ABSTRACT
Noninferiority randomized controlled trial (RCT) effectiveness may erode when results favor the active control over time and when a decreasingly effective control arm is used in serial trials. We analyzed 32 antifungal noninferiority RCTs (NI-RCTs) for these scenarios in this secondary analysis of a systematic review. Our exploratory analysis suggests that the erosion risk in the effectiveness of antifungal noninferiority trials is uncommon. Findings are limited by small sample size and overall risk of bias.
KEYWORDS: antifungal agents, anti-infective agents, noninferiority trials, systematic review, biocreep
INTRODUCTION
Since the 1990s, antimicrobial randomized controlled trials (RCTs) are typically conducted with a noninferiority design (1). This design implies that a treatment is considered at least equivalent to the comparator if its efficacy is no worse than the comparator by a predefined margin. Over time, serial noninferiority trials (NI-RCTs) may lead to loss of efficacy because of inherent weaknesses to this design in certain scenarios.
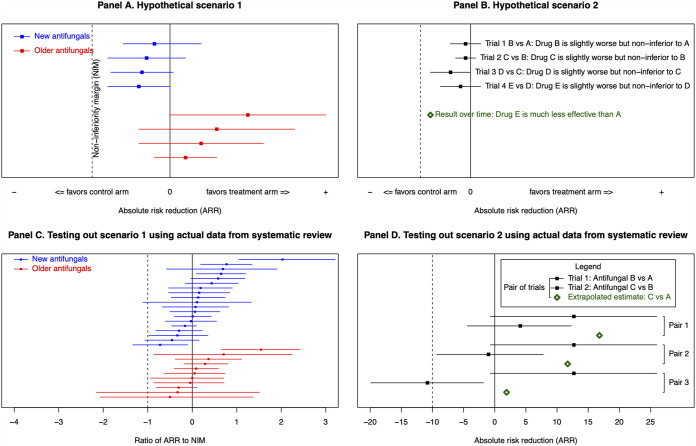
In “scenario 1” (Fig. 1a), the constancy assumption – that the treatment arm would be superior if tested in a placebo-controlled trial – is exploited. If results favor the control arm or the selected noninferiority margin (NIM) is too large, novel drugs appear falsely noninferior to older ones in serial trials.
FIG 1.
(A) In scenario 1, trials of novel antifungals violate the constancy assumption by selecting noninferiority margins that are too large or by results that favor the control arm. This leads to novel antifungal treatment effects that are consistently worse than older trials. (B) Scenario 2 illustrates the effect of biocreep in serial noninferiority trials. The antifungal used as the treatment arm in trial 1 becomes the control arm in trial 2, and so on. However, when the treatment arm in each successive trial is noninferior but the ARR < 0, the result is that over time the control effectiveness erodes to be no better than placebo. The green diamond in this panel represents the extrapolated ARR estimate for a trial comparing drug E to drug A when biocreep occurs in successive pairs of noninferiority trials. (C) Testing out scenario 1 using data from the systematic review. Only studies with a 95% CI are included in this panel. The ARR is divided by the NIM, since it allows the proportional risk reduction to be visualized. New antifungal trials (blue) represented in this panel, in order from top to bottom: Cornely 2007 (8), Reboli 2007 (27), Kohno 2010 (14), van Burik 2004 (30), Mora-Duarte 2002 (24), Rex 1994 (28), Chosidow 2003 (7), Maertens 2016 (20), Pappas 2007 (25), de la Paz Cota 2018 (9), Walsh 1999 (32), Walsh 2004 (34), Küse 2007 (18), de Wet 2005 (10), Krause 2004 (15), Vazquez 2010 (31), Kullberg 2005 (16), Walsh 2002 (33), and Kullberg 2019 (17). Older antifungal trials (red) represented in this panel, in order from top to bottom: Marks 2011 (21), Jeong 2016 (12), Molloy 2018 (23), Saliba 2015 (29), Le 2017 (19), Yim 2010 (35), Mersal 2013 (22), Kang 2020 (13), Huang 2012 (11), Yoshida 2020 (36), and Benjamin 2018 (5). Data from Buechner 2014 (6) and Queiroz-Telles 2008 (26) are not included in figure, as NIMs were published on a scale not amenable to comparison with other studies. (D) Testing out scenario 2 using data from serial noninferiority trials in the systematic review. The extrapolated point estimate is calculated by: ARR(B versus A) trial 1 + ARR(C versus B) trial 2 = ARR(C versus A). Pair 1 represented in this panel, from top to bottom, is Mora-Duarte 2002 (24) and the 100 mg micafungin arm of Pappas 2007 (25). Pair 2 represented in this panel, from top to bottom, is Mora-Duarte 2002 (24) and the 150 mg micafungin arm of Pappas 2007 (25). Pair 3 represented in this panel, from top to bottom, is Mora-Duarte 2002 (24) and Kullberg 2019 (17). ARR = absolute risk reduction; NIM = noninferiority margin. Figure adapted from Bai et al., with panels A and B reproduced with permission (2).
“Scenario 2” (Fig. 1b) requires successive NI-RCTs where the treatment in trial A becomes the control in trial B, and the treatment in trial B becomes the control in trial C, etc. If the treatment arms of each successive trial are statistically noninferior but in fact slightly inferior, the control arm's effectiveness degrades over time until it is equivalent to placebo, a phenomenon termed “biocreep.”
Our prior antibiotic NI-RCT analysis showed biocreep is rare (2). In this secondary analysis of a systematic review (40) we examined biocreep in antifungal NI-RCTs.
This study was reported per PRISMA guidelines (Text S1a, b) (3) and prospectively registered (PROSPERO CRD42020219497).
Medline, Embase, Cochrane Central, and the FDA drugs database were searched without language restrictions from inception to September 9, 2020 (Text S2). Retrieved article reference lists were screened.
We included NI-RCTs in humans comparing ≥2 antifungal regimens used for prophylaxis of an invasive fungal infection; or to treat a possible, probable, or proven fungal infection. Studies with invasive fungal infection (IFI) populations were included if the participants met the 2019 EORTC/MSG IFI consensus definition, regardless of publication year. Novel antifungal studies were defined as those conducted within 5 years of the FDA approval date for the treatment arm, whereas older antifungal studies were those occurring >5 years after – cutoffs chosen for consistency with prior literature (2). Phase I, II, and superiority design RCTs were excluded.
All assessments were performed independently in duplicate. Year of study, study centers, population, treatment and control arms, sample size calculation, NIM, and outcomes were captured. Risk of bias assessments were performed using the Cochrane RoB 2.0 tool. Disagreements were resolved by adjudication.
The coprimary outcomes were the occurrences of a consistently worse treatment effect in studies of novel antifungals compared to older ones (“scenario 1”); and of a decreasingly effective control arm in serial NI-RCTs (“scenario 2”).
We used previously described statistical analysis methods (2, 4). For scenario 1, study outcomes were converted to absolute risk reduction (ARR), where ARR <0 means the treatment arm is less effective than control. ARR was calculated as: Failure ratetreatment - failure ratecontrol. 95% confidence intervals were calculated using Miettinen and Nurminen's method. Fisher's exact test compared the proportion of novel antifungal studies with an ARR >0 to older antifungal studies. The ARR was divided by the NIM to allow visualization of a standardized proportional risk reduction across studies. Wilcoxon's rank-sum test compared novel and older antifungal ARRs.
For scenario 2, noninferiority trial pairs for the same infectious disease were identified where the treatment arm in an earlier trial was the subsequent control arm in a more recent trial. An extrapolated ARR estimate for the treatment arm in the later trial versus the control arm for the earlier trial was calculated: ARRantifungal B versus A in trial 1 + ARRantifungal C versus B in trial 2.
Statistical tests were two-sided, with statistical significance defined as P < 0.05. Analysis was performed using R, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). Meta-analysis was not performed due to heterogeneity. Post hoc subgroup analyses stratified by systemic treatment, systemic prophylaxis, and topical treatment NI-RCTs were performed to ensure results were not influenced by including these study types.
Of 700 screened abstracts, 44 full texts were assessed, and 32 full texts were included (Fig. S1; for studies excluded at full text, Table S1) (5–36). Study characteristics and consensus extracted data set are described in Table 1 and Table S2, respectively.
TABLE 1.
Study characteristics
| Characteristic | Novel antifungala studies (n = 21) | Older antifungalb studies (n = 11) |
|---|---|---|
| Study population exclusively adults | 14 | 7 |
| Multicentre | 21 | 11 |
| Pharmaceutical industry funding | 21 | 6 |
| Treatment arm median (IQR) | 200.5 (131.5-183.25) | 91.5 (64.75-183.25) |
| Infectious disease syndrome | ||
| Aspergillosis | 1 | 0 |
| Cutaneous mycosis | 0 | 1 |
| Febrile neutropenia | 3 | 2 |
| Invasive candidiasis (including candidemia) | 11 | 1 |
| Prophylaxis in nontransplant patients | 1 | 1 |
| Prophylaxis in transplant patients | 1 | 4 |
| Other | 5 | 2 |
| Method of diagnosis | ||
| Antigen detection | 1 | 1 |
| Expert opinion based on IFI consensus definition | 5 | 6 |
| Culture (excluding IFI)c | 12 | 1 |
| Microscopy | 1 | 1 |
| Other | 2 | 2 |
| Primary outcome | ||
| Clinical outcome | 7 | 4 |
| Microbiologic outcome | 0 | 2 |
| Composite clinical and microbiologic outcome | 14 | 5 |
| Reporting of adverse events | 21 | 11 |
| Conclusion by authors | ||
| Noninferiority shown | 14 | 8 |
| Superiority shown | 4 | 2 |
| Inferiority shown | 3 | 0 |
| Inconclusive | 0 | 1 |
Novel antifungal studies are defined as studies undertaken within 5 years of the FDA approval date of the antifungal in question.
Older antifungal studies are defined as studies undertaken ≥5 years from FDA approval date for the antifungal in the trial's treatment arm.
As culture is part of, but not singularly responsible for, the EORTC/MSG diagnostic criteria for IFI, culture for other fungal infections such as candidemia or superficial dermatophyte infection has been separated from that used as part of the diagnostic criteria for EORTC/MSG-defined IFI.
54.5% of older antifungal studies had an ARR > 0, compared with 61.9% of novel antifungal studies (Fig. 1c). The median ARR/NIM for older and novel antifungal studies was both 0.089 (IQR, older antifungal studies: −0.045–0.365; IQR, novel antifungal studies: −0.086–0.473).
Three pairs of serial NI-RCTs, all with invasive candidiasis populations, were found (Fig. 1d, Table S3). No pair had an ARR < 0 in both constituent trials; the probability of biocreep was low. Subgroup analyses stratified by systemic treatment, systemic prophylaxis, and topical prophylaxis NI-RCTs (Fig. S2) suggest results are not influenced by including these study types.
Overall risk of bias was low in 9.4%, drew some concern in 40.6%, and was high in 50% of included studies (Fig. S3). Risk of bias within individual studies is described in Table S4.
Noninferiority trials rely on assumptions of assay sensitivity – the ability of the trial to distinguish effective from ineffective treatment – and choice of an appropriate NIM and analysis set (37). They may therefore be at risk of eroding effectiveness over time. Our prior study of antibiotic RCTs (2) was congruent with others (38, 39) that biocreep is rare. Our current study suggests biocreep may be similarly uncommon with antifungal RCTs.
Our study has limitations: we examined many clinically heterogeneous syndromes, which may introduce noise. Biocreep conclusions are limited by small sample size, with three pairs of serial trials identified, all in invasive candidiasis populations. This small number of serial trials limits our conclusions to an exploratory analysis which should be interpreted cautiously. These conclusions may not be generalizable to other fungal populations. Trials were at moderate to high risk of overall bias.
Our systematic review suggests antifungal NI-RCTs may be at low risk of eroding effectiveness, supporting their continued use.
ACKNOWLEDGMENTS
We thank Neera Bhatnagar at the McMaster University Library for guiding our search strategy.
We declare no funding.
We report that we do not have any conflicts of interest in the public, commercial, or nonprofit sectors relevant to this publication.
ASK, DLL, DM, and DY conceived and designed the study. ASK, AC, and OM performed abstract screening. ASK, ADB, AC, OM, CKLL, XXL, VM, AF, DBD, and CF performed data extraction from full text. ASK and ADB performed the analysis, and ASK wrote a first draft of the manuscript. All authors reviewed the full data set prior to publication. All authors reviewed and revised the manuscript and approved a final version to be submitted for publication.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Infectious Diseases Society of America. 2012. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis 55:1031–1046. 10.1093/cid/cis688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai AD, Komorowski AS, Lo CKL, Tandon P, Li XX, Mokashi V, Cvetkovic A, Findlater A, Liang L, Loeb M, Mertz D. 2020. Novel antibiotics may be noninferior but are they becoming less effective? A systematic review. Antimicrob Agents Chemother 64:e01597-20. 10.1128/AAC.01597-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aberegg SK, Hersh AM, Samore MH. 2018. Do non-inferiority trials of reduced intensity therapies show reduced effects? A descriptive analysis. BMJ Open 8:e019494. 10.1136/bmjopen-2017-019494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin DK, Kaufman DA, Hope WW, Smith PB, Arrieta A, Manzoni P, Kovanda LL, Lademacher C, Isaacson B, Jednachowski D, Wu C, Kaibara A, Walsh TJ. 2018. A phase 3 Study of micafungin versus amphotericin B deoxycholate in infants with invasive candidiasis. Pediatr Infect Dis J 37:992–998. 10.1097/INF.0000000000001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buechner SA. 2014. Multicenter, double-blind, parallel group study investigating the non-inferiority of efficacy and safety of a 2% miconazole nitrate shampoo in comparison with a 2% ketoconazole shampoo in the treatment of seborrhoeic dermatitis of the scalp. J Dermatolog Treat 25:226–231. 10.3109/09546634.2013.782092. [DOI] [PubMed] [Google Scholar]
- 7.Chosidow O, Maurette C, Dupuy P. 2003. Randomized, open-labeled, non-inferiority study between ciclopiroxolamine 1% cream and ketoconazole 2% foaming gel in mild to moderate facial seborrheic dermatitis. Dermatology (Dermatology) 206:233–240. 10.1159/000068904. [DOI] [PubMed] [Google Scholar]
- 8.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh Y-T, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. Fluconazole or Itraconazole Prophylaxis in Patients with Neutropenia. N Engl J Med 356:348–359. 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 9.de la Paz Cota BR, Cepero Vega PP, Matus Navarrete JJ, Aguado Mulgado GE, Narváez Huerta JJ, Lamadrid Bautista E, Fiscal Chauteco E. 2018. Efficacy and safety of eberconazole 1% otic solution compared to clotrimazole 1% solution in patients with otomycosis. Am J Otolaryngol 39:307–312. 10.1016/j.amjoto.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 10.de Wet NTE, Bester AJ, Viljoen JJ, Filho F, Suleiman JM, Ticona E, Llanos EA, Fisco C, Lau W, Buell D. 2005. A randomized, double blind, comparative trial of micafungin (FK463) vs. fluconazole for the treatment of oesophageal candidiasis. Aliment Pharmacol Ther 21:899–907. 10.1111/j.1365-2036.2005.02427.x. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Chen H, Han M, Zou P, Wu D, Lai Y, Huang H, Chen X, Liu T, Zhu H, Wang J, Hu J. 2012. Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant 18:1509–1516. 10.1016/j.bbmt.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Jeong SH, Kim DY, Jang JH, Mun Y-C, Choi CW, Kim S-H, Kim JS, Park JS. 2016. Efficacy and safety of micafungin versus intravenous itraconazole as empirical antifungal therapy for febrile neutropenic patients with hematological malignancies: a randomized, controlled, prospective, multicenter study. Ann Hematol 95:337–344. 10.1007/s00277-015-2545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang W-H, Song G-W, Lee S-G, Suh K-S, Lee K-W, Yi N-J, Joh JW, Kwon CHD, Kim JM, Choi DL, Kim JD, Kim MS. 2020. A multicenter, randomized, open-label study to compare micafungin with fluconazole in the prophylaxis of invasive fungal infections in living-donor liver transplant recipients. J Gastrointest Surg 24:832–840. 10.1007/s11605-019-04241-w. [DOI] [PubMed] [Google Scholar]
- 14.Kohno S, Izumikawa K, Ogawa K, Kurashima A, Okimoto N, Amitani R, Kakeya H, Niki Y, Miyazaki Y, Japan Chronic Pulmonary Aspergillosis Study Group (JCPASG) . 2010. Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: a multicenter trial in Japan. J Infect 61:410–418. 10.1016/j.jinf.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Krause DS, Simjee AE, van Rensburg C, Viljoen J, Walsh TJ, Goldstein BP, Wible M, Henkel T. 2004. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin Infect Dis 39:770–775. 10.1086/423378. [DOI] [PubMed] [Google Scholar]
- 16.Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, Cleary JD, Rubinstein E, Church LWP, Brown JM, Schlamm HT, Oborska IT, Hilton F, Hodges MR. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet (London, England) 366:1435–1442. 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 17.Kullberg BJ, Viscoli C, Pappas PG, Vazquez J, Ostrosky-Zeichner L, Rotstein C, Sobel JD, Herbrecht R, Rahav G, Jaruratanasirikul S, Chetchotisakd P, Van Wijngaerden E, De Waele J, Lademacher C, Engelhardt M, Kovanda L, Croos-Dabrera R, Fredericks C, Thompson GR. 2019. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: the ACTIVE trial. Clin Infect Dis 68:1981–1989. 10.1093/cid/ciy827. [DOI] [PubMed] [Google Scholar]
- 18.Kuse E-R, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, Sekhon JS, Freire A, Ramasubramanian V, Demeyer I, Nucci M, Leelarasamee A, Jacobs F, Decruyenaere J, Pittet D, Ullmann AJ, Ostrosky-Zeichner L, Lortholary O, Koblinger S, Diekmann-Berndt H, Cornely OA. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet (London, England) 369:1519–1527. 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 19.Le T, Kinh NV, Cuc NTK, Tung NLN, Lam NT, Thuy PTT, Cuong DD, Phuc PTH, Vinh VH, Hanh DTH, Tam VV, Thanh NT, Thuy TP, Hang NT, Long HB, Nhan HT, Wertheim HFL, Merson L, Shikuma C, Day JN, Chau NVV, Farrar J, Thwaites G, Wolbers M. 2017. A trial of itraconazole or amphotericin B for HIV-associated talaromycosis. N Engl J Med 376:2329–2340. 10.1056/NEJMoa1613306. [DOI] [PubMed] [Google Scholar]
- 20.Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, Baddley JW, Giladi M, Heinz WJ, Herbrecht R, Hope W, Karthaus M, Lee D-G, Lortholary O, Morrison VA, Oren I, Selleslag D, Shoham S, Thompson GR, Lee M, Maher RM, Schmitt-Hoffmann A-H, Zeiher B, Ullmann AJ. 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet (London, England) 387:760–769. 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 21.Marks DI, Pagliuca A, Kibbler CC, Glasmacher A, Heussel C‐P, Kantecki M, Miller PJS, Ribaud P, Schlamm HT, Solano C, Cook G, for the IMPROVIT Study Group . 2011. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol 155:318–327. 10.1111/j.1365-2141.2011.08838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mersal A, Alzahrani I, Azzouz M, Alsubhi A, Alsawaigh H, Albshri N, Bajammal M, Avand G, Almahbosh A. 2013. Oral nystatin versus intravenous fluconazole as neonatal antifungal prophylaxis: non-inferiority trial. J Clin Neonatol 2:88–92. 10.4103/2249-4847.116408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molloy SF, Kanyama C, Heyderman RS, Loyse A, Kouanfack C, Chanda D, Mfinanga S, Temfack E, Lakhi S, Lesikari S, Chan AK, Stone N, Kalata N, Karunaharan N, Gaskell K, Peirse M, Ellis J, Chawinga C, Lontsi S, Ndong J-G, Bright P, Lupiya D, Chen T, Bradley J, Adams J, van der Horst C, van Oosterhout JJ, Sini V, Mapoure YN, Mwaba P, Bicanic T, Lalloo DG, Wang D, Hosseinipour MC, Lortholary O, Jaffar S, Harrison TS, ACTA Trial Study Team . 2018. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 378:1004–1017. 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 24.Mora-Duarte J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, Lupinacci R, Sable C, Kartsonis N, Perfect J, Caspofungin Invasive Candidiasis Study Group . 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 347:2020–2029. 10.1056/NEJMoa021585. [DOI] [PubMed] [Google Scholar]
- 25.Pappas PG, Rotstein CMF, Betts RF, Nucci M, Talwar D, De Waele JJ, Vazquez JA, Dupont BF, Horn DL, Ostrosky-Zeichner L, Reboli AC, Suh B, Digumarti R, Wu C, Kovanda LL, Arnold LJ, Buell DN. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 45:883–893. 10.1086/520980. [DOI] [PubMed] [Google Scholar]
- 26.Queiroz-Telles F, Berezin E, Leverger G, Freire A, van der Vyver A, Chotpitayasunondh T, Konja J, Diekmann-Berndt H, Koblinger S, Groll AH, Arrieta A. 2008. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J 27:820–826. 10.1097/INF.0b013e31817275e6. [DOI] [PubMed] [Google Scholar]
- 27.Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ, Anidulafungin Study Group . 2007. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 356:2472–2482. 10.1056/NEJMoa066906. [DOI] [PubMed] [Google Scholar]
- 28.Rex JH, Bennett JE, Sugar AM, Pappas PG, van der Horst CM, Edwards JE, Washburn RG, Scheld WM, Karchmer AW, Dine AP. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med 331:1325–1330. 10.1056/NEJM199411173312001. [DOI] [PubMed] [Google Scholar]
- 29.Saliba F, Pascher A, Cointault O, Laterre P-F, Cervera C, De Waele JJ, Cillo U, Langer RM, Lugano M, Göran-Ericzon B, Phillips S, Tweddle L, Karas A, Brown M, Fischer L, TENPIN Liver Transplant European Study Into the Prevention of Fungal Infection Investigators . 2015. Randomized trial of micafungin for the prevention of invasive fungal infection in high-risk liver transplant recipients. Clin Infect Dis 60:997–1006. 10.1093/cid/ciu1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Burik J-AH, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, Vesole DH, Bunin N, Wall DA, Hiemenz JW, Satoi Y, Lee JM, Walsh TJ, for the National Institute of Allergy and Infectious Diseases Mycoses Study Group . 2004. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 39:1407–1416. 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez JA, Patton LL, Epstein JB, Ramlachan P, Mitha I, Noveljic Z, Fourie J, Conway B, Lalla RV, Barasch A, Attali P, SMiLES Study Group . 2010. Randomized, comparative, double-blind, double-dummy, multicenter trial of miconazole buccal tablet and clotrimazole troches for the treatment of oropharyngeal candidiasis: study of miconazole Lauriad efficacy and safety (SMiLES). HIV Clin Trials 11:186–196. 10.1310/hct1104-186. [DOI] [PubMed] [Google Scholar]
- 32.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, Schuster M, Dismukes WE, Holcenberg JS. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med 340:764–771. 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 33.Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, Yanovich S, Stiff P, Greenberg R, Donowitz G, Schuster M, Reboli A, Wingard J, Arndt C, Reinhardt J, Hadley S, Finberg R, Laverdière M, Perfect J, Garber G, Fioritoni G, Anaissie E, Lee J. 2002. Voriconazole compared with liposomal amphotericin b for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med 346:225–234. 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 34.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, Cornely OA, Bourque MR, Lupinacci RJ, Sable CA, dePauw BE. 2004. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med 351:1391–1402. 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 35.Yim SM, Ko JH, Lee YW, Kim HW, Lee JY, Kim NI, Kye YC, Park KC, Choi JH, Lee KH, Kim MN, Kim KJ, Ro YS, Ahn KJ. 2010. Study to compare the efficacy and safety of fluconazole cream with flutrimazole cream in the treatment of superficial mycosis: a multicentre, randomised, double-blind, phase III trial. Mycoses 53:522–529. 10.1111/j.1439-0507.2009.01738.x. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida I, Saito AM, Tanaka S, Choi I, Hidaka M, Miyata Y, Inoue Y, Yamasaki S, Kagoo T, Iida H, Niimi H, Komeno T, Yoshida C, Tajima F, Yamamoto H, Takase K, Ueno H, Shimomura T, Sakai T, Nakashima Y, Yoshida C, Kubonishi S, Sunami K, Yoshida S, Sakurai A, Kaneko Y, Miyazaki Y, Nagai H. 2020. Intravenous itraconazole compared with liposomal amphotericin B as empirical antifungal therapy in patients with neutropaenia and persistent fever. Mycoses 63:794–801. 10.1111/myc.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumi J, Wittes JT. 2011. Through the looking glass: understanding non-inferiority. Trials 12:106. 10.1186/1745-6215-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everson-Stewart S, Emerson SS. 2010. Bio-creep in non-inferiority clinical trials. Stat Med 29:2769–2780. 10.1002/sim.4053. [DOI] [PubMed] [Google Scholar]
- 39.Odem-Davis K, Fleming TR. 2015. A simulation study evaluating bio-creep risk in serial non-inferiority clinical trials for preservation of effect. Stat Biopharm Res 7:12–24. 10.1080/19466315.2014.1002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komorowski AS, Bai AD, Cvetkovic A, Mourad O, Lo CKL, Li XX, Mokashi V, Findlater A, Duncan DB, Fuller C, Yamamura Y, Mertz D, for the McMaster Infectious Diseases Fellow Research Group . 2021. Methodological and reporting quality of non-inferiority randomized controlled trials comparing antifungal therapies: a systematic review. Clin Microbiol Infect 7:12–24. 10.1016/j.cmi.2021.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.01627-21-s0001.pdf, PDF file, 0.6 MB (603.3KB, pdf)