LETTER
With interest we read the Letter to the Editor of Wei and Sluis-Cremer about the role of human immunodeficiency virus type 1 (HIV-1) 3′-polypurine tract (3′PPT) mutations in dolutegravir (DTG) resistance (1). In particular, they report that the 3′PPT mutations identified by Wijting et al. (2) in a patient that failed on DTG monotherapy do not confer DTG resistance in a phenotypic drug resistance assay and conclude that genotypic analysis of the 3′PPT in HIV-1 resistance to integrase strand transfer inhibitors (INSTIs) is not warranted. However, we argue that it is too early for such a conclusion.
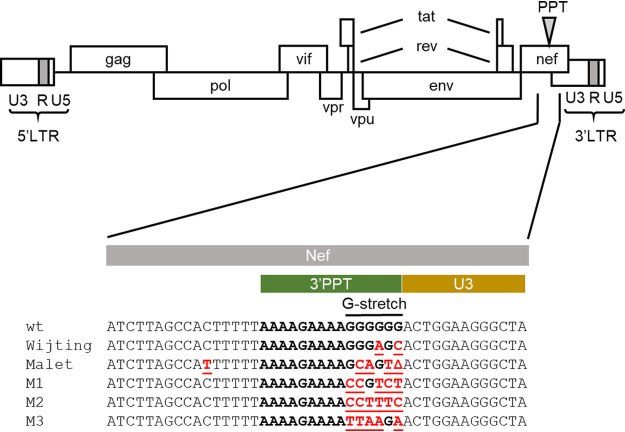
Previously, Malet et al. (3) selected an HIV-1 variant with a mutated 3′PPT upon long-term culturing of HIV-1 in the presence of DTG. Different mechanistic explanations have been suggested (4, 5). We recently identified several other 3′PPT-mutated viruses by creating a library of HIV-1 clones in which the G stretch of the 3′PPT sequence was randomized, followed by selection of replication-competent variants on the C8166 T-cell line in the presence of DTG (details to be presented elsewhere). The 3′PPT sequences in these viruses (M1 to M3) differ from those in the wild-type (wt) virus and the variants previously described by Malet et al. (3) and Wijting et al. (2) (Fig. 1).
FIG 1.
Mutations in the HIV-1 3′-polypurine tract (3′PPT) upon in vivo and in vitro dolutegravir (DTG) treatment. 3′PPT sequences observed upon DTG monotherapy (Wijting et al. [2]), upon long-term in vitro culturing with DTG (Malet et al. [3]) and upon a novel in vitro randomization/selection protocol (M1 to M3) are shown. The G stretch that was randomized in the latter analysis is indicated. Nucleotide mutations are indicated in red and underlined (Δ, nucleotide deletion).
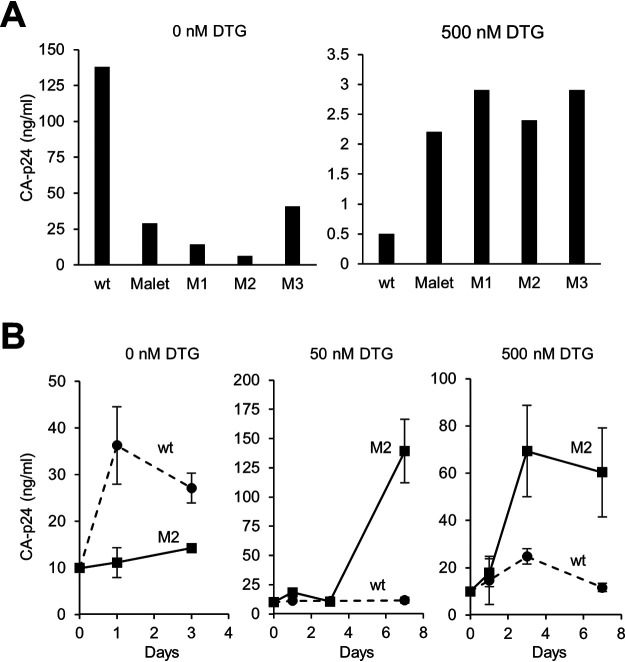
The selected 3′PPT sequences were cloned into the HIV-1 LAI molecular clone, and virus replication was analyzed in C8166 cells. Without DTG administration, wt LAI virus replicated more efficiently than all variants, resulting in a higher CA-p24 level in the culture supernatant (Fig. 2A, left). In contrast, when cultured with 500 nM DTG, infection resulted in higher CA-p24 levels for the variants compared to those in LAI (right), although the absolute levels were relatively low compared to values obtained without DTG. Replication of the M2 mutant at different DTG levels was also longitudinally compared with that of LAI upon infection with a high virus input (Fig. 2B). Again, the 3′PPT-mutated virus demonstrated reduced replication without DTG and improved replication with DTG compared to that of the wt virus. These results demonstrate that all selected 3′PPT mutations reduce viral fitness but improve replication in the presence of DTG.
FIG 2.
Replication of HIV-1 3′PPT variants. (A) C8166 cells were infected with an equal amount of the different viruses (corresponding to 1 ng CA-p24) and cultured without or with DTG. The CA-p24 level in the culture supernatant measured at 3 days (no DTG; left) or 5 days (500 nM DTG; right) after infection are shown. (B) C8166 cells were infected with a large amount of wild-type (wt) or M2 virus (corresponding to 10 ng CA-p24) and cultured with 0, 50, or 500 nM DTG. Infections were performed in triplicate, and the average CA-p24 level measured in the culture supernatant at different times after infection is shown (error bars indicate standard error).
As a phenotypic drug resistance test, we infected TZM-bl cells in the presence of different levels of DTG. These cells carry an HIV-1 Tat-inducible long terminal repeat (LTR)-luciferase reporter construct that allows quantification of Tat production as a measure of virus infectivity. As shown in Fig. 3A, infectivity was significantly reduced for all 3′PPT variants in the absence of DTG, which is in accordance with a profoundly reduced viral fitness. Interestingly, DTG (50 and 500 nM) reduced the infectivity of LAI more severely than the 3′PPT variants (Fig. 3B), which confirms their reduced sensitivity toward DTG. Notably, the residual activity of the wt virus at 500 nM DTG was similar to that of some of the 3′PPT variants in this assay, while replication of the wt virus was worse than that of all variants at this high DTG level in the replication assays (Fig. 2). This is likely due to the fact that the activity measured in this TZM-bl infection assay reflects Tat production from the viral DNA formed upon infection, which may not depend on integration, rather than that it reflects multiple complete cycles of virus replication.
FIG 3.
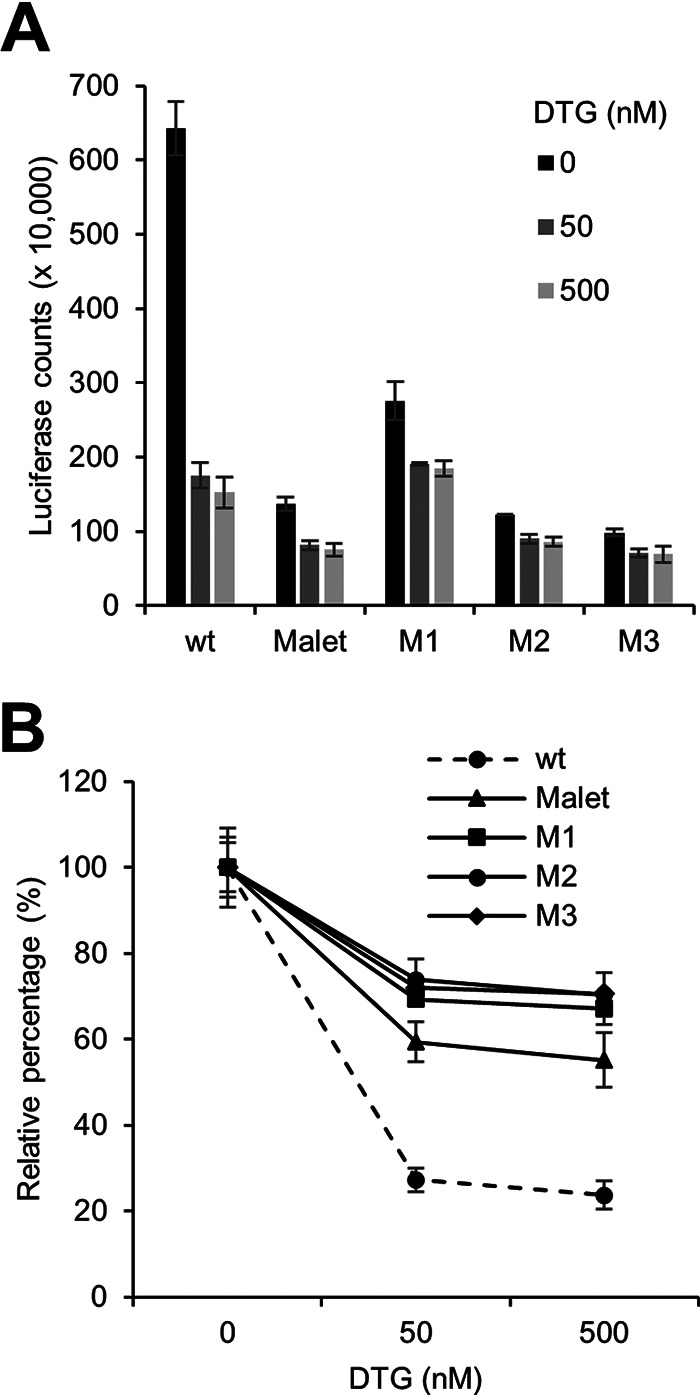
DTG sensitivity of 3′PPT variants. (A) TZM-bl cells were infected with equal amounts of wt and 3′PPT-mutated HIV-1 (0.25 ng CA-p24 virus) and cultured at different DTG concentrations. The Tat-induced luciferase activity was measured at 3 days after infection (n = 2), and average values (± standard error) are plotted. (B) Relative luciferase activity upon infection in the presence of DTG with the activity measured without DTG set at 100%.
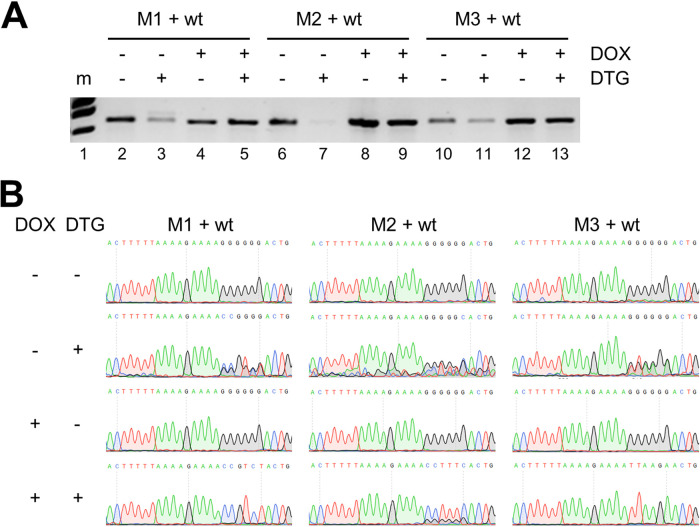
It was recently demonstrated that the human T-cell leukemia virus 1 (HTLV-1) Tax protein can rescue replication of integrase-deficient HIV in T cells (6, 7). Whereas the unintegrated HIV DNA, which forms episomal DNA circles, is normally silenced through epigenetic changes, HTLV-1 Tax was shown to induce recruitment of NF-κB to the viral LTR promoter region, which reverses epigenetic silencing and activates transcription (6, 7). As we selected our DTG-resistant 3′PPT-mutated HIV variants in C8166 cells, which are known to carry HTLV-1 sequences and express Tax (8), we investigated whether replication of these 3′PPT mutants was activated by Tax. We therefore compared replication of the wt and mutant viruses in CEM-SS T cells that carry a doxycycline (DOX)-inducible Tax gene (6). Cells were infected with an equal amount of the wt virus and one of the mutants (M1, M2, or M3) and cultured in the absence or presence of DTG and DOX. At 8 days after infection, when cytopathogenic effects due to virus replication were observed in all cultures except for the DTG-only culture, the intracellular DNA was isolated, and the 3′PPT region in the viral DNA was amplified by PCR (Fig. 4A). Analysis of the PCR products by gel electrophoresis showed a strongly reduced viral DNA level in cells cultured with only DTG compared to that in untreated cells (compare band intensity in lanes 3, 7, and 11 and lanes 2, 6, and 10, respectively, in Fig. 4A), indicating DTG inhibition of both wt and mutant virus replication. Coadministration of DOX, which activates Tax production, restored viral DNA production (lanes 5, 9, and 13). The PCR products were sequenced to determine the relative abundance of wt and mutant viruses in each culture. Without DTG and DOX, a wt 3′PPT sequence was obtained for all 3 mixed cultures (Fig. 4B), demonstrating a higher abundance of the wt virus and confirming the reduced fitness of the 3′PPT mutants. DOX-only administration did not change this pattern. Sequencing of the poorly detectable PCR product obtained for the DTG-only treated cultures yielded a mixed mutant/wt sequence. Probably, this PCR product resulted from the low level of viral DNA produced during reverse transcription of the input viruses rather than subsequent rounds of virus replication. Upon coadministration of DOX and DTG, the mutant 3′PPT sequence was observed for every mixed culture, demonstrating that Tax activated replication of the 3′PPT mutants but did not activate or activated significantly less wt virus in the presence of DTG.
FIG 4.
Tax-induced replication of 3′PPT-mutated HIV variants. (A) CEM-SS T cells that carry a doxycycline (DOX)-inducible Tax gene (6) were infected with an equal amount (corresponding to 2 ng CA-p24) of wt and 3′PPT-mutated HIV (M1, M2, or M3) and cultured with or without DOX (0.5 µg/ml) and DTG (500 nM). Intracellular DNA was isolated at 8 days after infection, and the 3′PPT region was amplified by PCR. PCR products were analyzed by agarose gel electrophoresis (A) and sequencing (B). m, DNA marker.
These results show that replication of the 3′PPT-mutated HIV variants, like that of the integrase-deficient virus (6), is activated by Tax. In the reverse transcription process, the 3′PPT normally dictates the start of the LTR U3 region and thereby the 5′ end of the viral DNA, which forms—together with the 3′ end—the substrate for integrase (4). Mutation of the 3′PPT may result in an alternative sequence at the 5′ end of the viral DNA that may prevent integration (4, 5). The nonintegrating viral DNA may form episomal circles, as observed for the integrase-deficient HIV (6), which could explain the observed Tax effect on replication. It should be noted that the DTG-resistant 3′PPT variant described by Malet et al. (3) was selected in HTLV-1-infected MT4 cells that, like C8166 cells, do express Tax (9).
Our results demonstrate that not only the 3′PPT mutations observed by Malet et al. but also several other 3′PPT mutations can confer DTG resistance, although these mutations do significantly reduce viral fitness. For the latter reason, Wei and Sluis-Cremer did not further analyze the effect of the mutations observed by Malet et al. on DTG resistance (1). Instead, they focused on the 3′PPT mutations observed upon virologic failure during DTG monotherapy reported by Wijting et al. (2), for which they demonstrate that the mutations neither reduce viral fitness nor increase DTG resistance significantly when recloned in the HIV-1 LAI background. Although no known INSTI resistance-associated mutations were detected in the integrase gene, the possibility cannot, however, be excluded that the patient’s virus had acquired additional mutations that contributed to DTG resistance. The 3′PPT sequence observed by Wijting et al. differs at only 2 nucleotide positions from the wt sequence, whereas both the DTG escape viruses described by Malet et al. (3) and by us deviate more from the wt sequence (Fig. 1). It thus seems possible that DTG resistance requires significant mutation of the 3′PPT, which likely affects the processes of HIV reverse transcription and integration, thus reducing viral fitness. We demonstrate that replication of the 3′PPT variants in T cells can be restored by the HTLV-1 Tax protein, but the possibility cannot be excluded that the virus can also improve replication capacity over time by acquiring compensatory mutations at the 3′PPT or elsewhere in the viral genome (10).
In conclusion, the data from Malet et al. (3) and this study indicate that several 3′PPT mutations can reduce DTG sensitivity, although such mutations by themselves may not be sufficient for virologic failure. As these findings are based on in vitro virus culture experiments, it remains important to continue the screening for similar DTG escape routes in patients. Further analysis of the mechanism by which 3′PPT mutations reduce viral fitness and increase DTG resistance is also important for a complete understanding of this potent drug class.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI147330.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Bryan Cullen and Hal Bogard (Duke University Medical Center, Durham, NC) for kindly providing us the CEM-SS cell line with DOX-controlled HTLV-1 Tax expression.
This letter is in memory of Charles Boucher.
Contributor Information
Ben Berkhout, Email: b.berkhout@amsterdamumc.nl.
Atze T. Das, Email: a.t.das@amsterdamumc.nl.
REFERENCES
- 1.Wei Y, Sluis-Cremer N. 2021. Mutations in the HIV-1 3′-polypurine tract and integrase strand-transfer inhibitor resistance. Antimicrob Agents Chemother 65:e02432-20. 10.1128/AAC.02432-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijting IEA, Lungu C, Rijnders BJA, van der Ende ME, Pham HT, Mesplede T, Pas SD, Voermans JJC, Schuurman R, van de Vijver DAMC, Boers PHM, Gruters RA, Boucher CAB, van Kampen JJA. 2018. HIV-1 resistance dynamics in patients with virologic failure to dolutegravir maintenance monotherapy. J Infect Dis 218:688–697. 10.1093/infdis/jiy176. [DOI] [PubMed] [Google Scholar]
- 3.Malet I, Subra F, Charpentier C, Collin G, Descamps D, Calvez V, Marcelin A-G, Delelis O. 2017. Mutations located outside the integrase gene can confer resistance to HIV-1 integrase strand transfer inhibitors. mBio 8:e00922-17. 10.1128/mBio.00922-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das AT, Berkhout B. 2018. How polypurine tract changes in the HIV-1 RNA genome can cause resistance against the integrase inhibitor dolutegravir. mBio 9:e00006-18. 10.1128/mBio.00006-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malet I, Subra F, Richetta C, Charpentier C, Collin G, Descamps D, Calvez V, Marcelin A-G, Delelis O. 2018. Reply to Das and Berkhout, “How polypurine tract changes in the HIV-1 RNA genome can cause resistance against the integrase inhibitor dolutegravir.” mBio 9:e00623-18. 10.1128/mBio.00623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irwan ID, Karnowski HL, Bogerd HP, Tsai K, Cullen BR. 2020. Reversal of epigenetic silencing allows robust HIV-1 replication in the absence of integrase function. mBio 11:e01038-20. 10.1128/mBio.01038-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwan ID, Cullen BR. 2021. Tax induces the recruitment of NF-κB to unintegrated HIV-1 DNA to rescue viral gene expression and replication. J Virol 95:e0028521. 10.1128/JVI.00285-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ongrádi J, Laird HM, Szilágyi JF, Horváth A, Bendinelli M. 2000. Unique morphological alterations of the HTLV-I transformed C8166 cells by infection with HIV-1. Pathol Oncol Res 6:27–37. 10.1007/BF03032655. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez MV, Delviks-Frankenberry KA, Scheiblin DA, Happel C, Pathak VK, Freed EO. 2019. Authentication analysis of MT-4 cells distributed by the National Institutes of Health AIDS Reagent Program. J Virol 93:e01390-19. 10.1128/JVI.01390-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Duyne R, Kuo LS, Pham P, Fujii K, Freed EO. 2019. Mutations in the HIV-1 envelope glycoprotein can broadly rescue blocks at multiple steps in the virus replication cycle. Proc Natl Acad Sci U S A 116:9040–9049. 10.1073/pnas.1820333116. [DOI] [PMC free article] [PubMed] [Google Scholar]