ABSTRACT
Hospitalized patients are at risk of developing serious multidrug resistant bacterial infections. This risk is heightened in patients who are on mechanical ventilation, are immunocompromised, and/or have chronic comorbidities. We report the case of a 52-year-old critically ill patient with a multidrug resistant Acinetobacter baumannii (MDR-A) respiratory infection who was successfully treated with antibiotics and intravenous and nebulized bacteriophage therapy.
KEYWORDS: multidrug-resistant bacterial infection, Acinetobacter baumannii, bacteriophage, mechanical ventilation, Acinetobacter, bacteriophage therapy, multidrug resistance
INTRODUCTION
Acinetobacter baumannii is a difficult-to-treat pathogen that has contributed to increased morbidity and mortality in high-risk patients. There is the potential for bacteriophage therapy, as an innovative adjunct to broad-spectrum antibiotics, to become a suitable pharmaceutical intervention for treating multidrug resistant Acinetobacter baumannii (MDR-A) infections (1, 2).
Bacteriophage therapy was first described in the early 1900s by Felix d’Herelle and Fredrick W. Twort and has since been subjected to scrutiny in terms of efficacy, pharmacokinetics, and immunogenicity (3). Until recently, therapy with bacteriophage has been overlooked in the West and was largely given orally in the former Soviet Union and Eastern Europe for over a century (4).
CASE PRESENTATION
A 52-year-old male presented to the emergency department (ED) following 1 day of cough, fever, generalized weakness, and decreased appetite. He had a history of hypertension, hyperlipidemia, mono-ocular blindness, poorly controlled type 2 diabetes, class II obesity, and bilateral diabetic foot ulcers requiring multiple debridement procedures in previous admissions. The patient also had a history of an episode of pericarditis from unknown etiology resulting in pericardiectomy and severe tracheal stenosis (dependent on tracheostomy). In the ED, the patient was febrile and ill-appearing but not in acute respiratory distress. A comprehensive physical examination was unremarkable except for a grossly, swollen, erythematous, right great toe with a volar ulcer exhibiting necrotic subcutaneous tissue and a similar-appearing dorsal wound on the left foot. Magnetic resonance imaging (MRI) revealed right foot osteomyelitis. Chest x-rays showed a tracheostomy tube in-situ and minimal left mid-lung subsegmental atelectasis. There was no evidence of acute cardiopulmonary disease. Laboratory values demonstrated hyperglycemia, hyperkalemia, hyponatremia, and hypoalbuminemia. Blood, sputum, and wound swab samples were collected for microscopy and culture and sensitivity (C/S) to evaluate the patient for an infectious process. Foot wound cultures were positive for mixed aerobic flora. Initial culture results were negative for Acinetobacter baumannii infection.
Based on the overall clinical presentation, it was concluded that the patient likely developed electrolyte imbalance and sepsis secondary to diabetic foot ulcers and was admitted. The patient declined surgical intervention and was treated with empirical broad-spectrum antibiotics including linezolid, meropenem, and piperacillin-tazobactam.
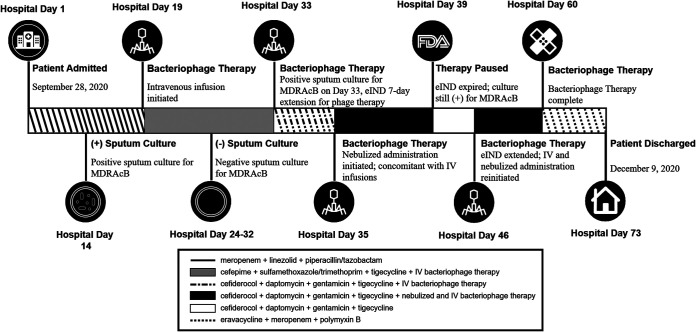
On hospital day (HD) 7, the patient experienced an episode of cardiac arrest with subsequent anoxic brain injury secondary to acute respiratory failure caused by displacement of the tracheostomy tube, which required emergent intubation and mechanical ventilation. Despite aggressive intensive care management, the patient developed ventilator-associated pneumonia 7 days after the initiation of mechanical ventilation. On HD 14, a sputum sample collected for microscopy and C/S grew carbapenem-resistant Acinetobacter baumannii (CRAB) and Pseudomonas aeruginosa (Fig. 1). Antimicrobial susceptibility testing for Acinetobacter baumannii demonstrated resistance to a broad spectrum of antibiotic classes and susceptibility to tigecycline and sulfamethoxazole/trimethoprim (Table 1). Antimicrobial susceptibility testing for Pseudomonas aeruginosa demonstrated susceptibility to cefepime, ceftazidime, and meropenem (Table 2). Accordingly, treatment with sulfamethoxazole/trimethoprim and tigecycline along with cefepime and fluconazole was initiated.
FIG 1.
Timeline for the treatment in a patient with multidrug-resistant Acinetobacter baumannii lung infection with strain-reactive bacteriophages and antibiotics.
TABLE 1.
Results of antibiotic susceptibility testing for Acinetobacter baumannii
| Antibiotic | MIC dilution | MIC interpretation |
|---|---|---|
| Cefepime | ≥64 | Resistant |
| Cefotaxime | ≥64 | Resistant |
| Ceftazidime | ≥64 | Resistant |
| Ceftriaxone | ≥64 | Resistant |
| Ciprofloxacin | ≥4 | Resistant |
| Gentamicin | ≥16 | Resistant |
| Imipenem | ≥16 | Resistant |
| Levofloxacin | ≥8 | Resistant |
| Meropenem | ≥16 | Resistant |
| Piperacillin-tazobactam | ≥128 | Resistant |
| Tetracycline | ≥16 | Resistant |
| Tigecycline | 2 | Susceptible |
| Tobramycin | ≥16 | Resistant |
| Trimethoprim/sulfametoxazole | ≤20 | Susceptible |
TABLE 2.
Results of antibiotic susceptibility test for Pseudomonas aeruginosa
| Antibiotic | MIC dilution | MIC interpretation |
|---|---|---|
| Cefepime | 8 | Intermediate |
| Ceftazidime | 16 | Intermediate |
| Ciprofloxacin | 0.5 | Susceptible |
| Gentamicin | ≤1 | Susceptible |
| Levofloxacin | 4 | Intermediate |
| Meropenem | 1 | Susceptible |
| Piperacillin-tazobactam | 32 | Susceptible |
Bacteriophage Selection and Production.
In response to Acinetobacter baumannii’s prevalence in the region, collaboration to produce bacteriophages began 6 weeks prior to this patient’s diagnosis. Tryptic Soy Agar (TSA) slants of CRAB isolated from sputum cultures were shared with the Walter Reed Army Institute of Research (WRAIR) and Adaptive Phage Therapeutics (APT) for whole genome sequencing and genome annotation of the Acinetobacter baumannii isolate (WRAIR) and bacteriophage sensitivity (APT).
With an initial screen of 23 CRAB isolates provided by DHR Health Institute for Research and Development (DHR-IRD), two location-specific bacteriophage sensitivity profiles were identified using the Host Range Quick Testing (HRQT) method (5). From this screen, Acinetobacter baumannii phage AbW4932ø1 lysed 19 of 23 (83%) shared isolates. A second Acinetobacter baumannii phage AbW4878ø1 lysed 3 of 23 (13%) of them.
The producer Acinetobacter baumannii strains AbW4932 and AbW4878 were grown to ∼0.1 OD600 at 37°C in Tryptic Soy Broth and subsequently infected with AbW4932ø1 and AbW4878ø1, respectively (0.22 μm filtered bacteriophage plate lysate) at a multiplicity of infection of ∼0.01. The infected cultures were incubated at 37°C while shaking. Culture growth was continually monitored until lysis was observed. The lysates were subsequently harvested, and residual contaminants were removed via centrifugation and sequential filtration. The filtered bacteriophage lysates were purified via proprietary methods described in APT’s Chemistry, Manufacturing, and Controls (CMC) on file with the agency under MF 18920.
The purified bacteriophages were formulated and aseptically filled into 1 ml single dose vials via proprietary methods described in APT’s Chemistry, Manufacturing, and Controls (CMC) on file (MF 18920) with the U.S. Food & Drug Administration. After filling, vials were cryopreserved and shipped at temperature controlled −80°C conditions and those stored for USP <71> sterility testing were shipped and stored at 2–8°C. The endotoxin and potency (phage titer) of the final bacteriophage vials were measured using Limulus Amoebocyte Lysate turbidimetric assay kit (Cape Cod Associates) and the solid media viral plaque assay method, respectively. The determined potency of the vials was 1 × 109 PFU/ml and the final endotoxin levels were 6 EU/ml. The vials arrived at the hospital site 2 weeks prior to the patient’s diagnosis.
The isolate collected from the patient’s sputum sample was shipped to the laboratory to determine if either of the two bacteriophages would be candidates for treating the patient’s infection. Acinetobacter baumannii isolate Ab059-R was screened using the HRQT. AbW4878ø1 inhibited the growth of the patient’s bacterial isolate in the HRQT for ∼40 h and was therefore considered suitable for therapeutic application. No inhibition of growth was observed for AbW4932ø1. An additional Acinetobacter baumannii isolate collected from the patient’s sputum sample on treatment day 14 confirmed the sensitivity against the selected bacteriophage. This isolate remained sensitive to AbW4878ø1. The strain-specific bacteriophage was selected from the inventory of bacteriophage that matched the patient’s Acinetobacter baumannii isolate.
An Emergency Investigational New Drug (eIND) application was submitted to and approved by the FDA to treat the patient with the identified bacteriophage. The DHR-IRD Institutional Review Board affirmed that the institutional procedure for emergency use reporting was followed and deemed the emergency use acceptable in accordance with 21CFR56.102(d) and 21CFR56.104(c).
CHALLENGE QUESTION
Studies have shown that inappropriate therapy of MDR-A may reach a 28-day mortality rate of 70%. Which of the following treatment strategies would be the most effective in treating a patient with MDR-A?
-
A.
Treatment with broad-spectrum cephalosporin
-
B.
A combination of beta-lactam/beta-lactamase inhibitor
-
C.
Tigecycline
-
D.
Combination antimicrobial therapy that includes both empiric and directed antibiotic therapy
TREATMENT AND OUTCOME
Despite 19 days of antibiotic therapy, including 5 days of treatment with sulfamethoxazole/trimethoprim and tigecycline to which this strain of MDR-A was deemed susceptible, the patient’s condition continued to deteriorate. The patient remained febrile (Tmax 100.4°F) and produced thick respiratory secretions. Laboratory results revealed elevated white blood cells (26.3 × 10E3/μl), leukocytosis, hypernatremia, and hyperkalemia. The ventilator settings were assist-control mode, tidal volume-500, respiratory rate-12, PEEP-5, FiO2-21% with O2 saturation at 99%. Patient was placed on PEG tube which was well tolerated. Patient remained unresponsive and no change in mental status following anoxic encephalopathy. Patient was deemed a poor candidate for hemodialysis despite volume overload. Sulfamethoxazole/trimethoprim was discontinued due to the patient’s hyperkalemic status and cefiderocol was added to the antibiotic cocktail.
On HD 18 (4 days poststrain-specific antibiotic therapy), a sputum culture collected yielded 1+ culture of Acinetobacter baumannii. Based on these findings and with few antibiotic options remaining, a decision was made to initiate bacteriophage therapy in combination with antibiotic therapy.
Twice daily intravenous administration of bacteriophage was initiated on HD 19, consisting of 1 × 109 plaque forming units/ml of reactive bacteriophage diluted in 50 ml of normal saline infused over 30 to 60 min. Antibiotic regimens for the treatment of CRAB were continued throughout the course of bacteriophage therapy (Fig. 1). Antibiotic infusions were timed to be given outside of a minimum of 2 h window before and after bacteriophage infusion with the goal of allowing residual bacteria to be robust enough to support exponential bacteriophage replication.
The patient was closely monitored for adverse reactions in accordance with Common Terminology Criteria for Adverse Events Version 5.0 published by the U.S Department of Health and Human Services in 2017 (6). Bilirubin were monitored daily. Bacteriophage therapy did not appear to result in end-organ liver function tests including aspartate aminotransferase, alanine aminotransferase, and total damage that would be reflected in these laboratory values and thus was continued.
Sputum was collected for C/S prior to initiating bacteriophage treatment and every alternate day thereafter. By bacteriophage treatment day (TD) 5, Acinetobacter baumannii was no longer grown from sputum culture until TD 14 when the patient’s sputum was again positive for Acinetobacter baumannii. However, the burden of infection, assessed using repeated sputum cultures, was reduced from 3+ to 1+. In light of these findings, continuation of bacteriophage treatment with concomitant antibiotic therapy was approved by the FDA allowing concurrent nebulized and intravenous bacteriophage infusion administered for an additional 21 days or until two consecutive sputum C/S tests were negative for Acinetobacter baumannii. For nebulization, a dose of 0.1 × 109 plaque forming units/ml twice a day of reactive bacteriophage diluted in 10 ml of normal saline in a vibrating mesh nebulizer was used. Nebulization was offered as an additional bacteriophage delivery modality as there is evidence that suggests the delivery of bacteriophages via nebulization may be effective in eradication of multidrug-resistant infections in the lungs (7). There was a temporary pause in bacteriophage treatment for a period of 7 days while approval of the modified eIND was sought (Fig. 1) during which time antibiotics were continued, and the patient’s clinical status remained stable.
After treatment with nebulized and intravenous bacteriophage therapy along with antibiotics for a total of 35 days, the patient’s condition improved significantly. He was weaned from mechanical ventilation on TD 27 (HD 52) and bacteriophage therapy was discontinued on HD 60 following two consecutive sputum cultures collected 6 days apart resulting in no growth of CRAB. The patient was discharged to a long-term rehabilitation facility for continuation of care on HD 73 with administration of eravacycline, meropenem, and polymyxin B as a precautionary measure.
In animal models of Acinetobacter baumannii infections, bacteriophage treatment appears to be both safe and efficacious (8). There is also evidence in an animal model that bacteriophage treatment may resensitize the drug-resistant Acinetobacter baumannii to antimicrobials which would be another advantage afforded by this treatment (9). Schooley et al. used bacteriophages to treat a critically ill 68-year-old diabetic patient with necrotizing pancreatitis and pancreatic pseudocyst infected by Acinetobacter baumannii (10). After 2 days, the patient's clinical condition improved. The therapy was continued for 8 weeks with a resolution of the infection and no subsequent recurrence. Nir-Paz et al. also used bacteriophage therapy to successfully treat a patient with a septic joint with knee aspirate growing both Acinetobacter baumannii and Klebsiella pneumoniae. A combination of bacteriophages and antibiotics were used to target each pathogen and the patient’s infection resolved after only 12 days of therapy (11).
This patient had ventilator-associated pneumonia caused by MDR-A. The prognosis of such patients is poor with mortality rates typically approaching 50% or greater (12). The patient was initially treated with an antibiotic cocktail to treat osteomyelitis (HD 2) to which sulfamethoxazole/trimethoprim and tigecycline were added after MDR-A diagnosis and susceptibility analysis (Fig. 1). Without clinical improvement and the discontinuation of sulfamethoxazole/trimethoprim, intravenous bacteriophage therapy in combination with broad-spectrum antibiotics was given for 14 days. Although the patient improved clinically, the treatment failed to eradicate the MDR-A infection as evidenced by positive sputum cultures. As a result, a combination of nebulized and intravenous bacteriophage therapy along with antibiotics was administered for an additional 21 days and was discontinued after two consecutive negative sputum samples were obtained, as required by the approved eIND. After significant clinical improvement, the patient was weaned off mechanical ventilation on HD 52 and bacteriophage treatment was discontinued after a total of 35 days. The clinical improvement of the patient after administering bacteriophage in a nebulized form suggests that it may be a more efficacious delivery method in order to directly target pathogens in the airways and lung parenchyma. This is similar to the success witnessed in eradication of Acinetobacter baumannii found in deep wound infections with both topical and intravenous bacteriophages as well as in the treatment of methicillin-resistant Staphylococcus aureus prosthetic joint infections with the combination of joint and intravenous infusions of bacteriophages (13).
The outcome of this case is encouraging and provides an alternative approach when treating patients with MDR-A. It also underscores the need to initiate appropriate clinical trials to unequivocally establish the safety and efficacy of bacteriophage therapy in patients with MDR-A and other antibiotic-resistant infections.
ACKNOWLEDGMENTS
This project was funded by a Seed Grant from the DHR Health Institute for Research & Development.
We thank Cristian Mercado in the DHR Health Institute for Research & Development for his help with data collection; Gavino Garza, Ronnie Ozuna, and Gabriel Garza in the DHR Health Pharmacy for their help with preparation of bacteriophage for infusion; Waqas Chaudhry, Viet Dang, Jarrar Haider, Stephen Johnson, Mark-Saint John Kerr, Martin Lee, Anjna Nair, Brittany Sisson, Joseph Tewell, Bryce Walker, and Ashley Williams in Adaptive Phage Therapeutics, who helped in purifying the bacteriophage and for completing the quality control process; and Damon Ellison, Patrick Mc Gann, Francois LeBreton, Yoon Kwak, and Derese Getnet at the Walter Reed Army Institute of Research for clinical and genetic characterization of the MDR-A and for aiding in bacteriophage identification. Special thanks to Subhendu Basu and Robert Hopkins at Adaptive Phage Therapeutics for their helpful discussions and advice.
The authors report no financial conflict of interest.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70-25.
Contributor Information
Sohail Rao, Email: s.rao@dhr-rgv.com.
Michael J. Brownstein, Email: mjbrownstein@gmail.com.
REFERENCES
- 1.Düzgüneş N, Sessevmez M, Yildirim M. 2021. Bacteriophage therapy of bacterial infections: The rediscovered frontier. Pharmaceuticals 14:34. doi: 10.3390/ph14010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, Dudeck MA, Pollock DA, Horan TC. 2009. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control 37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Merril CR, Scholl D, Adhya SL. 2003. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov 2:489–497. doi: 10.1038/nrd1111. [DOI] [PubMed] [Google Scholar]
- 4.Gelman D, Eisenkraft A, Chanishvili N, Nachman D, Coppenhagem Glazer S, Hazan R. 2018. The history and promising future of phage therapy in the military service. J Trauma Acute Care Surg 85:S18–S26. doi: 10.1097/TA.0000000000001809. [DOI] [PubMed] [Google Scholar]
- 5.Henry M, Biswas B, Vincent L, Mokashi V, Schuch R, Bishop-Lilly KA, Sozhamannan S. 2012. Development of a high throughput assay for indirectly measuring phage growth using OmniLogTM system. Bacteriophage 2:159–167. doi: 10.4161/bact.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2017. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. US Department of Health and Human Services, National Cancer Institute, Bethesda, MD. [Google Scholar]
- 7.Chang RYK, Wallin M, Lin Y, Leung SSY, Wang H, Morales S, Chan H-K. 2018. Phage therapy for respiratory infections. Adv Drug Deliv Rev 133:76–86. doi: 10.1016/j.addr.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagińska N, Pichlak A, Górski A, Jończyk-Matysiak E. 2019. Jończyk-Matysiak E. Specific and selective bacteriophages in the fight against multidrug-resistant Acinetobacter baumannii. Virol Sin 34:347–357. doi: 10.1007/s12250-019-00125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordillo Altamirano F, Forsyth JH, Patwa R, Kostoulias X, Trim M, Subedi D, Archer SK, Morris FC, Oliveira C, Kielty L, Korneev D, O’Bryan MK, Lithgow TJ, Peleg AY, Barr JJ. 2021. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat Microbiol 6:157–161. doi: 10.1038/s41564-020-00830-7. [DOI] [PubMed] [Google Scholar]
- 10.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:1–15. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nir-Paz R, Gelman D, Khouri A, Sisson BM, Fackler J, Alkalay-Oren S, Khalifa L, Rimon A, Yerushalmy O, Bader R, Amit S, Coppenhagen-Glazer S, Henry M, Quinones J, Malagon F, Biswas B, Moses AE, Merril G, Schooley RT, Brownstein MJ, Weil YA, Hazan R. 2019. Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination. Clin Infect Dis 69:2015–2018. doi: 10.1093/cid/ciz222. [DOI] [PubMed] [Google Scholar]
- 12.Inchai J, Pothirat C, Bumroongkit C, Limsukon A, Khositsakulchai W, Liwsrisakun C. 2015. Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care 3:1–8. doi: 10.1186/s40560-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doub JB, Ng VY, Johnson AJ, Slomka M, Fackler J, Horne B, Brownstein MJ, Henry M, Malagon F, Biswas B. 2020. Salvage bacteriophage therapy for a chronic MRSA prosthetic joint infection. Antibiotics 9:241–246. doi: 10.3390/antibiotics9050241. [DOI] [PMC free article] [PubMed] [Google Scholar]