderate/strong or strong biofilm formers treated with ATCC 23360-B1. It is not well understood whether these relatively weaker biofilm formers were more susceptible to phage than stronger biofilm formers because of lower starting cell densities, an ambiguity that may be attributed to the semiquantitative nature of the colorimetric assay. No test for association was performed between nonadherent or weak biofilm formers and moderate/strong or strong biofilm formers, or between nonadherent or weak biofilm formers and weak/moderate or moderate biofilm formers treated with ATCC 19685-B1; between nonadherent or weak biofilm formers and moderate/strong or strong biofilm formers, or between nonadherent or weak biofilm formers and weak/moderate or moderate biofilm formers treated with ATCC 23360-B1 because of the absence of corresponding isolates.
TABLE 4.
Correlational analysis of biofilm phage response and strength of biofilm formationa
| Phage | Levels of biofilm formation | No. of isolates | Levels compared | P value |
|---|---|---|---|---|
| ATCC 11988-B1 | 0 | 2 | 1−0 | 0.3502 |
| 1 | 4 | 2−1 | 0.1507 | |
| 2 | 16 | 2−0 | 0.0045 | |
| ATCC 19685-B1 | 0 | 0 | 2−1 | 0.1602 |
| 1 | 2 | |||
| 2 | 78 | |||
| ATCC 23360-B1 | 0 | 0 | 2−1 | 0.0462 |
| 1 | 1 | |||
| 2 | 61 |
The Wilcoxon signed rank test was used to relate strength of biofilm formation and biofilm phage response as measured by percent reduction in treated versus control conditions, α = 0.05. Replicates were assigned categories of strength of biofilm formation established by Stepanovic et al. (68), as 0 = nonadherent or weak biofilm formers; 1 = weak/moderate or moderate biofilm formers; or 2 = moderate/strong or strong biofilm formers. Isolates were analyzed if biological replicates yielded the same or adjacent categorical assignments. Statistics were calculated for differences in percent biomass reduction between biofilm formation levels per phage.
Associations were probed between biofilm phage activity and small versus normal colony variant phenotype (Table 5), with no differences found based on percent OD492 reduction between treated and untreated isolates for any phage tested.
TABLE 5.
Correlational analysis of biofilm phage response and small-colony-variant statusa
| Phage | No. of small-colony-variant isolates | No. of normal colony isolates | P value |
|---|---|---|---|
| ATCC 11988-B1 | 1 | 26 | 0.6635 |
| ATCC 19685-B1 | 2 | 78 | 0.9347 |
| ATCC 23360-B1 | 0 | 63 | NA |
The Wilcoxon rank sum test with normal approximation was used to query the relationship between normal or small-colony-variant status and biofilm phage response as measured by percent reduction under treated versus control conditions per phage, α = 0.05. NA = not applicable.
There was no association between biofilm phage susceptibility and susceptibility to oxacillin, linezolid, daptomycin, rifampin, clindamycin, or levofloxacin (Table 6).
TABLE 6.
Correlational analysis of biofilm phage susceptibility and oxacillin, linezolid, rifampin, daptomycin, clindamycin, and levofloxacin susceptibilitya
| Antibiotic | No. of antibiotic- susceptible isolates | No. of antibiotic intermediate isolates | No. of antibiotic- resistant isolates | Phage | P value |
|---|---|---|---|---|---|
| Oxacillin | 18 | NA | 7 | ATCC 11988-B1 | 0.3047 |
| 48 | NA | 26 | ATCC 19685-B1 | 0.6044 | |
| 42 | NA | 15 | ATCC 23360-B1 | 0.8444 | |
| Clindamycin | 16 | NA | 9 | ATCC 11988-B1 | 0.2620 |
| 52 | NA | 21 | ATCC 19685-B1 | 0.9879 | |
| 40 | NA | 17 | ATCC 23360-B1 | 0.1331 | |
| Rifampin | 25 | NA | 0 | ATCC 11988-B1 | NA |
| 72 | NA | 2 | ATCC 19685-B1 | 0.2638 | |
| 56 | NA | 1 | ATCC 23360-B1 | 0.5243 | |
| Daptomycin | 11 | NA | 0 | ATCC 11988-B1 | NA |
| 35 | NA | 0 | ATCC 19685-B1 | NA | |
| 24 | NA | 0 | ATCC 23360-B1 | NA | |
| Levofloxacin | 10 | 8 | 7 | ATCC 11988-B1 | 0.5772b |
| 29 | 22 | 23 | ATCC 19685-B1 | 0.4102b | |
| 23 | 18 | 16 | ATCC 23360-B1 | 0.7386b | |
| Linezolid | 12 | NA | 0 | ATCC 11988-B1 | NA |
| 24 | NA | 0 | ATCC 19685-B1 | NA | |
| 25 | NA | 0 | ATCC 23360-B1 | NA |
The Wilcoxon rank sum test with normal approximation was performed to assess correlation between oxacillin, linezolid, rifampin, daptomycin, clindamycin, or levofloxacin susceptibility and biofilm phage response as measured by percent reduction under treated versus control conditions in biological replicates, α = 0.05. NA, not applicable.
Kruskal-Wallis test with chi square approximation was performed to accommodate nonbinary levofloxacin susceptibility data.
DISCUSSION
Among few published in vitro phage screens in PJI-associated S. aureus, this study features the most extensive panel of clinical isolates. Phage susceptibility of planktonic bacteria was phage-dependent, ranging in susceptibility from 6% to 73% of isolates. This work did not define why such differential susceptibility patterns were observed. Possibilities might include mismatched phage depolymerase enzyme or EPS carbohydrate receptor (46) or suboptimal relative phage density (11), such that bacteria replicated faster than phage-mediated bacteriolysis could occur. Of the seven phages originally tested, three were active against ≥25% of isolates and advanced to biofilm studies. Among bacteria that demonstrated susceptibility to these phages planktonically, between 96 and 100% had significant reductions in biofilm-associated biomass when treated with planktonically active phages (Fig. 1). Available literature does not appear to suggest that antibiofilm activity would be observed in the absence of planktonic activity (47), although a lack of antibiofilm activity does not preclude planktonic activity (48). For instance, in the present study, 4% of isolates demonstrated planktonically susceptible to ATCC 11988-B1 were unaffected by the phage as biofilms compared with untreated controls (Fig. 1).
The three phages shown to have activity against both planktonic and biofilm-associated bacteria in the present study have been described to various degrees in the literature. Phage ATCC 23360-B1 was included in standardized phage typing sets for bovine staphylococci (49–54). Phage ATCC 19685-B1, or phage K, is a well-known staphylococcal phage in the family of Myoviridae of the order Caudovirales, having an icosahedral head and long, contractile tail (55). It utilizes the N-acetylglucosamine moiety of wall teichoic acid to adsorb to bacteria and its antibiofilm properties have been previously noted; in addition to S. aureus, coagulase-negative staphylococci may be also susceptible to phage K (56). Additionally, its lysin, LysK, has been studied for its bactericidal activity independently and recombinantly (57–61). Furthermore, phage K has been utilized for generation of mutants to expand phage diversity and host spectrum (62). Phage ATCC 11988-B1, or phage P14, is a Twort-like phage and genetic relative of phage K (63, 64). Studies of P14’s infection mechanisms historically contributed to functional understanding of lysin in phage-induced bacteriolysis and viral dissemination (65, 66). While phage K has been extensively utilized for its antibacterial potential, ATCC 23360-B1 and ATCC 11988-B1 (P14) have not been therapeutically deployed, though perhaps deserve consideration to this end given their broad spectrum of activity against planktonic and biofilm S. aureus clinical isolates.
Among planktonic phage susceptibility results, no correlation was found with the small versus normal colony phenotype (Table 2), although planktonic susceptibility to ATCC 11988-B1 was correlated with oxacillin susceptibility (Table 3). The significance of this finding, if any, is not clear, but could hypothetically implicate the involvement of penicillin binding proteins in phage ATCC 11988-B1 interactions with S. aureus. On the other hand, that another Twort-like staphylococcal phage has been shown to elicit synergistic activity when combined with oxacillin may suggest that different pathways entirely are utilized by these antimicrobial agents (67).
Of the established biofilms, analyses were conducted in subgroups based on an isolate’s ability to form biofilm (non-adherent/weak; weak/moderate or moderate; or moderate/strong or strong) using a method modified from Stepanovic et al. (68). The Wilcoxon signed rank test was used to measure the relationship between biofilm formation ability and percent reduction between the optical density of phage treated and untreated biofilms, revealing significantly increased percent reduction among non-adherent or weak biofilm formers treated with ATCC 11988-B1 versus moderate/strong or strong biofilm formers under the same conditions, as well as among weak/moderate or moderate biofilm formers treated with ATCC 23360-B1 versus moderate/strong or strong biofilm formers under the same conditions (Table 4). Further studies are needed to interpret the relationship between biofilm susceptibility to phage and biofilm robustness, preferably across larger, more balanced groups of strains with differing biofilm formation potential. It is possible that this result is attributed to differential initial cell densities as an artifact of the semiquantitative nature of optical density readings.
Beyond biofilm-associated infections, small-colony variants represent another form of phenotypic resistance implicated in PJI. Characterized by their reduced colony size and growth rate, with ultrastructural changes to the cell wall and/or altered nutrient synthesis capabilities, small-colony variants may be antibiotic tolerant and/or establish intracellular residence manifesting as chronic infection (69–71). In a retrospective study of PJI-associated staphylococci, we found that while there was no difference in the risk of two-stage exchange treatment failure in PJI cases caused by small-colony variant and wild-type bacteria, the former was associated with extended infection times and an increased frequency of antecedent antimicrobial interventions (72). Although one study demonstrated the activity of phage-derived HY-133 lysin against both small-colony variant and wild type planktonic and biofilm-associated S. aureus in vitro (70), our work is the first description of small-colony-variant response to phage. In this study, no differences were found in the activity of any phage tested against biofilms of small-colony variants compared to those of non-small-colony variants (Table 5).
Lastly, we probed for a correlation between isolate biofilm susceptibility and susceptibility to oxacillin, linezolid, daptomycin, rifampin, clindamycin, and levofloxacin. Oxacillin, daptomycin, and linezolid are potential antimicrobial therapies for PJI caused by methicillin-susceptible and -resistant staphylococci (23), with combination therapy including rifampin alongside clindamycin or levofloxacin, among others (73, 74, 77). The Wilcoxon exact test identified no significant relationship between biofilm biomass percent reduction secondary to phage and susceptibility or resistance to the above antibiotics (Table 6). This reassuring finding suggests that antibiotic-resistant bacteria may be as responsive to phage treatment as antibiotic-susceptible counterparts.
One limitation of this study is the range of concentrations at which phages were utilized for planktonic susceptibility testing above a threshold of 104 PFU/ml such that dose dependent effects could not be easily controlled in this step. Standardized phage susceptibility methods are needed to support potential clinical implementation of phage therapy, although no so such test yet exist. The variability observed in presently available methods must be addressed as standardized methods are developed. One approach for abrogating such variability is repeat testing, as performed herein. Another limitation of this study is the experimental model in which anti-biofilm activity of phage was assessed. The establishment of biofilms on a polystyrene substrate in nutrient broth bears little resemblance to the microenvironment in which biofilms become established in the setting of PJI. Future studies should examine whether phage activity is upheld against biofilms in plasma and synovial fluid, and on more clinically relevant surfaces, to better recapitulate the context of infection. Still, it should be noted there are not analogous standardized biofilm assays available in the clinical microbiology laboratory for antibiotics at this time. Finally, the small number of isolates comprising small-colony variants and nonadherent/weak and weak/moderate or moderate biofilm formers limits the statistical power of these respective tests such that additional studies are needed to draw definitive conclusions on the correlation between these phenotypes and phage susceptibility.
In summary, a screen of seven commercially available S. aureus phages against a large panel of PJI clinical isolates demonstrated phage-mediated activity against planktonic and biofilm-associated bacteria. Results show that up to 73% of clinical isolates are phage susceptible planktonically and up to 100% of those demonstrating planktonic activity underwent significant biomass reduction when treated with phage as in vitro biofilms. That nearly all isolates susceptible to phage planktonically attain significant biomass reduction begs the question as to whether biofilm phage susceptibility testing will be needed, at least for S. aureus. Collectively, these findings justify further studies of phage in the setting of PJI and suggest potential new phages for this approach.
MATERIALS AND METHODS
Bacterial isolates.
122 S. aureus PJI isolates collected at the Mayo Clinic between April 1999 and February 2018 were tested (Table 1). Susceptibility to oxacillin, linezolid, rifampin, clindamycin, levofloxacin, and daptomycin, performed as part of routine clinical practice, was recorded, where available. Bacteria, which had been stored in Microbank vials (Pro-Lab Diagnostics, Round Rock, TX) at −80°C, were subcultured twice onto BBL Trypticase Soy Agar with 5% Sheep Blood (TSA II) (Becton, Dickinson and Company, Sparks, MD) and incubated at 37°C in room air for 24 h. Small-colony variants were previously characterized (72).
Phage stocks.
Seven S. aureus phages from ATCC were studied (ATCC 23360-B1 [strain designation 15], ATCC 11988-B1 [strain designation P14], ATCC 27704-B1 [strain designation 6], ATCC 27703-B1 [strain designation 3C], ATCC 27695-B1 [strain designation 54], ATCC 27698-B1 [strain designation 75], and ATCC 19685-B1 [strain designation K]). Phages were propagated from stocks as recommended by ATCC. Briefly, lyophilized phages were reconstituted in 250 μl of overnight host culture suspended in tryptic soy broth (BD Bacto™ Tryptic Soy Broth [TSB] #211825, Franklin Lakes, NJ), and plated by the double agar assay (75): phage and host were inoculated in cooled, molten 10 mM MgSO4 TSB containing 0.5% agar, which was poured over solidified tryptic soy agar (TSA), and incubated overnight at 37°C in room air. To amplify phage, plaques were scraped and resuspended in saline, followed by centrifugation at 15,000 rpm for 4 min. Phage-containing supernatant was then 0.22 μm filter sterilized (Millex Millipore Sigma, Burlington, MA). Prior to susceptibility testing, phage titers were determined by spotting 1 μl of 1:10 serial dilutions onto double layer agar containing 250 μl log-phase host.
Planktonic phage susceptibility testing.
Plaque assay. Phage-bacteria combinations were evaluated by a spot assay modification of the double agar assay, in which 1 μl aliquots of each phage strain (≥104 PFU/ml) were plated on double layer agar containing log-phase bacteria (76). Each bacterium in the collection was exposed to each of the seven phages. Phage activity was recorded following overnight incubation at 37°C in room air. Isolates for which phage spotting yielded plaques (P) or a zone of clearance (ZC) were considered susceptible, while those for which phage spotting yielded no degree of observable activity (NA) or a zone of growth attenuation (ZGA) were considered non-susceptible. Scoring was conducted according to the following method (Fig. S1): NA, no activity; ZGA, some degree of nondiscrete growth reduction was denoted as a zone of growth attenuation; P, discrete, quantifiable puncta of total bacterial clearance were denoted as plaques; ZC, total bacterial clearance in a region of any size (excepting discrete, individual plaques) was denoted as a zone of clearance. Planktonic reproducibility was determined by comparing phage activity scores across two replicates on different days for each phage-bacterium combination. Isolates with any combination of ZC and/or P; or ZGA and/or NA across replicates were designated as having consistent levels of phage activity. Phages that yielded consistent activity in ≥25% of the collection were evaluated for anti-biofilm activity against isolates for which planktonic activity had been observed (Fig. 1).
Biofilm phage susceptibility testing.
Biofilm-associated cell quantitation. Three to five colonies were added to 2 ml TSB and grown to log phase at 37°C with shaking (120 rpm) for 2 h. Liquid cultures were diluted to 104 CFU/ml in cation-adjusted Mueller-Hinton Broth (CAMHB). One hundred fifty microliters of bacterial diluent were added to wells of a sterile Falcon non-tissue culture treated flat-bottom 96-well plate (Corning, Inc., Corning, NY) in triplicate per organism, and incubated under the same conditions as above for 4 h at 37°C room air. Wells were emptied and rinsed once with sterile saline to remove vegetative bacteria, then systematically scraped in 200 μl fresh 1X phosphate-buffered saline (PBS) and quantitatively cultured to enumerate biofilm-associated cellular density. Bacterial concentration expressed as the average CFU/well of each isolate tested in triplicate was used to compute the phage concentration needed per well to attain the desired multiplicity of infection (MOI).
Biofilm phage susceptibility testing. Biofilms were prepared and grown as described above. Following incubation, wells were rinsed once with PBS and treated with 200 μl phage (MOI =10; bacterial concentration was calculated as the average CFU/well) suspended in 10 mM MgSO4 TSB. Control wells per plate included a media sterility control, a phage sterility control, an antibiotic control (4 μg/ml rifampin), and a biofilm positive control (Staphylococcus epidermidis RP62A). Plates were incubated for 24 h at 37°C, emptied, rinsed once with saline, and dried overnight. Wells were stained for 1 min with 0.1% safranin, rinsed twice with sterile water, and dried overnight. Finally, stained cells were resuspended in 30% glacial acetic acid and the OD492 measured (accuSkan GO Fisher Scientific, Waltham, MA). All testing was performed in duplicate on 2 days (for a total of four tests per phage-bacterium combination in addition to control conditions). Prior to analysis, spectrophotometric data of each plate was normalized by subtracting the average reading of the media sterility wells from each replicate of each condition.
Visual comparison of staining patterns across treatment conditions (i.e., safranin color intensity of treated wells greater than, less than, or like that of untreated wells) performed on the same and different days was recorded for each isolate. If treated versus untreated conditions did not yield the same pattern across replicates from different days, inconsistent phage activity was recorded. Only combinations yielding consistent findings across technical and biological replicates in addition to a standard deviation of the average percent reduction across biological duplicates less than or equal to 5% of the value of average reduction were included in statistical analyses measuring phage-mediated density reduction as well as the relationship between phage response and strength of biofilm formation, small-colony-variant status, and antibiotic susceptibility. To exclude outliers of variability, isolates yielding inconsistent visual comparison results between technical replicates in two or more tests were excluded from final analysis.
Statistical analysis.
Planktonic phage susceptibility was correlated with small-colony-variant status as well as susceptibility to oxacillin, clindamycin, rifampin, daptomycin, levofloxacin and linezolid susceptibility using Fisher’s exact test. Statistical significance of the effect of phage treatment on biofilm biomass as measured by OD492 for each isolate was computed by the Wilcoxon rank sum test. All other analyses were performed according to the percent reduction of OD492 in treated versus control conditions, accounting for isolates whose standard deviation of the average percent reduction across biological duplicates was less than or equal to 5% of the value of average reduction. The Wilcoxon rank sum test with normal approximation was used to determine the strength of association between biofilm phage susceptibility and small-colony-variant status; the Wilcoxon rank sum test with normal approximation, as well as the Kruskal-Wallis test with chi square approximation, was used to determine the strength of association between biofilm phage susceptibility and antibiotic susceptibility. The Wilcoxon signed rank test was used to interrogate the association between biofilm forming status and phage susceptibility, based on the percent reduction of OD492 in treated versus control conditions, accounting for isolates whose standard deviation of the average percent reduction across biological duplicates was less than or equal to 5% of the value of average reduction. Replicates were assigned to categories of biofilm formation based on a method established by Stepanovic et al. (68), modified to accommodate the possibility that an isolate be assigned to adjacent categories among replicates due to experimental variation within normal range (0 = nonadherent or weak biofilm formers; 1 = weak/moderate or moderate biofilm formers; and 2 = moderate/strong or strong biofilm formers). Isolates whose biological replicates yielded the same categorical assignments were evaluated for statistical associations between biofilm formation strength and phage susceptibility. The Wilcoxon exact test was performed to assess correlation between oxacillin, clindamycin, rifampin, daptomycin, levofloxacin (in addition to the Kruskal Wallis test), and linezolid susceptibility and biofilm phage susceptibility as measured by average percent reduction under treated versus control conditions across biological replicates. Antibiotic susceptibilities were interpreted using the Clinical and Laboratory Standards Institute (CLSI) 2021 MIC breakpoints. All tests were two sided with α = 0.05 and P values ≤0.05 were considered statistically significant. Analyses were performed using JMP 14.1.0 software (SAS Inc., Cary, NC), and figures were prepared using GraphPad Prism 8.4.2 software (GraphPad Software, San Diego, CA).
ACKNOWLEDGMENTS
We thank Scott A. Cunningham, MS, and Kerryl E. Greenwood-Quaintance, MS, for their technical expertise and proofreading, and Suzannah M. Schmidt-Malan, MS, for her technical expertise. This work was supported by T32 AR56950 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and by UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Robin Patel is supported, in part, by UM1 AI104681 and R01 AR056647. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Loc-Carrillo C, Abedon ST. 2011. Pros and cons of phage therapy. Bacteriophage 1:111–114. 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caflisch KM, Patel R. 2019. Implications of bacteriophage- and bacteriophage component-based therapies for the clinical microbiology laboratory. J Clin Microbiol 57:e00229. 10.1128/JCM.00229-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doolittle MM, Cooney JJ, Caldwell DE. 1995. Lytic infection of Escherichia coli biofilms by bacteriophage T4. Can J Microbiol 41:12–18. 10.1139/m95-002. [DOI] [PubMed] [Google Scholar]
- 5.Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, Fiscarelli EV, Rossitto M, Cariani L, Briani F, Debarbieux L, Ghisotti D. 2018. Design of a broad-range bacteriophage cocktail that reduces Pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob Agents Chemother 62:e02573-17. 10.1128/AAC.02573-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam MS, Zhou Y, Liang L, Nime I, Liu K, Yan T, Wang X, Li J. 2019. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses 11:841. 10.3390/v11090841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melo LDR, Ferreira R, Costa AR, Oliveira H, Azeredo J. 2019. Efficacy and safety assessment of two enterococci phages in an in vitro biofilm wound model. Sci Rep 9:6643–6643. 10.1038/s41598-019-43115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siringan P, Connerton PL, Payne RJH, Connerton IF. 2011. Bacteriophage-mediated dispersal of Campylobacter jejuni biofilms. Appl Environ Microbiol 77:3320–3326. 10.1128/AEM.02704-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seth AK, Geringer MR, Nguyen KT, Agnew SP, Dumanian Z, Galiano RD, Leung KP, Mustoe TA, Hong SJ. 2013. Bacteriophage therapy for Staphylococcus aureus biofilm–infected wounds: a new approach to chronic wound care. Plast Reconstr Surg 131:225–234. 10.1097/PRS.0b013e31827e47cd. [DOI] [PubMed] [Google Scholar]
- 10.Maciejewska B, Olszak T, Drulis-Kawa Z. 2018. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application? Appl Microbiol Biotechnol 102:2563–2581. 10.1007/s00253-018-8811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan BK, Abedon ST. 2015. Bacteriophages and their enzymes in biofilm control. Curr Pharm Des 21:85–99. 10.2174/1381612820666140905112311. [DOI] [PubMed] [Google Scholar]
- 12.Sharma U, Vipra A, Channabasappa S. 2018. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov Today 23:848–856. 10.1016/j.drudis.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Hughes KA, Sutherland IW, Jones MV. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144:3039–3047. 10.1099/00221287-144-11-3039. [DOI] [PubMed] [Google Scholar]
- 14.Fernández L, González S, Campelo AB, Martínez B, Rodríguez A, García P. 2017. Downregulation of autolysin-encoding genes by phage-derived lytic proteins inhibits biofilm formation in Staphylococcus aureus. Antimicrob Agents Chemother 61. 10.1128/AAC.02724-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei R, Lamas-Samanamud GR. 2014. Inhibition of biofilm formation by T7 bacteriophages producing quorum-quenching enzymes. Appl Environ Microbiol 80:5340–5348. 10.1128/AEM.01434-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández L, González S, Campelo AB, Martínez B, Rodríguez A, García P. 2017. Low-level predation by lytic phage phiIPLA-RODI promotes biofilm formation and triggers the stringent response in Staphylococcus aureus. Sci Rep 7:40965–40965. 10.1038/srep40965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gödeke J, Paul K, Lassak J, Thormann KM. 2011. Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1. ISME J 5:613–626. 10.1038/ismej.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, Yu P, Wang Z, Alvarez PJJ. 2020. Hormetic promotion of biofilm growth by polyvalent bacteriophages at low concentrations. Environ Sci Technol 54:12358–12365. 10.1021/acs.est.0c03558. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng A, Tzeng TH, Vasdev S, Korth K, Healey T, Parvizi J, Saleh KJ. 2015. Treating periprosthetic joint infections as biofilms: key diagnosis and management strategies. Diagn Microbiol Infect Dis 81:192–200. 10.1016/j.diagmicrobio.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Gbejuade HO, Lovering AM, Webb JC. 2015. The role of microbial biofilms in prosthetic joint infections: a review. Acta Orthop 86:147–158. 10.3109/17453674.2014.966290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caflisch KM, Suh GA, Patel R. 2019. Biological challenges of phage therapy and proposed solutions: a literature review. Expert Rev Anti Infect Ther 17:1011–1041. 10.1080/14787210.2019.1694905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akanda ZZ, Taha M, Abdelbary H. 2018. Current review-The rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J Orthop Res 36:1051–1060. 10.1002/jor.23755. [DOI] [PubMed] [Google Scholar]
- 23.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barros J, Melo LDR, Poeta P, Igrejas G, Ferraz MP, Azeredo J, Monteiro FJ. 2019. Lytic bacteriophages against multidrug-resistant Staphylococcus aureus, Enterococcus faecalis and Escherichia coli isolates from orthopaedic implant-associated infections. Int J Antimicrob Agents 54:329–337. 10.1016/j.ijantimicag.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Kaur S, Harjai K, Chhibber S. 2014. Bacteriophage mediated killing of Staphylococcus aureus in vitro on orthopaedic K wires in presence of linezolid prevents implant colonization. PLoS One 9:e90411. 10.1371/journal.pone.0090411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobb LH, Park J, Swanson EA, Beard MC, McCabe EM, Rourke AS, Seo KS, Olivier AK, Priddy LB. 2019. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS One 14:e0220421. 10.1371/journal.pone.0220421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris JL, Letson HL, Elliott L, Grant AL, Wilkinson M, Hazratwala K, McEwen P. 2019. Evaluation of bacteriophage as an adjunct therapy for treatment of peri-prosthetic joint infection caused by Staphylococcus aureus. PLoS One 14:e0226574. 10.1371/journal.pone.0226574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doub JB, Ng VY, Johnson AJ, Slomka M, Fackler J, Horne B, Brownstein MJ, Henry M, Malagon F, Biswas B. 2020. Salvage bacteriophage therapy for a chronic MRSA prosthetic joint infection. Antibiotics (Basel) 9. 10.3390/antibiotics9050241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cevallos EC, Caflisch K, Bollyky P, Belleghem JV, Patel R, Fackler J, Brownstein M, Horne B, Biswas B, Henry M, Malagon F, Lewallen D, Suh G. 2021. Phage therapy for limb-threatening prosthetic knee Klebsiella pneumoniae infection: case report and in vitro characterization of anti-biofilm activity. Clin Infect Dis 73(1):e144–e151. 10.1093/cid/ciaa705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkhilaishvili T, Winkler T, Müller M, Perka C, Trampuz A. 2019. Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 64. 10.1128/AAC.00924-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferry T, Leboucher G, Fevre C, Herry Y, Conrad A, Josse J, Batailler C, Chidiac C, Medina M, Lustig S, Laurent F, Group LBS. 2018. Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infect Dis 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuts A-S, Berkhout HJ, Hartog A, Goosen JHM. 2021. Bacteriophage therapy cures a recurrent Enterococcus faecalis infected total hip arthroplasty? A case report. Acta Orthopaedica 10.1080/17453674.2021.1968714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doub JB, Ng VY, Wilson E, Corsini L, Chan BK. 2021. Successful treatment of a recalcitrant Staphylococcus epidermidis prosthetic knee infection with intraoperative bacteriophage therapy. Pharmaceuticals (Basel) 14 10.3390/ph14030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Sanchez C, Gonzales F, Buckley M, Biswas B, Henry M, Deschenes MV, Horne B, Fackler J, Brownstein MJ, Schooley RT, Aslam S. 2021. Successful treatment of Staphylococcus aureus prosthetic joint infection with bacteriophage therapy. Viruses 13 10.3390/v13061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patey O, McCallin S, Mazure H, Liddle M, Smithyman A, Dublanchet A. 2018. Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 11:18. 10.3390/v11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chanishvili N, Sharp R. 2008. Bacteriophage therapy: experience from the Eliava Institute, Georgia. Microbiol Aust 96–101. 10.1071/MA08096. [DOI] [Google Scholar]
- 37.Ferry T, Boucher F, Fevre C, Perpoint T, Chateau J, Petitjean C, Josse J, Chidiac C, L'Hostis G, Leboucher G, Laurent F. 2018. Innovations for the treatment of a complex bone and joint infection due to XDR Pseudomonas aeruginosa including local application of a selected cocktail of bacteriophages. J Antimicrob Chemother 73:2901–2903. 10.1093/jac/dky263. [DOI] [PubMed] [Google Scholar]
- 38.Albee FH. 1933. The treatment of osteomyelitis by bacteriophage. J Bone Joint Surg 15:58–66. [Google Scholar]
- 39.Fish R, Kutter E, Bryan D, Wheat G, Kuhl S. 2018. Resolving digital staphylococcal osteomyelitis using bacteriophage-A case report. Antibiotics (Basel, Switzerland) 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. 2018. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018:60–66. 10.1093/emph/eoy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aslam S, Pretorius V, Lehman SM, Morales S, Schooley RT. 2019. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J Heart Lung Transplant 38:475–476. 10.1016/j.healun.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Gilbey T, Ho J, Cooley LA, Petrovic Fabijan A, Iredell JR. 2019. Adjunctive bacteriophage therapy for prosthetic valve endocarditis due to Staphylococcus aureus. Med J Aust 211:142–143.e1. 10.5694/mja2.50274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Exarchos V, Tkhilaishvili T, Potapov E, Starck C, Trampuz A, Schoenrath F. 2020. Successful bacteriophage treatment of infection involving cardiac implantable electronic device and aortic graft: a Trojan horse concept. Europace 22:597–597. 10.1093/europace/euz319. [DOI] [PubMed] [Google Scholar]
- 44.Aslam S, Lampley E, Wooten D, Karris M, Benson C, Strathdee S, Schooley RT. 2020. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect Dis 7. 10.1093/ofid/ofaa389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris J, Kelly N, Elliott L, Grant AL, Wilkinson M, Hazratwala K, McEwen P. 2019. Evaluation of bacteriophage anti-biofilm activity for potential control of orthopedic implant-related infections caused by Staphylococcus aureus. Surg Infect (Larchmt) 20:16–24. 10.1089/sur.2018.135. [DOI] [PubMed] [Google Scholar]
- 46.Latka A, Maciejewska B, Majkowska-Skrobek G, Briers Y, Drulis-Kawa Z. 2017. Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl Microbiol Biotechnol 101:3103–3119. 10.1007/s00253-017-8224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen MF, Svenningsen SL, Røder HL, Middelboe M, Burmølle M. 2019. Big impact of the tiny: bacteriophage-bacteria interactions in biofilms. Trends Microbiol 27:739–752. 10.1016/j.tim.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Ferriol-González C, Domingo-Calap P. 2020. Phages for biofilm removal. Antibiotics (Basel, Switzerland) 9:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Contag CH. 1956. Use of bacteriophage in the study of staphylococcic bovine mastitis. Master of Science. Iowa State University. [Google Scholar]
- 50.Davidson I. 1972. A collaborative investigation of phages for typing bovine staphylococci. Bull Wld Hlth Org 46:81–98. [PMC free article] [PubMed] [Google Scholar]
- 51.Slanetz LW, Bartley CH. 1962. Bacteriophage and serological typing of staphylococci from bovine mastitis. J Infect Dis 110:238–245. 10.1093/infdis/110.3.238. [DOI] [PubMed] [Google Scholar]
- 52.Parker M. 1972. Phage-typing of Staphylococcus aureus. In Norris J, Ribbons D (ed), Methods in Microbiology, vol 7B. Academic Press. [Google Scholar]
- 53.Blair JE, Williams RE. 1961. Phage typing of staphylococci. Bull World Health Organ 24:771–784. [PMC free article] [PubMed] [Google Scholar]
- 54.Carroll P, Francis P. 1985. The basic phage set for typing bovine staphylococci. J Hyg (Lond) 95:665–669. 10.1017/s0022172400060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rees PJ, Fry BA. 1981. The morphology of staphylococcal bacteriophage K and DNA metabolism in infected Staphylococcus aureus. J Gen Virol 53:293–307. 10.1099/0022-1317-53-2-293. [DOI] [PubMed] [Google Scholar]
- 56.O'Flaherty S, Coffey A, Edwards R, Meaney W, Fitzgerald GF, Ross RP. 2004. Genome of staphylococcal phage K: a new lineage of Myoviridae infecting Gram-positive bacteria with a low G+C content. J Bacteriol 186:2862–2871. 10.1128/JB.186.9.2862-2871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenton M, Ross P, McAuliffe O, O'Mahony J, Coffey A. 2010. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:9–16. 10.4161/bbug.1.1.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. 2005. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol 187:7161–7164. 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ajuebor J, Buttimer C, Arroyo-Moreno S, Chanishvili N, Gabriel EM, O'Mahony J, McAuliffe O, Neve H, Franz C, Coffey A. 2018. Comparison of Staphylococcus Phage K with close phage relatives commonly employed in phage therapeutics. Antibiotics (Basel) 7. 10.3390/antibiotics7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker SC, Foster-Frey J, Donovan DM. 2008. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol Lett 287:185–191. 10.1111/j.1574-6968.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- 61.Kelly D, McAuliffe O, Ross RP, Coffey A. 2012. Prevention of Staphylococcus aureus biofilm formation and reduction in established biofilm density using a combination of phage K and modified derivatives. Lett Appl Microbiol 54:286–291. 10.1111/j.1472-765X.2012.03205.x. [DOI] [PubMed] [Google Scholar]
- 62.Botka T, Pantůček R, Mašlaňová I, Benešík M, Petráš P, Růžičková V, Havlíčková P, Varga M, Žemličková H, Koláčková I, Florianová M, Jakubů V, Karpíšková R, Doškař J. 2019. Lytic and genomic properties of spontaneous host-range Kayvirus mutants prove their suitability for upgrading phage therapeutics against staphylococci. Sci Rep 9:5475. 10.1038/s41598-019-41868-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ralston DJ, Krueger AP. 1952. Phage multiplication on two hosts. Isolation and activity of variants of Staphylococcus phage P1. Proc Soc Exp Biol Med 80:217–220. 10.3181/00379727-80-19575. [DOI] [PubMed] [Google Scholar]
- 64.Raiston DJ, Krueger AP. 1954. The isolation of a staphylococcal phage variant susceptible to an unusual host control. J Gen Physiol 37:685–716. 10.1085/jgp.37.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ralston DJ, Baer B, Lieberman M, Krueger AP. 1961. Virolysin, a virus-induced lysin: its appearance and function in phage-infected staphylococci. J Gen Microbiol 24:313–325. 10.1099/00221287-24-3-313. [DOI] [PubMed] [Google Scholar]
- 66.Ralston DJ, Lieberman M, Baer B, Krueger AP. 1957. Staphylococcal virolysin, a phage-induced lysin; its differentiation from the autolysis of normal cells. J Gen Physiol 40:791–807. 10.1085/jgp.40.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simon K, Pier W, Krüttgen A, Horz H-P. 2021. Synergy between phage Sb-1 and oxacillin against methicillin-resistant Staphylococcus aureus. Antibiotics 10:849. 10.3390/antibiotics10070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 69.Perez K, Patel R. 2017. Staphylococcus epidermidis small-colony variants are induced by low pH and their frequency reduced by lysosomal alkalinization. J Infect Dis 215:488–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schleimer N, Kaspar U, Knaack D, von Eiff C, Molinaro S, Grallert H, Idelevich EA, Becker K. 2019. In vitro activity of the bacteriophage endolysin HY-133 against Staphylococcus aureus small-colony variants and their corresponding wild types. Int J Mol Sci 20. 10.3390/ijms20030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuchscherr L, Kreis CA, Hoerr V, Flint L, Hachmeister M, Geraci J, Bremer-Streck S, Kiehntopf M, Medina E, Kribus M, Raschke M, Pletz M, Peters G, Loffler B. 2016. Staphylococcus aureus develops increased resistance to antibiotics by forming dynamic small colony variants during chronic osteomyelitis. J Antimicrob Chemother 71:438–448. 10.1093/jac/dkv371. [DOI] [PubMed] [Google Scholar]
- 72.Tande AJ, Osmon DR, Greenwood-Quaintance KE, Mabry TM, Hanssen AD, Patel R. 2014. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio 5:e01910-14–e01914. 10.1128/mBio.01910-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leijtens B, Elbers JBW, Sturm PD, Kullberg BJ, Schreurs BW. 2017. Clindamycin-rifampin combination therapy for staphylococcal periprosthetic joint infections: a retrospective observational study. BMC Infect Dis 17:321–321. 10.1186/s12879-017-2429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wouthuyzen-Bakker M, Tornero E, Morata L, Nannan Panday PV, Jutte PC, Bori G, Kampinga GA, Soriano A. 2018. Moxifloxacin plus rifampin as an alternative for levofloxacin plus rifampin in the treatment of a prosthetic joint infection with Staphylococcus aureus. Int J Antimicrob Agents 51:38–42. 10.1016/j.ijantimicag.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP. 2009. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol 501:69–76. 10.1007/978-1-60327-164-6_7. [DOI] [PubMed] [Google Scholar]
- 76.Mazzocco A, Waddell T, Lingohr E, Johnson R. 2009. Enumeration of bacteriophages using the samll drop plaque assay system, p 81–85. In Clokie M, Kropinski A (ed), Bacteriophages: methods and protocols, vol 1: isolation, characterization, and interactions. HumanaPress. [DOI] [PubMed] [Google Scholar]
- 77.Kim B-N, Kim ES, Oh M-D. 2014. Oral antibiotic treatment of staphylococcal bone and joint infections in adults. J Antimicrob Chemother 69(2):309–322. 10.1093/jac/dkt374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.01879-21-s0001.pdf, PDF file, 0.2 MB (251KB, pdf)