Abstract
Background
The Stockholm III trial randomly assigned 840 patients to short-course radiotherapy of 5 × 5 Gy with surgery within 1 week (SRT), short-course radiotherapy of 5 × 5 Gy with surgery after 4–8 weeks (SRT-delay), or long-course radiotherapy of 25 × 2 Gy with surgery after 4–8 weeks (LRT-delay). This study details the long-term oncological outcomes and health-related quality of life (HRQoL).
Methods
Patients with biopsy-proven resectable adenocarcinoma of the rectum were included. Primary outcome was time to local recurrence (LR), and secondary endpoints were distant metastases (DMs), overall survival (OS), recurrence-free survival (RFS), and HRQoL. Patients were analysed in a three-arm randomization and a short-course radiotherapy comparison.
Results
From 1998 to 2013, 357, 355, and 128 patients were randomized to the SRT, SRT-delay, and LRT-delay groups respectively. Median follow-up time was 5.7 (range 5.3–7.6) years. Comparing patients in the three-arm randomization, the incidence of LR was three of 129 patients, four of 128, and seven of 128, and DM 31 of 129 patients, 38 of 128, and 38 of 128 in the SRT, SRT-delay, and LRT-delay groups respectively. In the short-course radiotherapy comparison, the incidence of LR was 11 of 357 patients and 13 of 355, and DM 88 of 357 patients and 82 of 355 in the SRT and SRT-delay groups respectively. No comparisons showed statistically significant differences. Median OS was 8.1 (range 6.9–11.2), 10.3 (range 8.2–12.8), and 10.5 (range 7.0–11.3) years after SRT, SRT-delay, and LRT-delay respectively. Median OS was 8.1 (range 7.2–10.0) years after SRT and 10.2 (range 8.5–11.7) years after SRT-delay. There were no statistically significant differences in HRQoL.
Conclusion
After a follow-up of 5 years, delaying surgery for 4–8 weeks after radiotherapy treatment with 5 × 5 Gy was oncologically safe. Long-term HRQoL was similar among the treatment arms.
Trial registration number
NTC00904813
Analysis of long-term follow-up after radiotherapy and surgery for rectal cancer showed that delaying surgery for 4–8 weeks after radiotherapy treatment with 5 × 5 Gy seems oncologically safe. HRQoL does not seem to differ between treatment arms with radiotherapy and immediate surgery, compared with delayed surgery.
Introduction
Preoperative radiotherapy (RT) in rectal cancer is used to reduce local recurrences (LRs), with a demonstrable positive impact on overall survival (OS)1,2. Improved surgery, with the introduction of total mesorectal excision (TME), has improved outcomes and this has been enhanced by RT, reducing the rate of LRs by more than 50 per cent3,4.
Short-course RT (SRT) (5 × 5 Gy over 1 week), followed by surgery within 1 week, has been used in some European countries5,6. The alternative is to delay surgery for 4–8 weeks after SRT (SRT-delay) and this approach was included in the Stockholm III trial protocol due to local experiences where some tumours showed significant downsizing or even a complete response when surgery had been delayed inadvertently. This approach was predominantly used for patients who did not tolerate standard treatment with chemoradiotherapy (CRT) for locally advanced, non-resectable rectal cancers where downsizing/downstaging was necessary, described both from Sweden and UK7–9. A third option was long-course RT (LRT) with 2 Gy delivered in 25 fractions (LRT-delay). This was the standard preoperative treatment until three different trials showed that concomitant administration of a fluoropyrimidine to LRT, that is CRT, improved local control but not OS10–12.
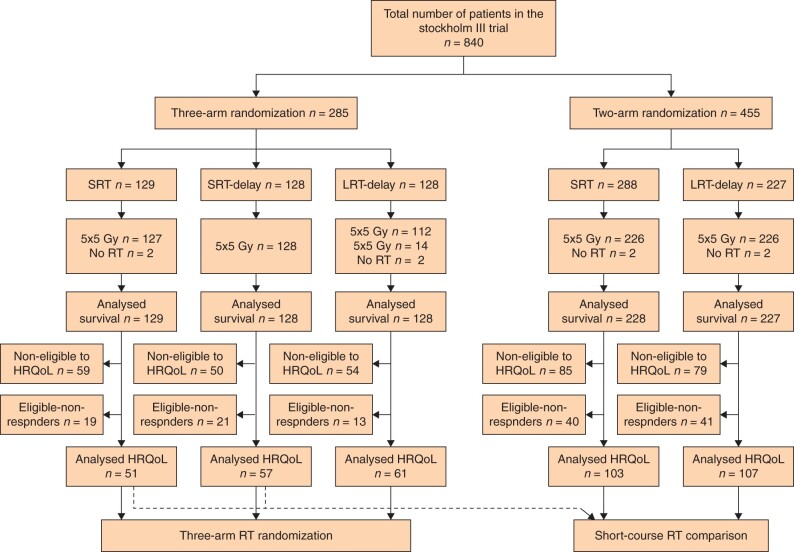
The Stockholm III trial randomly assigned patients with primarily resectable rectal cancer (with rigid sigmoidoscopy demonstrating a tumour ≤ 15 cm from the anal verge) to SRT, SRT-delay, or LRT-delay (Fig. 1). After a minimum follow-up of 2 years, oncological outcomes were similar in the three treatment groups, but with fewer postoperative complications in the groups with delayed surgery13.
Fig. 1.
Flow chart Stockholm III trial
SRT, short-course radiotherapy; LRT, long-course radiotherapy; RT, radiotherapy; HRQoL, health-related quality of life.
The benefits of RT must be balanced against the risks of both early and long-term side-effects. Early toxicity is higher after prolonged RT, particularly if chemotherapy (CT) is added5,14. Although patients report more pain, fatigue, and insomnia during the first 6 months after RT, most patients return to their pretreatment levels within 2 years15. However, long-term effects on gastrointestinal, urogenital, and sexual function clearly affect health-related quality of life (HRQoL), even decades after initial treatment16–18.
The aim of this phase of the study was to analyse local and distant recurrences and long-term survival in the Stockholm III trial after a minimum follow-up of 5 years, and to analyse long-term HRQoL after a minimum follow-up of 3 years.
Methods
The design and early results of the Stockholm III trial, which included patients enrolled from November 1998 to January 2013, have been presented previously9,13.
Patients with a biopsy-proven adenocarcinoma of the rectum, planned for a rectal resection, were included. Those with severe cardiovascular co-morbidities or previous RT to the pelvis were excluded.
The primary endpoint was time to LR, and other outcomes included distant metastases (DMs), OS, postoperative complications, and late morbidity. Early after initiation of the trial, an amendment to the protocol was proposed and endpoints regarding HRQoL and tumour regression were added. Tumour regression was analysed in 2019, with pathologists performing the reassessment blinded to treatment and previous staging19. Recruiting centres could choose to randomize patients to any of all three options, that is SRT, SRT-delay, or LRT-delay, or to apply a two-arm randomization between SRT and SRT-delay. Surgical options included anterior resection, abdominoperineal excision, or Hartmann’s procedure (all with the TME technique). All patients underwent surgery via an open approach. Patients were reported to the Swedish ColoRectal Cancer Registry (SCRCR), and reported data were used as the clinical reporting form. In the SCRCR, data are recorded prospectively by surgeons, pathologists, and oncologists. The registry has been validated several times and in 2018, it was shown to have a national coverage of more than 97 per cent20. Standard reporting intervals in the SCRCR is after surgery, at years 1, 3, and 5, or earlier if a recurrence is detected. In Sweden, the standard follow-up programme for rectal cancer is terminated if no events have occurred within 5 years after surgery. However, the SCRCR guideline states that any late recurrence detected after 5 years should be reported to the SCRCR. For patients in the trial, the participating centres were asked to verify that there were no recurrences in patients who did not have 5-year follow-up. Survival data in the SCRCR are linked to the Swedish Population Register and updated weekly when a patient has deceased. For recurrence and survival analyses, the last day of follow-up was set for 31 March 2018 when all patients had been followed up for at least 5 years after surgery.
HRQoL
In 2004, all patients without LR or DM and with a minimum follow-up of 3 years were invited to participate in a questionnaire survey. Patient invitation was by mail, and reminders sent if patients had not replied within 2 weeks. For those who were eligible and accepted to participate, the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire C30, version 3.0 (QLQ-C30) was sent out at one or two timepoints, before or after 6 years from the time of inclusion. For the analyses in this report, data from one questionnaire for each patient that was closest in time to 4–6 years were included in the analyses21,22.
The EORTC QLQ-C30 consists of 30 questions on global assessment, including two questions and five functional scales (physical, role, emotional, cognitive, and social) where high scores indicate high level of functioning, three symptom scales (fatigue, nausea and vomiting, and pain), and six single-symptom items (dyspnoea, insomnia, appetite loss, constipation, diarrhoea, and financial difficulties) where high scores indicate a high number of symptoms. Patients in the Stockholm III trial, as a whole group, were compared with EORTC QLQ-C30 data from a standard Swedish population matched for age and sex23.
Patients randomized to LRT-delay had tumours at a greater distance from the anal verge and consequently a higher frequency of anterior resections and less permanent stomas. Because of this and the permissive randomization protocol, patients randomized to SRT, SRT-delay, and LRT-delay in the three-arm comparison were analysed separately. Patients randomized to SRT and SRT-delay in both the three- and two-arm randomization were pooled and analysed in a short-course RT comparison.
Statistical methods
Sample sizes in the randomization arms were determined based on power calculation regarding the primary outcome time to LR. Incidence data were based on previous studies at the time of study planning when LR frequency was estimated to be about 15 per cent. The trial was designed as a non-inferiority study and the experimental arm (SRT-delay) was deemed non-inferior if the upper limit of a one-sided 90 per cent confidence interval of a hazard ratio (HR) did not exceed 1.7 regarding the primary outcome. However, after initiation of the trial, it became clear that the LR rates were significantly lower than initially estimated and a new power calculation was done. It was concluded that with the current sample size, non-inferiority could be decided at an upper confidence interval limit of 6.5. This was accepted, and sample sizes were not changed. The present study was analysed on an intention-to-treat (ITT) basis, that is patients remained in the groups to which they were allocated, independent of the therapy received. Continuous variables were presented as interquartile range (i.q.r.) and compared with the Kruskal–Wallis test. Dichotomous variables were analysed with the chi-square test and Fisher’s exact test when appropriate. OS was calculated as time between the date of randomization and death. Recurrence-free survival (RFS) was calculated from the date of randomization to the first event of LR, DM, or death. Survival data were analysed with the Kaplan–Meier method. HRs were calculated by Cox regression, stratified according to participating centres. Data are presented with 95 per cent confidence intervals, except for LR which are presented with 90 per cent confidence intervals.
The EORTC QLQ-C30 questionnaires were processed according to the scoring manual24. The scale used ranged from 0 to 100 points, with higher scores representing better HRQoL on the functional scales and lower HRQoL on the symptom scales and single-item measures. A difference of 5–9 points on the 100-point scale was considered a ‘small’ clinical difference, 10–19 points a ‘moderate’ clinical difference, and ≥ 20 points a ‘large’ clinical difference25. The chi-square test was used to compare baseline characteristics for the whole study population with those of the population participating in the HRQoL analyses, and Fisher’s test was used when appropriate. The expected mean for each of the scale scores was calculated by use of the age distribution in the whole HRQoL group, together with age-specific mean reference scale scores from the Swedish population23.
Statistical significance level was set as P < 0.050. STATA version 14.2 (StataCorp, College Station, Texas, USA) and R version 5.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) were used for statistical calculations and plotting of graphs.
Results
Baseline characteristics of 840 randomized patients in the trial are presented in Table 1, along with baseline characteristics of 379 patients included in the HRQoL analyses (Table 2).
Table 1.
Baseline characteristics, type of surgery and postoperative stage of the patients in the Stockholm III trial, sorted by three-arm randomization or pooled short-course radiotherapy comparison
| Patients included in the Stockholm III trial (n = 840) |
|||||
|---|---|---|---|---|---|
| Three-arm randomization |
Short-course radiotherapy comparison |
||||
| SRT (n = 129) | SRT-delay (n = 128) | LRT-delay (n = 128) | SRT (n = 357) | SRT-delay (n = 355) | |
| Age (years) | 67 (35–86) | 67 (41–85) | 66 (40–85) | 67 (35–89) | 67 (40–88) |
| Sex | |||||
| Men | 81 (62.8%) | 79 (61.7%) | 73 (57.0%) | 218 (61.1%) | 213 (60.0%) |
| Women | 48 (37.2%) | 49 (38.3%) | 55 (43.0%) | 139 (38.9%) | 142 (40.0%) |
| Height from anal verge (cm) | |||||
| 0–5 | 50 (38.8%) | 57 (44.5%) | 31 (24.2%) | 128 (35.9%) | 125 (35.2%) |
| 6–10 | 49 (38.0%) | 49 (38.3%) | 60 (46.9%) | 140 (39.2%) | 144 (4.6%) |
| 11–15 | 30 (23.3%) | 21 (16.4%) | 35 (27.3%) | 88 (24.6%) | 84 (23.7%) |
| Type of surgery | |||||
| AR | 79 (61.2%) | 68 (53.1%) | 93 (72.7%) | 218 (61.1%) | 204 (57.5%) |
| APE | 47 (36.4%) | 53 (41.4%) | 24 (18.8%) | 122 (34.2%) | 132 (37.2%) |
| Hartmann’s | 3 (2.3%) | 6 (4.7%) | 8 (6.3%) | 17 (4.8%) | 18 (5.1%) |
| LE | 0 (0%) | 1 (0.8%) | 1 (0.8%) | 0 (0%) | 1 (0.3%) |
| NR | 0 (0%) | 0 (0%) | 2 (1.6%) | 0 (0%) | 0 (0%) |
| ypStage | |||||
| I* | 38 (29.5%) | 55 (43.0%) | 37 (28.9%) | 96 (26.9%) | 138 (38.9%) |
| II | 43 (33.3%) | 31 (24.2%) | 46 (35.9%) | 118 (33.1%) | 86 (24.2%) |
| III | 48 (37.2%) | 31 (24.2%) | 37 (28.9%) | 134 (37.5%) | 107 (30.1%) |
| IV | 0 (0%) | 7 (5.5%) | 5 (3.9%) | 7 (2.0%) | 13 (3.7%) |
| X | 0 (0%) | 3 (2.3%) | 1 (0.8%) | 1 (0.3%) | 9 (2.5%) |
Data are median (range) or n (%).
*Includes patients with complete pathological response. SRT, short-course radiotherapy of 5 × 5 Gy, with surgery within 1 week; SRT-delay, short-course radiotherapy of 5 × 5 Gy, with a delay of 4–8 weeks to surgery; LRT-delay, long-course radiotherapy of 25 × 2 Gy, with surgery after 4–8 weeks; AR, anterior resection; APE, abdominal perineal excision; LE, local excision; NR, no resection.
Table 2.
Baseline characteristics, type of surgery, and postoperative stage of patients in HRQoL analyses, sorted by three-arm randomization and pooled short-course radiotherapy comparison
| Patients included in HRQoL analyses (n = 379) |
|||||
|---|---|---|---|---|---|
| Three-arm randomization |
Short-course radiotherapy comparison |
||||
| SRT (n = 51) | SRT-delay (n = 57) | LRT-delay (n = 61) | SRT (n = 154) | SRT-delay (n = 164) | |
| Age (years) | 64 (35–79) | 63 (41–83) | 65 (40–79) | 65 (35–81) | 64 (39–80) |
| Sex | |||||
| Men | 33 (64.7%) | 40 (70.2%) | 38 (62.3%) | 94 (61.0%) | 97 (59.1%) |
| Women | 18 (35.3%) | 17 (29.8%) | 23 (37.7%) | 60 (39.0%) | 67 (40.9%) |
| Height from anal verge (cm) | |||||
| 0–5 | 20 (39.2%) | 25 (43.9%) | 18 (29.5%) | 53 (34.4%) | 55 (33.5%) |
| 6–10 | 20 (39.2%) | 20 (35.1%) | 28 (45.9%) | 60 (39.0%) | 60 (36.9%) |
| 11–15 | 11 (21.6%) | 12 (21.1%) | 15 (24.6%) | 41 (26.6%) | 49 (29.9%) |
| Type of surgery | |||||
| AR | 33 (64.7%) | 34 (59.6%) | 41 (67.2%) | 98 (63.6%) | 105 (64.0%) |
| APE | 17 (33.3%) | 22 (38.6%) | 15 (24.6%) | 50 (32.5%) | 56 (34.1%) |
| Hartmann's | 1 (2.0%) | 1 (1.8%) | 3 (4.9%) | 6 (3.9%) | 3 (1.8%) |
| LE | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| NR | 0 (0%) | 0 (0%) | 2 (3.3%) | 0 (0%) | 0 (0%) |
| yPStage | |||||
| I* | 28 (54.9%) | 30 (52.6%) | 23 (37.7%) | 65 (42.2%) | 74 (45.1%) |
| II | 14 (27.5%) | 15 (26.3%) | 20 (32.8%) | 49 (31.8%) | 38 (23.2%) |
| III | 9 (17.6%) | 10 (17.5%) | 17 (27.9%) | 39 (25.3%) | 44 (26.8%) |
| IV | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.6%) |
| X | 0 (0%) | 2 (3.5%) | 1 (1.6%) | 1 (0.6%) | 7 (4.3%) |
Data are median (range) or n (%).
*Includes patients with complete pathological response. HRQoL, health-related quality of life; SRT, short-course radiotherapy of 5 × 5 Gy, with surgery within 1 week; SRT-delay, short-course radiotherapy of 5 × 5 Gy, with a delay of 4–8 weeks to surgery; LRT-delay, long-course radiotherapy of 25 × 2 Gy, with surgery after 4–8 weeks; AR, anterior resection; APE, abdominal perineal excision; LE, local excision; NR, no resection.
Seven patients did not receive any RT, and 14 patients allocated to receiving 25 × 2 Gy had 5 × 5 Gy. Median follow-up time for OS was 9.8 (i.q.r. 7.7–12.6) years. Median follow-up time for patients in the HRQoL analyses was 4.3 (i.q.r. 3.1–11.6) years.
Three-arm randomization (SRT, SRT-delay, and LRT-delay)
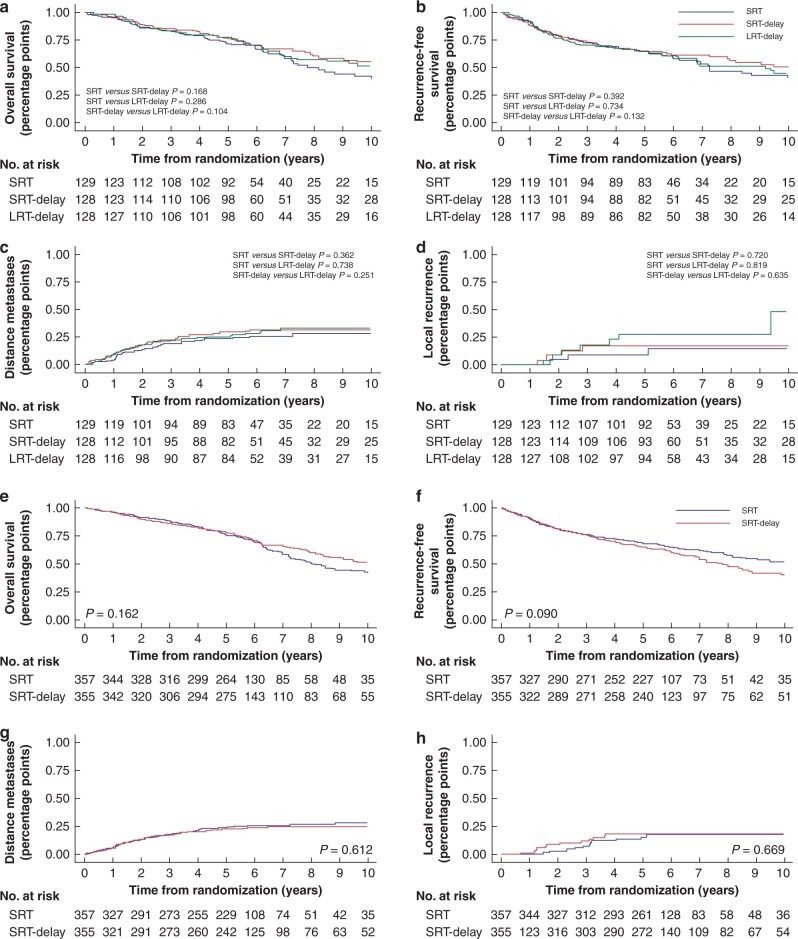
There were no statistically significant differences among the treatment groups regarding LR, DM, RFS, or OS rates (Fig. 2). Oncological outcomes are presented in Table 3. Results on HRQoL in the three-arm randomization groups are presented in Table 4. Neither the functioning or symptom scales nor the single-item measures or global measure demonstrated any statistically significant differences among the groups. Baseline characteristics of eligible patients who did not respond to HRQoL questionnaires are presented in Table 5.
Fig. 2.
Oncological outcomes
Three-arm randomization and short-course comparison.
Table 3.
Oncological outcomes and survival in the three-arm randomization and short-course radiotherapy comparison
| Three-arm randomization | |||||
|---|---|---|---|---|---|
| SRT (n = 129) | SRT-delay (n = 128) | LRT-delay (n = 128) | |||
| Local recurrence | 3 (2.3%) | 4 (3.1%) | 7 (5.5%) | ||
| Distant metastases | 31 (24.0%) | 38 (29.7%) | 38 (29.7%) | ||
| Any death | 65 (50.4%) | 58 (45.3%) | 56 (43.8%) | ||
| Intercurrent death | 37 (28.7%) | 24 (18.8%) | 23(18.0%) | ||
|
| |||||
| Hazard ratio | SRT (reference) | SRT-delay | P | LRT-delay | P |
|
| |||||
| LR HR* | 1.0 | 1.18 (0.33, 4.15) | 0.830 | 2.23 (0.70, 7.14) | 0.236 |
| DM HR | 1.0 | 1.47 (0.90, 2.42) | 0.123 | 1.23 (0.76, 1.99) | 0.390 |
| OS HR | 1.0 | 0.75 (0.51, 1.09) | 0.137 | 0.88 (0.61, 1.29) | 0.512 |
| RFS HR | 1.0 | 0.90 (0.61, 1.21) | 0.589 | 0.99 (0.63, 1.30) | 0.956 |
| Short-course radiotherapy comparison | |||
|---|---|---|---|
| SRT (n = 357) | SRT-delay (n = 355) | ||
|
| |||
| Local recurrence | 11 (3.1%) | 13 (3.7%) | |
| Distant metastases | 88 (24.7%) | 82 (23.1%) | |
| Any death | 200 (56.0%) | 211 (59.4%) | |
| Intercurrent death | 84 (23.5%) | 68 (19.2%) | |
|
| |||
| Hazard ratio | SRT (reference) | SRT-delay | P |
|
| |||
| LR HR* | 1.0 | 1.31 (0.66,2.61) | 0.518 |
| DM HR | 1.0 | 0.94 (0.69,1.27) | 0.692 |
| OS HR | 1.0 | 0.84 (0.66,1.06) | 0.146 |
| RFS HR | 1.0 | 0.85 (0.68,1.06) | 0.144 |
Data are n (%) or hazard ratio (HR) with 95 per cent confidence intervals, unless otherwise specified.
*90 per cent confidence intervals. SRT, short-course radiotherapy and surgery within 1 week; SRT-delay, short-course radiotherapy, with surgery after 4–8 weeks; LRT-delay, long-course radiotherapy of 25 × 2 Gy, with surgery after 4–8 weeks; LR, local recurrence; DM, distant metastases; OS, overall survival; RFS, recurrence-free survival.
Table 4.
EORTC QLQ-C30 three-arm randomization
| EORTC QLQ-C30 | SRT (n = 51) |
SRT-delay (n = 57) |
LRT-delay (n = 61) |
P |
|---|---|---|---|---|
| Mean scale score (s.d.) | Mean scale score (s.d.) | Mean scale score (s.d.) | ||
| Global health status | 75 (19) | 71(23) | 71(21) | 0.494 |
| Functional scales | ||||
| Physical functioning | 86 (19) | 85 (19) | 84 (18) | 0.851 |
| Role functioning | 81 (28) | 82 (27) | 79 (27) | 0.868 |
| Emotional functioning | 86 (19) | 82 (19) | 84 (19) | 0.525 |
| Cognitive functioning | 88 (16) | 85 (17) | 91 (17) | 0.698 |
| Social functioning | 74 (28) | 76 (29) | 76 (29) | 0.905 |
| Symptoms | ||||
| Fatigue | 21 (21) | 24 (23) | 24 (24) | 0.626 |
| Nausea and vomiting | 6 (12) | 6 (16) | 2 (7) | 0.091 |
| Pain | 19 (26) | 19 (26) | 17 (26) | 0.812 |
| Single items | ||||
| Dyspnoea | 16 (22) | 19 (24) | 19 (25) | 0.714 |
| Insomnia | 20 (25) | 21 (29) | 21 (29) | 0.963 |
| Appetite loss | 5 (12) | 6 (18) | 10 (24) | 0.397 |
| Constipation | 17 (27) | 14 (24) | 15 (29) | 0.791 |
| Diarrhoea | 23 (30) | 22 (31) | 19 (30) | 0.752 |
| Financial difficulties | 2 (11) | 10 (23) | 9 (20) | 0.074 |
EORTC, European Organization for Research and Treatment of Cancer; QLQ-C30, Quality of Life Core Questionnaire C30, version 3.0; SRT, short-course radiotherapy and surgery within 1 week; SRT-delay, short-course radiotherapy, with surgery after 4–8 weeks; LRT-delay, long-course radiotherapy of 25 × 2 Gy, with surgery after 4–8 weeks.
Table 5.
Baseline characteristics of eligible non-responders (alive and recurrence-free patients) who were invited to, but did not, participate in HRQoL analyses
| Three-arm randomization |
Short-course radiotherapy comparison |
||||
|---|---|---|---|---|---|
| SRT (n = 19) | SRT-delay (n = 21) | LRT-delay (n = 13) | SRT (n = 59) | SRT-delay (n = 62) | |
| Age (years) | 68 (56–77) | 69 (60–78) | 63 (48–85) | 67 (49–81) | 66 (42–86) |
| Sex | |||||
| Men | 12 (63.2%) | 7 (33.3%) | 5 (38.5%) | 34 (57.6%) | 32 (51.6%) |
| Women | 7 (36.8%) | 14 (66.7%) | 8 (61.5%) | 25 (42.4%) | 30 (48.4%) |
| Height from anal verge (cm) | |||||
| 0–5 | 6 (31.6%) | 10 (47.6%) | 5 (38.5%) | 22 (37.3%) | 19 (30.6%) |
| 6–10 | 8 (42.1%) | 9 (42.9%) | 8 (61.5%) | 21 (35.6%) | 31 (50.0%) |
| 11–15 | 5 (26.3%) | 2 (9.5%) | 0 (0%) | 16 (27.1%) | 12 (19.4%) |
| Type of surgery | |||||
| AR | 12 (63.2%) | 10 (47.6%) | 9 (69.2%) | 38 (64.4%) | 36 (58.1%) |
| APE | 6 (31.6%) | 10 (47.6%) | 4 (30.8%) | 20 (34%) | 24 (38.7%) |
| Hartmann’s | 1 (5.3%) | 1 (4.8%) | 0 (0%) | 1 (21.7%) | 2 (3.2%) |
| LE | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| NR | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| YpStage | |||||
| I* | 3 (15.8%) | 11 (52.4%) | 7 (53.9%) | 12 (20.3%) | 27 (43.5%) |
| II | 7 (36.8%) | 6 (28.6%) | 5 (38.5%) | 24 (40.7%) | 17 (27.4%) |
| III | 9 (47.4%) | 3 (14.3%) | 1 (7.7%) | 23 (39.0%) | 17 (27.4%) |
| IV | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| X | 0 (0%) | 1 (4.8%) | 0 (0%) | 0 (0%) | 1 (1.6%) |
Data are median (range) or n (%). *Includes patients with complete response. HRQoL, health-related quality of life; SRT, short-course radiotherapy of 5 × 5 Gy, with surgery within 1 week; SRT-delay, short-course radiotherapy of 5 × 5 Gy, with a delay of 4–8 weeks to surgery; LRT-delay, long-course radiotherapy of 25 × 2 Gy, with surgery after 4–8 weeks; AR, anterior resection; APE, abdominal perineal excision; LE, local excision; NR, no resection.
Short-course RT comparison (SRT and SRT-delay)
There were no statistically significant differences between the two groups regarding LR, DM, RFS or OS rates (Fig. 2). HRs from Cox regression analysis are presented in Table 3.
Overall, 72 per cent of eligible patients completed the EORTC QLQ-C30 questionnaire. No statistically significant differences were found between the SRT and SRT-delay groups (data not shown).
HRQoL comparison between recurrence-free rectal cancer patients and a Swedish reference population
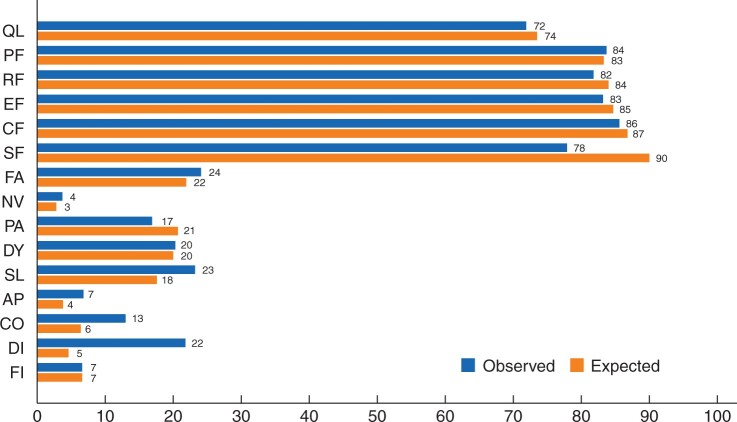
Comparison of results from 379 study participants completing the EORTC QLQ-C30 questionnaire versus a reference population showed clinical differences in social functioning (‘moderate’), sleep disturbances (‘small’), and diarrhoea (‘moderate’), better functioning, and lower levels of symptoms in the reference population. None of the other scales showed clinical differences between the two populations (Fig. 3).
Fig. 3.
Mean values for EORTC QLQ-C30 subscales for the study sample (observed) and the Swedish reference population (expected)
Expected scale scores are calculated by indirect standardization with age- and sex-specific scores from the Swedish population. EORTC, European Organization for Research and Treatment of Cancer; QLQ-C30, Quality of Life Core Questionnaire C30, version 3.0; QL, global quality of life; PF, physical functioning; RF, role functioning; EF, emotional functioning; CF, cognitive functioning; SF, social functioning; FA, fatigue; NV, nausea and vomiting; PA, pain; DY, dyspnoea; SL, insomnia; AP, appetite loss; CO, constipation; DI, diarrhoea; FI, financial difficulties.
In the trial, data on CT received were not validated. Medical oncological treatment was not reported to the SCRCR before 2007. In the period from 2007 to 2013, 489 patients were included in the trial and 72 patients (14.7 per cent) received postoperative CT, including two patients with ypStage I, six patients with ypStage II, and 64 patients with ypStage III13.
Discussion
Delaying surgery after preoperative treatment in rectal cancer has some major advantages: time for patient optimization, possibility of a complete clinical response, and lower risk of postoperative complications. However, it may delay the start of adjuvant chemotherapy or increase the risk of tumour progression, with a potentially worse oncological outcome. In this long-term follow-up of the Stockholm III trial, no statistically significant differences in oncological outcomes regarding LR, DM, RFS, OS, or HRQoL were found on comparison of the SRT, SRT-delay, and LRT-delay groups. Delaying surgery after short-course RT seems safe, at least when use of postoperative CT is limited, as it was in Sweden during the trial period.
The main advantage of this study is the randomized patient cohort with minimal differences in patient characteristics. All patients were followed up in the SCRCR, and data were validated in the patients’ medical charts. However, one possible risk is that late recurrences after 5 years were not reported to the SCRCR, especially if the recurrence was not diagnosed at a surgical or oncological department. This could impact the absolute number of recurrences; however, it is unlikely that there would be a difference in registry reporting, depending on the allocated treatment. In the present report, about 7 per cent of local or distant recurrences were diagnosed more than 5 years from randomization. Most recurrences are diagnosed within the first 2 years after surgery, although late recurrences can be seen after rectal cancer surgery26,27. Furthermore, the cumulative incidences of LR and DM are in line with what is expected considering the postoperative tumour stage.
An obvious limitation is the long inclusion period. Surgical treatment, preoperative staging, and postoperative care continually evolve28. The impact of these improvements on the different treatment strategies is unknown. In Sweden, initial treatment in rectal cancer is recommended at a multidisciplinary conference where MRI is the standard imaging for local tumour staging. However, data on the preoperative local tumour stage were only available in the SCRCR from 2007 and could not be analysed in this study. Treatment guidelines during the inclusion period emphasized the importance of categorizing tumours into three different groups, according to the ‘good, bad, or ugly’ concept29. Most patients included in the Stockholm III trial had tumours classified as ‘bad or intermediate risk’, although patients with stage I disease were included early in the trial. Since 2003 when MRI was implemented for rectal cancer staging, with a more precise preoperative staging, only patients with stage II and III disease were included. Patients with more advanced or ‘ugly’ tumours usually received CRT outside the Stockholm III trial30.
As improved surgical techniques and selective neoadjuvant treatments have reduced LR rates, the present focus on rectal cancer recurrence should be on DM. In the present study, no statistically significant differences in the rates of DM could be found between the arms, neither in the ITT analyses nor when comparing the as-treated groups (data not shown). Other trials comparing different RT regimens have found similar results. Local tumour treatment, including different overall treatment times (OTTs), does not seem to affect the rate of DM, at least not at a group level31–34. In addition, there were no differences in survival among the treatment arms in the three-arm randomization nor in comparing time to surgery after SRT.
The potential downside of delaying surgery after preoperative RT is prolongation of OTT. RT toxicity can be an issue and 6 per cent of patients in the arms with a delay to surgery required in-hospital care13. In patients with no or minor tumour regression following radiation, the prognosis is inferior, compared with those achieving an excellent response35. Whether 4–8 weeks of delay to surgery matters with respect to recurrence or survival in patients with non-responding tumours is unknown. The optimal waiting time before clinical and radiological evaluation after RT is not clear. The first signs of tumour regression following SRT can be found after an OTT of 10 days, but full regression effect may take several weeks to months31,36–38.
Tumour repopulation using [18F]fluorodeoxyglucose PET (FDG-PET) can be seen after 6–12 weeks in about half of the population treated with CRT39. The beneficial effect of adjuvant CT in patients who have received preoperative treatment remains controversial40; however, if postoperative CT is indicated, a prolongation of the time to start what may negatively influence outcome41. The RAPIDO trial included patients with locally advanced rectal cancer treated with SRT followed by CT for 4–5 months—this was well tolerated and reduced DM rates, compared with standard treatment with conventional CRT42.
In this study, there were no significant differences among the treatment arms in overall HRQoL after a minimum follow-up of 3 years. However, patients in the Stockholm III trial overall had worse scores related to diarrhoea and social functioning, compared with the reference population. Worse bowel function would be anticipated in a patient group that have been treated for rectal cancer.
In the present trial, it was not possible to detect any differences in age or other characteristics between responders and eligible non-responders.
There are limitations associated with qualitative research relating to HRQoL, especially if response rates differ among patients who are the most symptomatic compared with those satisfied with their quality of life and function. There could be inherent bias from patients who have been cured from cancer being thankful, thus impacting how they respond to the questions. Responding to a questionnaire at home may differ from providing responses in a clinical setting with a healthcare professional.
The present results have provided some reassurance for patients requiring rectal cancer treatment during the coronavirus disease (Covid-19) pandemic. A prolonged time between RT and surgery seems acceptable when good response to preoperative treatment has been demonstrated. SRT-delay has therefore been recommended, instead of CRT, to decrease the number of fractions, and thus to reduce hospital visits, in addition to the beneficial effect on the risk of developing postoperative complications43.
Funding
This study was funded by the Swedish Research Council, Swedish Cancer Society and Stockholm Cancer Society, and the Stockholm County Council and Karolinska Institutet through regional agreement on medical training and clinical research.
Disclosure. The authors declare no conflict of interest.
Data availability
The data underlying this article are available on request.
Acknowledgements
The authors thank the Stockholm Colorectal Cancer study group, T. Singnomklao for helping with registry data and validation, and all hospitals for recruiting patients to the Stockholm III Trial, in particular: B. Cedermark (Karolinska University Hospital, Stockholm), M. Goldinger (St Göran’s Hospital, Stockholm), M. Bragmark, F. Hjern (Danderyd Hospital, Stockholm), G. Lindgren (Södertälje Hospital, Södertälje), N. Lundqvist, (Norrtälje Hospital, Norrtälje), Y. Raab (South Hospital, Stockholm), M. Machado (Ersta Hospital, Stockholm), Å. Berglund, P. Nygren (Uppsala Akademiska Hospital), A. Nihlberg (Falu Hospital, Falun), R. Heuman (Mora Hospital, Mora), I. Syk (Skåne University Hospital, Malmö), G. Lindmark (Helsingborg Hospital, Helsingborg), G. Ljung (Mälarsjukhuset Hospital, Eskilstuna), O. Hallböök (Linköping University Hospital, Linköping), P. Loftås (Vrinnevi Hospital, Norrköping), and P. Gustavsson (Visby Hospital, Visby). J.E. and S.F. contributed equally as first authors. B.G. and A.M. contributed equally as last authors.
References
- 1. Swedish Rectal Cancer Trial. Improved survival with pre-operative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997;336:980–987. [DOI] [PubMed] [Google Scholar]
- 2. Martling A, Holm T, Johansson H, Rutqvist LE, Cedermark B. ; Stockholm Colorectal Cancer Study Group. The Stockholm II trial on pre-operative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 2001;92:896–902. [DOI] [PubMed] [Google Scholar]
- 3. Kapiteijn E, Marijnen CAM, Nagtegaal ID, Putter H, Steup WH, Wiggers T. et al. ; Dutch Colorectal Cancer Group. Pre-operative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 4. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S. et al. Pre-operative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Pudełko M. et al. Sphincter preservation following pre-operative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72:15–24. [DOI] [PubMed] [Google Scholar]
- 6. van den Broek CB, van Gijn W, Bastiaannet E, Møller B, Johansson R, Elferink MA. et al. ; EURECCA Consortium. Differences in pre-operative treatment for rectal cancer between Norway, Sweden, Denmark, Belgium and the Netherlands. Eur J Surg Oncol 2014;40:1789–1796. [DOI] [PubMed] [Google Scholar]
- 7. Radu C, Berglund A, Pahlman L, Glimelius B.. Short-course pre-operative radiotherapy with delayed surgery in rectal cancer – a retrospective study. Radiother Oncol 2008;87:343–349. [DOI] [PubMed] [Google Scholar]
- 8. Hatfield P, Hingorani M, Radhakrishna G, Cooper R, Melcher A, Crellin A. et al. Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiother Oncol 2009;92:210–214. [DOI] [PubMed] [Google Scholar]
- 9. Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A.. Pre-operative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg 2012;99:577–583. [DOI] [PubMed] [Google Scholar]
- 10. Gérard A, Buyse M, Nordlinger B, Loygue J, Pène F, Kempf P. et al. Pre-operative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg 1988;208:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L. et al. ; EORTC Radiotherapy Group Trial 22921. Chemotherapy with pre-operative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 12. Brændengen M, Glimelius B.. Pre-operative radiotherapy or chemoradiotherapy in rectal cancer – is survival improved? An update of the “Nordic” LARC study in non-resectable cancers. Radiother Oncol 2018;127:392–395. [DOI] [PubMed] [Google Scholar]
- 13. Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C. et al. Optimal fractionation of pre-operative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336–346. [DOI] [PubMed] [Google Scholar]
- 14. Ansari N, Solomon MJ, Fisher RJ, Mackay J, Burmeister B, Ackland S. et al. Acute adverse events and postoperative complications in a randomized trial of pre-operative short-course radiotherapy versus long-course chemoradiotherapy for T3 adenocarcinoma of the rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04). Ann Surg 2017;265:882–888. [DOI] [PubMed] [Google Scholar]
- 15. Couwenberg AM, Burbach JPM, van Grevenstein WMU, Smits AB, Consten ECJ, Schiphorst AHW. et al. Effect of neoadjuvant therapy and rectal surgery on health-related quality of life in patients with rectal cancer during the first 2 years after diagnosis. Clin Colorectal Cancer 2018;17:e499–e512. [DOI] [PubMed] [Google Scholar]
- 16. Wiltink LM, Marijnen CA, Meershoek-Klein Kranenbarg E, van de Velde CJ, Nout RA.. A comprehensive longitudinal overview of health-related quality of life and symptoms after treatment for rectal cancer in the TME trial. Acta Oncol 2016;55:502–508. [DOI] [PubMed] [Google Scholar]
- 17. Birgisson H, Pahlman L, Gunnarsson U, Glimelius B; Swedish Rectal Cancer Trial Group. Adverse effects of pre-operative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol 2005;23:8697–8705. [DOI] [PubMed] [Google Scholar]
- 18. Birgisson H, Påhlman L, Gunnarsson U, Glimelius B.. Late adverse effects of radiation therapy for rectal cancer – a systematic overview. Acta Oncol 2007;46:504–516. [DOI] [PubMed] [Google Scholar]
- 19. Erlandsson J, Lörinc E, Ahlberg M, Pettersson D, Holm T, Glimelius B. et al. Tumour regression after radiotherapy for rectal cancer – results from the randomised Stockholm III trial. Radiother Oncol 2019;135:178–186. [DOI] [PubMed] [Google Scholar]
- 20. Moberger P, Sköldberg F, Birgisson H.. Evaluation of the Swedish Colorectal Cancer Registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol 2018;57:1611–1621. [DOI] [PubMed] [Google Scholar]
- 21. Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK.. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res 1993;2:287–295. [DOI] [PubMed] [Google Scholar]
- 22. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 23. Michelson H, Bolund C, Nilsson B, Brandberg Y.. Health-related quality of life measured by the EORTC QLQ-C30. Reference values from a large sample of the Swedish population. Acta Oncol 2000;39:477–484. [DOI] [PubMed] [Google Scholar]
- 24. Fayers AN, Bjordal K, Groenvold M, Curran D, Bottomley A.. EORTC QLQ-C30 Scoring Manual (3rd edn). Brussels: European Organization for Research & Treatment of Cancer, 2001. [Google Scholar]
- 25. Osoba D, Rodrigues G, Myles J, Zee B, Pater J.. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–144. [DOI] [PubMed] [Google Scholar]
- 26. Adams K, Higgins L, Beazley S, Papagrigoriadis S.. Intensive surveillance following curative treatment of colorectal cancer allows effective treatment of recurrence even if limited to 4 years. Int J Colorectal Dis 2015;30:1677–1684. [DOI] [PubMed] [Google Scholar]
- 27. Grossmann I, Doornbos PM, Klaase JM, de Bock GH, Wiggers T.. Changing patterns of recurrent disease in colorectal cancer. Eur J Surg Oncol 2014;40:234–239. [DOI] [PubMed] [Google Scholar]
- 28. Kodeda K, Johansson R, Zar N, Birgisson H, Dahlberg M, Skullman S. et al. Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Colorectal Dis 2015;17:O168–O179. [DOI] [PubMed] [Google Scholar]
- 29. Blomqvist L, Glimelius B.. The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncol 2008;47:5–8. [DOI] [PubMed] [Google Scholar]
- 30. Glimelius B. On a prolonged interval between rectal cancer (chemo)radiotherapy and surgery. Ups J Med Sci 2017;122:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R. et al. ; German Rectal Cancer Study Group. Pre-operative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 32. Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D. et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–3833. [DOI] [PubMed] [Google Scholar]
- 33. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M.. Long-term results of a randomized trial comparing pre-operative short-course radiotherapy with pre-operative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215–1223. [DOI] [PubMed] [Google Scholar]
- 34. Glimelius B, Myklebust TA, Lundqvist K, Wibe A, Guren MG.. Two countries – two treatment strategies for rectal cancer. Radiother Oncol 2016;121:357–363. [DOI] [PubMed] [Google Scholar]
- 35. Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C. et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, Hermans J, van de Velde CJ, Leer JW. et al. ; Pathology Review Committee and the Cooperative Clinical Investigators. No downstaging after short-term pre-operative radiotherapy in rectal cancer patients. J Clin Oncol 2001;19:1976–1984. [DOI] [PubMed] [Google Scholar]
- 37. Graf W, Dahlberg M, Osman MM, Holmberg L, Pahlman L, Glimelius B.. Short-term pre-operative radiotherapy results in down-staging of rectal cancer: a study of 1316 patients. Radiother Oncol 1997;43:133–137. [DOI] [PubMed] [Google Scholar]
- 38. Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA. et al. ; Dutch Surgical Colorectal Audit. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013;100:933–939. [DOI] [PubMed] [Google Scholar]
- 39. Perez RO, Habr-Gama A, São Julião GP, Gama-Rodrigues J, Sousa AH Jr, Campos FG. et al. Optimal timing for assessment of tumor response to neoadjuvant chemoradiation in patients with rectal cancer: do all patients benefit from waiting longer than 6 weeks? Int J Radiat Oncol Biol Phys 2012;84:1159–1165. [DOI] [PubMed] [Google Scholar]
- 40. Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L. et al. Adjuvant chemotherapy after pre-operative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200–207. [DOI] [PubMed] [Google Scholar]
- 41. Glimelius B, Martling A.. What conclusions can be drawn from the Stockholm III rectal cancer trial in the era of watch and wait? Acta Oncol 2017;56:1139–1142. [DOI] [PubMed] [Google Scholar]
- 42. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM. et al. ; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus pre-operative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 43. Marijnen CAM, Peters FP, Rödel C, Bujko K, Haustermans K, Fokas E. et al. International expert consensus statement regarding radiotherapy treatment options for rectal cancer during the COVID 19 pandemic. Radiother Oncol 2020;148:213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available on request.