ABSTRACT
Terbinafine is used as the first-line therapy for dermatophytosis, but the incidence of terbinafine resistance is increasing. A combination of terbinafine with itraconazole was tested using the checkerboard method based on the EUCAST methodology for antifungal susceptibility testing against 9 terbinafine-susceptible and 7 terbinafine-resistant clinical isolates of Trichophyton spp. from India. Synergistic interactions were observed for 4/9 of the susceptible isolates, with fractional inhibitory concentration index (FICI) values of 0.3125 to 0.5, and for 4/7 of the resistant isolates, with FICI values of 0.032 to 0.3125.
KEYWORDS: antifungal combination, terbinafine, itraconazole, EUCAST, dermatophytes, Trichophyton
INTRODUCTION
Dermatophytosis is the most common superficial fungal infection worldwide. In recent years, superficial mycoses have become increasingly resistant to current antifungals; specifically, high incidences of chronic infection, reinfection, and treatment failure have been reported in India (1–3). Furthermore, antifungal-resistant dermatophytosis is spreading globally because of widespread travel and migration and has recently been described in Europe (4). Strains from the Trichophyton rubrum and Trichophyton mentagrophytes complexes are the most commonly isolated, but their incidence varies in different geographical locations. In India, a unique population of T. mentagrophytes complex strains has been reported to be the cause of terbinafine-resistant epidemics of tinea corporis and tinea cruris. Recently, these highly terbinafine-resistant Indian Trichophyton strains have been described as a new species called Trichophyton indotineae, according to their clinical and mycological features, multigene phylogeny, and genome sequencing (5–7).
Terbinafine is an allylamine antifungal administered orally or topically as a first-line therapy for the treatment of dermatophyte cutaneous infections and onychomycosis (8). The mechanism of action of terbinafine is the inhibition of the squalene epoxidase. This enzyme converts squalene to 2,3-oxydosqualene, an important step in the biosynthesis of ergosterol. (8). Inhibition leads to the toxic intracellular accumulation of squalene, responsible for the primary fungicidal activity of the drug (9). In dermatophytes, the molecular mechanisms of terbinafine resistance involve point mutations in the fungal squalene epoxidase gene (SQLE) (10). Resistance to terbinafine has been reported in T. rubrum and T. mentagrophytes/Trichophyton interdigitale complex strains. The most common amino acid substitutions in the SQLE protein are Leu393Phe, Phe397Leu, Phe415Ser, and His440Tyr (1, 11, 12). The use of combination therapy is an effective strategy to overcome the emergence of antifungal resistance (13). Moreover, antifungal combination can increase the efficacy of the treatment in case of synergistic interactions between the two partner drugs. Combination therapy can also reduce toxicity by decreasing the antifungal dosages and improve the pharmacokinetics of one or both molecules (14). The aim of the present study was to evaluate the combination of terbinafine with itraconazole against terbinafine-susceptible and terbinafine-resistant clinical isolates of T. interdigitale recently proposed as T. indotineae, independent of T. interdigitale to avoid confusion in the taxonomy of the T. mentagrophytes/T. interdigitale complex.
A total of 16 clinical strains of T. indotineae, including 9 terbinafine-susceptible and 7 terbinafine-resistant isolates from India, were tested. As the clinical breakpoints are not yet determined, resistance was defined by the presence of SQLE mutations previously known to confer resistance to terbinafine. All strains were previously identified by internal transcribed spacer (ITS) region sequencing (2). In the resistant isolates, the amino acid substitutions Leu393Phe and Phe397Leu (Table 1) were identified as a source of terbinafine resistance (2, 6). The strains were stored as frozen stocks at −80°C and were subcultured on Sabouraud agar slants (Bio-Rad, Marnes-la-Coquette, France) to ensure their viability and purity.
TABLE 1.
In vitro interaction of itraconazole with terbinafine against 16 isolates of Trichophytona
| Isolate | Amino acid substitution in SQLEp | MIC (µg/ml) of drugs alone |
MIC (µg/ml) of drugs in combination |
Lowest FICI in combination | Interpretation | ||
|---|---|---|---|---|---|---|---|
| ITZ | TER | ITZ | TER | ||||
| TER-resistant | |||||||
| VPCI 62/P/14 | L393F | 0.5 | 64 | 0.125 | 4 | 0.31 | SYN |
| VPCI 63/P/16 | L393F | 0.125 | 2 | 0.004 | 0.06 | 0.10 | SYN |
| VPCI 64/P/15 | L393F | 0.06 | 8 | 0.03 | 2 | 0.75 | IND |
| VPCI 65/P/16 | L393F | 0.25 | 64 | 0.125 | 8 | 0.62 | IND |
| VPCI 66/P/16 | F397L | 0.125 | 4 | 0.008 | 0.06 | 0.10 | SYN |
| VPCI 67/P/16 | F397L | 0.25 | 8 | 0.004 | 0.125 | 0.03 | SYN |
| VPCI 68/P/16 | ND | 0.25 | 16 | 0.125 | 4 | 0.75 | IND |
| TER-susceptible | |||||||
| VPCI 69/P/16 | WT | 0.5 | 0.125 | 0.015 | 0.06 | 0.53 | IND |
| VPCI 70/P/15 | WT | 0.125 | 0.125 | 0.008 | 0.03 | 0.31 | SYN |
| VPCI 71/P/14 | WT | 0.25 | 0.125 | 0.06 | 0.03 | 0.50 | SYN |
| VPCI 72/P/16 | WT | 0.5 | 0.25 | 0.03 | 0.125 | 0.56 | IND |
| VPCI 73/P/16 | WT | 0.25 | 0.06 | 0.06 | 0.03 | 0.75 | IND |
| VPCI 74/P/17 | WT | 0.5 | 0.125 | 0.06 | 0.06 | 0.62 | IND |
| VPCI 75/P/16 | WT | 0.5 | 0.5 | 0.03 | 0.125 | 0.31 | SYN |
| VPCI 76/P/16 | WT | 0.125 | 0.03 | 0.06 | 0.008 | 0.75 | IND |
| VPCI 77/P/16 | WT | 0.25 | 0.06 | 0.06 | 0.015 | 0.50 | SYN |
SQLEp, squalene epoxidase protein; ND, not done; WT, wild type; ITZ, itraconazole; TER, terbinafine; IND, indifferent; SYN, synergy; FICI, fractional inhibitory concentration index.
The interactions of terbinafine (Sigma, St. Quentin Fallavier, France) with itraconazole (Sigma) were evaluated using a microdilution checkerboard method (15), based on the EUCAST reference technique for antifungal susceptibility testing (16). The final concentrations ranged from 0.004 to 0.25 µg/ml for itraconazole. For terbinafine, depending on the susceptibility of the isolates, the concentration ranges were 0.002 to 1 µg/ml for the susceptible isolates and 0.06 to 32 µg/ml for the resistant isolates.
Before the experiments, the isolates were subcultured a second time on Sabouraud agar slants (bioMérieux) for 5 days at 25°C. Using a sterile cotton swab, spores were transferred to a sterile tube containing 5 ml water supplemented with Tween 20. The suspension was counted in a hemocytometer and adjusted to 2 to 5 × 106 conidia/ml with sterile distilled water. After dilution by 1/10 in water, each well of the plate was inoculated with 100 µl of the spore suspension, resulting in a final inoculum size of 1 to 2.5 × 105 CFU/ml.
The plates were incubated at 25°C for 5 days and read using an automated Dynex MRX spectrophotometer (Dynex Technology, Chantilly, VA, USA) at 550 nm. A stringent growth inhibition endpoint of 90% was used for both the drugs tested alone and in combination. Three reference strains were used as the quality controls, Candida parapsilosis ATCC 22019, Candida krusei ATCC 6258, and Aspergillus fumigatus ATCC 204305. All experiments were performed in duplicate and yielded similar results (the MICs of the two runs were within 2 log2 dilutions for 100% and 93.7% of the isolates for itraconazole and terbinafine, respectively).
The interactions were evaluated using the fractional inhibitory concentration index (FICI). The FICI was defined as FICA + FICB = (CA/MICA) + (CB/MICB), where MICA and MICB are the MICs of drugs A and B alone, and CA and CB are the concentrations of drugs A and B in combination, corresponding to the lowest FICI (or highest FICI, in the case of antagonism). The interaction was defined as synergistic when the FICI was ≤0.5, indifferent at >0.5 to ≤4.0, and antagonistic at >4 (17).
The MIC results of the drugs alone and in combination against the 16 Trichophyton isolates are summarized in Table 1. For the susceptible isolates, the MIC ranges (geometric means [GM]) of the drugs alone were 0.125 to 0.5 µg/ml (0.30 µg/ml) and 0.03125 to 0.5 µg/ml (0.12 µg/ml) for itraconazole and terbinafine, respectively. For the resistant isolates, the MIC ranges (GM) of the drugs alone were 0.0625 to 0.5 µg/ml (0.19 µg/ml) and 2 to 64 µg/ml (11.9 µg/ml) for itraconazole and terbinafine, respectively.
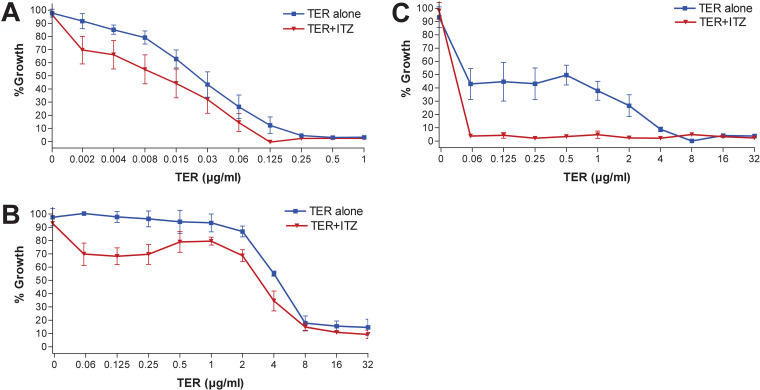
When the drugs were tested in combination against the susceptible isolates, the MICs were 1- to 5-fold lower for itraconazole and 1- to 2-fold lower for terbinafine. Synergy was observed for 4/9 isolates (FICI ranging from 0.3125 to 0.5), and indifferent interactions were observed for 5/9 isolates (FICI ranging from 0.53 to 0.75). When the drugs were tested in combination against the resistant isolates, the MICs were 1- to 6-fold lower for itraconazole and 2- to 6-fold lower for terbinafine. For the lowest FICIs for the drug combination against the resistant isolates, the MIC ranges decreased to 0.004 to 0.125 µg/ml and 0.0625 to 8 µg/ml for itraconazole and terbinafine, respectively. Synergy was observed for 4/7 isolates (FICI ranging from 0.032 to 0.3125), and indifferent interactions were observed for 3/7 isolates (FICI ranging from 0.625 to 0.75). Analysis of the growth inhibition curves showed that combination with itraconazole, even at low concentrations (e.g., 0.03 µg/ml), improved the activity of terbinafine, both for the terbinafine-susceptible and -resistant isolates (Fig. 1). Nevertheless, among the resistant isolates, this effect was more prominent for isolates with moderately increased terbinafine MICs (2 to 8 µg/ml) than for those isolates with high MICs (16 to 64 µg/ml). Antagonism was never observed for any of the tested isolates.
FIG 1.
Growth inhibition curves for terbinafine (TER) alone and in combination with itraconazole (ITZ) at 0.03 µg/ml against T. indotineae clinical isolates. (A) TER-susceptible isolates (n = 9). (B) Isolates with high TER MICs (n = 3; MIC, 16 to >32 µg/ml). (C) Isolates with moderately increased TER MICs (n = 4; MIC, 2 to 8 µg/ml). For all curves, the mean and SD are shown.
The emergence of terbinafine resistance is increasingly reported and has been linked to the widespread use of this drug alone, or in combination with topic steroids (18). Combination therapy may be an option to improve the outcome of terbinafine-resistant dermatophytosis. Several combinations have previously been tested against dermatophytes in vitro. Itraconazole in combination with amorolfine or cyclopiroxolamine was synergistic against 19/21 and 100% of the tested dermatophytes, respectively (19, 20). However, when itraconazole was combined with efinaconazole or luliconazole, synergy was present only against 2/16 and 3/16 of the tested strains, respectively (21). Terbinafine in combination with efinaconazole (7/16 strains) or clioquinol led to partial synergy (5/12 strains) (21, 22), while terbinafine in combination with luliconazole exhibited only indifference (21). To our knowledge, the combination of terbinafine with itraconazole has not been tested in vitro so far. This combination may be of particular interest, as both drugs have efficacy against dermatophytosis. Although itraconazole may have more side effects and drug interactions than terbinafine, the potential synergism of the combination may be beneficial for infections due to terbinafine-resistant isolates.
In this study, we found that the combination of itraconazole with terbinafine was synergistic for 50% of the tested isolates, even when the tested isolate was resistant to terbinafine (57% of synergy). The concentrations of terbinafine at which synergistic effects were observed are in the range of skin concentrations and therefore clinically relevant (8). There is a theoretical synergism between the two drugs, as both act at different steps of the same biosynthesis pathway (inhibition of ergosterol biosynthesis) (8, 23–25). The combination of itraconazole with terbinafine has been tested against other fungi. Synergistic or indifferent interactions were found against Aspergillus spp. in two studies, with FICI ranging from 0.02 to 0.25 in the first study and from 0.15 to 1 in the other one (26, 27). A limited synergy was found for the combination against Fusarium species isolates (25%, 8/32 isolates) (28) and against Candida glabrata (21%, 5/24) (29).
Combinations of terbinafine with other azoles have been used successfully in patients to treat refractory scedosporiosis (30, 31). Interestingly, a recent study reported that terbinafine in combination with itraconazole was better than either drug used alone for 152 patients with fungal skin diseases, including 110 patients with tinea or onychomycosis (32). The terbinafine with itraconazole group exhibited a 100% cure rate with a shorter cure time and increased number of cured patients than for terbinafine or itraconazole monotherapy. In conclusion, the in vitro results of synergistic interactions of terbinafine and itraconazole against terbinafine-resistant Trichophyton in the present study are promising. However, further studies on diverse geographical strains, including investigations in animal models, are warranted to confirm the utility of combination therapy in difficult-to-treat, terbinafine-resistant superficial dermatophytosis.
ACKNOWLEDGMENT
This work was supported in part by a research grant from the Science and Engineering Research Board (SERB file no. CRG/2020/001735 to A.C.), Department of Science and Technology, Government of India, New Delhi, India.
REFERENCES
- 1.Ebert A, Monod M, Salamin K, Burmester A, Uhrlaß S, Wiegand C, Hipler U-C, Krüger C, Koch D, Wittig F, Verma SB, Singal A, Gupta S, Vasani R, Saraswat A, Madhu R, Panda S, Das A, Kura MM, Kumar A, Poojary S, Schirm S, Gräser Y, Paasch U, Nenoff P. 2020. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: a multicentre study. Mycoses 63:717–728. doi: 10.1111/myc.13091. [DOI] [PubMed] [Google Scholar]
- 2.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, Meis JF, Chowdhary A. 2018. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 61:477–484. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 3.Panda S, Verma S. 2017. The menace of dermatophytosis in India: the evidence that we need. Indian J Dermatol Venereol Leprol 83:281–284. doi: 10.4103/ijdvl.IJDVL_224_17. [DOI] [PubMed] [Google Scholar]
- 4.Saunte DML, Pereiro-Ferreirós M, Rodríguez-Cerdeira C, Sergeev AY, Arabatzis M, Prohić A, Piraccini BM, Lecerf P, Nenoff P, Kotrekhova LP, Bosshard PP, Padovese V, Szepietowski JC, Sigurgeirsson B, Nowicki RJ, Schmid-Grendelmeier P, Hay RJ. 2021. Emerging antifungal treatment failure of dermatophytosis in Europe: take care or it may become endemic. J Eur Acad Dermatol Venereol 35:1582–1586. doi: 10.1111/jdv.17241. [DOI] [PubMed] [Google Scholar]
- 5.Kano R, Kimura U, Kakurai M, Hiruma J, Kamata H, Suga Y, Harada K. 2020. Trichophyton indotineae sp. nov.: a new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 185:947–958. doi: 10.1007/s11046-020-00455-8. [DOI] [PubMed] [Google Scholar]
- 6.Singh A, Masih A, Monroy-Nieto J, Singh PK, Bowers J, Travis J, Khurana A, Engelthaler DM, Meis JF, Chowdhary A. 2019. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: genomic insights and resistance profile. Fungal Genet Biol 133:103266. doi: 10.1016/j.fgb.2019.103266. [DOI] [PubMed] [Google Scholar]
- 7.Tang C, Kong X, Ahmed SA, Thakur R, Chowdhary A, Nenoff P, Uhrlass S, Verma SB, Meis JF, Kandemir H, Kang Y, de Hoog GS. 2021. Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex harboring the highly virulent, multiresistant genotype T. indotineae. Mycopathologia 186:315–326. doi: 10.1007/s11046-021-00544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan-Natesan S. 2009. Terbinafine: a pharmacological and clinical review. Expert Opin Pharmacother 10:2723–2733. doi: 10.1517/14656560903307462. [DOI] [PubMed] [Google Scholar]
- 9.Ryder NS. 1992. Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol 126:2–7. doi: 10.1111/j.1365-2133.1992.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharjee R, Dogra S. 2018. “End of the road for terbinafine” in dermatophytosis: is it a valid conclusion? Indian J Dermatol Venereol Leprol 84:706–707. doi: 10.4103/ijdvl.IJDVL_717_18. [DOI] [PubMed] [Google Scholar]
- 11.Saunte DML, Hare RK, Jørgensen KM, Jørgensen R, Deleuran M, Zachariae CO, Thomsen SF, Bjørnskov-Halkier L, Kofoed K, Arendrup MC. 2019. Emerging terbinafine resistance in Trichophyton: clinical characteristics, squalene epoxidase gene mutations, and a reliable EUCAST method for detection. Antimicrob Agents Chemother 63:e01126-19. doi: 10.1128/AAC.01126-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khurana A, Masih A, Chowdhary A, Sardana K, Borker S, Gupta A, Gautam RK, Sharma PK, Jain D. 2018. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother 62:e01038-18. doi: 10.1128/AAC.01038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kontoyiannis DP, Lewis RE. 2004. Toward more effective antifungal therapy: the prospects of combination therapy. Br J Haematol 126:165–175. doi: 10.1111/j.1365-2141.2004.05007.x. [DOI] [PubMed] [Google Scholar]
- 14.Vitale RG, Afeltra J, Dannaoui E. 2005. Antifungal combinations. Methods Mol Med 118:143–152. doi: 10.1385/1-59259-943-5:143. [DOI] [PubMed] [Google Scholar]
- 15.Bidaud A-L, Schwarz P, Herbreteau G, Dannaoui E. 2021. Techniques for the assessment of in vitro and in vivo antifungal combinations. J Fungi 7:113. doi: 10.3390/jof7020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendrup MC, Kahlmeter G, Guinea J, Meletiadis J, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2021. How to: perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0, exemplified by Trichophyton. Clin Microbiol Infect 27:55–60. doi: 10.1016/j.cmi.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 18.Khurana A, Sardana K, Chowdhary A. 2019. Antifungal resistance in dermatophytes: recent trends and therapeutic implications. Fungal Genet Biol 132:103255. doi: 10.1016/j.fgb.2019.103255. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Asahara M, Yamamoto M, Yamaura M, Matsumura M, Goto K, Rezaei-Matehkolaei A, Mirhendi H, Makimura M, Makimura K. 2014. In vitro susceptibility of dermatomycoses agents to six antifungal drugs and evaluation by fractional inhibitory concentration index of combined effects of amorolfine and itraconazole in dermatophytes. Microbiol Immunol 58:1–8. doi: 10.1111/1348-0421.12109. [DOI] [PubMed] [Google Scholar]
- 20.Santos DA, Hamdan JS. 2006. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol 44:357–362. doi: 10.1080/13693780500536893. [DOI] [PubMed] [Google Scholar]
- 21.Sugiura K, Masumoto A, Tachibana H, Tatsumi Y. 2021. In vitro combination effect of topical and oral anti-onychomycosis drugs on Trichophyton rubrum and Trichophyton interdigitale. J Fungi 7:208. doi: 10.3390/jof7030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Costa B, Pippi B, Kaminski TFA, Andrade SF, Fuentefria AM. 2020. In vitro antidermatophytic synergism of double and triple combination of clioquinol with ciclopirox and terbinafine. Mycoses 63:993–1001. doi: 10.1111/myc.13127. [DOI] [PubMed] [Google Scholar]
- 23.Sardana K, Khurana A, Singh A. 2020. Scientific rationale of antifungal drug combination, including oral itraconazole and terbinafine, in recalcitrant dermatophytoses. J Dermatolog Treat 31:43–45. doi: 10.1080/09546634.2019.1675857. [DOI] [PubMed] [Google Scholar]
- 24.Ryder NS, Wagner S, Leitner I. 1998. In vitro activities of terbinafine against cutaneous isolates of Candida albicans and other pathogenic yeasts. Antimicrob Agents Chemother 42:1057–1061. doi: 10.1128/AAC.42.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehan DJ, Hitchcock CA, Sibley CM. 1999. Current and emerging azole antifungal agents. Clin Microbiol Rev 12:40–79. doi: 10.1128/CMR.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryder NS, Leitner I. 2001. Synergistic interaction of terbinafine with triazoles or amphotericin B against Aspergillus species. Med Mycol 39:91–95. doi: 10.1080/mmy.39.1.91.95. [DOI] [PubMed] [Google Scholar]
- 27.Mosquera J, Sharp A, Moore CB, Warn PA, Denning DW. 2002. In vitro interaction of terbinafine with itraconazole, fluconazole, amphotericin B and 5-flucytosine against Aspergillus spp. J Antimicrob Chemother 50:189–194. doi: 10.1093/jac/dkf111. [DOI] [PubMed] [Google Scholar]
- 28.Spader TB, Venturini TP, Rossato L, Denardi LB, Cavalheiro PB, Botton SA, Santurio JM, Alves SH. 2013. Synergysm of voriconazole or itraconazole with other antifungal agents against species of Fusarium. Rev Iberoam Micol 30:200–204. doi: 10.1016/j.riam.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Perea S, Gonzalez G, Fothergill AW, Sutton DA, Rinaldi MG. 2002. In vitro activities of terbinafine in combination with fluconazole, itraconazole, voriconazole, and posaconazole against clinical isolates of Candida glabrata with decreased susceptibility to azoles. J Clin Microbiol 40:1831–1833. doi: 10.1128/JCM.40.5.1831-1833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenks JD, Seidel D, Cornely OA, Chen S, van Hal S, Kauffman C, Miceli MH, Heinemann M, Christner M, Jover Sáenz A, Burchardt A, Kemmerling B, Herbrecht R, Steinmann J, Shoham S, Gräber S, Pagano L, Deeren D, Aslam S, Taplitz R, Revankar SG, Baddley J, Mehta SR, Reed S, Slavin MA, Hoenigl M. 2020. Voriconazole plus terbinafine combination antifungal therapy for invasive Lomentospora prolificans infections: analysis of 41 patients from the FungiScope registry 2008–2019. Clin Microbiol Infect 26:784.e1–784-e5. doi: 10.1016/j.cmi.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Howden BP, Slavin MA, Schwarer AP, Mijch AM. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur J Clin Microbiol Infect Dis 22:111–113. doi: 10.1007/s10096-002-0877-z. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Liao W, Chen C, Lai H, Liu S. 2021. Terbinafine hydrochloride combined with itraconazole for fungal skin diseases: a randomized controlled trial. Am J Ther 28:e179–e186. doi: 10.1097/MJT.0000000000001075. [DOI] [PubMed] [Google Scholar]