Introduction
Headache is a common symptom with a wide variety of potential causes. More than 70% of the U.S. population are estimated to experience headaches1,2, with the vast majority of headaches being caused by benign primary headache disorders and not significant pathological conditions1,3. Migraine is a severe, disabling brain condition that ranks 6th most disabling disorder globally according to the World Health Organization (WHO)4,5. Migraine is the most frequent neurological disorder in adults, affecting up to 12% of the general population6. The annual costs of migraine – including lost productivity – are more than $19.6B in the U.S.7 and €27B in Europe8, making it a significant public health issue.
The current pathophysiological concepts of headache formation, including migraine, implicates vascular changes including changes in vessel diameter and cerebral blood flow as part of the migraine phenomenon9; however, to date no single diagnostic tool is able to define, ensure, or differentiate the various headache syndromes. Clinical use of neuroimaging in diagnosing headache varies widely, and the overall yield of neuroimaging studies to identify significant abnormalities in patients presenting with headache has been reported to be less than 8%2,3,10–14. Recommendations regarding when to perform imaging for headache have been published by the U.S. Headache Consortium, American Academy of Neurology (AAN)15, American College of Emergency Physicians (ACEP)16, and the American College of Radiology (ACR) based on the current level of scientific and clinical evidence1,17. Consistent with most of these suggestions, the European Federation of Neurological Societies (EFNS) Task Force does not recommend routine use of neuroimaging in adult and pediatric patients with migraine with no recent change in attack pattern, seizures, or other focal neurological symptoms18. highlighting the often contradictory and non-specific findings in the literature. Despite these recommendations, MRI and PET imaging appear to have significant potential for exploring the pathophysiology of headaches and potentially determine the effects of therapeutic interventions18.
Pain Circuits in Migraine
During acute pain resulting from migraine or other pain conditions, focal increases in CBF have been found bilaterally within the anterior insula, contralateral thalamus, ipsilateral anterior cingulate cortex (ACC) and the cerebellum19. Activation of ACC has been reported in PET studies on sensation of somatic or visceral pain as well as emotional responses to pain20–22. Activation of the insula has been demonstrated in a variety of sensory and pain inducing paradigms20,21,23–26. The insula has been suggested as a relay station for sensory information into the limbic system and is known to play an important role in regulation of autonomic responses. The thalamus has also been shown to be critically important in acute pain processing; activation of the thalamus has been shown in both animals27 and human functional imaging pain studies21,28. Together, these regions are thought to make up the “Pain Matrix”29,30 (Fig. 1), which are of significant consequence in migraine.
Fig 1. The Pain Matrix.
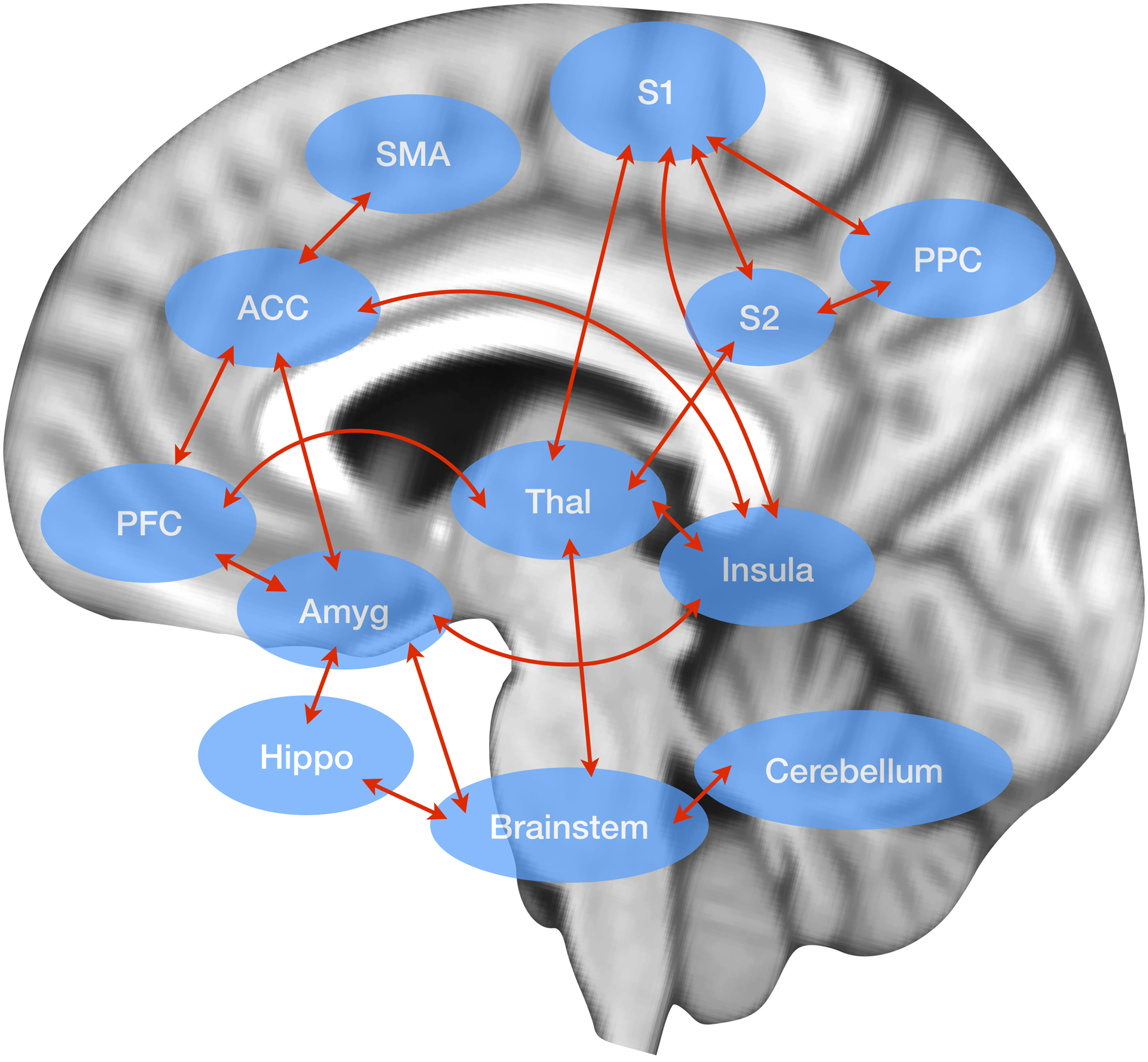
The pain matrix consists of the thalamus (Thal), insula, amygdala (Amyg), brainstem (including the pons and paraquiductal gray), hippocampus (Hippo), prefrontal cortex (PFC), anterior cingulate cortex (ACC), cerebellum, supplemental motor area (SMA), primary somatosensory cortex (S1), secondary somatosensory cortex (S2), and the posterior parietal cortex (PPC). For review, see29,30.
Anatomic Imaging in Migraine
While standard anatomic imaging appears to be of limited diagnostic value in migraine, recent studies have suggested significant cortical thinning may occur within regions within the pain matrix. Additionally, patients with migraine appear to be at higher risk for T2 hyperintense lesions, suggesting ischemic or degenerative processes may be involved. Early voxel based morphometry (VBM) studies focusing on gray matter thickness and density did not observe significant differences in cortical density in patients with migraine31. However, subsequent larger studies have noted significant reductions in gray matter density in cortical areas involved in pain processing32–35, as well as an increase in gray matter density within the PAG in patients with visible T2 lesions32. Interestingly, in patients with migraine with visual aura, studies have identified thicker visual cortex36, presumably due to more frequent activation in these areas.
In addition to changes in gray matter density, a number of studies have suggested migraine is an independent risk factor for deep white matter lesions37–40. Migraine patients appear to have an increased risk of ischemic vascular disease41 and approximately double the risk of ischemic stroke42. In a cohort of 186 patients with migraine, significant associations were found between T2 hyperintense lesions and longer disease duration and higher headache frequency43. Prevalence of white matter T2 hyperintense lesions appears to be higher in migraine with aura compared to migraine without aura40. Although largely unknown, a number of pathological processes have been proposed as an explanation for this higher incidence including focal hypoperfusion, oligemia, glutamatergic excitotoxicity, immune-related demyelination, inflammation, and mitochondrial dysfunction39,44–46.
Diffusion Imaging in Migraine
Diffusion MR changes have also been observed in patients with migraine. In particular, studies have shown higher apparent diffusion coefficient (ADC), or mean diffusivity (MD), and lower fractional anisotropy (FA) in the frontal lobe47 along with the genu, splenium, and body of the corpus callosum48, consistent with microstructural alterations along these pathways. In migraine patients with aura, a reduced FA along the thalamocortical tract and reduced FA along ventral trigeminothalamic tract have been observed49, whereas a reduced FA in the ventrolateral PAG has been observed in migraine patients without aura49. Additionally, diffusion MR has revealed enhanced connectivity between temporal pole and entorhinal cortex50, as well as high connectivity between frontal lobe regions with reduced FA and regions within the pain network (orbitofrontal cortex, insula, thalamus, and dorsal midbrain/pons)47. Interestingly, a lower ADC has been observed in migraine patients with T2 hyperintense lesions51 and transient diffusion changes in the thalamus (increased FA and lower MD) have been observed during migraine without aura, which were normalized after attack52. Together, these observations suggest dynamic changes in water mobility may occur during the various stages of attacks.
Perfusion and Vascular Imaging in Migraine
Most notably, cerebral blood flow (CBF) in patients with migraine appears significantly impaired, although the temporal changes in CBF before, during, and after migraine appear complex (Table 1). Cortical spreading depression (CSD)53 is thought to underlie migraine visual aura54,55, and the early depolarization or activation phase of CSD has been shown to be associated with a transient change in CBF54,55 and blood oxygenation56,57. This appears to be in conflict with work by Olesen et al.58,59 using SPECT to show focal reduction in CBF in migraine attacks with an aura. Subsequent dynamic susceptibility contrast (DSC) perfusion MRI studies have confirmed the characteristic hypoperfusion (lower CBF) and collapsed vasculature (low CBV with increased MTT) that occurs during aura in patients with migraine60–62. Dynamic contrast enhanced (DCE) perfusion MRI, which can provide additional information about blood-brain barrier (BBB), has similarly shown an increased CBF without increased BBB disruption in the pons/brainstem in patients with migraine63. These results are consistent with arterial spin labeling (ASL)64 and perfusion studies using SPECT demonstrating similarly decreased CBF in patients with migraine65,66.
Table 1:
Summary of brain perfusion studies in migraine headaches.
| Authors | Technique | Headache | Participants (N) | Conclusion | References |
|---|---|---|---|---|---|
| Gutschalk et al. (2002) | DWI, MR Perfusion, PET | Hemiplegic Migraine | 1 | DWI and MR perfusion: normal. PET: reduced relative tracer uptake. | Gutschalk A, et al. Neurosci Lett. 2002; 332:115–118. |
| Hsu et al. (2008) | MR Perfusion | Hemiplegic Migraine | 11 | Hyperperfusion | Hsu DA, et al. Brain Dev. 2008; 30:86–90. |
| Masuzaki et al. (2001) | MR Perfusion | Hemiplegic Migraine | 1 | Hyperperfusion | Masuzaki M, et al. AJNR Am J Neuroradiol. 2001; 22:1795–1797. |
| Jacob et al. (2006) | MR Perfusion | Hemiplegic Migraine | 1 | Hyperperfusion | Jacob A, et al. Cephalalgia. 2006; 26:1004–1009. |
| Oberndorfer et al. (2004) | MR Perfusion | Hemiplegic Migraine | 1 | Hyperperfusion | Oberndorfer S, Cephalalgia. 2004; 24:533–539. |
| Lindahl et al. (2002) | MR Perfusion | Hemiplegic Migraine | 1 | Hyperperfusion, DWI normal | Lindahl AJ, et al. J Neurol Neurosurg Psychiatry. 2002; 73:202–203. |
| Yilmaz et al. (2009) | MR Perfusion, DWI | Hemiplegic Migraine | 1 | Hypoperfusion, DWI normal | Yilmaz A, et al. Cephalalgia. 2009; 30:615–619. |
| Kraus et al. (2007) | MR Perfusion, DWI | Hemiplegic Migraine | 2 | Hypoperfusion, DWI normal | Kraus J, et al. Nervenarzt. 2007; 78:1420–1424. |
| Altinok et al. (2010) | MR Perfusion, DWI | Hemiplegic Migraine | 1 | Hypoperfusion, small area of restricted diffusion | Altinok D, et al. Pediatr Radiol. 2010; 40:1958–1961. |
| Gonzalez-Alegre (2003) | MRA | Hemiplegic Migraine | 1 | MRA normal | Gonzalez-Alegre P, Tippin J. Headache. 2003; 43:72–75. |
| De Sanctis et al. (2011) | MRA, DWI | Hemiplegic Migraine | 2 | MRA, DWI were normal | De Sanctis S, et al. Headache. 2011; 51:447–450. |
| Barbour et al. (2001) | MRI and MRA | Hemiplegic Migraine | 1 | Normal | Barbour PJ. Headache. 2001; 41:310–316. |
| Kumar et al. (2009) | MRI, DWI | Hemiplegic Migraine | 1 | DWI normal | Kumar G, et al. Headache. 2009; 49:139–142. |
| Friberg et al. (1987) | SPECT | Hemiplegic Migraine | 3 | Hypoperfusion, preceded by focal hyperperfusion | Friberg L, et al. Brain. 1987; 110:917–934. |
| Cheng et al. (2010) | SPECT | Hemiplegic Migraine | 30 | During aura: hypoperfusion. During headache: hyperperfusion. | Cheng MF, et al. Clin Nucl Med. 2010; 35:456–458. |
This reduced CBF during the onset of prolonged migraine with aura is contrasted with a substantial increase in CBF during the late stages of migraine67 (Table 1). Using ASL, lower CBF has been observed in brain regions consistent with symptoms in childhood migraine within 14 hours of aura, and higher CBF was observed after 14 hours from symptoms68. Similarly, ASL studies have well documented hyperperfusion during migraine headaches after aura has occurred, but during symptom presentation69. DCE perfusion MRI case reports have suggested possible hemispheric increase in vascular permeability in migraine with aura70 and increased CBF in the visual cortex and posterior white matter regions in migraineurs with visual aura63. These studies support the hypothesis that migraine with aura results in a transient, early decrease in CBF during aura formation and a characteristic increase in CBF occurs during the late stages of migraine.
In migraine without aura, CBF changes appear less consistent across studies (Table 1). Many studies have shown no changes in hemodynamics in migraine patients without aura60,71–73, while other studies have shown reduced CBF during migraine attack without aura74,75 or significantly higher CBF values during the acute headache attack in the brainstem76 or the dorsal pons77,78. Underscoring this inconsistency may be the spatial heterogeneity of CBF changes during migraine. For example, a study by Arkink et al.79 observed complex changes in CBF throughout the brain, including an increase in CBF during attack the inferior and middle temporal gyrus in migraine without aura, while also observing a decrease in CBF within the inferior frontal gyrus in migraine without aura. Taken together, current literature suggest less pronounced and more complex changes in CBF may occur in migraine patients without aura.
Some studies have suggested a possible link between vascular anomalies within the circle of Willis and decreased CBF during migraine with aura80. While early MR angiography studies appeared to show no difference in incompleteness of the circle of Willis in migraine patients compared with healthy controls81, subsequent studies have shown a higher than expected prevalence of incomplete circle of Willis in patients with migraine82,83. In particular, migraine patients experiencing aura appear to have a higher prevalence of an incomplete circle of Willis84, whereas no elevated incidence in migraine without aura has been observed84,85.
Functional Imaging in Migraine
The most crucial observation in functional neuroimaging in migraine has been that brainstem areas are active during pain and that after successful treatment this activation persists, while it is not present between attacks76–78,86,87 (Table 2). Activated areas in the brainstem include the dorsal midbrain and dorsolateral pons, and hypothalamic activation has been described as well87. Increased activation of dorsolateral pons is also observed in chronic migraine88 and dorsal midbrain activation is consistent with reports of migraine-like headaches following stimulation in patients with implanted electrodes for chronic pain control89,90 as well as reports of patients with lesions in these areas producing migraine symptoms91,92.
Table 2:
Summary of brain functional imaging studies in migraine.
| Authors | Technique | Headache | Participants (N) | Conclusion | References |
|---|---|---|---|---|---|
| Kim et al. (2010) | 18FDG-PET | Migraine | 40 | Hypometabolism in regions known to be involved in central pain processing (bilateral insula, bilateral ACC and PCC, left premotor and PFP, and left primary SSC). | Kim JH, Kim S, Suh SI, et al. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia. 2010; 30(1):53–61. |
| Shin et al. (2014) | 18FDG-PET | Spontaneous Migraine | 2 | Activation of the vestibulo-thalamocortical pathway and decreased metabolism in the VC. | Shin JH, Kim YK, Kim HJ, Kim JS. Altered brain metabolism in vestibular migraine: comparison of interictal and ictal findings. Cephalalgia. 2014; 34(1):58–67. |
| Kassab et al. (2009) | 18FDG-PET | Migraine | 25 | Metabolic disturbance in posterior white matter of cerebrum and cerebellum in migraneurs. | Kassab M, Bakhtar O, Wack D, Bednarczyk E. Resting brain glucose uptake in headache-free migraineurs. Headaches, 2009; 49(1):90–97. |
| Hadjikhani et al. (2001) | Event-related fMRI | Migraine with Aura | 3 | Focal increase in BOLD signal within extrastriate cortex progressing contiguously and slowly over occipital cortex during visual aura. | Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001; 98(8):4687–92. |
| Moulton et al. (2008) | Event-related fMRI | Migraine | 24 | Hypo-function of nucleus cuneiforms in migraine patients. | Moulton EA, Burstein R, Tully S, et al. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799. |
| Moulton et al. (2011) | Event-related fMRI | Migraine | 17 | Increase BOLD response to trigeminal painful stimulation in TP and EC in migraine patients, during the interictal period. | Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine states. Cereb Cortex. 2011; 21:435–48. |
| Russo et al. (2012) | Event-related fMRI | Migraine without Aura | 32 | Increasing BOLD response in perigenual part of ACC at 51 degrees C, and divergent response in pons in migraine patients. | Russo A, Tessitore A, Esposito F, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol. 2012; 259:1903–12. |
| Stankewitz (2011) | Event-related fMRI | Migraine | 40 | Increased BOLD response in PFC, ACC, red nucleus, and ventral medulla in migraine patients and a decreasein HC, without changes in pain perception. | Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011; 77(5):476–82. |
| Schulte et al. (2017) | Event-related fMRI | Migraine | 54 | Significantly stronger activation of the anterior right hypothalamus in chronic migraine patients compared to HC. | Schulte LH, Allers A, May A. Hypothalamus as a mediator of chronic migraine evidence from high-resolution fMRI. Neurology. 2017; 88(21):2011–6. |
| Russo et al. (2017) | Event-related fMRI | Migraine without Aura | 60 | Increased BOLD response in the MFG. | Russo A, Esposito F, Conte F, et al. Functional interictal changes of pain processing in migraine with ictal cutaneous allodynia. Cephalalgia. 2017; 37(4):305–14. |
| Schwedt et al. (2014) | Event-related fMRI | Migraine without Aura | 51 | Greater activation in cortical and subcortical areas involved in pain processing in migraine patients within the interictal period. | Schwedt TJ, Chong CD, Chiang CC, et al. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014; 34(12):947–58. |
| Schulte et al. (2016) | Event-related fMRI | Migraine without Aura | 1 | Hypothalamic activity increases towards the next migraine attack. Altered functional coupling between the hypothalamus, spinal trigeminal nuclei, and the dorsal rostral pons. | Schulte LH, May A. The migraine generator revisted: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016; 139 (Pt 7):1987–93. |
| Martin et al. (2011) | Event-related fMRI | Migraine | 38 | Hyperexcitability of theVC with a wider photoresponsive area in migraine patients during interictal periods. | Martin H, Sanchez del Rio M, de Silanes CL, et al. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependant in migraine patients and healthy volunteers: pathophysiological implications. Headache. 2011; 51(10):1520–8. |
| Datta et al. (2013) | Event-related fMRI | Migraine | 75 | Greater response to visual stimulation within primary VC and lateral geniculate nuclei in patients with MwA compared to patients with MwoA and HC. | Datta R, Aguirre GK, Hu S, et al. Interictal cortical hyperresponsiveness in mingraine is directly relatedto the presence of aura. Cephalalgia. 2013; 33:365–74. |
| Hougaard et al. (2014) | Event-related fMRI | Migraine with Aura | 20 | Greater response in cortical area which belong to an advanced visual network (i.e., inferor parietal and frontal gyrus, superior parietal lobule). | Hougaard A, Amin FM, Hoffman MB, et al. Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp. 2014; 35(6):2714–23. |
| Stankewitz et al. (2010) | Event-related fMRI | Migraine | 20 | Greater BOLD response in limbic structures as well as in the RoP in migraine patients during spontaneous and untreated attacks. | Stankewitz A, Voit HL, Bingel U, Peschke C, May A. A new trigemino-nociceptive stimulation model for event-related fMRI. Cephalalgia. 2010; 30:475–85. |
| Russo et al. (2014) | Event-related fMRI | Migraine without Aura | 24 | Greater response to vestibular stimuli in mediodorsal thalamusin patients with VM relative to both patients with MwoA and HC. | Russo A, Marcelli V, Esposito F, et al. Abnormal thalamic function in patients with vestibular migraine. Neurology. 2014; 82(23):2120–6. |
| Weiller et al. (1995) | H215O-labeled PET | Spontaneous Migraine | 9 | Activation in brainstem DoP, persistent even after injection of sumatriptan. | Weiller C, May A, Limmroth V, et al. Brainstem activation in spontaneous human migraine attacks. Nat Med. 1995: 1(7):658–60. |
| Afridi et al. (2005) | H215O-labeled PET | Induced Migraine | 6 | Activation in DoP during migraine attack, ipsilateral to side of pain, in migraine patients. | Afridi SK, Giffin NJ, Kaube H, et al. A positron emission tmographic study in spontaneous migraine. Arch Neurol. 2005; 62(8):1270–5. |
| Denuelle et al. (2007) | H215O-labeled PET | Migraine | 7 | Activations in midbrain, pons and hypothalamus during migrine attack and headache relief by sumatriptan. | Denuelle M, Fabre N, Payoux P, et al. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007; 47(10):1418–26. |
| Denuelle et al. (2007) | H215O-labeled PET | Migraine | 7 | Activation in VC by luminous stimulation during migraine attack and aftr headache relief but not during interictal period. | Denuelle M, Fabre N, Payoux P, et al. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007; 47(10):1418–26. |
| Maniyar et al. (2014) | H215O-labeled PET | Induced Migraine | 8 | Activations in the posterolateral hypothalamus, midbrain tegnental area, PAG, DP, and various cortical areas. | Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014; 137(Pt 1):232–41. |
| Maniyar et al. (2014) | H215O-labeled PET | Induced Migraine | 27 | Activation in brain circuits mediating nausea such rostal dorsal medulla and PAG. | Maniyar FH, Sprenger T, Schankin C, et al. The origin of nausea in migraine-a PET study. J Headache Pain. 2014; 15:84. |
| Boulloche et al. (2010) | H215O-labeled PET | Migraine | 14 | VC hyperexcitability potentiated by the concomitant heat pain stimulation. | Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2010; 81(9):978–84. |
| Denuelle et al. (2008) | H215O-labeled PET | Migraine | 7 | Activations in midbrain, pons and hypothalamus during migrine attack and headache relief by sumatriptan. | Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Posterior cerebral hypoperfusion in migraine without aura. Cephalalgia. 2008; 28(8):856–862. |
| Denuelle et al. (2011) | H215O-labeled PET | Migraine | 8 | Activation in visual cortex by luminous stimulation during migraine attack and after headache relief but not during interictal period. | Denuelle M, Boulloche N, Payoux P, Fabre N, Trotter Y, Geraud G. A PET study of photophobia during spontaneous migraine attacks. Neurology. 2011; 76(3):213–218. |
| Dermarquay et al. (2008) | H215O-labeled PET | Migraine | 23 | Activation in piriform cortex and anterosuperior temporal gyrus in olfactory hypersensitivity and odor-triggered headache attack in migraineurs. | Demarquay G, Royet JP, Mick G, Ryvlin P. Olfactory hypersensitivity in migraineurs: a H(2)(15)O-PET study. Cephalalgia. 2008; 28(10):1069–1080. |
| Coppola et al. (2017) | Resting-state fMRI | Migraine | 32 | Increased FC between MPFC and and both PCC and bilateral insula. | Coppola G, Di Renzo A, Tinelli E, et al. Resting state connectivity between default mode network and insula and encodes acute migraine headache. Cephalalgia. 2017;1:333102417715230. |
| Mainero et al. (2010) | Resting-state fMRI | Migraine | 34 | Decrease functional resting-state connectivity between PAG and brain regions invovled in pain processing. | Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011; 70(5):838–45. |
| Tessitore et al. (2013) | Resting-state fMRI | Migraine | 40 | Decreased FC in prefrontal and temporal regions of DMN in migraine patients. | Tessitore A, Russo A, Giordano A, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013; 14:89. |
| Russo et al. (2012) | Resting-state fMRI | Migraine without Aura | 28 | Reduction in the MFG and the ACC in migraine patients with MwoA. | Russo A, Tessitore A, Giordano A, et al. Executive resting-state network connectivity in migraine without aura. Cephalalgia. 2012; 32(14):1041–8. |
| Tessitore et al. (2015) | Resting-state fMRI | Migraine with Aura | 60 | Reduction in the MFG and the ACC in migraine patients with MwA. | Tessitore A, Russo A, Conte F, et al. Abnormal connectivity within executive resting-state network in migraine with aura. Headache. 2015; 55(6):794–805. |
| Tedeschi et al. (2016) | Resting-state fMRI | Migraine with Aura | 60 | Significant increased FC in the right lingual gyrus within the RS visual in patients with MwA. | Tedeschi G, Russo A, Conte F, et al. Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia. 2016; 36(2):139–47. |
| Niddam et al. (2015) | Resting-state fMRI | Migraine with Aura | 78 | Reduced FC between salience and visual networks in patients with MwA. | Niddam DM, Lai KL, Fuh JL, et al. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia. 2015; 36(1):53–66. |
| Hougaard et al. (2015) | Resting-state fMRI | Migraine with Aura | 80 | No abnormalities of intrinsic brain connectivity in the interictal phase of MwA. | Hougaard A, Amin FM, Magon S, et al. No abnormalities of intrinsic brain connectivity in the interictal phase of migraine with aura. Eur J Neurol. 2015; 22(4):702-e46. |
| Zhao et al. (2013) | Resting-state fMRI | Migraine without Aura | 60 | Neuronal dsyfunction in the thalamus, brainstem, and temporal pole in patients with long-term disease duration compared with patients with short-term disease duration and HC. | Zhao L, Liu J, Dong X, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain. 2013; 14:85. |
| Moulton et al. (2014) | Resting-state fMRI | Migraine without Aura | 24 | Increased FC between the hypothalamus and brain areas that regulate sympathetic and parasympathetic functions. | Moulton EA, Becerra L, Johnson A, et al. Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS One. 2014; 9(4):e95508. |
| Hadjikhani et al. (2013) | Resting-state fMRI | Migraine | 82 | Increased FC between the amygdala and visceroceptive insula in migraine patients. | Hadjikhani N, Ward N, Boshyan J, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia. 2013; 33(15):1264–8. |
| Yu et al. (2012) | Resting-state fMRI | Migraine without Aura | 52 | Decreased ReHo values in supplementary motor area, rostral anterior cingulate, prefrontal and orbitofrontal cortices in migraineurs. | Yu D, Yuan K, Zhao L, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed. 2012; 25(5):806–812. |
| Cao et al. (1999) | Task-related fMRI | Migraine | 18 | Activation of the red nucleus and substantia nigra in association with visually triggered symptions of migraine | Cao Y, Welch KM, Aurora S, Vikingstad EM. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol. 1999; 56(5):548–554. |
| Antal et al. (2011) | Task-related fMRI | Migraine | 36 | Hyperresponsivness of the VC beyond visual areas in migrainous even in the interictal period. | Antal A, Polania R, Saller K, et al. Differential activation of the middle-temporal complex to visual stimulation in migraineurs. Cephalagia. 2011; 31(3):338–345. |
| Vincent et al.(2003) | Task-related fMRI | Migraine with Aura | 10 | Activation in extrastriate visual cortex contralaterally to the side of stimulation in migraineurs. | Vincent M, Pedra E, Mourao-Miranda J, Bramati IE, Henrique AR, Moll J. Ehanced interictal responsiveness of the migraineous visual cortex to incongruent activation bar stimulation: a funcational MRI visual activation study. Cephalalgia. 2003; 23(9): 860–868. |
| Bramanti et al. (2005) | Task-related fMRI | Migraine with Aura | 1 | Different activation patterns in occipital cortex during headache attack and interictal. | Bramanti P, Grugno R, Vitetta A, Marino S, Di Bella P, Nappi G. Ictal and interictal hypoactivation of the occiptial cortex in migraine with aura. A neuroimaging and electrophysiological study. Funct Neurol. 2005; 20(4):169–171. |
| Huang et al. (2006) | Task-related fMRI | Migraine with Aura | 20 | No differences in visual cortical activation in migraineurs compared with HC. | Huang J, DeLano M, Cao Y. Visual cortical inhibitory function in migraine is not generally impaired: eveidence from a combined psychophysical test with an fMRI study. Cephalalgia. 2006; 26(5):554–560. |
| Stankewitz et al. (2011) | Task-related fMRI | Migraine | 40 | Lower activations in trigeminal nuclei during interictal; increased activation in dorsal pons. | Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci. 2011; 31(6):1937–1943. |
Task-based fMRI as a clinical tool for assessing migraine is relatively limited due to the spontaneous, transient nature of these attacks and the imaging need for a planned experimental paradigm. Therefore, most functional studies in migraine have induced migraines to image the resulting changes (Table 2). Functional tasks performed during H215O-labled positron emission tomography (PET) showed increased activation in the dorsal pons76,86,87, midbrain87, brainstem, hypothalamus87, periaqueductal gray93,94, midbrain trigeminal area, and visual cortex87 during painful stimulation. An increase in blood oxygen level dependent (BOLD) functional MRI signal has been observed in the extrastriate cortex and occipital cortex during induction of migraine with visual aura57, and increased BOLD signal in temporal pole and entorhinal cortex has been observed in both ictal and interictal migraine periods50. Functional MRI during visual, olfactory, or vestibular stimuli result in greater BOLD response and hyperexcitability in visual cortex and visual network95–98, limbic structures99, and mediodorsal thalamus100, respectively. Additionally, increased BOLD signal in anterior cingulate cortex (ACC)101,102, prefrontal cortex or middle frontal gyrus102,103, brainstem and medulla102, and hypothalamus104,105 has also been observed, consistent with an increase in activation in areas involved in pain processing during invoked migraine106.
Functional connectivity measures using MRI at rest are less consistent and appear more complex, with some regions demonstrating increased connectivity and other areas reduced connectivity as a result of migraine. For example, decreased functional connectivity within the pain-processing networks107, the default mode network (DMN)108, and fronto-parietal regions of the executive network (middle frontal gyrus and dorsal ACC)109,110 have all been observed. Additionally, increased connectivity between PAG, hypothalamus, and/or amygdala and other brain areas within nociceptive and somatosensory processing pathways have also been detected111–114.
Metabolic and Molecular Imaging in Migraine
In addition to functional alterations, patients with migraine also appear to have altered brain metabolism and biochemistry (Table 3). [18F]-fluorodeoxyglucose (FDG) PET studies have demonstrated increased activation of the vestibulo-thalamo-cortical pathway and decreased metabolism in the visual cortex during spontaneous migraine attacks115. At rest, significant hypometabolism has been observed in regions involved in pain processing116, including bilateral insula, bilateral ACC and PPC, premotor, PFC, and primary somatosensory cortex. These results suggest migraine may be intimately linked with primary metabolic dysfunction as well as potential secondary effects of brain regions involved in pain processing due to repetitive headache attacks.
Table 3:
Summary of brain molecular and metabolic imaging in migraine.
| Authors | Technique | Headache | Participants (N) | Conclusion | References |
|---|---|---|---|---|---|
| Aguila et al. (2015) | 1H-MRS | Migraine | 38 | GABA+ increased in MX vs. controls. Suggest altered excitability of cortical neurons during the interictal period. GABA+ included detection of nonspecified macromolecules, which might cause contamination of the results. | Aguila M-ER, et al. NMR Biomed. 2015; 28:890–897. |
| Arngrim et al. (2016) | 1H-MRS | Migraine | 29 | No differences in MA during hypoxiainduced headaches vs. controls. Suggest no mitochondrial dysfunction. | Arngrim N, et al. Brain. 2106; 139:723–737. |
| Becerra et al. (2016) | 1H-MRS | Migraine | 65 | No differences in MO+MA vs. controls. Posthoc analysis: Cross-validation test using quadratic discriminant analysis model showed that glutamine, NAA and aspartate as a group differentiate MO+MA from control. Suggest a ‘complex’ of metabolite alterations, which may underlie changes in neuronal chemistry in the migraine brain, supporting the theory of the hyperexcitable migraine brain. | Becerra L, et al. Neuroimage Clin. 2016; 11:588–594. |
| Bigal et al. (2008) | 1H-MRS | Migraine | 28 | GABA decreased in MO+MA with severe migraine attacks in the month prior to MRS vs. controls. We suggest that it may indicate reduced inhibition. | Bigal ME, et al. Neurology. 2008; 70:2078–2080. |
| Bridge et al. (2015) | 1H-MRS | Migraine | 26 | GABA ~10% decreased in MA vs. controls. Suggest reduced inhibition occipitally in MA consistent with occipital hyperexcitability. Positive correlation between glutamate and BOLD activation in the visual cortex during visual stimulation in MA vs. controls. Suggests enhanced glutamate activation. Altogether, the results suggest an abnormal excitation-inhibition coupling in the occipital cortex. The MA cohort reported visual stimuli as a migraine trigger. | Bridge H, et al. Cephalalgia. 2015; 35:1025–1030. |
| Dichgans et al. (2005) | 1H-MRS | Migraine | 32 | Differences measured in cerebellum for FHM1 vs. controls. Suggest neuronal impairment (NAA), altered glial cell proliferation (myoinositol) and impaired glutamatergic neurotransmission. | Dichgans M, et al. Neurology. 2005; 64:608–613. |
| Fayed et al. (2014) | 1H-MRS | Migraine | 216 | No differences in MX vs. controls. | Fayed N, et al. Acad Radiol. 2014; 21:1211–1217. |
| Gonzales de la Aleja et al. (2013) | 1H-MRS | Migraine | 46 | Glutamate increased in MA+MO vs. controls in the anterior paracingulate cortex. Suggests altered excitability and increased susceptibility to migraine triggers. Glutamate/glutamine-ratio abnormal in MO+MA vs. controls in the occipital cortex. Suggests abnormal neuronal–glial coupling of glutamatergic metabolism or increased neuron/astrocyte ratio in the occipital cortex. | Gonzales de la Aleja J, et al. Headache. 2013; 53:365–375. |
| Grimaldi et al. (2010) | 1H-MRS | Migraine | 14 | No differences in FHM2 vs. controls. | Grimaldi D, et al. Cephalalgia. 2010; 30:522–559. |
| Gu et al. (2008) | 1H-MRS | Migraine | 34 | NAA/Choline decreased in left thalamus in MO vs. controls. Suggest mitochondrial and neuronal dysfunction due to neuronal deafferentation in the thalamus. | Gu T, et al. Neurol Res 2008; 30:229–233. |
| Lai et al. (2011) | 1H-MRS | Migraine | 88 | NAA increased in EM in pons bilaterally compared to CM and controls. No differences between CM vs. controls. Suggest neuronal hypertrophy at the dorsal pons in EM. 23/53 CM patients were diagnosed with MOH. | Lai T, et al. J Headache Pain. 2011; 12:295–302. |
| Lirng et al. (2015) | 1H-MRS | Migraine | 30 | Myo-inositol increased in MX with depression vs. MX without depression. No healthy controls. Suggest glial dysfunction in dorsolateral prefrontal cortex in migraineurs with depression. | Lirng J, et al. Cephalagia. 2015; 35:702–709. |
| Macri et al. (2003) | 1H-MRS | Migraine | 15 | Choline decreased in MA vs. controls. Suggest membrane composition alterations. Only study to report choline alterations. | Macri M, et al. J Magn Reson Imaging. 2003; 21:1201–1206. |
| Mohamed et al. (2013) | 1H-MRS | Migraine | 32 | NAA decreased in MO vs. controls. NAA more decreased in right thalamus vs. left thalamus in MO. NAA decreased, lactate and myoinositol increased with increased duration and attack frequency in MO. Suggest altered energy metabolism correlated to severity of disease. | Mohamed RE, et al. Egypt J Radiol Nucl Med. 2013; 44:859–870. |
| Prescot et al. (2009) | 1H-MRS | Migraine | 18 | No differences in MX vs. controls. Linear discriminant analysis showed a separation between MX and controls based on NAAG and glutamine in anterior cingulate cortex and insula. Suggest glutamatergic abnormalities in anterior cingulate cortex and insula. | Prescot A, et al. Mol Pain. 2009; 5:34. |
| Reyngoudt et al. (2011) | 1H-MRS | Migraine | 40 | No differences in MO vs. controls before or after visual stimulation. Argue against a significant switch to nonaerobic glucose metabolism during long-lasting photic stimulation of the visual cortex in MO. | Reyngoudt H, et al. J Headache Pain. 2011; 12:295–302. |
| Sandor et al. (2005) | 1H-MRS | Migraine | 21 | Lactate increased in MA with visual aura vs. FHM/SHM and vs. controls before, during and after visual stimulation in visual cortices. Suggest mitochondrial dysfunction. | Sandor P, et al. Cephalalgia. 2005; 25:507–518. |
| Sarchielli et al. (2005) | 1H-MRS | Migraine | 54 | NAA decreased at baseline and after visual stimulation in MA vs. MO and vs. controls No differences in MO vs. controls. Suggest less efficient mitochondrial function in MA. | Sarichielli P, et al. Neuroimage. 2005; 24:1025–1031. |
| Schulz et al. (2007) | 1H-MRS | Migraine | 37 | No difference between MA and SHM+FHM vs. controls. Lactate peaks undetectable. | Schulz UG, et al. Brain. 2007; 130:3102–3110. |
| Siniatchkin et al. (2012) | 1H-MRS | Migraine | 20 | Glx increased at baseline in MA vs. controls. Suggests excessive glutamate mediated excitation in migraine. Both anodal and cathodal transcranial direct current stimulation caused Glx decrease in MA, which did not increase to baseline after visual stimulation as in controls. Suggest abnormal cortical information processing and excitability in migraineurs mediated by altered glutamatergic neurotransmission. | Siniatchkin M, et al. Cereb Cortex. 2012; 22:2207–2216. |
| Wang et al. (2006) | 1H-MRS | Migraine | 37 | No differences in CM vs. controls. Suggest that the hypothalamus might not play a pivotal role in chronic migraine. | Wang S, et al. J Neurol Neurosurg Psychiatry. 2006; 77:622–625. |
| Watanabe et al. (1996) | 1H-MRS | Migraine | 12 | Lactate increased in a small heterogeneous group of patients: migraine with visual aura (N = 3), basilar type migraine (N = 1) and migrainous infarction (N = 2) vs. controls (N = 6). Suggest mitochondrial dysfunction. The participants had last attack within 2 months prior to testing. | Watanabe H, et al. Neurology. 1996; 47:1093–1095. |
| Zielman et al. (2014) | 1H-MRS | Migraine | 37 | NAA decreased in SHM+FHM1+FHM2 vs. controls in cerebellum. NAA more decreased in FHM1 vs. controls than SHM and FHM2. Suggest neuronal loss or dysfunction in the cerebellum and/or less efficient mitochondrial function. Glx and myo-inositol not measured in pons. | Zielman R, et al. Cephalalgia. 2014; 34:959–967. |
| Barbiroli et al. (1990) | 31P-MRS | Migraine | 23 | PCr decreased in MpA+MS vs. controls. Suggest mitochondrial abnormalities as apotential cause to defects in oxidative metablosim, making cells not meet energy demand. | Barbiroli B, Montagna P, Cortelli P, et al. Complicated migraine studied by phosphorus magnetic resonance spectroscopy. Cephalalgia 1990: 10:263–272. |
| Barbiroli et al. (1992) | 31P-MRS | Migraine | 24 | Significant changes in MA vs. controls. Only 31P-MRS study to report decreased pHi. Indicates increased lactate levels. Collectively, the data suggest less freely available energy in the cell and abnormal oxidative metabolism due to mitochondrial dysfunction. | Barbiroli B, et al. Neurology. 1992; 42:1209–1214. |
| Boska et al. (2002) | 31P-MRS | Migraine | 86 | Magnesium decreased in FHM+SHM vs. controls in the posterior region including the occipital lobe. Suggested to contribute to the cortical hyperexcitability. PDE increased in MO vs. controls in the posterior region including the occipital lobe. Suggested that this might be a compensatory mechanism to maintain membrane stability. Data not shown for ADP. | Boska MD, et al. Neurology. 2002; 58;1227–1233. |
| Lodi et al. (1997) | 31P-MRS | Migraine | 27 | Significant changes in MA vs. controls. Collectively, these data suggest impaired cerebral oxidative metabolism and abnormal mitochondrial function, unable to meet increased energy demand. Only 31P-MRS study to report increased pHi. Indicates decreased lactate levels. Suggested to be due to ionic abnormalities in the brain causing dysfunction of proton pumps. The cohorts were juvenile. | Lodi R, et al. Pediatr Res. 1997; 42:866–871. |
| Lodi et al. (2001) | 31P-MRS | Migraine | 107 | Magnesium and deltaGATPhyd decreased in all groups vs. controls. The data suggest reduced release of free energy by ATP hydrolysis due to mitochondrial dysfunction, thus magnesium levels are downregulated to re-equilibrate the rapidly available free energy. deltaGATPhyd is defined as the freely available energy released by ATP hydrolysis in the intact cell, calculated based on ATP, ADP, Pi and magnesium. Data not shown for PCr, Pi, and ADP. | Lodi R, et al. Brain Res Bull. 2001; 54:437–441. |
| Montagna et al. (1994) | 31P-MRS | Migraine | 40 | Significant changes in MO vs. controls. Collectively, the data suggest a defect and altered energy metabolism. | Montagna P, et al. Neurology. 1994; 44:666–669. |
| Ramadan et al. (1989) | 31P-MRS | Migraine | 44 | Magnesium decreased ictally in MO þMA (N = 10) vs. controls. Suggest that low magnesium promotes cortical spreading depression, thus initiating the migraine attack. No patient was tested both ictally and interictally. None had aura during testing. Data not shown for PCr, Pi, ADP, and ATP. | Ramadan NM, et al. Headache. 1989; 29:590–593. |
| Reyngoudt et al. (2010) | 31P-MRS | Migraine | 44 | PCr, PP and ATP decreased in MO vs. controls. Collectively, the data suggest an impaired energy metabolism and the decreased ATP level further suggest presence of a mitochondrial component in migraine. ATP was more decreased in a subgroup with the highest attack frequency. Only 31P-MRS study to determine the absolute [ATP] and not calculate other metabolite concentrations based on assumed [ATP] = 3.0 mmol/L. | Reyngoudt H, et al.Cephalalgia. 2010; 31:1243–1253. |
| Schulz et al. (2007) | 31P-MRS | Migraine | 37 | Cr/Pi decreased and Pi/ATP increased in sporadic and familiar hemiplegic migraine (N ¼ 9) vs. migraine with nonmotor aura (N ¼ 10) in gray matter. Suggest alterations in the energy metabolism. | Schulz UG, et al. Brain. 2007; 130:3102–3110. |
| Uncini et al. (1995) | 31P-MRS | Migraine | 35 | Significant changes in family members (N = 5) vs. controls. Suggest abnormal energy metabolism due to mitochondrial dysfunction. Differences reported for 5 family members, whereof 2 have no history of migraine, vs. controls. | Unicini A, et al. J Neurol Sci. 1995; 129:214–222. |
| Welch et al. (1988) | 31P-MRS | Migraine | 47 | No differences in pHi ictally (N = 11) or interictally (N = 9) in MO+MA vs. controls. Indicates no lactate alteration. Data not shown for ADP and ATP. Data for PCr and Pi are reported in Welch et al. [37]. No ictal vs. interictal state comparison. No patient had aura during testing, and the results were compared to controls who were not age- and gender-matched. | Welch KMA, et al. Cephalalgia. 1988; 8:273–277. |
| Welch et al. (1989) | 31P-MRS | Migraine | 47 | PCr decreased and Pi increased ictally (N = 11) in MO þMA vs. controls. Pi increased interictally (N = 9) in MO þMA vs. controls. Suggest an altered energy metabolism during migraine attacks. No difference in pHi suggests no lactate alteration. Data not shown for ADP and ATP. No ictal vs. interictal state comparison. No patient had aura during testing, and the results were compared to controls who were not age and gender matched. | Welch KMA, et al. Neurology. 1989; 39:538–541. |
| Wall et al. (2005) | PET and [carbonyl-11C] zolmitriptan | Migraine | 8 | Rapid dose-proportional uptake of 11C-zolmitriptan into the brain. | Wall A, Kågedal M, Bergstrom M, et al. Distribution of zolmitriptan into the CNS in healthy volunteers: a positron emission tomography study. Drugs R.D. 2005; 6(3);139–147. |
| Da Silva et al. (2014) | PET with 11C-carfentanil | Spontaneous Migraine | 12 | micro-OR activation in the ictal phase in the medial PFC, strongly associated with the microp-OR availability level during the interictal phase. | Da Silva AF, Nascimento TD, DosSantos MF, et al. Association of micro-opioid activation in the prefrontal cortex with spontaneous migraine attacks-brief report I. Ann Clin Transl Neurol. 2014; 1(6):439–44 |
| Da Silva et al. (2014) | PET with 11C-carfentanil | Spontaneous Migraine | 1 | Reduction in micro-OR in the pain-modulatory regions of the endogenous micro-opiod system during the migraine attack. | Da Silva AF, Nascimento TD, Love T, et al. 3D-neuronavigation in vivo through a patient’s brain during a spontaneous migraine headache. J Vis Exp. 2014;(88). |
| Chabriat et al. (1995) | PET with 18F-fuorosetoperone | Migraine | 12 | No differences of cortical 5-HT2 receptors’ distribution volumes in migraine patients when compared with HC. | Chabriat H, Tehindrazanarivelo A, Vera P, et al. 5HT2 receptors in cerebral cortex of migraineurs studied using PET and 18F-fluorosetoperone. Cephalalgia. 1995; 15(2):104–8. |
| Demarquay et al. (2011) | PET with a 5HT1A radioligand | Migraine | 20 | Increased 5-HT1A receptors’ in the pontine raphe during odor-triggered migraine attack. | Demarquay G, Lothe A, Royet JP, et al. Brainstem changes in 5-HT1A receptor availability during migraine attack. Cephalalgia. 2011; 3(1):84–94. |
| Chugani et al. (1999) | PET with alpha-[11C] methyl-L-tryptophan tracer | Migraine without Aura | 19 | Increased rate of brain serotonin synthesis in the ictal phase of migraine attack. | Chugani DC, Niimura K, Chaturvedi S, et al. Increased brain serotonin synthesis in migraine. Neurology. 1999; 53(7):1473–9. |
MR spectroscopy (MRS), a noninvasive method of investigating the biochemical composition of the brain and infer metabolic information, has been used to highlight various biological changes within the brain in patients with migraine. Phosphorous (31P)-MRS studies have implied abnormal energy metabolism and potential mitochondrial dysfunction may occur in patients with migraine. Multiple studies have observed decreased phosphocreatine (PCr)117–123, suggesting mitochondrial abnormalities and impaired cerebral oxidative metabolism as potential causes of migraine. Using proton (1H)-MRS, reports have shown altered excitability of the brain in patients with migraine, including abnormal levels of neurotransmitters glutamate and γ-aminobutyrate (GABA) in the occipital cortex, cingulate cortex, and other areas implicated in pain processing124–129. Additionally, lower levels of N-acetyl aspartate (NAA), a neuronal marker, was observed in migraineurs with aura who had T2 hyperintense lesions51 within the occipital lobe128,130, as well as the thalamus131 and cerebellum132.
Additionally, serotonergic function and 5HT receptors have been implicated in migraine pathogenesis133, leading to a number of PET studies examining 5HT having been performed134. PET imaging using [18F]-fluorosetoperone (a 5-HT2-specific radioligand), however, did not show any differences of the distribution of 5HT2 receptors in the cortex135. A study using an α-[11C]methyl-L-tryptophan tracer did show an increased rate of brain serotonin synthesis during the acute phase of migraine attacks136, which was subsequently verified using a specific antagonist of serotonin receptors137, suggesting that increased 5-HT1A receptor availability is present during migraine attacks, particularly within the pontine raphe135,138.
Conclusions
In summary, the use of advanced imaging in routine diagnostic practice appears to provide only limited value in adult and pediatric patients with migraine who have not experienced changes in headache quality or associated symptoms. However, advanced imaging may have potential for studying the biological manifestations and pathophysiology of migraine headaches. Migraine with aura appears to have characteristic spatiotemporal changes in structural anatomy, function, hemodynamics, metabolism, and biochemistry, while migraine without aura appears more complex. Large, controlled, multicenter imaging-based observational trials are needed to confirm the abundant anecdotal evidence in the literature and test the variety of scientific hypotheses thought to underscore migraine pathophysiology.
Synopsis.
The use of advanced imaging in routine diagnostic practice appears to provide only limited value in patients with migraine who have not experienced recent changes in headache characteristics or symptoms. However, advanced imaging may have potential for studying the biological manifestations and pathophysiology of migraine headaches. Migraine with aura appears to have characteristic spatiotemporal changes in structural anatomy, function, hemodynamics, metabolism, and biochemistry, whereas migraine without aura produces more subtle and complex changes. Advanced imaging studies in migraine with aura reveal a decrease in cortical density, altered microstructural connectivity, an increase in functional activation in common pain processing pathways, and a characteristic hypoperfusion followed by hyperperfusion after aura onset. Altered glucose metabolism, neurotransmitter concentration, and receptor density have also been observed. Large, controlled, multicenter imaging-based observational trials are needed to confirm the abundant anecdotal evidence in the literature and test the variety of scientific hypotheses thought to underscore migraine pathophysiology.
Key Points.
Clinical use of advanced imaging in headaches is not standardized; no single diagnostic imaging technique is able to define and/or differentiate idiopathic headache syndromes.
In migraine with aura, cortical thinning, microstructural changes, spatiotemporal fluctuations in blood flow, adaptations in brain function, and alterations in both metabolism and biochemistry have been observed in pain processing areas of the brain (thalamus, insula, amygdala, brainstem, hippocampus, prefrontal cortex, anterior cingulate cortex, cerebellum, supplemental motor area, primary and secondary somatosensory areas, and the posterior parietal cortex).
A characteristic decrease in blood flow has been observed during aura, while a significant increase in blood flow has been observed in subsequent stages of migraine attacks.
Imaging changes in migraine patients who do not experience aura are subtler and more complex, with studies varying widely in the literature.
References
- 1.Medina LS, D’Souza B & Vasconcellos E Adults and children with headache: evidence-based diagnostic evaluation. Neuroimaging Clin N Am 13, 225–235 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Evans RW Diagnostic testing for the evaluation of headaches. Neurol Clin 14, 1–26 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Jordan JE & Expert Panel on Neurologic, I. Headache. AJNR Am J Neuroradiol 28, 1824–1826 (2007). [PMC free article] [PubMed] [Google Scholar]
- 4.Goadsby PJ, Lipton RB & Ferrari MD Migraine--current understanding and treatment. N Engl J Med 346, 257–270, doi: 10.1056/NEJMra010917 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Study, C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800, doi: 10.1016/S0140-6736(15)60692-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lance JW Current concepts of migraine pathogenesis. Neurology 43, S11–15 (1993). [PubMed] [Google Scholar]
- 7.Stewart WF, Ricci JA, Chee E, Morganstein D & Lipton R Lost productive time and cost due to common pain conditions in the US workforce. JAMA 290, 2443–2454, doi: 10.1001/jama.290.18.2443 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Andlin-Sobocki P, Jonsson B, Wittchen HU & Olesen J Cost of disorders of the brain in Europe. Eur J Neurol 12 Suppl 1, 1–27, doi: 10.1111/j.1468-1331.2005.01202.x (2005). [DOI] [PubMed] [Google Scholar]
- 9.May A A review of diagnostic and functional imaging in headache. J Headache Pain 7, 174–184, doi: 10.1007/s10194-006-0307-1 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demaerel P, Boelaert I, Wilms G & Baert AL The role of cranial computed tomography in the diagnostic work-up of headache. Headache 36, 347–348 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Sotaniemi KA, Rantala M, Pyhtinen J & Myllyla VV Clinical and CT correlates in the diagnosis of intracranial tumours. J Neurol Neurosurg Psychiatry 54, 645–647 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HZ, Simonson TM, Greco WR & Yuh WT Brain MR imaging in the evaluation of chronic headache in patients without other neurologic symptoms. Acad Radiol 8, 405–408, doi: 10.1016/S1076-6332(03)80548-2 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Detsky ME et al. Does this patient with headache have a migraine or need neuroimaging? JAMA 296, 1274–1283, doi: 10.1001/jama.296.10.1274 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Sempere AP et al. Neuroimaging in the evaluation of patients with non-acute headache. Cephalalgia 25, 30–35, doi: 10.1111/j.1468-2982.2004.00798.x (2005). [DOI] [PubMed] [Google Scholar]
- 15.Silberstein SD Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 55, 754–762 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Edlow JA et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 52, 407–436, doi: 10.1016/j.annemergmed.2008.07.001 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Becker WJ et al. Guideline for primary care management of headache in adults. Can Fam Physician 61, 670–679 (2015). [PMC free article] [PubMed] [Google Scholar]
- 18.Sandrini G et al. Neurophysiological tests and neuroimaging procedures in non-acute headache: guidelines and recommendations. Eur J Neurol 11, 217–224, doi: 10.1111/j.1468-1331.2003.00785.x (2004). [DOI] [PubMed] [Google Scholar]
- 19.May A et al. Experimental cranial pain elicited by capsaicin: a PET study. Pain 74, 61–66 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Hsieh JC, Hannerz J & Ingvar M Right-lateralised central processing for pain of nitroglycerin-induced cluster headache. Pain 67, 59–68 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Casey KL et al. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol 71, 802–807, doi: 10.1152/jn.1994.71.2.802 (1994). [DOI] [PubMed] [Google Scholar]
- 22.Jones AK, Friston K & Frackowiak RS Localization of responses to pain in human cerebral cortex. Science 255, 215–216 (1992). [DOI] [PubMed] [Google Scholar]
- 23.Coghill RC et al. Distributed processing of pain and vibration by the human brain. J Neurosci 14, 4095–4108 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh JC et al. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain 64, 303–314 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Burton H, Videen TO & Raichle ME Tactile-vibration-activated foci in insular and parietal-opercular cortex studied with positron emission tomography: mapping the second somatosensory area in humans. Somatosens Mot Res 10, 297–308 (1993). [DOI] [PubMed] [Google Scholar]
- 26.Derbyshire SW et al. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg Psychiatry 57, 1166–1172 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goadsby PJ, Zagami AS & Lambert GA Neural processing of craniovascular pain: a synthesis of the central structures involved in migraine. Headache 31, 365–371 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Rosen SD et al. Central nervous pathways mediating angina pectoris. Lancet 344, 147–150 (1994). [DOI] [PubMed] [Google Scholar]
- 29.Peyron R, Laurent B & Garcia-Larrea L Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 30, 263–288 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Forss N, Raij TT, Seppa M & Hari R Common cortical network for first and second pain. Neuroimage 24, 132–142, doi: 10.1016/j.neuroimage.2004.09.032 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Matharu MS, Good CD, May A, Bahra A & Goadsby PJ No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol 10, 53–57 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Rocca MA et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 37, 1765–1770, doi: 10.1161/01.STR.0000226589.00599.4d (2006). [DOI] [PubMed] [Google Scholar]
- 33.Valfre W, Rainero I, Bergui M & Pinessi L Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 48, 109–117, doi: 10.1111/j.1526-4610.2007.00723.x (2008). [DOI] [PubMed] [Google Scholar]
- 34.Kim JH et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 28, 598–604, doi: 10.1111/j.1468-2982.2008.01550.x (2008). [DOI] [PubMed] [Google Scholar]
- 35.Yu ZB et al. Different mean thickness implicates involvement of the cortex in migraine. Medicine (Baltimore) 95, e4824, doi: 10.1097/MD.0000000000004824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaist D et al. Migraine with visual aura associated with thicker visual cortex. Brain, doi: 10.1093/brain/awx382 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Kruit MC et al. Migraine as a risk factor for subclinical brain lesions. JAMA 291, 427–434, doi: 10.1001/jama.291.4.427 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Kruit MC, Launer LJ, Ferrari MD & van Buchem MA Infarcts in the posterior circulation territory in migraine. The population-based MRI CAMERA study. Brain 128, 2068–2077, doi: 10.1093/brain/awh542 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Kruit MC, Launer LJ, Ferrari MD & van Buchem MA Brain stem and cerebellar hyperintense lesions in migraine. Stroke 37, 1109–1112, doi: 10.1161/01.STR.0000206446.26702.e9 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Kruit MC, van Buchem MA, Launer LJ, Terwindt GM & Ferrari MD Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia 30, 129–136, doi: 10.1111/j.1468-2982.2009.01904.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bigal ME, Kurth T, Hu H, Santanello N & Lipton RB Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology 72, 1864–1871, doi: 10.1212/WNL.0b013e3181a71220 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurth T, Chabriat H & Bousser MG Migraine and stroke: a complex association with clinical implications. Lancet Neurol 11, 92–100, doi: 10.1016/S1474-4422(11)70266-6 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Trauninger A et al. Risk factors of migraine-related brain white matter hyperintensities: an investigation of 186 patients. J Headache Pain 12, 97–103, doi: 10.1007/s10194-011-0299-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodick DW & Roarke MC Crossed cerebellar diaschisis during migraine with prolonged aura: a possible mechanism for cerebellar infarctions. Cephalalgia 28, 83–86, doi: 10.1111/j.1468-2982.2007.01458.x (2008). [DOI] [PubMed] [Google Scholar]
- 45.Longoni M & Ferrarese C Inflammation and excitotoxicity: role in migraine pathogenesis. Neurol Sci 27 Suppl 2, S107–110, doi: 10.1007/s10072-006-0582-2 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Sparaco M, Feleppa M, Lipton RB, Rapoport AM & Bigal ME Mitochondrial dysfunction and migraine: evidence and hypotheses. Cephalalgia 26, 361–372, doi: 10.1111/j.1468-2982.2005.01059.x (2006). [DOI] [PubMed] [Google Scholar]
- 47.Szabo N et al. White matter microstructural alterations in migraine: a diffusion-weighted MRI study. Pain 153, 651–656, doi: 10.1016/j.pain.2011.11.029 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Li XL et al. A diffusion tensor magnetic resonance imaging study of corpus callosum from adult patients with migraine complicated with depressive/anxious disorder. Headache 51, 237–245, doi: 10.1111/j.1526-4610.2010.01774.x (2011). [DOI] [PubMed] [Google Scholar]
- 49.DaSilva AF et al. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport 18, 301–305, doi: 10.1097/WNR.0b013e32801776bb (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moulton EA et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex 21, 435–448, doi: 10.1093/cercor/bhq109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aradi M et al. Quantitative MRI studies of chronic brain white matter hyperintensities in migraine patients. Headache 53, 752–763, doi: 10.1111/head.12013 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Coppola G et al. Dynamic changes in thalamic microstructure of migraine without aura patients: a diffusion tensor magnetic resonance imaging study. Eur J Neurol 21, 287–e213, doi: 10.1111/ene.12296 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Leao AAP SPREADING DEPRESSION OF ACTIVITY IN THE CEREBRAL CORTEX. Journal of Neurophysiology 7, 359–390, doi: 10.1152/jn.1944.7.6.359 (1944). [DOI] [PubMed] [Google Scholar]
- 54.Lauritzen M Regional cerebral blood flow during cortical spreading depression in rat brain: increased reactive hyperperfusion in low-flow states. Acta Neurol Scand 75, 1–8 (1987). [DOI] [PubMed] [Google Scholar]
- 55.Mraovitch S, Calando Y, Goadsby PJ & Seylaz J Subcortical cerebral blood flow and metabolic changes elicited by cortical spreading depression in rat. Cephalalgia 12, 137–141; discussion 127, doi: 10.1046/j.1468-2982.1992.1203137.x (1992). [DOI] [PubMed] [Google Scholar]
- 56.Cao Y, Welch KM, Aurora S & Vikingstad EM Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol 56, 548–554 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Hadjikhani N et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 98, 4687–4692, doi: 10.1073/pnas.071582498 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olesen J, Larsen B & Lauritzen M Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 9, 344–352, doi: 10.1002/ana.410090406 (1981). [DOI] [PubMed] [Google Scholar]
- 59.Friberg L, Olesen J, Lassen NA, Olsen TS & Karle A Cerebral oxygen extraction, oxygen consumption, and regional cerebral blood flow during the aura phase of migraine. Stroke 25, 974–979 (1994). [DOI] [PubMed] [Google Scholar]
- 60.Sanchez del Rio M et al. Perfusion weighted imaging during migraine: spontaneous visual aura and headache. Cephalalgia 19, 701–707, doi: 10.1046/j.1468-2982.1999.019008701.x (1999). [DOI] [PubMed] [Google Scholar]
- 61.Blicher JU, Tietze A, Donahue MJ, Smith SA & Ostergaard L Perfusion and pH MRI in familial hemiplegic migraine with prolonged aura. Cephalalgia 36, 279–283, doi: 10.1177/0333102415586064 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Forster A, Wenz H, Kerl HU, Brockmann MA & Groden C Perfusion patterns in migraine with aura. Cephalalgia 34, 870–876, doi: 10.1177/0333102414523339 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Hougaard A et al. Increased brainstem perfusion, but no blood-brain barrier disruption, during attacks of migraine with aura. Brain 140, 1633–1642, doi: 10.1093/brain/awx089 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Kato Y, Araki N, Matsuda H, Ito Y & Suzuki C Arterial spin-labeled MRI study of migraine attacks treated with rizatriptan. J Headache Pain 11, 255–258, doi: 10.1007/s10194-010-0215-2 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oberndorfer S et al. Familial hemiplegic migraine: follow-up findings of diffusion-weighted magnetic resonance imaging (MRI), perfusion-MRI and [99mTc] HMPAO-SPECT in a patient with prolonged hemiplegic aura. Cephalalgia 24, 533–539, doi: 10.1111/j.1468-2982.2003.00706.x (2004). [DOI] [PubMed] [Google Scholar]
- 66.Takase Y, Nakano M, Tatsumi C & Matsuyama T Clinical features, effectiveness of drug-based treatment, and prognosis of new daily persistent headache (NDPH): 30 cases in Japan. Cephalalgia 24, 955–959, doi: 10.1111/j.1468-2982.2004.00771.x (2004). [DOI] [PubMed] [Google Scholar]
- 67.Mourand I et al. Perfusion-weighted MR imaging in persistent hemiplegic migraine. Neuroradiology 54, 255–260, doi: 10.1007/s00234-011-0946-z (2012). [DOI] [PubMed] [Google Scholar]
- 68.Boulouis G et al. Magnetic resonance imaging arterial-spin-labelling perfusion alterations in childhood migraine with atypical aura: a case-control study. Dev Med Child Neurol 58, 965–969, doi: 10.1111/dmcn.13123 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Pollock JM et al. Migraine associated cerebral hyperperfusion with arterial spin-labeled MR imaging. AJNR Am J Neuroradiol 29, 1494–1497, doi: 10.3174/ajnr.A1115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rotstein DL, Aviv RI & Murray BJ Migraine with aura associated with unilateral cortical increase in vascular permeability. Cephalalgia 32, 1216–1219, doi: 10.1177/0333102412462286 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Olesen J, Lauritzen M, Tfelt-Hansen P, Henriksen L & Larsen B Spreading cerebral oligemia in classical- and normal cerebral blood flow in common migraine. Headache 22, 242–248 (1982). [DOI] [PubMed] [Google Scholar]
- 72.Olesen J et al. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann Neurol 28, 791–798, doi: 10.1002/ana.410280610 (1990). [DOI] [PubMed] [Google Scholar]
- 73.Lauritzen M & Olesen J Regional cerebral blood flow during migraine attacks by Xenon-133 inhalation and emission tomography. Brain 107 (Pt 2), 447–461 (1984). [DOI] [PubMed] [Google Scholar]
- 74.Friberg L et al. Interictal “patchy” regional cerebral blood flow patterns in migraine patients. A single photon emission computerized tomographic study. Eur J Neurol 1, 35–43, doi: 10.1111/j.1468-1331.1994.tb00048.x (1994). [DOI] [PubMed] [Google Scholar]
- 75.Friberg L, Olesen J, Iversen HK & Sperling B Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet 338, 13–17 (1991). [DOI] [PubMed] [Google Scholar]
- 76.Weiller C et al. Brain stem activation in spontaneous human migraine attacks. Nat Med 1, 658–660 (1995). [DOI] [PubMed] [Google Scholar]
- 77.Bahra A, Matharu MS, Buchel C, Frackowiak RS & Goadsby PJ Brainstem activation specific to migraine headache. Lancet 357, 1016–1017 (2001). [DOI] [PubMed] [Google Scholar]
- 78.Afridi SK et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 128, 932–939, doi: 10.1093/brain/awh416 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Arkink EB et al. Cerebral perfusion changes in migraineurs: a voxelwise comparison of interictal dynamic susceptibility contrast MRI measurements. Cephalalgia 32, 279–288, doi: 10.1177/0333102411435985 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cucchiara B & Detre J Migraine and circle of Willis anomalies. Med Hypotheses 70, 860–865, doi: 10.1016/j.mehy.2007.05.057 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Paemeleire K, Proot P, De Keyzer K, Achten E & Crevits L Magnetic resonance angiography of the circle of Willis in migraine patients. Clin Neurol Neurosurg 107, 301–305, doi: 10.1016/j.clineuro.2004.09.017 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Bugnicourt JM et al. Incomplete posterior circle of willis: a risk factor for migraine? Headache 49, 879–886 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Cavestro C et al. Anatomical variants of the circle of willis and brain lesions in migraineurs. Can J Neurol Sci 38, 494–499 (2011). [DOI] [PubMed] [Google Scholar]
- 84.Cucchiara B et al. Migraine with aura is associated with an incomplete circle of willis: results of a prospective observational study. PLoS One 8, e71007, doi: 10.1371/journal.pone.0071007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ezzatian-Ahar S et al. Migraine without aura is not associated with incomplete circle of Willis: a case-control study using high-resolution magnetic resonance angiography. J Headache Pain 15, 27, doi: 10.1186/1129-2377-15-27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Afridi SK et al. A positron emission tomographic study in spontaneous migraine. Arch Neurol 62, 1270–1275, doi: 10.1001/archneur.62.8.1270 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Denuelle M, Fabre N, Payoux P, Chollet F & Geraud G Hypothalamic activation in spontaneous migraine attacks. Headache 47, 1418–1426, doi: 10.1111/j.1526-4610.2007.00776.x (2007). [DOI] [PubMed] [Google Scholar]
- 88.Matharu MS et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain 127, 220–230, doi: 10.1093/brain/awh022 (2004). [DOI] [PubMed] [Google Scholar]
- 89.Raskin NH, Hosobuchi Y & Lamb S Headache may arise from perturbation of brain. Headache 27, 416–420 (1987). [DOI] [PubMed] [Google Scholar]
- 90.Veloso F, Kumar K & Toth C Headache secondary to deep brain implantation. Headache 38, 507–515, doi: 10.1046/j.1526-4610.1998.3807507.x (1998). [DOI] [PubMed] [Google Scholar]
- 91.Haas DC, Kent PF & Friedman DI Headache caused by a single lesion of multiple sclerosis in the periaqueductal gray area. Headache 33, 452–455 (1993). [DOI] [PubMed] [Google Scholar]
- 92.Goadsby PJ Neurovascular headache and a midbrain vascular malformation: evidence for a role of the brainstem in chronic migraine. Cephalalgia 22, 107–111, doi: 10.1046/j.1468-2982.2002.00323.x (2002). [DOI] [PubMed] [Google Scholar]
- 93.Maniyar FH, Sprenger T, Monteith T, Schankin C & Goadsby PJ Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 137, 232–241, doi: 10.1093/brain/awt320 (2014). [DOI] [PubMed] [Google Scholar]
- 94.Maniyar FH, Sprenger T, Schankin C & Goadsby PJ The origin of nausea in migraine-a PET study. J Headache Pain 15, 84, doi: 10.1186/1129-2377-15-84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boulloche N et al. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 81, 978–984, doi: 10.1136/jnnp.2009.190223 (2010). [DOI] [PubMed] [Google Scholar]
- 96.Martin H et al. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache 51, 1520–1528, doi: 10.1111/j.1526-4610.2011.02013.x (2011). [DOI] [PubMed] [Google Scholar]
- 97.Datta R, Aguirre GK, Hu S, Detre JA & Cucchiara B Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia 33, 365–374, doi: 10.1177/0333102412474503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hougaard A et al. Interhemispheric differences of fMRI responses to visual stimuli in patients with side-fixed migraine aura. Hum Brain Mapp 35, 2714–2723, doi: 10.1002/hbm.22361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stankewitz A, Voit HL, Bingel U, Peschke C & May A A new trigemino-nociceptive stimulation model for event-related fMRI. Cephalalgia 30, 475–485, doi: 10.1111/j.1468-2982.2009.01968.x (2010). [DOI] [PubMed] [Google Scholar]
- 100.Russo A et al. Abnormal thalamic function in patients with vestibular migraine. Neurology 82, 2120–2126, doi: 10.1212/WNL.0000000000000496 (2014). [DOI] [PubMed] [Google Scholar]
- 101.Russo A et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol 259, 1903–1912, doi: 10.1007/s00415-012-6438-1 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Stankewitz A & May A Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology 77, 476–482, doi: 10.1212/WNL.0b013e318227e4a8 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Russo A et al. Functional interictal changes of pain processing in migraine with ictal cutaneous allodynia. Cephalalgia 37, 305–314, doi: 10.1177/0333102416644969 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Schulte LH, Allers A & May A Hypothalamus as a mediator of chronic migraine: Evidence from high-resolution fMRI. Neurology 88, 2011–2016, doi: 10.1212/WNL.0000000000003963 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Schulte LH & May A The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 139, 1987–1993, doi: 10.1093/brain/aww097 (2016). [DOI] [PubMed] [Google Scholar]
- 106.Schwedt TJ et al. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia 34, 947–958, doi: 10.1177/0333102414526069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Colombo B, Rocca MA, Messina R, Guerrieri S & Filippi M Resting-state fMRI functional connectivity: a new perspective to evaluate pain modulation in migraine? Neurol Sci 36 Suppl 1, 41–45, doi: 10.1007/s10072-015-2145-x (2015). [DOI] [PubMed] [Google Scholar]
- 108.Tessitore A et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain 14, 89, doi: 10.1186/1129-2377-14-89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Russo A et al. Executive resting-state network connectivity in migraine without aura. Cephalalgia 32, 1041–1048, doi: 10.1177/0333102412457089 (2012). [DOI] [PubMed] [Google Scholar]
- 110.Tessitore A et al. Abnormal Connectivity Within Executive Resting-State Network in Migraine With Aura. Headache 55, 794–805, doi: 10.1111/head.12587 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Mainero C, Boshyan J & Hadjikhani N Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 70, 838–845, doi: 10.1002/ana.22537 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao L et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain 14, 85, doi: 10.1186/1129-2377-14-85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moulton EA, Becerra L, Johnson A, Burstein R & Borsook D Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS One 9, e95508, doi: 10.1371/journal.pone.0095508 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hadjikhani N et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia 33, 1264–1268, doi: 10.1177/0333102413490344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shin JH, Kim YK, Kim HJ & Kim JS Altered brain metabolism in vestibular migraine: comparison of interictal and ictal findings. Cephalalgia 34, 58–67, doi: 10.1177/0333102413498940 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Kim JH et al. Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia 30, 53–61, doi: 10.1111/j.1468-2982.2009.01890.x (2010). [DOI] [PubMed] [Google Scholar]
- 117.Barbiroli B et al. Complicated migraine studied by phosphorus magnetic resonance spectroscopy. Cephalalgia 10, 263–272, doi: 10.1046/j.1468-2982.1990.1005263.x (1990). [DOI] [PubMed] [Google Scholar]
- 118.Barbiroli B et al. Abnormal brain and muscle energy metabolism shown by 31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology 42, 1209–1214 (1992). [DOI] [PubMed] [Google Scholar]
- 119.Lodi R et al. Deficient energy metabolism is associated with low free magnesium in the brains of patients with migraine and cluster headache. Brain Res Bull 54, 437–441 (2001). [DOI] [PubMed] [Google Scholar]
- 120.Montagna P et al. 31P-magnetic resonance spectroscopy in migraine without aura. Neurology 44, 666–669 (1994). [DOI] [PubMed] [Google Scholar]
- 121.Reyngoudt H, Paemeleire K, Descamps B, De Deene Y & Achten E 31P-MRS demonstrates a reduction in high-energy phosphates in the occipital lobe of migraine without aura patients. Cephalalgia 31, 1243–1253, doi: 10.1177/0333102410394675 (2011). [DOI] [PubMed] [Google Scholar]
- 122.Schulz UG et al. Association between cortical metabolite levels and clinical manifestations of migrainous aura: an MR-spectroscopy study. Brain 130, 3102–3110, doi: 10.1093/brain/awm165 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Uncini A et al. Abnormal brain and muscle energy metabolism shown by 31P-MRS in familial hemiplegic migraine. J Neurol Sci 129, 214–222 (1995). [DOI] [PubMed] [Google Scholar]
- 124.Bridge H et al. Altered neurochemical coupling in the occipital cortex in migraine with visual aura. Cephalalgia 35, 1025–1030, doi: 10.1177/0333102414566860 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez de la Aleja J et al. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache 53, 365–375, doi: 10.1111/head.12030 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Siniatchkin M et al. Abnormal changes of synaptic excitability in migraine with aura. Cereb Cortex 22, 2207–2216, doi: 10.1093/cercor/bhr248 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Aguila ME et al. Elevated levels of GABA+ in migraine detected using (1) H-MRS. NMR Biomed 28, 890–897, doi: 10.1002/nbm.3321 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Dichgans M, Herzog J, Freilinger T, Wilke M & Auer DP 1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1. Neurology 64, 608–613, doi: 10.1212/01.WNL.0000151855.98318.50 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Fayed N, Andres E, Viguera L, Modrego PJ & Garcia-Campayo J Higher glutamate+glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad Radiol 21, 1211–1217, doi: 10.1016/j.acra.2014.04.009 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Sarchielli P et al. Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage 24, 1025–1031, doi: 10.1016/j.neuroimage.2004.11.005 (2005). [DOI] [PubMed] [Google Scholar]
- 131.Gu T, Ma XX, Xu YH, Xiu JJ & Li CF Metabolite concentration ratios in thalami of patients with migraine and trigeminal neuralgia measured with 1H-MRS. Neurol Res 30, 229–233, doi: 10.1179/016164107X235473 (2008). [DOI] [PubMed] [Google Scholar]
- 132.Zielman R et al. Biochemical changes in the brain of hemiplegic migraine patients measured with 7 tesla 1H-MRS. Cephalalgia 34, 959–967, doi: 10.1177/0333102414527016 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Hamel E Serotonin and migraine: biology and clinical implications. Cephalalgia 27, 1293–1300, doi: 10.1111/j.1468-2982.2007.01476.x (2007). [DOI] [PubMed] [Google Scholar]
- 134.Aurora SK, Barrodale PM, Tipton RL & Khodavirdi A Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache 47, 996–1003; discussion 1004–1007, doi: 10.1111/j.1526-4610.2007.00853.x (2007). [DOI] [PubMed] [Google Scholar]
- 135.Chabriat H et al. 5HT2 receptors in cerebral cortex of migraineurs studied using PET and 18F-fluorosetoperone. Cephalalgia 15, 104–108; discussion 177, doi: 10.1046/j.1468-2982.1995.015002104.x (1995). [DOI] [PubMed] [Google Scholar]
- 136.Chugani DC et al. Increased brain serotonin synthesis in migraine. Neurology 53, 1473–1479 (1999). [DOI] [PubMed] [Google Scholar]
- 137.Demarquay G et al. Brainstem changes in 5-HT1A receptor availability during migraine attack. Cephalalgia 31, 84–94, doi: 10.1177/0333102410385581 (2011). [DOI] [PubMed] [Google Scholar]
- 138.Russo A, Tessitore A, Giordano A, Salemi F & Tedeschi G The pain in migraine beyond the pain of migraine. Neurol Sci 33 Suppl 1, S103–106, doi: 10.1007/s10072-012-1052-7 (2012). [DOI] [PubMed] [Google Scholar]


