ABSTRACT
Telacebec (Q203) is a potent drug candidate under clinical development for the treatment of drug-naïve and drug-resistant tuberculosis. The first-in-human randomized, placebo-controlled, double-blind, dose-escalation Phase 1A trial (Q203-TB-PI-US001) was conducted to evaluate the safety, tolerability, and pharmacokinetics of telacebec. A total of 56 normal, healthy, male and female subjects (42 active and 14 placebo) were enrolled in the study. The doses of telacebec were 10 mg (Cohort 1), 30 mg (Cohort 2), 50 mg (Cohort 3), 100 mg (Cohort 4), 200 mg (Cohort 5), 400 mg (Cohort 6), and 800 mg (Cohort 7) in a fasted state. Subjects participating in Cohort 4 were also enrolled in Cohort 8 to investigate the food effect on the pharmacokinetics of telacebec after a high-fat meal. In all subjects dosed with telacebec (10 to 800 mg), telacebec was well tolerated and did not lead to any significant or serious adverse events. Following a single oral administration of telacebec (10 to 800 mg), telacebec plasma concentration reached the maximal plasma concentration (Cmax) in average 2.0 to 3.5 h and showed multi-exponential decline thereafter. The area under the plasma concentration versus time curve (AUC) was approximately dose-proportional. A significant increase in plasma concentrations was observed in the fed condition compared with the fasted condition with the geometric mean ratio of 3.93 for Cmax. Moderate delay in Tmax (4.5 h) was also observed in the fed condition. These results, combined with the demonstrated activity against drug-sensitive and multidrug-resistant Mycobacterium tuberculosis, support further investigation of telacebec for the treatment of tuberculosis.
KEYWORDS: telacebec, Q203, tuberculosis, safety, tolerability, pharmacokinetics, phase 1 trial
TEXT
Tuberculosis is a severe and life-threatening infectious disease that infects and kills more than a million lives globally every year (1, 2). Tuberculosis, when untreated, has an approximately 70% mortality rate in sputum smear-positive people and is estimated to have killed one billion people worldwide in the past two centuries. Thus, it has been considered as one of the most critical public health threats of the 21st century. According to the World Health Organization (WHO), tuberculosis remains one of the top 10 causes of death and the top infections killer worldwide, with a total death of 1.4 million from tuberculosis in 2019 (3).
Although public health approaches have saved a lot of lives from tuberculosis, the progress in the fight against tuberculosis is not satisfactory toward ending tuberculosis. A new challenge facing countries around the world is the advent of multi-drug-resistant tuberculosis and extensively drug-resistant tuberculosis (1, 2). In 2019, it was estimated that there were approximately half a million new cases of tuberculosis resistant to rifampin, of which 78% were multi-drug-resistant tuberculosis (3). The rise of these drug-resistant strains of tuberculosis can be attributed to the improper or discontinued use of first-line antibiotic treatments before their completion. Treatment for drug-resistant tuberculosis and multi-drug-resistant tuberculosis takes a longer time and requires more expensive and toxic drugs. While the treatment regimens for drug-susceptible tuberculosis needed 6 months and cost approximately $860 per patient, the cost per patient for multi-drug-resistant tuberculosis was approximately $5,659 in 2019 and took 6–20 months (3). Many factors contribute to the spread of multi-drug-resistant and extensively drug-resistant tuberculosis. Among others, some of the most important factors include a lack of rapid, cheap, point-of-care diagnostics to diagnose multi-drug-resistant tuberculosis and insufficient second-line drug options (1).
Moreover, the current COVID-19 pandemic threatens to undermine the global efforts to combat tuberculosis. The impact of the COVID-19 pandemic on tuberculosis services has been severe, which worsens the tuberculosis epidemics worldwide (3, 4). In 2020, there were sharp decreases in tuberculosis case notifications, which may, in turn, exacerbate the mortality of tuberculosis (3). It is evident that the COVID-19 pandemic has imposed an additional burden on public health systems and weakens the national tuberculosis services. Evidence also suggests the potential biological interaction between COVID-19 and tuberculosis (5, 6), and research is ongoing for a better understanding of the effect of COVID-19 on the treatment outcomes of patients with tuberculosis (4).
Telacebec, previously known as Q203, is a novel first-in-class drug candidate for the treatment of tuberculosis with the proof of efficacy achieved in humans (7). Telacebec blocks the growth of Mycobacterium tuberculosis (M. tuberculosis) by inhibiting the cytochrome bc1 complex, resulting in the inhibition of ATP synthesis (8). The potency of telacebec has been well demonstrated by in vitro as well as in vivo studies (8, 9). Telacebec inhibited the growth of multi-drug-resistant and extensively drug-resistant M. tuberculosis clinical isolates in the low nanomolar range, which were otherwise resistant to standard anti-microbial treatment. It also showed activity in the in vivo murine model of tuberculosis at a dose less than 1 mg/kg. Moreover, in the recently completed Phase 2 clinical trial, telacebec reduced vial mycobacterial sputum load in a dose-dependent manner, demonstrating the activity of telacebec in patients with tuberculosis (7).
Pharmacokinetics of telacebec in humans, however, have not been reported so far. This is the first report of the clinical pharmacokinetics, safety, and tolerability of telacebec in healthy human volunteers following administration at escalating doses of telacebec in Phase 1 clinical trial. This study would provide useful information for further studies to evaluate telacebec as a promising antituberculosis agent in various nonclinical and clinical development.
RESULTS
Subjects.
A total of 56 healthy male and female volunteers were enrolled in the present study to investigate the safety, tolerability, and pharmacokinetics of telacebec, with 42 subjects receiving telacebec and 14 receiving placebos. Eight subjects who received 100 mg in fasted condition (Cohort 4) returned for 100 mg in fed state to assess food effect (Cohort 8). The demographic features and baseline characteristics of the subjects are summarized in Table 1. Most of the subjects were black/African American (39 of 56; 69.6%) or white (15 of 56; 26.8%). The average age of the subjects was 38.1 ± 9.1 years with an average body mass index (BMI) of 26.2 ± 2.5 kg/m2 across the treatment cohorts.
TABLE 1.
Baseline demographicsa
| Statistic | Placebo (n = 12) | 10 mg (n = 6) | 30 mg (n = 6) | 50 mg (n = 6) | 200 mg (n = 6) | 400 mg (n = 6) | 800 mg (n = 6) | Fasted vs fed |
|
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 2) | 100 mg (n = 6) | ||||||||
| Age, yrs | 38.3 (9.2) | 43.5 (8.0) | 39.3 (9.2) | 32.2 (5.8) | 36.7 (7.0) | 34.3 (8.6) | 34.5 (11.2) | 35.5 (14.9) | 46.2 (7.4) |
| Gender, male:female | 9:3 | 4:2 | 4:2 | 5:1 | 4:2 | 4:2 | 5:1 | 2:0 | 4:2 |
| Ethnicity, n (%) | |||||||||
| Hispanic or Latino | 2 (16.7) | 0 | 0 | 0 | 1 (16.7) | 1 (16.7) | 0 | 0 | 3 (50.0) |
| Not Hispanic or Latino | 10 (83.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 5 (83.3) | 5 (83.3) | 6 (100.0) | 2 (100.0) | 3 (50.0) |
| Race, n (%) | |||||||||
| White | 4 (33.3) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 0 | 0 | 4 (66.7) |
| American Indian or Alaska Native | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| Asian | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 |
| Black or African American | 8 (66.7) | 5 (83.3) | 3 (50.0) | 5 (83.3) | 3 (50.0) | 5 (83.3) | 6 (100.0) | 2 (100.0) | 2 (33.3) |
| Height, cm | 176.9 (6.5) | 174.2 (7.9) | 170.5 (8.4) | 173.8 (8.2) | 171.8 (8.4) | 172.7 (13.1) | 179.0 (8.0) | 174.5 (2.1) | 173.2 (7.2) |
| Weight, kg | 81.3 (8.0) | 80.0 (10.8) | 76.5 (4.8) | 82.6 (13.5) | 75.3 (7.1) | 77.9 (17.3) | 80.2 (10.2) | 78.3 (10.3) | 82.4 (8.1) |
| BMI, kg/m2 | 26.0 (2.5) | 26.3 (2.3) | 26.4 (1.6) | 27.2 (3.0) | 25.5 (2.2) | 26.0 (3.9) | 25.0 (2.1) | 25.7 (2.8) | 27.5 (2.2) |
Data are mean (standard deviation) unless otherwise specified; BMI, body mass index.
Safety and tolerability.
Telacebec was well tolerated after single oral administration at doses from 10 mg to 800 mg in the present study. There were no serious adverse events, deaths, or discontinuations due to adverse events. No systematic or drug-related effects on 12-lead electrocardiogram (ECG) parameters such as heart rate, QT, and corrected QT were reported. There were no clinically significant findings in the safety assessments of vital signs, including heart rate, blood pressure, temperature, or respiration during the study. Headache was the most common adverse event across all dose cohorts, followed by elevated abdominal discomfort. The adverse events, i.e., headache and abdominal discomfort, were less noted in subjects taking placebo. Mild headache was experienced by one subject (7.1%) in the placebo fasted cohort, one subject (16.7%) each in the 10 mg cohort and 200 mg cohort, and two subjects (33.3%) in the 800 mg cohort. Moderate headache was experienced by two subjects (33.3%) in the 100 mg fasted cohort. Abdominal discomfort was reported by one subject (16.7%) in the 100 mg fasted cohort.
Pharmacokinetics.
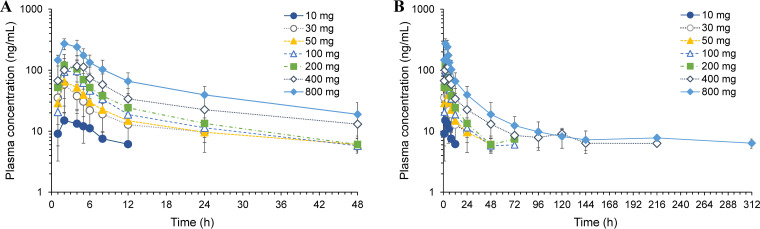
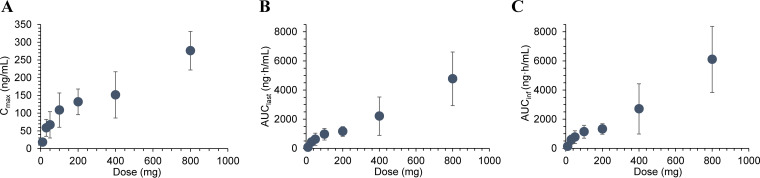
Average plasma concentrations of telacebec versus time profile following a single oral dose administration of telacebec at 10–800 mg in the fasted state are shown in Fig. 1. The non-compartmental pharmacokinetic parameters of telacebec are summarized in Table 2. Following the oral dose of telacebec, telacebec was rapidly absorbed, and the plasma concentration of telacebec reached the maximum plasma concentrations (Cmax) within an average 2.0–3.5 h after drug administration and showed a multi-exponential decline after that. As shown in Fig. 1A, all the plasma concentrations were below the lower limit of quantification (BLQ) from 24 h after the drug administration at 10 mg of telacebec (Cohort 1). The time at which 100% of samples showed BLQ was 48 h for 30 mg (Cohort 2), 72 h for 50 mg (Cohort 3), 96 h for 100 mg (Cohort 4) and 200 mg (Cohort 5), and 312 h for 400 mg (Cohort 6) after administration of telacebec. Plasma concentration was detected until the last observed time, i.e., 312 h after administration of telacebec at 800 mg (Cohort 7) and 100 mg at fed state (Cohort 8). Thus, the terminal phases in the plasma concentration-time profile were not fully characterized in the lower dose cohorts, i.e., 10 mg–50 mg. The estimated apparent terminal half-life (t1/2) of telacebec ranged from 21.13 h (100 mg dose group) to 150.79 h (800 mg dose group) with the increase of telacebec dose. The average half-life estimated from 100 mg to 800 mg dose cohort was 62.50 ± 71.86 h. The mean Cmax increased with the dose and ranged from 18.0 ng/mL to 276.0 ng/mL. In parallel, the area under the plasma concentration versus time curve (AUC) increased with the dose increase (Fig. 2). The mean AUC from time zero to last measured concentration (AUClast) and AUC from time zero to infinity (AUCinf) increased from 70.55 ng·h/mL to 4768.11 ng·h/mL and from 160.79 ng·h/mL to 6116.62 ng·h/mL, respectively, as telacebec dose increased from 10 mg to 800 mg.
FIG 1.
Plasma concentration versus time profiles (A) for 48 h and (B) for 312 h after escalating single dose administration of telacebec from 10 mg to 800 mg in the fasted state. Data represent the mean ± SD (n = 6).
TABLE 2.
Pharmacokinetic parameters of telacebec (Q203) following oral administration of telacebec (Q203-TB-PI-US001)a
| Fasting state | Cohort | Dose (mg) | Tmax (h) | t1/2 (h) | Cmax (ng/mL) | AUClast (ng·h/mL) | AUCinf (ng·h/mL) |
|---|---|---|---|---|---|---|---|
| Fasted | 1 (n = 6) | 10 | 2.17 ± 0.98 | 6.32 ± 1.64 | 18.03 ± 6.93 | 70.55 ± 44.74 | 160.79 ± 38.14 |
| 2 (n = 6) | 30 | 2.00 ± 0.00 | 11.42 ± 6.48 | 58.52 ± 23.94 | 430.12 ± 225.82 | 590.38 ± 314.50 | |
| 3 (n = 6) | 50 | 2.33 ± 0.82 | 15.81 ± 3.14 | 66.97 ± 37.08 | 618.79 ± 417.76 | 764.65 ± 437.55 | |
| 4 (n = 6) | 100 | 3.00 ± 1.10 | 21.13 ± 6.73 | 108.62 ± 48.42 | 965.89 ± 397.09 | 1138.57 ± 444.12 | |
| 5 (n = 6) | 200 | 2.67 ± 1.03 | 18.60 ± 5.81 | 131.85 ± 36.30 | 1156.98 ± 331.82 | 1332.94 ± 355.34 | |
| 6 (n = 6) | 400 | 3.50 ± 1.64 | 59.47 ± 51.41 | 151.50 ± 65.30 | 2206.56 ± 1317.82 | 2715.14 ± 1721.38 | |
| 7 (n = 6) | 800 | 2.67 ± 1.03 | 150.79 ± 85.44 | 276.00 ± 54.41 | 4768.11 ± 1842.01 | 6116.62 ± 2272.77 | |
| Fed | 8 (n = 6) | 100 | 4.50 ± 1.22 | 321.12 ± 227.29 | 435.00 ± 201.51 | 5407.79 ± 2257.65 | 8814.22 ± 4146.16 |
Data represent the mean ± SD.
FIG 2.
Systemic exposure represented by (A) Cmax, (B) AUClast, and (C) AUCinf versus dose of telacebec after single administration of telacebec in the fasted state. Data represent the mean ± SD (n = 6).
Dose proportional increase of the systemic exposure to telacebec was observed with respect to AUClast over the dose range of 10 mg to 800 mg with the slope estimate of 0.867 [95% confidence interval (CI); 0.730–1.004]. AUCinf and Cmax also increased with the dose with the slope estimate of 0.717 [95% CI; 0.585–0.850] and 0.560 [95% CI; 0.457–0.664], respectively. Dose proportionality was thus statistically significant only for AUClast over the entire telacebec dose range of 10 mg to 800 mg. For AUCinf, dose proportionality was statistically significant over the range of 100 mg to 800 mg with the slope estimate of 0.808 [95% CI; 0.514–1.101]. Dose proportionality was not statistically significant for Cmax.
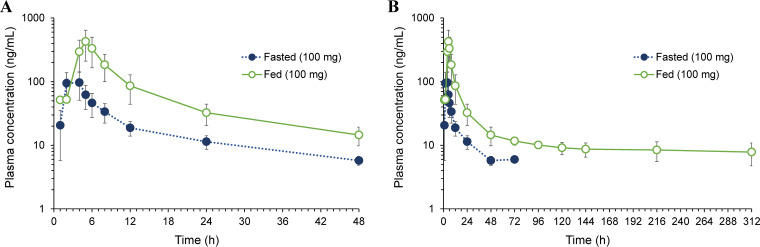
The plasma concentration versus time profiles following oral administration of telacebec at 100 mg in the fed state compared to that in the fasted state are shown in Fig. 3. The non-compartmental pharmacokinetic parameters of telacebec in the fed state are summarized in Table 2. In the fed state, plasma concentrations of telacebec were significantly higher than those in the fasted state. The peak plasma concentration (Cmax) was observed at an average Tmax of 4.5 h following a single 100 mg dose of telacebec in the fed state. While telacebec was not detected in the plasma for all subjects after 96 h of drug administration at 100 mg in the fasted state with t1/2 of 21.13 ± 6.73 h, the plasma concentration of telacebec after administration of 100 mg in the fed state exhibited a multi-exponential decline with the extended t1/2 of 321.12 ± 227.29 h. The mean Cmax in the fed state was 435.0 ng/mL, which was significantly higher than that in the fasted state, i.e., 108.6 ng/mL. Significantly higher AUClast and AUCinf were found in the fed state compared with fasted state. Compared with the fasted state, the geometric mean ratio for Cmax in the fed state was 3.93 [90% CI; 2.60–5.92]. The geometric mean ratio was 5.22 [90% CI; 2.73–10.01] for AUClast and 6.74 [90% CI; 3.02–15.04] for AUCinf, indicating the increased bioavailability of telacebec when food is administered.
FIG 3.
Plasma concentration versus time profiles (A) for 48 h and (B) for 312 h after administration of telacebec (100 mg) under fasted and fed conditions. Data represent the mean ± SD (n = 6).
DISCUSSION
A Phase 1A, randomized, placebo-controlled, double blind, dose-escalation study (Q203-TB-PI-US001) evaluated the safety, tolerability, and pharmacokinetics of telacebec in healthy subjects. In 42 normal, healthy, male and female volunteers administered telacebec at 10–800 mg, telacebec was well tolerated without any serious adverse events. Following oral administration, telacebec was relatively rapidly observed and the exposure was approximately dose-proportional. Exposure to telacebec was significantly increased in the fed condition compared to in the fasted condition.
Telacebec (Q203) is a potent drug candidate with the minimum concentration to inhibit the growth of 50% of organisms (MIC50) of 2.7 nM in cultured broth medium and 0.28 nM inside macrophages (8). Excellent killing profile of telacebec was observed in chronic M. tuberculosis infection models (8, 10). In an acute mouse model of tuberculosis, telacebec promoted a reduction in bacterial load of more than 90% at a dose of 10 mg/kg, which is comparable to that of bedaquiline or isoniazid. Favorable nonclinical pharmacokinetic properties, including excellent oral bioavailability (90.7%) and long half-life (11), also supported the further evaluation of telacebec in clinical trials.
Consistent with the results from nonclinical studies, telacebec showed good safety and pharmacokinetic profiles in the present clinical trial in healthy human volunteers. There were no serious or severe adverse events reported after telacebec administration in the present study. Since the conventional treatment duration is usually more than half a year, the safety profile is the most important property for a new drug development for tuberculosis. Particularly, potential cardiac toxicity associated with QT prolongation is a recognized safety concern for antituberculosis drugs (12, 13). Thus, it is encouraging that no abnormalities in ECG was observed after telacebec administration in this study. The low risk for cardiotoxicity of telacebec has also been indicated by the preclinical results that telacebec did not inhibit hERG potassium channel (8).
The maximum plasma levels (Cmax) of telacebec observed in this study after a single oral administration at 10–800 mg in the fasted and 100 mg in the fed states were approximately 12.0- to 289.2-fold higher than MIC50 determined in cultured broth medium, 2.7 nM (8). The average plasma concentration of telacebec after 24 h of drug administration also remained above the MIC50, i.e., 6.4–26.2 times higher, except for the lowest dose. These findings indicate that the systemic exposure to telacebec is sufficient to exhibit its activity against tuberculosis following a once daily dosing regimen. After oral administration at doses of 10–800 mg, the absorption of telacebec was relatively rapid with the mean Tmax of 2.00–3.50 h. The systemic exposure represented by AUClast and AUCinf was approximately dose-proportional, and dose proportionality was statistically significant over the dose range of 10–800 mg for AUClast and 100–800 mg for AUCinf (Fig. 2). Extended terminal half-life (t1/2) was observed in the high dose cohorts, which is likely associated with the multi-exponential decline of plasma concentration (Fig. 1). Due to the limitation of the analytical sensitivity, the multiexponential pattern is less distinct in the lower dose cohort, resulting in the variable apparent terminal half-life estimates depending on the dose. However, the terminal half-life may represent only a minimal fraction of the total clearance of the drug, which is less significant to predict the accumulation upon multiple dosing (14). The operational or effective half-life may be comparable among different dose cohorts, which will be confirmed by further studies following multiple administrations. The half-life of telacebec was estimated to be 10.92–15.24 h by using the plasma concentration-time data from time zero to 24 h (Fig. 1A).
In the fed condition, the systemic exposure to telacebec significantly increased compared to that in the fasted state (Fig. 3). Moderate delay in Tmax was also observed in the fed state compared to the fasted state (4.50 ± 1.22 h versus 3.00 ± 1.10 h). However, despite the increased exposure, there were no safety differences between fasted and fed groups. Food may affect the pharmacokinetics of a drug via various mechanisms including delaying gastric emptying, altering the pH of the gastrointestinal tract, stimulating bile flow, changing metabolism of a drug, and physicochemical interaction with the drug. However, the low potential for drug–drug or drug–food interaction of telacebec has been indicated by in vitro metabolism studies. It has been shown that telacebec was not an inhibitor of any cytochrome P450 (CYP450) nor an inducer of human pregnane X receptor (hPXR) (8, 11). Moreover, telacebec was not a substrate or an inhibitor of P-glycoprotein (8, 11). Thus, the enhanced absorption of telacebec may be attributed to the favorable gastrointestinal environment for dissolution and solubility by stimulating the bile flow in the fed state. Preliminary dissolution studies using the biorelevant dissolution media also indicated that the rate and extent of telacebec dissolution increased in the fed state simulated intestinal fluid (FeSSIF) compared to those in the fasted state simulated intestinal fluid (FaSSIF). Further nonclinical animal studies may provide a better understanding of the food effect on the pharmacokinetics of telacebec.
In conclusion, telacebec was well tolerated after oral administration at doses as high as 800 mg with no safety concerns. The pharmacokinetics of telacebec was generally dose-proportional and suitable for a once-a-day dosing regimen. These results, combined with the activity of telacebec demonstrated in vitro and in vivo studies, support the further investigation of telacebec for the promising antitubercular drug candidate. Other nonclinical studies are also under way to characterize the effect and interactions of telacebec in novel drug combinations (8). Moreover, the potential benefits of telacebec in other indications such as Buruli ulcer and viral acute respiratory distress syndrome (ARDS) have been of recent interest. The present results may provide valuable information for further evaluation of telacebec in various nonclinical and clinical studies.
MATERIALS AND METHODS
Study design.
A Phase 1 study (Q203-TB-PI-US001) was a randomized, double-blind, placebo-controlled, and single escalating dose study of telacebec. The ascending doses of telacebec and matching placebo were administered to separate groups of telacebec-naïve subjects. The doses of telacebec were 10 mg (Cohort 1), 30 mg (Cohort 2), 50 mg (Cohort 3), 100 mg (Cohort 4), 200 mg (Cohort 5), 400 mg (Cohort 6), and 800 mg (Cohort 7) in a fasted state (Period 1) and 100 mg with food (Cohort 8, Period 2). Subjects who received 100 mg in fasted condition (Cohort 4) in Period 1 returned after 6 weeks of washout period and received 100 mg in fed condition to assess the food effect (Cohort 8) in Period 2. In each cohort, eight subjects were randomized 6:2 to receive either telacebec or placebo, respectively. The study protocols were approved by institutional review boards (IRB) and the study was conducted at Parexel in Baltimore, MD.
Participants.
Normal, healthy, male and female subjects were enrolled in the present study. All subjects provided written informed consents before initiation of the study. The participants were aged 19 to 55 years, and in good health with no clinically significant findings from their medical history, clinical laboratory assessments, 12-lead electrocardiograms (ECG), and physical examination. Subjects were excluded if they reported use of any prescription or over-the-counter drug, herbal products, vitamin supplements, drug-metabolizing enzyme-altering drug (inducers or inhibitors), or supplement (e.g., St. John’s wort) within 14 days before drug administration and throughout the study. Subjects who had taken any known QT-prolonging agents within 28 days before drug administration and throughout the study were also excluded. Subject safety was ensured by monitoring adverse events, vital signs, 12-lead ECG, and standard clinical laboratory tests including serum chemistry, hematology, coagulation, and urinalysis.
Sampling.
Blood samples were collected before dosing (0 h) and at 1, 2, 4, 5, 6, 8, 12, 24, 48, 72, 96, 120, 144, 216, and 312 h after telacebec administration for the determination of plasma concentrations of telacebec. Plasma samples were obtained by centrifugation of the collected blood samples and stored at −20°C until analysis.
Clinical laboratory assessments (serum chemistry, hematology, and urinalysis) were performed at screening, check-in (Day −1), Day 2, Day 8, Day 14, and Day 16. A coagulation test was performed at screening, check-in (Day −1), Days 1–16, 21, 28, and 44. Vital signs were measured at screening, Day −1, each day on Days 1–14 (predose [within 2 h prior to dosing], 1, 2, 4, 6, 8, 12, and 16 h after dosing), plus Day 15 and Day 16. Triplicate 12-lead ECG readings were collected at screening, Day −1, each day on Days 1–14 (predose [within 2 h prior to dosing], 2, 4, 6, and 8 h after dosing), plus Day 16. In addition, triplicate ECG readings were extracted from the Holter monitor on Days 1, 7, and 14 at scheduled times. Full physical examinations were performed at screening, Day −1, predose, and Days 2, 8, 14, and 16.
Bioanalytical methods.
Plasma concentrations of telacebec were determined by using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method by using PerkinElmer Series 200 Micro Pump equipped with an AB SCIEX API 4000 triple quadrupole mass spectrometer. D8-Q203 was used as an internal standard. The electrospray ionization source was operated in a positive mode. The mass spectrometric analysis was performed in the multiple reaction monitoring mode. The standard curve range was from 5 to 5,000 ng/mL for telacebec using a sample volume of 0.025 mL. The lower limit of quantitation (LLOQ) was 5.0 ng/mL. The intra- and inter-batch accuracy (% bias) was −2.7 to 11.2% and −0.7 to 5.0%, respectively. The intra- and inter-batch precisions were all within 8.82%.
Pharmacokinetic analysis.
The pharmacokinetic parameters of telacebec were determined by non-compartmental analysis using Phoenix WinNonlin Version 6.3 (Certara, L.P., Princeton, NJ). The following pharmacokinetic parameters were calculated: terminal half-life (t1/2), area under the plasma concentration versus time curve from time zero to the last measurable non-zero concentration (AUClast), and the area under the plasma concentration versus time curve from time zero to infinity (AUCinf). Maximum plasma concentration (Cmax) and the time to reach Cmax (Tmax) were directly obtained from the observed plasma concentration versus time data.
Statistical analysis.
Dose proportionality was tested for the pharmacokinetic parameters of Cmax, AUClast, and AUCinf for telacebec by applying a power model (15). It was assumed that the natural logarithm of the pharmacokinetic parameter is linearly related to the natural logarithm of dose as in the following equation: ln(PK parameter) = b0 + b1·ln(dose). The slope coefficient (b1) and its two-sided 95% confidence intervals (CI) were estimated via linear regression. The dose proportionality was accepted if the 95% CI of the slope coefficient (b1) contained 1.00.
To test the significance of the food effect, natural log-transformed pharmacokinetic parameters of Cmax, AUClast, and AUCinf were evaluated separately using a linear mixed effects model with fixed effects terms for treatment condition, i.e., fasted or fed. An unstructured covariance matrix was used to allow for unequal treatment variances and model the correlation between the treatment measurements within each subject. Geometric means, ratio of geometric means, and their confidence 90% CI were estimated and presented on the original scale of measurements. An absence of food effect was concluded if the 90% CI for all comparisons were contained within the interval [0.8, 1.25].
ACKNOWLEDGMENT
Jeongjun Kim, Jinho Choi, Hwankyu Kang, Jiye Ahn, Jane Hutchings, Christo van Niekerk, Dongsik Park, Jaeseung Kim, Yeejin Jeon, and Kiyean Nam are employees of Qurient Co., Ltd.
Contributor Information
Soyoung Shin, Email: shins@wku.ac.kr.
Beom Soo Shin, Email: bsshin@skku.edu.
REFERENCES
- 1.Abubakar I, Zignol M, Falzon D, Raviglione M, Ditiu L, Masham S, Adetifa I, Ford N, Cox H, Lawn SD, Marais BJ, McHugh TD, Mwaba P, Bates M, Lipman M, Zijenah L, Logan S, McNerney R, Zumla A, Sarda K, Nahid P, Hoelscher M, Pletschette M, Memish ZA, Kim P, Hafner R, Cole S, Migliori GB, Maeurer M, Schito M, Zumla A. 2013. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis 13:529–539. 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 2.Gunther G. 2014. Multidrug-resistant and extensively drug-resistant tuberculosis: a review of current concepts and future challenges. Clin Med (Lond) 14:279–285. 10.7861/clinmedicine.14-3-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2020. Global tuberculosis report 2020. WHO, Geneva, Switzerland.
- 4.Visca D, Ong CWM, Tiberi S, Centis R, D'Ambrosio L, Chen B, Mueller J, Mueller P, Duarte R, Dalcolmo M, Sotgiu G, Migliori GB, Goletti D. 2021. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology 27:151–165. 10.1016/j.pulmoe.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadolini M, Codecasa LR, García-García J-M, Blanc F-X, Borisov S, Alffenaar J-W, Andréjak C, Bachez P, Bart P-A, Belilovski E, Cardoso-Landivar J, Centis R, D'Ambrosio L, Luiza De Souza-Galvão M, - Dominguez-Castellano A, Dourmane S, Fréchet Jachym M, Froissart A, Giacomet V, Goletti D, Grard S, Gualano G, Izadifar A, Le Du D, Marín Royo M, Mazza-Stalder J, Motta I, Ong CWM, Palmieri F, Rivière F, Rodrigo T, Silva DR, Sánchez-Montalvá A, Saporiti M, Scarpellini P, Schlemmer F, Spanevello A, Sumarokova E, Tabernero E, Tambyah PA, Tiberi S, Torre A, Visca D, Zabaleta Murguiondo M, Sotgiu G, Migliori GB. 2020. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J 56:2001398. 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motta I, Centis R, D'Ambrosio L, García-García J-M, Goletti D, Gualano G, Lipani F, Palmieri F, Sánchez-Montalvá A, Pontali E, Sotgiu G, Spanevello A, Stochino C, Tabernero E, Tadolini M, van den Boom M, Villa S, Visca D, Migliori GB. 2020. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology 26:233–240. 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jager VR, Dawson R, van Niekerk C, Hutchings J, Kim J, Vanker N, van der Merwe L, Choi J, Nam K, Diacon AH. 2020. Telacebec (Q203), a new antituberculosis agent. N Engl J Med 382:1280–1281. 10.1056/NEJMc1913327. [DOI] [PubMed] [Google Scholar]
- 8.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim SA, Nam J, Kang H, Kwon H, Oh CT, Cho Y, Jang Y, Kim J, Chua A, Tan BH, Nanjundappa MB, Rao SP, Barnes WS, Wintjens R, Walker JR, Alonso S, Lee S, Kim J, Oh S, Oh T, Nehrbass U, Han SJ, No Z, et al. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157–1160. 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 9.Lu P, Asseri AH, Kremer M, Maaskant J, Ummels R, Lill H, Bald D. 2018. The anti-mycobacterial activity of the cytochrome bcc inhibitor Q203 can be enhanced by small-molecule inhibition of cytochrome bd. Sci Rep 8:2625. 10.1038/s41598-018-20989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoagland DT, Liu J, Lee RB, Lee RE. 2016. New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv Drug Deliv Rev 102:55–72. 10.1016/j.addr.2016.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang S, Kim RY, Seo MJ, Lee S, Kim YM, Seo M, Seo JJ, Ko Y, Choi I, Jang J, Nam J, Park S, Kang H, Kim HJ, Kim J, Ahn S, Pethe K, Nam K, No Z, Kim J. 2014. Lead optimization of a novel series of imidazo[1,2-a]pyridine amides leading to a clinical candidate (Q203) as a multi- and extensively-drug-resistant anti-tuberculosis agent. J Med Chem 57:5293–5305. 10.1021/jm5003606. [DOI] [PubMed] [Google Scholar]
- 12.Guglielmetti L, Tiberi S, Burman M, Kunst H, Wejse C, Togonidze T, Bothamley G, Lange C. 2018. QT prolongation and cardiac toxicity of new tuberculosis drugs in Europe: a Tuberculosis Network European Trialsgroup (TBnet) study. Eur Respir J 52:1800537. 10.1183/13993003.00537-2018. [DOI] [PubMed] [Google Scholar]
- 13.Tadolini M, Lingtsang RD, Tiberi S, Enwerem M, Ambrosio L, Sadutshang TD, Centis R, Migliori GB. 2016. First case of extensively drug-resistant tuberculosis treated with both delamanid and bedaquiline. Eur Respir J 48:935–938. 10.1183/13993003.00637-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahin S, Benet LZ. 2008. The operational multiple dosing half-life: a key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm Res 25:2869–2877. 10.1007/s11095-008-9787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, Forgue ST. 2000. Confidence interval criteria for assessment of dose proportionality. Pharm Res 17:1278–1283. 10.1023/A:1026451721686. [DOI] [PubMed] [Google Scholar]