Abstract
Background:
Studies have shown that air pollution exposures during pregnancy are associated with an increased risk of autism spectrum disorder (ASD) in children, and the risk appears to be greater for boys. However, studies assessing gestational windows of susceptibility have been mostly limited by trimesters.
Objective:
We identified sensitive windows of exposure to regional air pollution and risk of ASD and examined sex differences in a large birth cohort.
Methods:
This population-based retrospective cohort study included 294,937 mother–child pairs with singleton deliveries in Kaiser Permanente Southern California (KPSC) hospitals from 2001 to 2014. Children were followed using electronic medical records until clinical ASD diagnosis, non-KPSC membership, death, or 31 December 2019, whichever came first. Weekly mean fine particulate matter [PM with an aerodynamic diameter of ()], nitrogen dioxide (), and ozone () pregnancy exposures were estimated using spatiotemporal prediction models. Cox proportional hazard models with distributed lags were used to estimate weekly pollutant exposure associations with ASD risk for the entire cohort, and separately for boys and for girls. Models were adjusted for child sex (for full cohort), maternal race/ethnicity, maternal age at delivery, parity, maternal education, maternal comorbidities, medical center, census tract median household income, birth year, and season.
Results:
There were 5,694 ASD diagnoses (4,636 boys, 1,058 girls). Sensitive exposure windows associated with ASD were found early in pregnancy, statistically significant throughout the first two trimesters [1–27 wk of gestation, cumulative [95% confidence interval (CI): 1.06, 1.23] per interquartile range (IQR) () increase]. exposure during 34–37 wk of gestation was associated with increased risk [ (95% CI: 1.01, 1.11) per IQR () increase] but with reduced risk during 20–28 wk of gestation [ (95% CI: 0.89, 0.98)]. No associations were observed with . Sex-stratified early gestational associations were stronger among boys [boys (95% CI: 1.08, 1.26); girls (95% CI: 0.89, 1.26)]. associations in later gestation were observed only in boys [boys (95% CI: 1.04, 1.16); girls (95% CI: 0.84, 1.05)].
Conclusions:
Exposures to in the first two gestational trimesters were associated with increased ASD risk in children, with stronger associations observed for boys. The role of exposure on ASD risk merits further investigation. https://doi.org/10.1289/EHP9509
Introduction
Autism spectrum disorder (ASD), a complex neurodevelopmental condition that disproportionately affects boys (Xu et al. 2019), imposes substantial lifetime social and economic costs on affected families and communities (Werling and Geschwind 2013). Although ASD risk is heritable, only of diagnoses are due to spontaneous single gene or chromosomal mutations (Bai et al. 2019; Rylaarsdam and Guemez-Gamboa 2019); the remaining causes are not well understood and are likely multifactorial. Prenatal factors are associated with increased risk of ASD (Stoner et al. 2014); a growing number of epidemiological studies have reported associations between prenatal exposure to fine particulate matter [PM with an aerodynamic diameter of ()] and increased risk for ASD (Chun et al. 2020; Lam et al. 2016). Possible mechanisms may include maternal systemic oxidative stress and pro-inflammatory cytokine production (Leni et al. 2020; Xu et al. 2012), resulting in placental and endothelial dysfunction and increased fetal oxidative stress that may disrupt differentiation and organization of the fetal brain (Block et al. 2012; Block and Calderón-Garcidueñas 2009).
Fetal brain development occurs through a sequence of carefully orchestrated histogenic events (Fox et al. 2010; Levitt 2003; Molnár et al. 2019), and environmental exposure that disrupts specific processes can have variable impacts, depending on the timing of the exposure as well as dose (Block et al. 2012). Despite this understanding, epidemiological studies have examined associations between air pollution and ASD using pregnancy average exposure or trimester-specific average exposure (Jo et al. 2019b; Pagalan et al. 2019; Raz et al. 2015; Ritz et al. 2018); results from these studies may be biased owing to seasonal trends in air pollution exposure (Wilson et al. 2017) and provide limited insight into sensitive exposure windows that may not coincide precisely with the commonly used trimester average exposure (Fox et al. 2010; Tau and Peterson 2010; Wright 2017). For example, neurulation continues up to 6 wk of gestation and cortical neurogenesis begins at 7 wk of gestation and continues through midpregnancy, whereas synaptogenesis begins during mid-gestation and extends through birth (Kast and Levitt 2019; Keeney et al. 2015). Weekly exposure resolution is now available for assessment of effects on transient fetal brain developmental susceptibility (Di et al. 2019, 2020; Requia et al. 2020).
The effects of in utero air pollution exposure on ASD risk also may vary by sex. We have reported statistically significant sex differences of first trimester association with ASD risk, stronger among boys (Jo et al. 2019b); other studies also reported sexual dimorphic trends in effect estimates on ASD (Pagalan et al. 2019; Raz et al. 2015; Ritz et al. 2018). Animal studies have shown sexual dimorphism in the developmental neurotoxicity of gestational exposure (Bolton et al. 2013, 2014; McCarthy 2016). The male fetus may be more vulnerable to oxidative injury (Minghetti et al. 2013); thus boys may have an exaggerated response to in utero exposure. Sex-specific prenatal effects are also observed for other neurodevelopmental and health outcomes (Chiu et al. 2016; Hsu et al. 2015; Rosa et al. 2017); decreased intelligence quotient scores in boys, but not in girls, were associated with late gestational exposure (Chiu et al. 2016).
The purpose of the present study was to address limitations of previous studies by examining the associations of weekly gestational air pollution [, nitrogen dioxide (), and ozone ()] exposure with ASD risk and sex differences in a large population-based pregnancy cohort. Air pollution exposure was estimated at maternal residential addresses during pregnancy using validated satellite-based spatiotemporally resolved () hybrid models. Results will help provide insight into vulnerable exposure windows and sexual dimorphism potentially related to the 4-fold larger ASD burden among boys (Maenner et al. 2020).
Materials and Methods
Study Population
This is a population-based retrospective pregnancy cohort study that included mothers with singleton deliveries at Kaiser Permanente Southern California (KPSC) hospitals between 1 January 2001 and 31 December 2014. KPSC is a large integrated health care system with over members across Southern California. KPSC membership is diverse and similar in socioeconomic characteristics to the region’s census demographics (Koebnick et al. 2012). Information related to the mother, including maternal address history, and to the child was extracted from high-quality, integrated electronic medical records (EMRs) maintained by KPSC. Addresses were geocoded using ArcGIS, and geocodes with SCORE of 90–100 and ADDRESS TYPE of point address or street address were considered suitable. Addresses based only on street name, postal code, locality, or administrative unit usually had SCORES and were considered too uncertain to be geolocated into the correct grid used for exposure assignments. Derivation of study sample size is shown in Figure S1. To ensure complete weekly air pollution exposures up to 37 wk of gestation and the opportunity to be screened for potential ASD risk starting at 18 months of age during their regular well-child visits at KPSC, children who were born singleton at 37–44 wk of gestation at KPSC hospitals and were covered by the KPSC health plan at 1 year of age were eligible (). A total of 48,876 births were excluded; 599 due to missing or data errors in birth weight, sex, maternal race/ethnicity, or age at delivery; 141 due to maternal age at delivery or of age; and 48,136 due to incomplete maternal residential address history in pregnancy or with geocodes not suitable for exposure assignment. The final data analysis included 294,937 mother–child pairs. The excluded children had more missing or incomplete covariates and maternal address history; excluded children’s mothers had more missing medical history before pregnancy and were younger, less educated, and more likely to be nulliparous, suggesting that these mothers were less likely to have joined KPSC before pregnancy (Table S1). Eligible children were followed using EMRs until 31 December 2019. The institutional review boards of both KPSC and the University of Southern California approved this study with waiver of individual subject consent.
Outcome ASD
Outcome measures were presence or absence of ASD during the follow-up period, which were identified according to the International Classification of Diseases, Ninth Revision (CDC 2013) codes 299.0, 299.1, 299.8, and 299.9 for EMR records before 1 October 2015 [date of the KPSC implementation of the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) (WHO 2016)] and subsequently according to ICD-10 codes F84.0, F84.3, F84.5, F84.8, and F84.9. These codes included autistic disorders, Asperger’s syndrome, or pervasive developmental disorder not otherwise specified and excluded childhood disintegrative disorder and Rett’s syndrome. Codes from at least two separate visits were required for ASD diagnosis. This approach has been validated with a positive predictive value of 88% (Coleman et al. 2015) and used in previous studies of ASD in this cohort (Jo et al. 2019b; Xiang et al. 2015, 2018).
Exposure Assessment
Air pollution exposures were assigned accounting for change in addresses for each participant during pregnancy. KPSC maintained a consolidated address history table recording changes of address from EMRs. Each address record has a corresponding start and end date. Addresses for each participant corresponding to each address update were interpolated from start to end date. Exposure models are described elsewhere in detail (Di et al. 2019, 2020; Requia et al. 2020). Briefly, three machine learning algorithms (neural network, random forest, and gradient boosting) and multiple predictor variables, including Moderate Resolution Imaging Spectroradiometer–derived aerosol optical depth measurements at a spatial resolution, measuring instrument and measurements, meteorological variables, land-use variables, chemical transport model predictions, emission inventories, and other variables were used to estimate daily 24-h average , 1-h maximum , and 8-h maximum at a resolution of across the contiguous United States. Performance of each model was evaluated by applying a 10-fold cross-validation technique. Ensemble models produced very good cross-validated values of 0.86 for , 0.79 for , and 0.90 for . To provide stable estimates of exposure, weekly averages of mother’s daily gestational exposure estimates were used for analysis.
Covariates
We selected covariates a priori based on expert knowledge and past literature on air pollution exposures and ASD, and included child sex, maternal race/ethnicity in the KPSC EMR (self-reported from multiple options), maternal age at delivery, parity, education, maternal history of comorbidity [ diagnosis of heart, lung, kidney, liver disease, or cancer], medical center, median family household income in census tract of residence, birth year, and an indicator variable for season (dry from April to October; wet from November to March)] (Jo et al. 2019b; Pagalan et al. 2019; Ritz et al. 2018). An indicator variable was created for missing covariates (parity: ; education: ; census tract household income: ). Birth year and season of conception were included to control for potential confounding due to omitting variables correlated with seasonal or time trends in ASD incidence and pollution levels. KPSC medical centers were used to control for spatial confounding or any physician differences in diagnosis between medical centers. ASD is more common in urban areas (Becker 2010), and the population of this cohort is largely urban. We adjusted our models for KPSC medical centers that broadly capture the spatial variation in urbanicity in Southern California. Gestational age and birth weight were not considered as covariates because they may be on the casual pathway between air pollution and ASD (Bekkar et al. 2020; Gardener et al. 2011).
Statistical Analyses
In recent years, distributed lag models (DLMs) have been used in pregnancy studies to examine associations between prenatal weekly air pollution exposures during gestation and child health outcomes (Chiu et al. 2016; Hsu et al. 2015; Wu et al. 2018). To identify sensitive windows for effects of air pollution on ASD, we constructed an exposure lag space by incorporating weekly averages of daily , , and estimates at each mother’s residence throughout the 37-wk gestational period (Gasparrini et al. 2010). Specially, we fit Cox proportional hazard models with a DLM for each pollutant to estimate weekly ASD hazard ratios (HRs) associated with estimated , , and while adjusting for the abovementioned covariates. Follow-up time was calculated from 1 year of age to a clinical diagnosis of ASD, last date of KPSC health plan membership, death, or 31 December 2019, whichever occurred first. Standard errors were estimated using robust sandwich estimators to control for potential correlation for families. Models incorporated data from all weeks simultaneously and estimated associations between outcome and exposure for a given week after controlling for exposure at all other weeks, assuming association varies smoothly as a function of week. We modeled this smooth function using natural splines (Raz et al. 2018; Wu et al. 2018). The degree of freedom (df) for the natural spline was chosen based on the lowest Akaike information criterion (AIC) value. Based on AIC, the linear model provided the best fit for and ; a model with 3 df provided the best fit for . A sensitive window was defined as the 95% confidence interval (CI) of HR that did not contain 1. We ran DLMs stratified by sex to identify sensitive windows of prenatal air pollution exposure on ASD separately for both boys and girls. As a post hoc data analysis, we fitted a multivariable Cox regression model and tested for interaction with sex by including an air interaction term using air pollution level averaged over the DLM-identified sensitive window. The proportional hazards assumption of the Cox proportional hazard model was assessed using the Schoenfeld residual plot of the average entire pregnancy and sensitive window exposures. No clear nonrandom patterns against follow-up time were observed.
We conducted several sensitivity analyses: a) censoring follow-up time up to 5 years of age such that all children reached the minimum of 5 years of age with a maximum follow-up until 5 years of age, b) extending prenatal air pollution exposure to full term by restricting the sample to children delivered at wk of gestation, and c) adjusting for prepregnancy obesity and prepregnancy diabetes, which were associated with ASD in this cohort in a previous study (Xiang et al. 2015).
DLMs were implemented using the dlnm package (Gasparrini 2011) and all statistical analyses were performed in R (version 3.5; R Development Core Team). The annotated syntax of the R codes are provided in the Supplemental Material in the section “Example R code of DLM modeling.”
Results
Over a median of 9.0 [interquartile range (IQR: ) y of follow-up after birth, 5,694 children (1.9%) were diagnosed with ASD (Table 1). The follow-up time for ASD children (i.e., the median age of first ASD diagnosis) was 3.5 y (IQR: ). Boys were 4.4 times more likely to be diagnosed with ASD than girls (4,636 boys; 1,058 girls). Children diagnosed with ASD were more likely to be born to slightly older with mothers who were nulliparous, had a history of comorbidities, and lived in a census tract with a lower median income (Table 1).
Table 1.
Characteristics of children, with and without autism spectrum disorder (ASD), Kaiser Permanente Southern California (KPSC) pregnancy cohort 2001–2014.
| Characteristics | Children | ||
|---|---|---|---|
| Overall () | With ASD () | Without ASD () | |
| Sex [ (%)] | |||
| Male | 150,009 (50.9) | 4,636 (81.4) | 145,373 (50.3) |
| Female | 144,928 (49.1) | 1,058 (18.6) | 143,870 (49.7) |
| Follow-up after birth {y [median (IQR)]} | 9.0 (5.9–13.1) | 3.5 (2.6–5.3) | 9.2 (6.0–13.2) |
| Gestational age at birth {wk of gestation [median (IQR)] | 39.0 (38.6–40.0) | 39.0 (38.0–40.0) | 39.0 (38.7–40.0) |
| Maternal age at delivery {y [median (IQR)]} | 30.3 (26.2–34.2) | 31.3 (27.5–35.2) | 30.3 (26.2–34.2) |
| Parity [ (%)] | |||
| 0 | 103,132 (35.0) | 2,351 (41.3) | 100,781 (34.8) |
| 1 | 97,611 (33.1) | 1,858 (32.6) | 95,753 (33.1) |
| 77,866 (26.4) | 1,137 (20.0) | 76,729 (26.5) | |
| Unknown | 16,328 (5.5) | 348 (6.1) | 15,980 (5.5) |
| Maternal Education [ (%)] | |||
| High school or lower | 103,723 (35.2) | 1,710 (30.0) | 102,013 (35.3) |
| Some college | 87,087 (29.5) | 1,802 (31.6) | 85,285 (29.5) |
| College graduate or higher | 101,296 (34.3) | 2,143 (37.6) | 99,153 (34.3) |
| Unknown | 2,831 (1.0) | 39 (0.7) | 2,792 (1.0) |
| Census tract median household annual income [ (%)]a | |||
| 7,590 (2.6) | 265 (4.7) | 7,325 (2.5) | |
| 71,950 (24.4) | 1,666 (29.3) | 70,284 (24.3) | |
| 98,525 (33.4) | 1905 (33.5) | 96,620 (33.4) | |
| 65,372 (22.2) | 1,077 (18.9) | 64,295 (22.2) | |
| 50,930 (17.3) | 770 (13.5) | 50,160 (17.3) | |
| Unknown | 570 (0.2) | 11 (0.2) | 559 (0.2) |
| Race/ethnicity [ (%)] | |||
| Non-Hispanic white | 75,814 (25.7) | 1,304 (22.9) | 74,510 (25.8) |
| Non-Hispanic black | 26,933 (9.1) | 576 (10.1) | 26,357 (9.1) |
| Hispanic | 149,536 (50.7) | 2,838 (49.8) | 146,698 (50.7) |
| Asian/Pacific Islander | 36,629 (12.4) | 833 (14.6) | 35,796 (12.4) |
| Other | 6,025 (2.0) | 143 (2.5) | 5,882 (2.0) |
| History of maternal comorbidity [ (%)]b | 42,452 (14.4) | 986 (17.3) | 41,466 (14.3) |
| Prepregnancy diabetes [ (%)]c | 8,565 (2.9) | 282 (5.0) | 8,283 (2.9) |
| Prepregnancy obesity [ (%)]d | 48,410 (16.4) | 1,205 (21.2) | 47,205 (16.3) |
| Year of birth [ (%)] | |||
| 2001–2005 | 91,714 (31.1) | 1,632 (28.7) | 90,082 (31.1) |
| 2005–2010 | 105,611 (35.8) | 1900 (33.4) | 103,711 (35.9) |
| 2010–2014 | 97,612 (33.1) | 2,162 (38.0) | 95,450 (33.0) |
Note: BMI, body mass index; IQR, interquartile range.
Based on census tract median household income.
diagnosis of heart, lung, kidney, liver disease, or cancer.
Type I and type II diabetes diagnosed before pregnancy.
Prepregnancy .
and concentrations during the study period decreased over time, whereas concentration remained relatively stable (Figure S2). exposure was higher in the wet season [ (IQR: ) vs. ] as was [ (IQR: ) vs. 32.2 (IQR: ) ppb] (Table S2). In contrast, exposure was higher during the dry season [ (IQR: ) vs. 50.1 ]. The average weekly , , and levels at mothers’ residential addresses across all years were (), (), and (), respectively (Table S2). Moderate correlation was observed between weekly and levels () (Table S3). No correlation was observed between yearly average and levels (), although there was a moderate negative correlation in the wet season () (Table S3).
Weekly was positively associated with ASD: The association was strongest early in pregnancy and decreased linearly to birth; associations were significant throughout the first two trimesters (1–27 wk) of pregnancy (Figure 1). The cumulative HR for the first two trimesters was 1.14 per IQR () increase in (95% CI: 106, 123) (Table 2). In sex-stratified analyses, we observed a sensitive exposure window between gestation weeks 1 and 28 among boys [ (95% CI: 1.08, 1.26) per IQR increase in ], but smaller and wider CIs and statistically nonsignificant estimates among girls [ (95% CI: 0.89, 1.26)] (Table 2). From a post hoc Cox analysis using an interaction term for sex and averaged exposure over this sensitive window, we found significant interaction between sex and (). Table 2 also provides the cumulative HR (95% CI) estimates associated with for the entire pregnancy: (95% CI: 1.08, 1.27) overall, (95% CI: 1.09, 1.31) for boys, and (95% CI: 0.86, 1.29) for girls.
Figure 1.
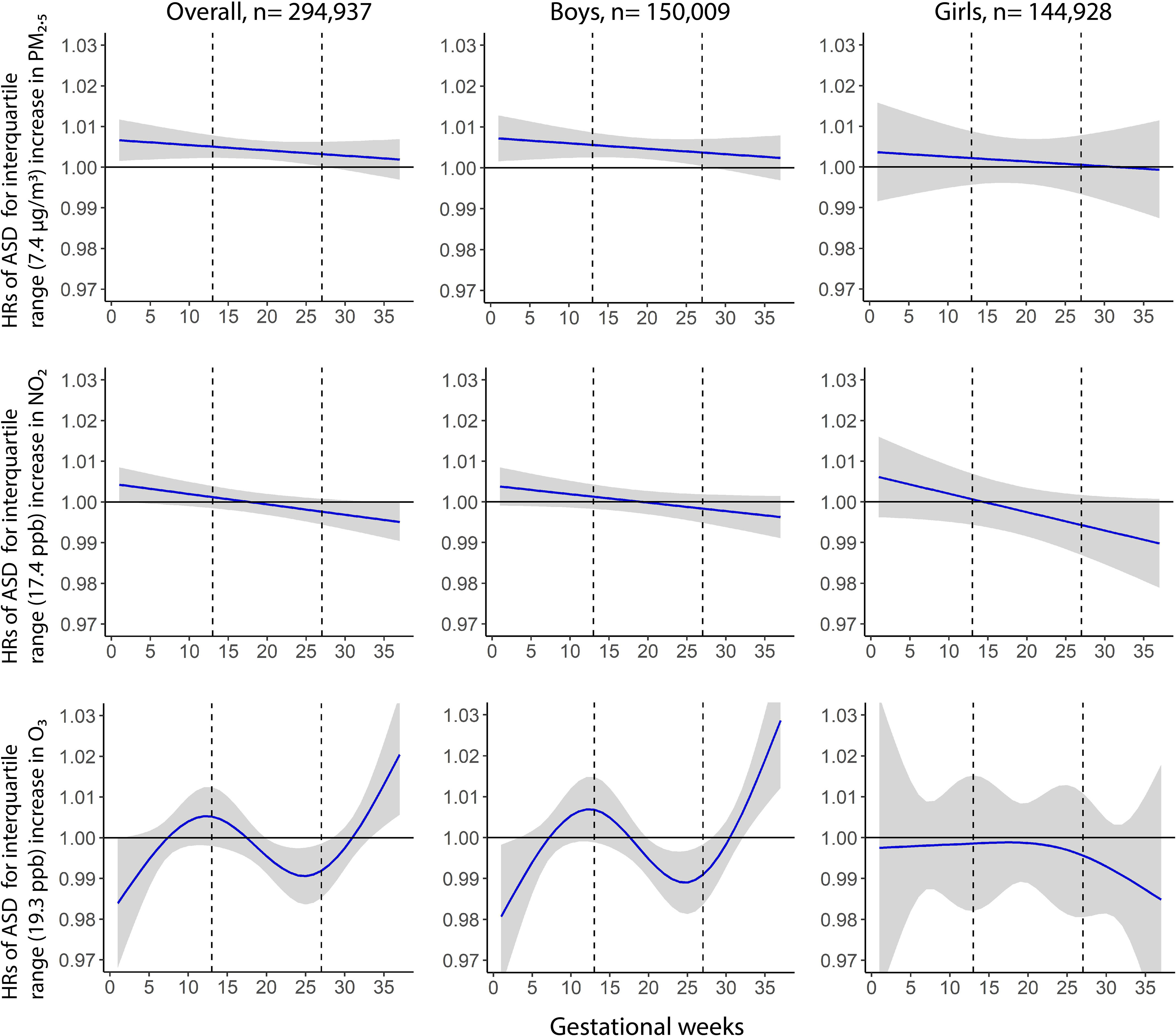
Weekly associated hazard ratios (HRs) associated with weekly , , and exposures over gestation with risk of ASD in the overall cohort (), and separately among boys () and girls (). Gray shade indicates 95% confidence intervals; dashed vertical lines demarcate trimesters. All the models were adjusted for maternal race/ethnicity, maternal age at delivery, parity, education, maternal comorbidities, medical centers, household income, birth year, and season; the model for the Overall cohort was further adjusted for child sex. Note: ASD, autism spectrum disorder; , nitrogen dioxide; , ozone; , fine particulate matter.
Table 2.
Cumulative hazard ratios (HRs) and 95% confidence intervals (CIs) of ASD for prenatal exposures to , , and for the entire pregnancy and DLM-identified sensitive windows.
| Pollutant | HR (95% CI) | |||
|---|---|---|---|---|
| Overall () | Boys () | Girls () | a | |
| (per increase) | ||||
| Entire pregnancy | 1.17 (1.08, 1.27) | 1.19 (1.09, 1.31) | 1.06 (0.86, 1.29) | — |
| Sensitive windows | 1.14 (1.06, 1.23)b | 1.16 (1.08, 1.26)c | 1.06 (0.89, 1.26)d | 0.01 |
| (per increase) | ||||
| Entire pregnancy | 0.99 (0.90, 1.09) | 1.00 (0.90, 1.11) | 0.93 (0.73, 1.15) | — |
| Sensitive windowse | — | — | — | — |
| (per increase) | ||||
| Entire pregnancy | 0.97 (0.85, 1.10) | 0.99 (0.86, 1.15) | 0.86 (0.64, 1.15) | — |
| Sensitive windows | 1.06 (1.01, 1.11)f | 1.10 (1.04, 1.16)g | 0.94 (0.84, 1.05)h | 0.007 |
| 0.93 (0.89, 0.98)i | 0.92 (0.87, 0.98)j | 0.98 (0.87, 1.10)k | 0.68 | |
Note: All the models were adjusted for maternal race/ethnicity, maternal age at delivery, parity, education, maternal comorbidities, medical centers, census tract household income, birth year, and season; the model for the Overall cohort was further adjusted for child sex. Results were scaled to per IQR increase in (), (), and (). —, Not applicable; ASD, autism spectrum disorder; DLM, distributed lag model; IQR, interquartile range; , nitrogen dioxide; , ozone; , fine particulate matter;
was calculated by including an air interaction term using air pollution level averaged over the DLM-identified sensitive window.
HR for the DLM-identified overall sensitive windows 1–27 wk of gestation.
HR for the DLM-identified sensitive windows 1–28 wk of gestation for boys.
HR for the sensitive windows identified for boys (1–28 wk of gestation). No sensitive windows were identified for girls for exposure.
No sensitive windows were identified for exposure.
HR for the DLM-identified sensitive windows 34–37 wk of gestation.
HR for the DLM-identified sensitive windows 33–37 wk of gestation for boys.
HR for the sensitive windows identified for boys (33–37 wk of gestation). No sensitive windows were identified for girls for exposure.
HR for the DLM-identified sensitive windows 20–28 wk of gestation.
HR for the DLM-identified sensitive windows 20–28 wk of gestation for boys.
HR for the sensitive windows identified for boys (20–28 wk of gestation). No sensitive windows were identified for girls for exposure.
No sensitive window of exposure was identified for exposure (Figure 1). The cumulative HR over entire pregnancy for exposure was 0.99 per IQR () increase (95% CI: 0.90, 1.09) (Table 2). We did not find any sensitive windows within any subgroup in sex-stratified analyses (Figure 1).
The weekly exposure association with ASD was curvilinear, with statistically significant increased risk between gestation weeks 34 and 37 but reduced risk between gestation weeks 20 and 28 (Figure 1). The exposure during entire pregnancy was not associated with ASD [cumulative per IQR () increase (95% CI: 0.85, 1.10)] (Table 2). However, the cumulative HR for the sensitive window of 34–37 wk of gestation was 1.06 per IQR increase (95% CI: 1.01, 1.11). The cumulative HR for the sensitive window of 20–28 wk of gestation was 0.93 per IQR increase (95% CI: 0.89, 0.98) (Table 2). In sex-stratified analyses, we observed a sensitive exposure window between gestation weeks 33 and 37 among boys [ (95% CI: 1.04, 1.16) per IQR increase in ], but an inverse association between gestation weeks 20 and 28, and no overall association (Table 2, Figure 1). No association was seen in girls. Post hoc analysis using interaction terms between sex and level averaged over DLM-identified windows (33–37 wk of gestation) also showed significant interaction between sex and (), but not for windows of 20–28 wk of gestation ().
Sensitivity analysis censoring follow-up time at 5 years of age produced results similar to those from the primary analyses (Figure S3). Sensitivity analysis including only births at wk of gestation (total 220,981 singleton children) also identified substantively similar critical windows of exposure (Figure S4). Results from a sensitivity analysis adjusting for prepregnancy obesity and diabetes were similar to those from the primary analyses (Figure S5).
Discussion
Data from this large and population-based multiethnic birth cohort study showed that increased prenatal exposure early in pregnancy was associated with increased risk of ASD in childhood; risk declined throughout pregnancy, and this association was statistically significant through 27 wk of gestation. The significant associations were primarily observed in boys. exposure had a varying relationship with ASD risk throughout pregnancy; was associated with increased risk of ASD in late pregnancy (34–37 wk of gestation) but with reduced risk in midpregnancy (20–28 wk of gestation); and the significant increased risk associated with was observed only in boys. We did not find associations between ASD and exposure.
To our knowledge, this is the first study to combine temporally resolved ambient prenatal , , and exposure estimates at maternal residential addresses with DLM to identify sex-specific susceptible exposure windows associated with ASD risk. The cumulative HR observed in this study for increased exposure during pregnancy was larger than reported in a recent meta-analysis [ (95% CI: 1.08, 1.27) vs. 1.04 (95% CI: 1.00, 1.08) for a increase in ] (Chun et al. 2020), but it was consistent with the results of our previous analysis in a subgroup of this cohort (Jo et al. 2019b). The absence of an association with ASD is consistent with some prior studies (Becerra et al. 2013; Goodrich et al. 2018; Guxens et al. 2016; Jo et al. 2019b; Pagalan et al. 2019) but not with others that observed prenatal exposure was associated with increased ASD risk (Ritz et al. 2018; Volk et al. 2013). One study also using temporally resolved exposure data in a DLM found an inverse association of gestational exposure with ASD during the first and second trimester (Raz et al. 2018).
Although previous studies have linked prenatal exposure with increased ASD risk in children (Jo et al. 2019b; Pagalan et al. 2019; Raz et al. 2015), fetal sex-specific windows of vulnerability have not been well defined. Most prior studies used trimester average exposure, which can introduce bias in estimates because season can covary with these predefined periods (Wilson et al. 2017). Susceptible trimesters identified by prior studies have not been consistent (Becerra et al. 2013; Jo et al. 2019b; Pagalan et al. 2019; Raz et al. 2015; Talbott et al. 2015; Volk et al. 2013). Two studies reported significant association across all three trimesters, with the largest association in the third trimester (Raz et al. 2015; Volk et al. 2013). Two studies did not identify a susceptible trimester (Pagalan et al. 2019; Talbott et al. 2015). However, trimester-specific average exposures may not align well with the critical timing of specific histogenic events, such as neuron production, cell migration, and the initial laying of major fiber tracts by mid-gestation or with narrow windows of developmental susceptibility (Chiu et al. 2016; Levitt 2003). In contrast, the DLMs produced weekly risk estimates that varied smoothly over time and demonstrated the largest estimated effects of exposure early in pregnancy.
Fetal brain development begins early in utero, with neuron production evident by 4–6 wk of gestation, and then proceeds through a sequence of orchestrated, orderly events (Levitt 2003). Thus, identifying the timing of fetal brain development perturbation may clarify the underlying mechanisms of air pollution-mediated disruptions. The observed association between exposure and ASD—strongest early in pregnancy and declining over the course of pregnancy—suggests that the association, if causal, occurred early during gestation but continued at least during 1–27 wk of gestation, spanning neurulation early in pregnancy, neurogenesis, and synaptogenesis (Rice and Barone 2000; Tau and Peterson 2010). These results also suggest that there was no discrete neurodevelopment event occurring in a specific narrow window of exposure that was the target for effects. Disruption by PM exposure has been shown in rodent studies to lead to neuropathological and morphological changes in the brain that adversely impact behavioral function (Cory-Slechta et al. 2018; Zheng et al. 2019). Microglial cells, which serve in part as innate immune cells of the brain, colonize the developing brain by 4 wk of gestation and proliferate extensively through mid-gestation (Menassa and Gomez-Nicola 2018). There is substantial experimental evidence that exposure results in disrupted brain development and neuroinflammatory responses (Kulas et al. 2018; Woodward et al. 2015; Zhang et al. 2018). This aligns with human molecular and anatomical neuropathology studies of brains from individuals with ASD, identifying reactive microglial accumulation and an increase in related gene expression (Gupta et al. 2014; Morgan et al. 2012; Pinto et al. 2014; Vargas et al. 2005; Voineagu et al. 2011). This convergence on microglia and developmental neuroinflammation as risks for ASD reflects an important role for this immunocompetent cell in typical brain development, with disruption altering connectivity and functions that are related to characteristics of ASD (Hong and Stevens 2016; Prinz and Priller 2014; Zhan et al. 2014). exposure also may increase ASD risk by its impact on placental health, which is essential for proper fetal brain development (Zeltser and Leibel 2011). Pathological cellular and molecular placental responses to early pregnancy exposure have been reported (Familari et al. 2019; Maghbooli et al. 2018; Saenen et al. 2015).
is a reactive gas, so developing fetal tissue is not exposed directly during gestation (Frampton et al. 1999). However, a rodent study demonstrated that respiratory oxidative stress and inflammation caused by can affect distant organs (Martínez-Lazcano et al. 2018). Increased exposure has resulted in oxidative stress in brains and altered nervous system function in adult rats (Kodavanti et al. 2021; Martínez-Lazcano et al. 2018). Therefore, associations of with increased ASD risk are biologically plausible. At least five previous epidemiological studies have examined ASD associations with (Becerra et al. 2013; Jo et al. 2019a, 2019b; Kaufman et al. 2019; Volk et al. 2013). Only one reported statistically significant associations: largest with third trimester exposure, consistent with our results (Becerra et al. 2013). One other study reported the strongest associations in the third trimester, albeit with wide CIs (Kaufman et al. 2019). We previously reported that among mothers with gestational diabetes, first trimester exposure was associated with increased ASD risk; however, among the general population of nondiabetic mothers, the third trimester exposure had the largest effect estimate (Jo et al. 2019a). In the present study, we also observed an inverse association between ASD risk and exposure during 20–28 wk of gestation; the biological plausibility of this association is not clear. However, if caused early fetal loss among fetuses that were at high risk for ASD, it is possible that an artifactual protective association with ASD might be observed among surviving children. These results warrant additional epidemiological and animal studies to identify the potential role of gestational exposure timing on risk of ASD.
Our study did not identify sensitive windows of exposure for ASD risk among girls, consistent with prior studies that found larger associations between prenatal exposure and ASD risk in boys (Jo et al. 2019b; Pagalan et al. 2019; Raz et al. 2015; Ritz et al. 2018). Animal studies also support sexual dimorphism in the neurotoxicity of prenatal exposure (Bolton et al. 2013, 2014). Higher rates of ASD in boys compared with girls is a central feature of ASD (Werling and Geschwind 2013). Our results are consistent with exposure contributing to this dimorphism; however, mechanisms of dimorphism for ASD, and specific contributions to this sex difference, remain to be determined.
This study has several strengths that provide additional evidence for the occurrence of exposure during prenatal development being a key contributor to ASD risk. Genetic variation is also a strong determinant of ASD risk, and the combination of the three elements () likely underlies many pediatric disorders (Boyce et al. 2021). The present study leverages a) a large population-based pregnancy cohort with standard diagnostic criteria for ASD; b) high-quality EMRs providing relevant confounders in an ethnically diverse sample of children; c) state-of-the-art hybrid spatiotemporal exposure models with weekly and resolution; and d) change of addresses during pregnancy used to assign air pollution exposures at home. We also acknowledge some limitations. Data on dietary foliate, which has been associated with ASD (Surén et al. 2013), was unavailable. To our knowledge, there is no evidence that maternal folate intake is associated with air pollution, and thus folate is unlikely to be a confounder of air pollution effects on ASD risk. There may be other environmental factors that covary with air pollution, such as noise and temperature; however, to our knowledge, their association with ASD have not been assessed. Mothers’ time–activity patterns were not available. Although we took advantage of the EMRs to identify ASD cases in a large birth cohort, we acknowledge some diagnostic misclassification cannot be avoided.
In conclusion, increased exposure in the first two trimesters and exposure in the late third trimester of pregnancy were associated with ASD risk in children, largely among boys. These results are consistent with experimental animal and human molecular and neuropathology epidemiological studies. The identified sensitive exposure window coincides with early sensitive periods of brain development when the massive diversity of neuronal populations and regional specialization are being established. This early temporal vulnerability to environmental toxicants, when foundational brain architecture is being established, is likely to have the greatest impact on disruption of complex behavior and function. exposure in midpregnancy was found to be associated with reduced ASD risk; the role of exposure on ASD risk merits further investigation. A more definitive characterization of the windows of susceptibility for neurotoxicants will enhance insight into underlying mechanisms.
Supplementary Material
Acknowledgments
The contributions of the authors were as follows. Study conceptualization and design: A.H.X. and R.M.; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: M.M.R. with assistance from A.H.X. and R.M.; statistical analysis: M.M.R., Y-H.S., T.C., S.P.E., A.H.X.; critical revision of the manuscript for important intellectual content: all authors; obtained funding: A.H.X. and R.M.; administrative, technical, or material support: M.P.M., T.C., A.H.X.; supervision: A.H.X. and R.M.; and all authors approved the final draft of the manuscript.
We thank the patients of Kaiser Permanente for helping us improve care through the use of information collected through our electronic health record systems, as well as the Kaiser Permanente and the Utility for Care Data Analysis team within Kaiser Permanente for creating the GEMS Datamart with consolidated addresses histories available to facilitate our research.
This research was supported by National Institutes of Health/National Institute of Environmental Health Sciences [R01 ES029963 (to A.H.X. and R.M.); R56ES028121 (to A.H.X.); P30ES007048 (to R.M. and S.P.E.)] and partly supported by Kaiser Permanente Southern California Direct Community Benefit Funds. J.S. was supported by the U.S. Environmental Protection Agency (RD-835872). F.W.L. is employed by Sonoma Technology, Inc., Petaluma, California. The funding agencies had no role in the design or conduct of the study; in the analysis or interpretation of the data; or in the preparation, review, or approval of the manuscript.
References
- Bai D, Yip BHK, Windham GC, Sourander A, Francis R, Yoffe R, et al. . 2019. Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry 76(10):1035–1043, PMID: , 10.1001/jamapsychiatry.2019.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. 2013. Ambient air pollution and autism in Los Angeles County, California. Environ Health Perspect 121(3):380–386, PMID: , 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KG. 2010. Autism and urbanization. Am J Public Health 100(7):1156–1157, PMID: , 10.2105/AJPH.2009.191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkar B, Pacheco S, Basu R, DeNicola N. 2020. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open 3(6):e208243, PMID: , 10.1001/jamanetworkopen.2020.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: , 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, et al. . 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33(5):972–984, PMID: , 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Auten RL, Bilbo SD. 2014. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav Immun 37:30–44, PMID: , 10.1016/j.bbi.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, et al. . 2013. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect 121(9):1075–1082, PMID: , 10.1289/ehp.1306560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Levitt P, Martinez FD, McEwen BS, Shonkoff JP. 2021. Genes, environments, and time: the biology of adversity and resilience. Pediatrics 147(2):e20201651, PMID: , 10.1542/peds.2020-1651. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2013. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm [accessed 15 January 2020]. [Google Scholar]
- Chiu YHM, Hsu HHL, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. . 2016. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int 87:56–65, PMID: , 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HK, Leung C, Wen SW, McDonald J, Shin HH. 2020. Maternal exposure to air pollution and risk of autism in children: a systematic review and meta-analysis. Environ Pollut 256:113307, PMID: , 10.1016/j.envpol.2019.113307. [DOI] [PubMed] [Google Scholar]
- Coleman KJ, Lutsky MA, Yau V, Qian Y, Pomichowski ME, Crawford PM, et al. . 2015. Validation of Autism spectrum disorder diagnoses in large healthcare systems with electronic medical records. J Autism Dev Disord 45(7):1989–1996, 10.1007/s10803-015-2358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Allen JL, Conrad K, Marvin E, Sobolewski M. 2018. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology 69:217–231, PMID: , 10.1016/j.neuro.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. . 2019. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int 130:104909, PMID: , 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. . 2020. Assessing NO2 concentration and model uncertainty with high spatiotemporal resolution across the contiguous United States using ensemble model averaging. Environ Sci Technol 54(3):1372–1384, PMID: , 10.1021/acs.est.9b03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familari M, Nääv Å, Erlandsson L, de Iongh RU, Isaxon C, Strandberg B, et al. . 2019. Exposure of trophoblast cells to fine particulate matter air pollution leads to growth inhibition, inflammation and ER stress. PLoS One 14(7):e0218799, PMID: , 10.1371/journal.pone.0218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Levitt P, Nelson CA III.. 2010. How the timing and quality of early experiences influence the development of brain architecture. Child Dev 81(1):28–40, PMID: , 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton MW, Pryor WA, Cueto R, Cox C, Morrow PE, Utell MJ. 1999. Aldehydes (nonanal and hexanal) in rat and human bronchoalveolar lavage fluid after ozone exposure. Res Rep Health Eff Inst 90:1–8, PMID: . [PubMed] [Google Scholar]
- Gardener H, Spiegelman D, Buka SL. 2011. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 128(2):344–355, PMID: , 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 2011. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw 43(8):1–20, PMID: , 10.18637/jss.v043.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29(21):2224–2234, PMID: , 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL, et al. . 2018. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res 11(1):69–80, PMID: , 10.1002/aur.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Ellis SE, Ashar FN, Moes A, Bader JS, Zhan J, et al. . 2014. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun 5:5748, PMID: , 10.1038/ncomms6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Ghassabian A, Gong T, Garcia-Esteban R, Porta D, Giorgis-Allemand L, et al. . 2016. Air pollution exposure during pregnancy and childhood autistic traits in four European population-based cohort studies: the ESCAPE project. Environ Health Perspect 124(1):133–140, PMID: , 10.1289/ehp.1408483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Stevens B. 2016. Microglia: phagocytosing to clear, sculpt, and eliminate. Dev Cell 38(2):126–128, PMID: , 10.1016/j.devcel.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Hsu HHL, Chiu YHM, Coull BA, Kloog I, Schwartz J, Lee A, et al. . 2015. Prenatal particulate air pollution and asthma onset in urban children: identifying sensitive windows and sex differences. Am J Respir Crit Care Med 192(9):1052–1059, PMID: , 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Eckel SP, Chen JC, Cockburn M, Martinez MP, Chow T, et al. . 2019a. Gestational diabetes mellitus, prenatal air pollution exposure, and autism spectrum disorder. Environ Int 133(pt A):105110, PMID: , 10.1016/j.envint.2019.105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Eckel SP, Wang X, Chen JC, Cockburn M, Martinez MP, et al. . 2019b. Sex-specific associations of autism spectrum disorder with residential air pollution exposure in a large Southern California pregnancy cohort. Environ Pollut 254(pt A):113010, PMID: , 10.1016/j.envpol.2019.113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast RJ, Levitt P. 2019. Precision in the development of neocortical architecture: from progenitors to cortical networks. Prog Neurobiol 175:77–95, PMID: , 10.1016/j.pneurobio.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JA, Wright JM, Rice G, Connolly N, Bowers K, Anixt J. 2019. Ambient ozone and fine particulate matter exposures and autism spectrum disorder in metropolitan Cincinnati, Ohio. Environ Res 171:218–227, PMID: , 10.1016/j.envres.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney JG, Davis JM, Siegenthaler J, Post MD, Nielsen BS, Hopkins WD, et al. . 2015. DUF1220 protein domains drive proliferation in human neural stem cells and are associated with increased cortical volume in anthropoid primates. Brain Struct Funct 220(5):3053–3060, PMID: , 10.1007/s00429-014-0814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PRS, Valdez M, Richards JE, Agina-Obu DI, Phillips PM, Jarema KA, et al. . 2021. Ozone-induced changes in oxidative stress parameters in brain regions of adult, middle-age, and senescent Brown Norway rats. Toxicol Appl Pharmacol 410:115351, PMID: , 10.1016/j.taap.2020.115351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, et al. . 2012. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J 16(3):37–41, PMID: , 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulas JA, Hettwer JV, Sohrabi M, Melvin JE, Manocha GD, Puig KL, et al. . 2018. In utero exposure to fine particulate matter results in an altered neuroimmune phenotype in adult mice. Environ Pollut 241:279–288, PMID: , 10.1016/j.envpol.2018.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, et al. . 2016. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS One 11(9):e0161851–27, PMID: , 10.1371/journal.pone.0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leni Z, Künzi L, Geiser M. 2020. Air pollution causing oxidative stress. Curr Opin Toxicol 20–21:1–8, 10.1016/j.cotox.2020.02.006. [DOI] [Google Scholar]
- Levitt P. 2003. Structural and functional maturation of the developing primate brain. J Pediatr 143(4 suppl):S35–S45, PMID: , 10.1067/S0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. . 2020. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2016. MMWR Surveill Summ 69(4):1–12, PMID: , 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghbooli Z, Hossein-Nezhad A, Adabi E, Asadollah-Pour E, Sadeghi M, Mohammad-Nabi S, et al. . 2018. Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS One 13(7):e0199772, PMID: , 10.1371/journal.pone.0199772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Lazcano JC, González-Guevara E, Custodio V, Pérez-Severiano F, Olvera-Pérez K, Salgado-Mozo S, et al. . 2018. Activity of nitric oxide synthase isoforms in acute brain oxidative damage induced by ozone exposure. Nitric Oxide 75:42–52, PMID: , 10.1016/j.niox.2018.02.004. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. 2016. Sex differences in the developing brain as a source of inherent risk. Dialogues Clin Neurosci 18(4):361–372, PMID: , 10.31887/DCNS.2016.18.4/mmccarthy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menassa DA, Gomez-Nicola D. 2018. Microglial dynamics during human brain development. Front Immunol 9:1014, PMID: , 10.3389/fimmu.2018.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Zanardo V, Suppiej A. 2013. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Neonatal Med 26(3):259–262, PMID: , 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Clowry GJ, Šestan N, Alzu’bi A, Bakken T, Hevner RF, et al. . 2019. New insights into the development of the human cerebral cortex. J Anat 235(3):432–451, PMID: , 10.1111/joa.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP. 2012. Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res 1456:72–81, PMID: , 10.1016/j.brainres.2012.03.036. [DOI] [PubMed] [Google Scholar]
- Pagalan L, Bickford C, Weikum W, Lanphear B, Brauer M, Lanphear N, et al. . 2019. Association of prenatal exposure to air pollution with autism spectrum disorder. JAMA Pediatr 173(1):86–92, PMID: , 10.1001/jamapediatrics.2018.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. . 2014. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet 94(5):677–694, PMID: , 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. 2014. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15(5):300–312, PMID: , 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Raz R, Levine H, Pinto O, Broday DM, Yuval , Weisskopf MG. 2018. Traffic-related air pollution and autism spectrum disorder: a population-based nested case-control study in Israel. Am J Epidemiol 187:717–725, PMID: , 10.1093/aje/kwx294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, et al. . 2015. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case–control analysis within the Nurses’ Health Study II cohort. Environ Health Perspect 123(3):264–270, PMID: , 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requia WJ, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, et al. . 2020. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol 54(18):11037–11047, PMID: , 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S Jr.. 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(suppl 3):511–533, PMID: , 10.2307/3454543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Liew Z, Yan Q, Cuia X, Virk J, Ketzel M, et al. . 2018. Air pollution and autism in Denmark. Environ Epidemiol 2(4):e028, PMID: , 10.1097/EE9.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa MJ, Just AC, Guerra MS, Kloog I, Hsu HHL, Brennan KJ, et al. . 2017. Identifying sensitive windows for prenatal particulate air pollution exposure and mitochondrial DNA content in cord blood. Environ Int 98:198–203, PMID: , 10.1016/j.envint.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylaarsdam L, Guemez-Gamboa A. 2019. Genetic causes and modifiers of autism spectrum disorder. Front Cell Neurosci 13:385, PMID: , 10.3389/fncel.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenen ND, Plusquin M, Bijnens E, Janssen BG, Gyselaers W, Cox B, et al. . 2015. In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an ENVIRONAGE birth cohort study. Environ Health Perspect 123(8):834–840, PMID: , 10.1289/ehp.1408549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, et al. . 2014. Patches of disorganization in the neocortex of children with autism. N Engl J Med 370(13):1209–1219, PMID: , 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. . 2013. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 309(6):570–577, PMID: , 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott EO, Arena VC, Rager JR, Clougherty JE, Michanowicz DR, Sharma RK, et al. . 2015. Fine particulate matter and the risk of autism spectrum disorder. Environ Res 140:414–420, PMID: , 10.1016/j.envres.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. 2010. Normal development of brain circuits. Neuropsychopharmacology 35(1):147–168, PMID: , 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. 2005. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57(1):67–81, PMID: , 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. . 2011. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474(7351):380–384, PMID: , 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. 2013. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70(1):71–77, PMID: , 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Geschwind DH. 2013. Sex differences in autism spectrum disorders. Curr Opin Neurol 26(2):146–153, PMID: , 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2016. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 10 January 2020].
- Wilson A, Chiu YHM, Hsu HHL, Wright RO, Wright RJ, Coull BA. 2017. Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol 186(11):1281–1289, PMID: , 10.1093/aje/kwx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N, Finch CE, Morgan TE. 2015. Traffic-related air pollution and brain development. AIMS Environ Sci 2(2):353–373, PMID: , 10.3934/environsci.2015.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO. 2017. Environment, susceptibility windows, development, and child health. Curr Opin Pediatr 29(2):211–217, PMID: , 10.1097/MOP.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Jiang B, Zhu P, Geng X, Liu Z, Cui L, et al. . 2018. Associations between maternal weekly air pollutant exposures and low birth weight: a distributed lag non-linear model. Environ Res Lett 13:024023, 10.1088/1748-9326/aaa346. [DOI] [Google Scholar]
- Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. . 2015. Association of maternal diabetes with autism in offspring. JAMA 313(14):1425–1434, PMID: , 10.1001/jama.2015.2707. [DOI] [PubMed] [Google Scholar]
- Xu G, Strathearn L, Liu B, O’Brien M, Kopelman TG, Zhu J, et al. . 2019. Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatr 173(2):153–159, PMID: , 10.1001/jamapediatrics.2018.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Deng F, Guo X, Lv P, Zhong M, Liu C, et al. . 2012. Association of systemic inflammation with marked changes in particulate air pollution in Beijing in 2008. Toxicol Lett 212(2):147–156, PMID: , 10.1016/j.toxlet.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltser LM, Leibel RL. 2011. Roles of the placenta in fetal brain development. Proc Natl Acad Sci U S A 108(38):15667–15668, PMID: , 10.1073/pnas.1112239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. . 2014. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17(3):400–406, PMID: , 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zheng X, Wang X, Zhao H, Wang T, Zhang H, et al. . 2018. Maternal exposure to PM2.5 during pregnancy induces impaired development of cerebral cortex in mice offspring. Int J Mol Sci 19(1):257, PMID: , 10.3390/ijms19010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang X, Wang T, Zhang H, Wu H, Zhang C, et al. . 2019. Gestational exposure to particulate matter 2.5 (PM2.5) leads to spatial memory dysfunction and neurodevelopmental impairment in hippocampus of mice offspring. Front Neurosci 12:1000, PMID: , 10.3389/fnins.2018.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


