Abstract
Over the past two decades, oncology has seen growing use of newly developed targeted therapies, with less emphasis on traditional chemotherapy based regimens. Although this has resulted in dramatic improvements in progression free and overall survival, challenges in the management of toxicities related to longer term treatment of these therapies have also become evident. Although a targeted approach often exploits the differences between cancer cells and non-cancer cells, overlap in fundamental signaling pathways necessary for the maintenance of function and survival in multiple cell types has resulted in systemic toxicities. In particular, cardiovascular toxicities of targeted therapies are of important concern. In this review, we highlight a number of targeted therapies commonly used across a variety of cancer types, including HER2+ targeted therapies, tyrosine kinase inhibitors, immune checkpoint inhibitors, proteasome inhibitors, androgen deprivation therapies, and MEK/BRAF inhibitors. We present the oncologic indications, heart failure incidence, hypothesized mechanisms of cardiotoxicity and potential mechanistic rationale for specific cardioprotective strategies (Table 1). Regarding our overview of the mechanisms of cardiotoxicity, we focused primarily on studies that evaluated the specific cardiotoxicity of these therapies, rather than the basic science evidence to support effects of a particular signaling pathway on the cardiovascular system.
Keywords: cardio-oncology, cardiotoxicity, heart failure, cardioprotection
Introduction
Over the past two decades, oncology has seen growing use of targeted therapies, with lesser emphasis on traditional chemotherapy-based regimens. Although this has resulted in improvements in progression-free and overall survival, challenges in the management of toxicities related to longer term treatment of these therapies have also become evident. Although a targeted approach often exploits the differences between cancer cells and non-cancer cells, overlap in fundamental signaling pathways necessary for the maintenance of function and survival in multiple cell types has resulted in systemic toxicities. In particular, cardiovascular toxicities, and specifically heart failure (HF) with targeted therapies are of important concern. In this review, we highlight a number of targeted therapies commonly used across a variety of cancer types, including HER2+ targeted therapies, tyrosine kinase inhibitors, immune checkpoint inhibitors, proteasome inhibitors, androgen deprivation therapies, and MEK/BRAF inhibitors. We present the oncologic indications, HF incidence, hypothesized mechanisms of cardiotoxicity and potential mechanistic rationale for specific cardioprotective strategies (Table 1). Regarding our overview of the mechanisms of cardiotoxicity, we focused primarily on studies that evaluated the specific cardiotoxicity of these therapies, rather than the basic science evidence to support effects of a particular signaling pathway on the cardiovascular system.
Table 1:
Cardiotoxicity of Targeted Therapies and Proposed Mechanistic Basis
| Targeted Therapy | Signaling Pathways Hypothesized to be Central to Cardiotoxicity Mechanisms | Cardiovascular Toxicities Directly or Indirectly Related to Heart Failure | Potential Cardioprotective Strategies |
|---|---|---|---|
| HER2+ Targeted Therapies | NRG-1 HER2/ERBB2 HER4/ERRB4 PI3K/Akt MEK/ERK AMPK mTOR |
LVEF declines Heart failure |
Beta-blockers (BB) RAAS inhibitors Metformin Bivalent NRG-1 |
| VEGF Signaling Pathway Inhibitors | Raf/MEK/ERK VEGFR PDGFR c-kit fms-like tyrosine kinase 3 AMPK PI3K/Akt |
Hypertension Heart failure |
Afterload reduction Beta-blockers (BB) Dihydropyridine Calcium channel blockers ACE-I Endothelin receptor antagonists Mitochondrial ROS scavengers |
| Immunotherapy | CTLA-4 PD-1 PD-L1 T cell and macrophage infiltration of myocardium |
Myocarditis Heart Failure Arrhythmias Heart block Accelerated atherosclerosis Cardiac arrest |
Steroids Statins CTLA-4 agonists |
| Proteasome inhibitors | Ubiquitin/proteasome system | Heart Failure LVEF declines Cardiac arrest Arrhythmia |
Neurohormonal therapy Possible effects with metformin |
| Androgen Deprivation Therapy | Androgen receptors Effects on FSH levels Mineralocorticoid excess Cardiometabolic effects |
Ischemic heart disease Hypertension Metabolic syndrome Heart failure |
Anti-hypertensive therapy Statins Metformin Anti-platelet therapy Aldosterone antagonists |
| MEK/BRAF Inhibitor | MEK/ERK | LVEF declines Heart failure Hypertension |
Beta-blockers (BB) RAAS inhibitors |
HER2- human epidermal growth factor receptor 2; NRG-1- Neuregulin-1; PI3K- phosphoinositide 3-kinase; Akt- Ak transforming factor; MEK- Ras/Raf/mitogen-activated-protein kinase kinase; ERK- extracellular signal-regulated kinase; AMPK- adenosine monophosphate-activated protein kinase; mTOR- mammalian target of rapamycin; LVEF- left ventricular ejection fraction; BB- beta-blockers; RAAS- renin-angiotensin-aldosterone system; VEGF- vascular endothelial growth factor; RAF- Raf-1; VEGFR- vascular endothelial growth factor receptor; PDGFR- platelet derived growth factor receptor; ACE-I- Angiotensin converting enzyme inhibitors; ROS- reactive oxygen species; CTLA-4- cytotoxic T lymphocyte associated antigen 4; PD-1- programmed death 1; PD-L1- programmed death 1 ligand; FSH- follicle-stimulating hormone; BRAF v-raf murine sarcoma viral oncogene homolog B1
Anthracyclines
Anthracyclines are amongst the most well established classes of cancer therapies to cause HF. The incidence of HF and asymptomatic left ventricular ejection fraction [LVEF] declines is approximately ~10 to 15% with systematic screening.1, 2 Mechanisms of cardiotoxicity include direct cardiomyocyte cytotoxicity via topoisomerase II-mediated DNA damage, generation of reactive oxygen species, and impaired mitochondrial function. 3, 4. Potential strategies for cardioprotection include concurrent treatment with dexrazoxane, 5 liposomal doxorubicin, or slow infusion. 6 Small studies with neurohormonal therapy, including angiotensin converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARB), aldosterone antagonists, and beta blockers have shown benefit in reducing declines in LVEF.7, 8 Expert consensus recommend prophylactic use of ACEi/ARB and/or beta blockers in patients with normal LVEF and established cardiovascular risk factors who will receive known cardiotoxic agents. 9 Once patients develop a LVEF decline, guideline directed medical therapy for HF is indicated.
HER2+ Targeted Therapies
Oncologic Indications
Amplification and overexpression of the human epidermal growth factor receptor 2 (HER2) drives tumorigenesis through downstream oncogenic signaling and promotion of cellular proliferation, survival, and angiogenesis.10 HER2 is an established target in breast and gastric/gastroesophageal cancers, and is an emerging target in other tumor types. Approximately 1 in 5 patients with breast cancer have HER2-overexpressing tumors; HER2-directed therapies have led to dramatic improvements in oncologic outcomes.11
Multiple classes of HER2-targeted therapies are currently in clinical use. The monoclonal antibodies trastuzumab and pertuzumab are approved for both localized and advanced breast cancer. The antibody-drug conjugate ado-trastuzumab emtansine (T-DM1), which consists of a HER2 monoclonal antibody connected to a cytotoxic antimicrotubule agent, and small molecule tyrosine kinase inhibitors lapatinib, tucatinib, and neratinib, are most commonly used in later-line settings for HER2+ metastatic breast cancer. Trastuzumab deruxtecan is another HER2 monoclonal antibody connected to a cytotoxic topoisomerase I inhibitor that is used in metastatic breast cancer.
Heart Failure Events
Cardiotoxicity with targeted anti-HER2 therapy was recognized in the seminal trials of trastuzumab in breast cancer, with a marked increase risk of HF and LVEF declines with concurrent administration of trastuzumab and anthracyclines. The incidence of LVEF declines has been reported to be 19% in patients with sequential anthracycline therapy.12 A meta-analysis of nearly 12,000 patients enrolled in 10 randomized clinical trials of trastuzumab noted the incidence of asymptomatic LVEF declines was 7.5% and symptomatic HF 1.9%;13 and others have shown that LVEF declines do place patients at an increased risk of subsequent HF.14 Generally speaking, with careful clinical management, there is a high likelihood of recovery from cardiotoxicity related to trastuzumab alone. The HF risk with trastuzumab deruxtecan appears to be low but measurable;15 clinical experience with this drug is still evolving.
Mechanisms of Cardiotoxicity
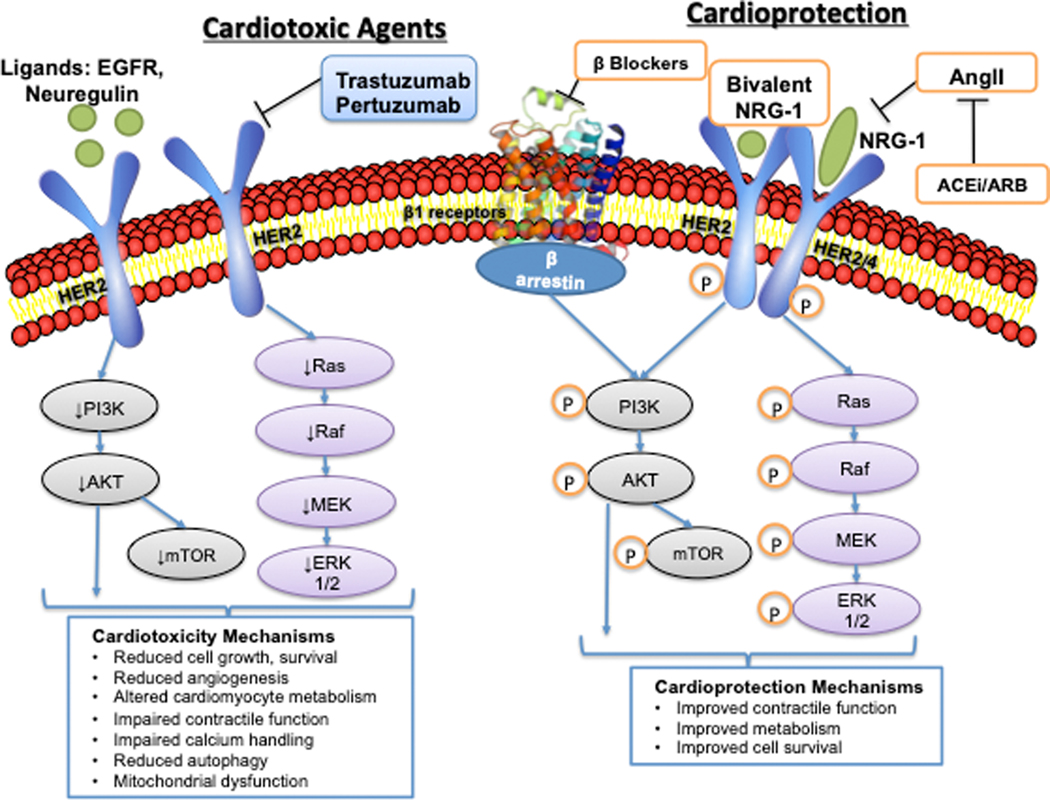
HER2 is a transmembrane tyrosine kinase receptor that belongs to the epidermal growth factor receptor (EGFR) family. Homo or hetero-dimerization leads to downstream signaling that promotes cell growth, proliferation, survival, and angiogenesis (Figure 1). These effects are largely mediated via augmented phosphoinositide 3-kinase (PI3K)/Ak transforming factor (Akt) and Ras/Raf/mitogen-activated-protein kinase kinase (MEK)/extracellular signal-regulated kinases (ERK) signaling (Figure 1). Trastuzumab and pertuzumab bind to the extracellular domain of HER2 and disrupt downstream HER2 signaling via reduced dimerization, antibody-dependent cytotoxicity, and downregulation of the HER2 receptor.16 Neuregulin-1 (NRG-1) is a HER2 ligand secreted by the endothelium that activates pro-survival and growth signaling in cardiomyocytes.17
Figure 1:
Human Epidermal Growth Factor Receptor Targeted Therapies: Mechanisms of Cardiotoxicity and Potential Cardioprotective Mechanisms. Left side: Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase that dimerizes with other members of the epidermal growth factor receptor family to activate downstream signaling that promotes cell survival and proliferation. Ligands of HER2 include epidermal growth factor (EGF) and neuregulin-1 (NRG-1). Trastuzumab and pertuzumab inhibit HER2 signaling, leading to downstream downregulation of PI3K/AKT, mTOR, and Ras/RAF/MEK/ERK signaling. In the cardiovascular system this leads to reduced growth and survival of cardiomyocytes, altered metabolism, reduced angiogenesis, altered calcium handling, reduced autophagy, and mitochondrial dysfunction. Right side: Potential cardioprotective mechanisms include beta-blockers that transactivate β-arrestin, promoting PI3K/Akt signaling in the cardiomyocyte. Additional cardioprotective mechanisms include ACE inhibitors/ARBs that reduce angiotensin-II mediated downregulation of NRG-1, and bivalent NRG-1. HER2- human epidermal growth factor receptor 2; EGF- epidermal growth factor; NRG-1- Neuregulin-1; PI3K- phosphoinositide 3-kinase; Akt- Ak transforming factor; mTOR- mammalian target of rapamycin; RAS- Ras GTPase; RAF- Raf-1; MEK- Ras/Raf/mitogen-activated-protein kinase kinase; ERK- extracellular signal-regulated kinase; ACEi- angiotensin converting enzyme inhibitor; ARB- angiotensin II receptor blocker (Illustration credit: Ben Smith).
The cardioprotective role of NRG-1/HER2 signaling has been studied extensively in animal models and induced pluripotent stem-cell derived cardiomyocytes (iPSC-CM). HER2 and HER4 levels are stable early after pressure overload in vivo, but later decrease by 40–50% as decompensated cardiomyopathy develops. 18 Mice with cardiomyocyte-specific HER2 knockdown developed dilated cardiomyopathy and isolated cardiomyocytes were more susceptible to anthracyclines.19 Recent studies using human iPSC-CMs have demonstrated altered metabolism as a potential mechanism of trastuzumab cardiotoxicity 20. iPSC-CMs treated with trastuzumab or lapatinib, a small molecule inhibitor of HER1/HER2, downregulated genes involved in small molecule metabolism, and had reduced glucose uptake. iPSC-CMs treated with trastuzumab and iPSC-CMs from patients treated with trastuzumab showed impaired contractile and calcium handling after trastuzumab treatment, but no change in sarcomere organization or cell viability as seen in anthracycline cardiotoxicity.21 Functional assays in trastuzumab treated iPSC-CMs demonstrated reduced autophagy, glucose uptake, and mitochondrial function as measured by Seahorse assay. RNAseq identified differentially expressed genes that were enriched in mitochondrial oxidative phosphorylation and cardiac dysfunction/enlargement pathways, including key metabolism genes.21 Treatment with the mTOR inhibitor rapamycin did not ameliorate trastuzumab-induced contractile dysfunction, while multiple pharmacologic agents that augment adenosine monophosphate-activated protein kinase (AMPK) activity (e.g. metformin) improved contraction velocity and metabolism. Other studies have supported the role of reduced autophagy22 and mitochondrial dysfunction23 in trastuzumab-mediated cardiotoxicity. These findings highlight potential mechanisms that can be targeted to prevent and treat trastuzumab cardiotoxicity.
Rationale for Cardioprotective Therapies
Angiotensin converting enzyme inhibitors (ACE-I) and beta-blockers (BB) are the most well-studied agents in trastuzumab cardiotoxicity, and there is mechanistic rationale for their use. The sympathetic nervous and renin-angiotensin-aldosterone systems (RAAS), specifically angiotensin II, downregulate NRG-1 expression in vitro.17 Reduction in angiotensin II levels with RAAS inhibition may promote cardiomyocyte survival pathways in patients receiving HER2 inhibitor therapy. Certain BB (carvedilol, nebivolol, alprenolol) block the beta-1 adrenergic receptor and transactivate β-arrestin.24 β-arrestin, an intracellular protein that transactivates epidermal growth factor receptors (EGFR) such as HER2 in cardiomyocytes, promotes activation of ERK1/2 and AKT kinase and signals through HER1/EGFR.25 β-arrestin-mediated EGFR transactivation has been associated with reduced cardiomyocyte apoptosis under in vivo conditions of cardiac stress. Carvedilol increases recruitment of β-arrestin to cardiac β-adrenergic receptors in vitro thereby stimulating β-arrestin-mediated cellular signaling and cardioprotective pathways. Beta-1 receptors are predominantly found in the heart, kidney, and adipose tissue, representing an opportunity to exploit tissue-specific differences. Interestingly, BB usage has been associated with improved disease specific survival in a meta-analysis including 46,000 breast cancer patients.26 To date, clinical studies of RAAS inhibitors and BB for prevention of HER2 inhibitor-related cardiac dysfunction have shown mixed results, though clinical practice guidelines support their use in high risk patients.27–30 There are also data to support the continuation of HER2+ therapy with neurohormonal therapy in the setting of modest LVEF declines. 31
Novel cardioprotective agents deserve further study. As noted above, multiple pharmacologic agents that augment adenosine monophosphate-activated protein kinase (AMPK) activity have resulted in improved contractile function and metabolism in vitro. Moreover, recombinant NRG-1 merits further investigation as a novel therapeutic for HER2 inhibitor-related cardiac dysfunction. Recombinant NRG-1 has been tested as treatment for systolic HF, and improves cardiac function in animal models of HF.32 Small, early phase clinical trials of recombinant NRG-1 in patients with chronic systolic HF have shown improvement in LV ejection fraction and LV volumes over short-term follow-up.34 Dedicated clinical studies are needed in patients with HER2-inhibitor cardiac dysfunction, with careful attention to cancer outcomes.
EGFR Signaling Pathway Tyrosine Kinase Inhibitors
Oncologic Indications
Since the introduction of imatinib for the molecularly-targeted treatment of chronic myelogenous leukemia nearly two decades ago, use of receptor tyrosine kinase inhibitors (TKIs) has rapidly expanded.35 Small molecule TKIs share potent and efficient competitive inhibition of catalytic binding sites of tyrosine kinase enzymes.36 However, TKI therapies target a wide variety of oncogenic receptor tyrosine kinases, including the vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR), platelet derived growth factor receptor (PDGFR), c-kit, bcr-abl, and fms like tyrosine kinase 3 (FLT3), among others.
TKIs targeting the EGFR include gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib. They are generally used to treat lung cancers that have EGFR mutations. Osimertinib is a third generation EGFR TKI that irreversibly targets the EGFR. This therapy prolongs progression-free survival in patients with EGFR mutation-positive advanced lung cancer.37, 38
Heart Failure Events
In the initial clinical trials for efficacy, 3–5% percent of patients treated with osimertinib had a significant LVEF decline.37, 38 Review of the Federal Drug Administration Adverse Events Reporting System demonstrated a reporting odds ratio of 2.2 (1.5–3.2) for cardiac failure with osimertinib versus first- and second-generation EGFR TKIs.39 Another study noted LVEF declines in 3% and HF in 2%, 40 while case reports have noted HF with reduced ejection fraction.41, 42
Mechanisms of Cardiotoxicity and Rationale for Cardioprotective Therapies
The mechanisms of EGFR TKI cardiotoxicity are hypothesized to be shared with that of HER2-targeted therapies, as they affect the same pro-survival signaling pathways. However, more data are needed regarding the unique mechanisms of osimertinib cardiotoxicity and efficacy of cardioprotective therapies. Currently, there is dearth of data specific to appropriate screening and cardioprotective strategies.
VEGF Signaling Pathway Tyrosine Kinase Inhibitors
Oncologic Indications
TKIs targeting the VEGFR, including sunitinib, pazopanib, axitinib, and cabozantinib, remain a fundamental therapy for many patients with clear cell renal cell carcinoma (ccRCC). The vast majority of ccRCC tumors harbor inactivating mutations in the von Hippel-Lindau (VHL) gene, resulting in accumulation of hypoxia inducible factor (HIF) and inappropriate upregulation of hypoxia-responsive angiogenic genes, including VEGF. Moreover, owing to their multi-kinase inhibitory properties, such TKIs are also commonly utilized for the treatment of thyroid cancers, hepatocellular carcinoma, and pancreatic neuroendocrine tumors.43, 44 In addition to these biomarker agnostic settings, TKI therapies have similarly improved clinical outcomes for molecularly-defined advanced malignancies, including Philadelphia chromosome-positive leukemias, EGFR mutation-positive non small-cell lung cancer, and FLT3-mutated acute myeloid leukemia.45, 46
Heart Failure Events
The most common CV side effect of VEGF signaling pathway inhibitors (VSPI) is hypertension, with an estimated incidence of 20–25% with sunitinib or sorafenib.47, 48 In a prospective study of vascular function during sunitinib treatment, sunitinib led to increased large artery stiffness, resistive load, and pulsatile load.49 Baseline resistive load was associated with diastolic function and elevation of estimated filling pressures over time. Asymptomatic LVEF declines and clinical HF due to left ventricular (LV) dysfunction are prevalent, with an estimated incidence of symptomatic HF and LVEF declines in 2–15%.50 A meta-analysis of ~10,000 patients treated with VEGF signaling pathway inhibitors (VSPI) noted an incidence of all grade HF of 2.4% in those who received TKIs, and 0.75% in those who did not.51 This study included trials of sorafenib, sunitinib, vandetanib, axitinib, and pazopanib. A recent clinical trial of 1900 patients with non-metastatic renal cell carcinoma treated with sunitinib, sorafenib, or placebo in the adjuvant setting noted asymptomatic LV dysfunction was relatively rare with an incidence of 1.9% with sunitinib, 1.4% with sorafenib, and 0.9% with placebo.52 Most of the patients (76%) recovered their LVEF. The lower incidence of LV dysfunction in this study may be secondary to differences in patient population, as most studies of VSPI have been performed in patients with metastatic disease.
Mechanisms of Cardiotoxicity
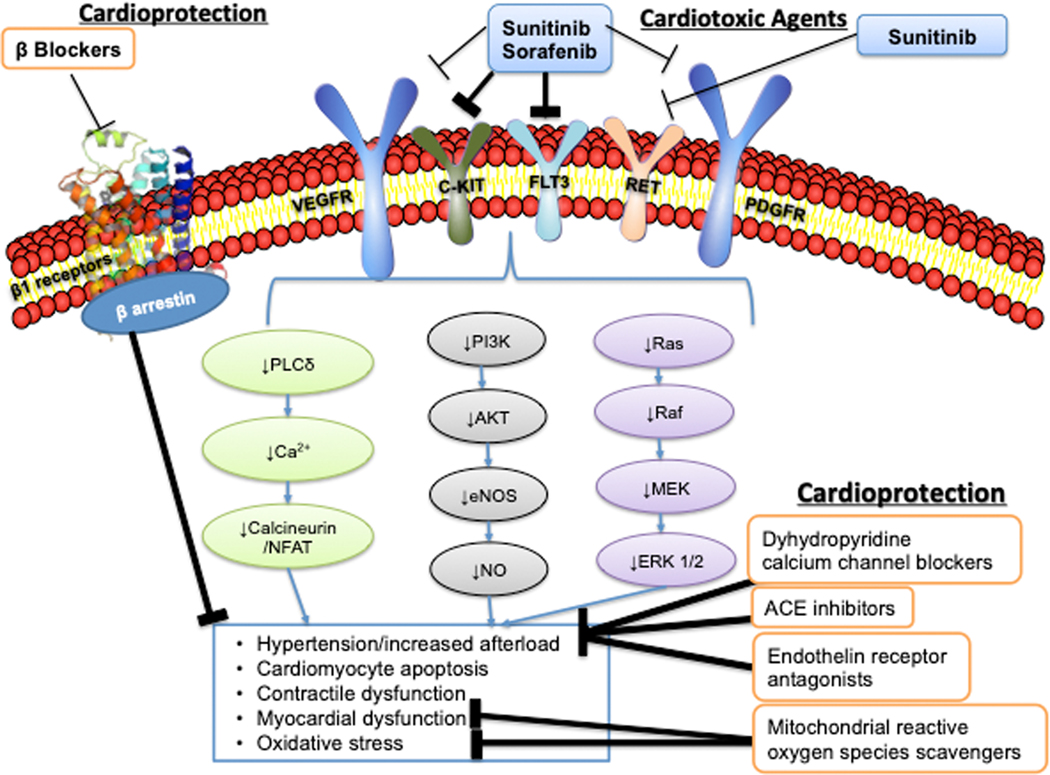
LV dysfunction observed with VSPI is hypothesized to be due to a combination of increased LV pressure overload, combined with the reduction of cardioprotective signaling mechanisms. The most well-studied VSPI in the context of HF are sorafenib and sunitinib. Sorafenib was originally developed as an inhibitor of Raf-1, a member of the RAF/MEK/ERK signaling pathway, but was found to also inhibit the VEGF receptor, the platelet derived growth factor (PDGF) receptor, c-Kit, and fms-like tyrosine kinase-3 (Figure 2).53 Sunitinib inhibits at least 50 kinases in vitro, including the VEGF receptor, PDGF receptor, c-Kit, colony-stimulating factor 1, RET, and fms-like tyrosine kinase-3.54
Figure 2:
Vascular Endothelial Growth Factor Signaling Pathway Inhibitors: Mechanisms of Cardiotoxicity and Potential Cardioprotective Mechanisms. Sunitinib and sorafenib are the most commonly used targeted VEGF pathway signaling inhibitors. They both target multiple tyrosine kinase receptors including the VEGFR, PDGFR, cKit, FLT3, and sunitinib targets RET. Inhibition of these receptors leads to downregulation of pro-survival signaling and angiogenesis, with additional effects on nitric oxide (NO) and calcineurin/NFAT. This leads to hypertension, cardiomyocyte apoptosis, contractile dysfunction, mitochondrial dysfunction, and oxidative stress. Potential cardioprotective mechanisms include dihydropyridine calcium channel blockers, ACE inhibitors, endothelin receptor antagonists, and scavengers of mitochondrial ROS. VEGFR- vascular endothelial growth factor receptor receptor; PDGFR- platelet derived growth factor receptor; FLT3- fms like tyrosine kinase 3; PLCgamma- phospholipase C gamma; NFAT- nuclear factor of activated T cells; PI3K- phosphoinositide 3-kinase; Akt- Ak transforming factor; eNOS- endothelial nitric oxide synthase; NO- nitric oxide; RAS- Ras GTPase; RAF- Raf-1; MEK- Ras/Raf/mitogen-activated-protein kinase kinase; ERK- extracellular signal-regulated kinase; ACEi- angiotensin converting enzyme inhibitor. (Illustration credit: Ben Smith).
Inhibition of the VEGF signaling pathway in animal models of pressure overload contributes to capillary rarefaction, reduced contractile function, and development of HF. Upregulation of VEGF in a large animal model of tachycardia-induced cardiomyopathy led to reduced apoptosis, reduced oxidative stress, prevention of capillary rarefaction, and delayed HF progression. 55 Animals treated with sunitinib developed hypertension and reduced LVEF, and demonstrated proteomic signatures that suggested impaired oxidative metabolism, reduced fatty acid beta-oxidation, and increased reliance on glycolysis.56 These metabolic changes occurred early in the treatment course and improved over time. Histologic analysis demonstrated lipid accumulation in the cardiomyocytes, while mitochondrial structure was preserved. Early in the treatment course, myocardial perfusion defined by myocardial positron emission tomography (PET) imaging was reduced, but improved over time. In other studies, human iPSC-CMs treated with sunitinib exhibited increased apoptosis, decreased contractile force generation, and mitochondrial dysfunction.57 Apoptosis was increased in the setting of increased afterload. Reduced AMPK activity has been postulated as an off-target effect of sunitinib leading to cardiotoxicity, though treatment with an AMPK activator did not improve mitochondrial function. Others have demonstrated worsened mitochondrial function, increased mitochondrial reactive oxygen species, and cardiomyocyte apoptosis with sunitinib, which improved after treatment with a mitochondrial ROS scavenger. 58
PDGF can be cardioprotective, especially in the setting of CV stress. Expression of PDGF increases after pressure overload and genetic deletion of PDGF leads to worsened left ventricular remodeling, HF, apoptosis, and reduction in cardioprotective Akt and ERK signaling.59 ERK is the downstream target of MEK, and plays an important role in proliferation, survival, and cardiac hypertrophy (both pathologic and physiologic). Neurohormonal activation upregulates MEK/ERK signaling, most likely as a protective stress response.60
Rationale for Cardioprotective Therapies
Treatment with afterload reducing agents is the mainstay of therapy for VSPI related hypertension and prevention and management of VSPI related cardiac dysfunction. There are limited data regarding the most effective agents for management of VSPI-induced hypertension. Based on the data noting increased cardiomyocyte apoptosis with sunitinib, beta-blockers that transactivate β-arrestin and augment pro-survival MEK/ERK signaling might have mechanistic rationale. Efforts to target oxidative stress in HF have thus far been unsuccessful, but two strategies to target mitochondrial derived ROS (mitoTEMPO and thioredoxin-2 signaling) have shown promise in animal models of HF.61, 62
In vivo and clinical studies suggest that dihydropyridine calcium channel blockers (CCB), such as amlodipine or felodipine, and ACE-I may be preferred. Sunitinib therapy led to increased arterial stiffness, worse resistive load, and abnormal arterial compliance suggesting that an arterial vasodilators may be beneficial.49 In mice treated with sunitinib, amlodipine mitigated VSPI induced hypertension while sildenafil did not.63 Nondihydropyridine CCBs are not recommended for treatment of VSPI related hypertension due to a CYP3A4 drug interaction. A separate in vivo study found that increases in afterload, concentric LV remodeling, and plasma angiotensin II levels on VSPI were all prevented by treatment with ramipril.64 Retrospective clinical studies suggest dihydropyridine CCBs and ACE-I are effective for treatment of VEGF-inhibitor related hypertension,65–68 though prospective studies are needed. While the endothelin receptor antagonists macitentan and tezosentan have prevented VSPI related hypertension in vivo, these agents have not been studied in patients.69
Immunotherapy
Oncologic Indications
Immunotherapy including immune checkpoint inhibitors (ICIs) activate the immune system to recognize cancer cells, and have revolutionized the management of many cancers. Many tumors express immune checkpoint molecules including cytotoxic T lymphocyte associated antigen 4 (CTLA-4), programmed death 1 (PD-1) and its ligand (PD-L1) in order to evade anti-tumor immunosurveillance and immune-mediated cytotoxicity. ICIs are monoclonal antibodies against these molecules that disrupt this inhibitory signaling and “unleash the brakes” on anti-tumor immunity 70. These agents have been incorporated into standard first-line therapy for advanced melanoma, head and neck squamous cell carcinoma, lung cancer, and renal cell carcinoma.71, 72 For other solid malignancies, ICIs are approved for use in later line or restricted to patients with biomarkers that predict for efficacy of immunotherapy, such as PD-L1 expression, mismatch repair deficiency, or mutations in DNA repair pathway genes.
Chimeric antigen receptor T cell therapies (CAR-T), another type of immune cancer-directed therapy, have shown remarkable success in treating highly refractory hematologic malignancies. In this process, autologous T cells are collected from a patient and genetically modified via a viral vector and transduction of a chimeric receptor antibody (CAR) that recognizes a tumor-specific antigen such as CD19. After ex-vivo expansion, this genetically modified CAR-T cell population is infused back to the patient. Currently, two CD19-specific CAR-T cell therapies (axicabtagene ciloleucel/Yescarta, Gilead Sciences; tisangenleclucel/Kymriah, Novartis) have been approved for use in relapsed/refractory lymphomas,73, 74 and others are in development. CAR-T therapy is generally administered in a monitored setting due to high risk for serious toxicities including neurotoxicity and cytokine release syndrome, which can require intensive care.
Heart Failure Events
CV toxicity from ICIs include myocarditis, pericarditis and pericardial effusion, HF, arrhythmias, heart block, accelerated atherosclerosis and cardiac arrest. Studies of the incidence of CV toxicities are heterogeneous due to differences in cardiotoxicity monitoring. A recent meta-analysis reported a cumulative incidence of cardiotoxicity as 7%, including an atrial fibrillation incidence of 4.6%.75 Other studies have reported a myocarditis incidence as ~1%. 76 Though myocarditis is rare, it accounted for 25% of deaths due to combination ICI therapy in a recent meta-analysis. 77
CV toxicities with CAR-T still remain to be fully elucidated, but arrhythmias, systolic dysfunction, and hypotension have been reported.78 In a retrospective review of 145 patients with B cell malignancies, 31 patients developed adverse CV events, including 21 who developed HF, and 11 atrial fibrillation. 79 The development of CV events typically was associated with severe cytokine release syndrome (CRS).
Mechanisms of Cardiotoxicity
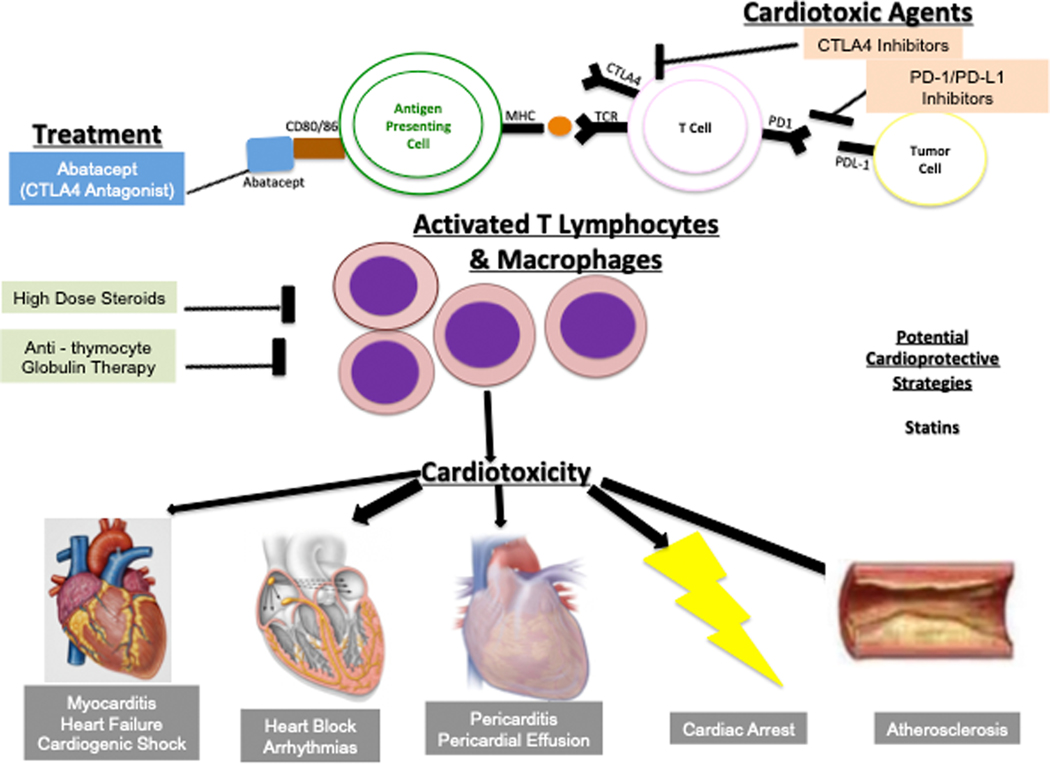
ICIs work by driving an anti-tumor CD4+ and CD8+ T cell response, by antagonizing the inhibitory function of CTLA-4 and PD-1 (Figure 3). Several mechanisms work in concert to ensure immune tolerance. Central tolerance is the process by which T cells that recognize self undergo apoptosis in the thymus. Peripheral tolerance is mediated by apoptosis of autoreactive T cells, and regulation of the co-stimulatory signals required to activate T cells in the periphery. Both CTLA-4 and PD-1 inhibit co-stimulatory signals necessary for activation of T cells.
Figure 3:
Immune Checkpoint Inhibitors: Mechanisms of Cardiotoxicity and Potential Treatments. Inhibitors of CTLA-4 and PD-1/PD-L1 are designed to activate anti-tumor T lymphocytes to harness the immune system to attack cancer cells. This leads to several autoimmune off-target effects including cardiotoxicity affecting the myocardium, pericardium, and conduction system. Immune checkpoint inhibitors can cause myocarditis, heart failure, cardiogenic shock, heart block, arrhythmias, pericarditis, pericardial effusion, cardiac arrest, and accelerated atherosclerosis. Potential treatment options include high dose steroids, abatacept (a CTLA-4 agonist), and anti-thymocyte globulin, while statins may be cardioprotective via pleiotropic effects. CTLA-4- cytotoxic T lymphocyte associated antigen 4; PD-1- programmed death 1; PD-L1- programmed death 1 ligand; TCR- T cell receptor; MHC- major histocompatibility complex. (Illustration credit: Ben Smith)
The impact of ICIs on the heart and other organs in animal models has been known for decades. Mice lacking CTLA-4 develop profound T cell and macrophage infiltration of multiple organs, leading to severe myocarditis and increased mortality.80 While CTLA-4 deficient mice develop a fatal lymphoproliferative disorder with systemic organ involvement, mice with PD-1 deficiency exhibit more targeted autoimmunity, particularly in the heart. Mice with genetic knockdown of PD-1 or PD-L1 spontaneously develop dilated cardiomyopathy and expressed autoantibodies to cardiac troponin I.81 Mice had increased mortality and exhibited myocarditis with dense infiltration of macrophages and T cells.82
Early case reports of fulminant ICI myocarditis noted significant infiltration of the myocardium with T lymphocytes (both CD4+ and CD8+) and macrophages, affecting the conduction system as well.83 Skeletal muscle, but not smooth muscle, were also affected. Transcriptomics demonstrated expression of muscle-specific transcripts in the tumors, suggesting that there may be shared epitopes between the tumor and striated muscle.
Rationale for Cardioprotective Therapies
Optimal strategies to prevent ICI-induced cardiac dysfunction and CV adverse events are currently unknown, although it is hypothesized that vasculoprotective therapies may be beneficial. The PD-1/PD-L1 pathway downregulates proatherogenic T cell responses within atherosclerotic plaque, and PD-1 deficiency is associated with increased aortic atherosclerotic burden and lesional T cell infiltration in murine models.84 Similarly, CTLA-4 overexpression is associated with reduced atherosclerotic lesion formation and reduced lesional accumulation of macrophages and T-cells.85 Studies of ICI on atherosclerosis in animal models have suggested that combination ICI therapy results in T-cell mediated plaque progression. 86 A retrospective study reported an increased risk of atherosclerotic CV events on ICI therapy, and statins or corticosteroids reduced plaque volume.87 Statins reduce production of pro-inflammatory cytokines and increase regulatory T cells in atherosclerotic plaques,88 while dexamethasone reduces aortic atherosclerotic plaque burden and lesional T-cell burden.89 Prospective studies are needed to determine the clinical and translational relevance of these findings. Treatment of ICI-induced myocarditis consists of potent anti-inflammatory therapy, as detailed in recent reviews.70
CAR-T cell therapy has been associated with adverse CV events including symptomatic HF in observational studies, particularly in patients who suffer CRS after CAR-T cell infusion.79,91 Treatment of severe CRS can include tocilizumab, an anti-IL6 monoclonal antibody, approved for treatment of CRS.92 Early treatment of CRS with tocilizumab and/or corticosteroids has been shown to improve outcomes and mitigate the toxicity profile without compromising anti-cancer efficacy. Additionally, delay in tocilizumab administration from CRS onset is associated with increased risk of adverse CV events.91
Proteasome Inhibitors
Oncologic Indications
The proteasome, a multicatalytic protein complex, is involved in the degradation of a majority of intracellular proteins. Through the highly regulated ubiquitin/proteasome system (UPS) pathway, targeted proteins are tagged with ubiquitin, recognized by the proteasome complex, unfolded, and cleaved into peptide products. Many oncogenic proteins involved in tumorigenesis and cancer progression, including p53, cyclins, and cyclin-dependent kinases, are degraded by the proteasome complex.93 Proteasome inhibitors (PIs), which target the catalytic subunits of the proteasome, are believed to significantly affect protein turnover, which proves toxic to malignant cells. While the anti-cancer effects of PIs remain unclear, this may occur through the faulty degradation of tumor suppressors, impairment of cell division and resultant cell death, and the accumulation of misfolded proteins which incite the unfolded protein response and cellular apoptosis.93 Over the last two decades, multiple PIs, including bortezomib, carfilzomib, and ixazomib, have been approved for the treatment of hematologic malignancies, including multiple myeloma and mantle cell lymphoma.94–96
Heart Failure Events
The incidence of cardiotoxicity with bortezomib is 2.6–5.6% for all-grade cardiotoxicity, based on a recent meta-analysis of 25 clinical trials and 5718 patients.97 Cardiotoxicity included LVEF declines, LV dysfunction, HF, cardiomyopathy, cardiac arrest, and arrhythmia. The majority of the events were HF related. A recent meta-analysis of 29 clinical trials of 4164 patients noted an incidence of carfilzomib cardiotoxicity, defined as acute coronary syndromes, acute HF or cardiomyopathy, arrhythmia, and cardiac arrest, of 6.1–11.6%, with an odds ratio of 2 for any cardiotoxicity. 98 The investigators separately reported the incidence of hypertension as 7.7–16.0% based on an analysis of 1905 patients. The incidence of HF alone secondary to carfilzomib is 4–7%100,101, although a more precise understanding of the predominant HF subtype (i.e., reduced or preserved LVEF) is needed.102,103 The risk with carfilzomib versus bortezomib appears to be greater, potentially due to carfilzomib’s irreversible inhibition. Ixazomib is an oral, reversible PI and is not known to increase the risk of HF; however, clinical experience is more limited.
Mechanisms of Cardiotoxicity
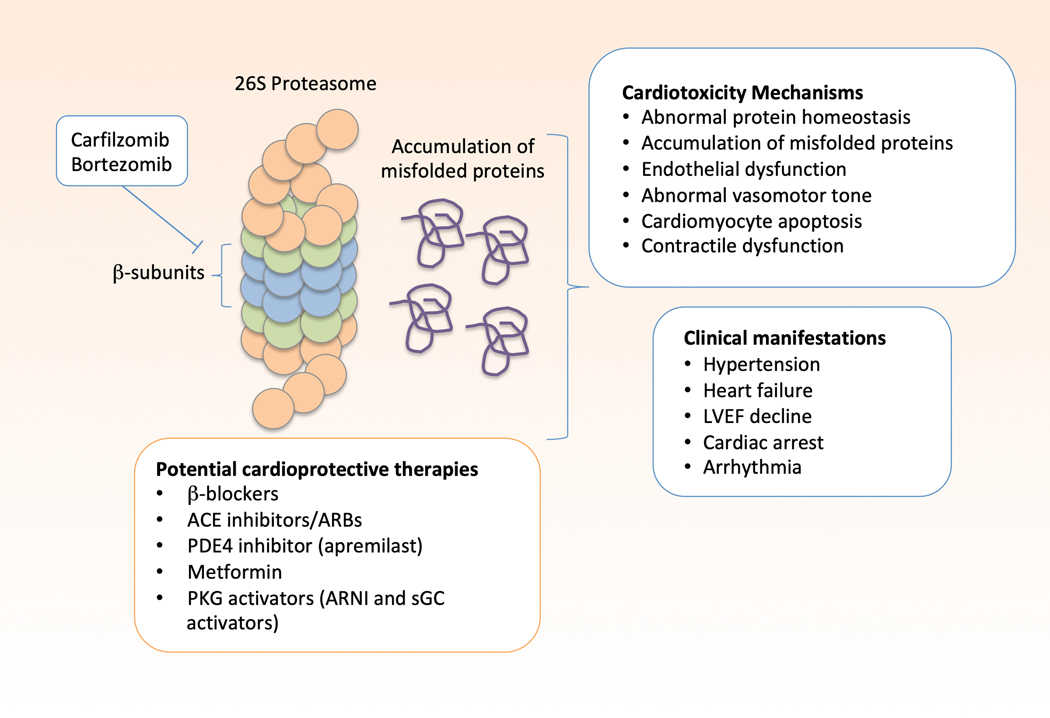
Bortezomib is a reversible inhibitor of the 26S proteasome, specifically targeting the proteolytic β-type subunits of the core particle, 104 while carfilzomib is an irreversible inhibitor of the same complex (Figure 4). The UPS plays an important role in protein homeostasis, degrading misfolded or damaged proteins. Patients with end-stage HF and hypertrophic cardiomyopathy have reduced proteasome activity in the myocardium, particularly patients with a sarcomere mutation. 105 Unloading of the LV with a ventricular assist device improved proteasome activity. 106 PIs may lead to endothelial dysfunction that underlies the vascular and blood pressure effects. 107 Cardiomyocytes are particularly susceptible to proteotoxicity due to their high protein turnover of the contractile proteins in the sarcomere. In vitro studies demonstrate direct cardiomyocyte toxicity after treatment with carfilzomib or bortezomib, leading to cardiomyocyte apoptosis. 108 In vivo studies of mice treated with carfilzomib have shown a reduction in proteasome activity in the heart and peripheral blood cells, along with a reduction in left ventricular function. Carfilzomib significantly increased PP2A phosphatase activity and reduced activation or expression of adenosine monophosphate kinase (AMPK), Raptor, LC3-II, PI3K, Akt, and eNOS. Other studies of genetically modified proteasome activity have demonstrated similar contractile dysfunction, particularly in the setting of other stressors. 109, 110 Many patients treated with proteasome inhibitors have pre-existing CV comorbidities, likely increasing susceptibility to cardiotoxic effects of PIs.
Figure 4:
Proteasome Inhibitors: Mechanisms of Cardiotoxicity and Potential Treatments. Carfilzomib and bortezomib inhibit the catalytically active beta-subunits of the 26S proteasome, impairing its ability to break down and recycle misfolded proteins. This leads to the accumulation of misfolded proteins and cellular proteotoxicity. This leads to abnormal protein homeostasis, accumulation of misfolded proteins, endothelial dysfunction, abnormal vasomotor tone, cardiomyocyte apoptosis, and contractile dysfunction. Clinical manifestations of proteasome inhibitor cardiotoxicity include hypertension, heart failure with preserved or reduced ejection fraction, left ventricular ejection fraction decline, arrhythmias, and cardiac arrest. Activators of protein kinase G (PKG) may counter these effects by activating the proteasome. Other potentially cardioprotective therapies include beta-blockers, ACE inhibitors, ARBs, apremilast, and metformin. PKG-protein kinase G; ARNI- angiotensin receptor-neprilysin inhibitor; sGC-soluble guanylate cyclase; ACEi- angiotensin converting enzyme inhibitor; ARB- angiotensin II receptor blocker; PDE4-phosphodiesterase 4; LVEF, left ventricular ejection fraction
Rationale for Cardioprotective Therapies
Multiple myeloma and light chain amyloidosis are among the most common indications for PIs, and patients with these conditions are at increased risk for HF in part due to light chain deposition within the myocardium. BB and RAAS inhibitors are currently the standard of care for patients with proteasome inhibitor-related cardiac dysfunction but these therapies are typically not well tolerated by patients with light chain amyloidosis. There are no data to support their use for primary prevention of LVEF declines. Carfilzomib, an irreversible inhibitor of the proteasome, is associated with increased vascular tone and impaired vasodilation,111 and strict blood pressure control is warranted though the optimal antihypertensive agent is not known. In mouse models, metformin prevented carfilzomib-induced cardiac dysfunction and improved vascular function by restoring AMPKα phosphorylation and autophagic signaling in cardiomyocytes and smooth muscle cells.112,113 However, the effect of metformin in humans receiving carfilzomib has not been studied. The bioflavinoid rutin and phosphodiesterase-4 inhibitor apremilast were found in vivo to mitigate carfilzomib-induced increases in expression of caspase-3, which participates in apoptosis signaling, suggesting possible utility for cardioprotection.114, 115
Protein kinase G is a cardioprotective kinase with pleotropic effects on the CV system, including enhancement of proteasome activity via phosphorylation and activation of key components of the proteasome.116,117 This signaling pathway is manipulated by multiple HF therapies, including vericiguat and neprilysin inhibitors, both of which have demonstrated a beneficial effect in the reduction of CV events in the general HF population, however these have never been tested in proteasome inhibitor cardiotoxicity.
Androgen Deprivation Therapy and Androgen Receptor Signaling Inhibitors
Oncologic Indications
Systemic androgen deprivation, most commonly through the use of gonadotropin-releasing hormone (GnRH) agonists or antagonists, has remained the mainstay therapy for men with advanced prostate cancer and those receiving curative-intent radiation therapy. Testosterone, and its potent metabolite dihydrotestosterone, bind to prostate cancer intracellular androgen receptors, upregulating expression of oncogenic target genes.119 Given this androgen-dependence, lifelong androgen deprivation therapy (ADT) remains standard practice for most men with advanced prostate cancer.
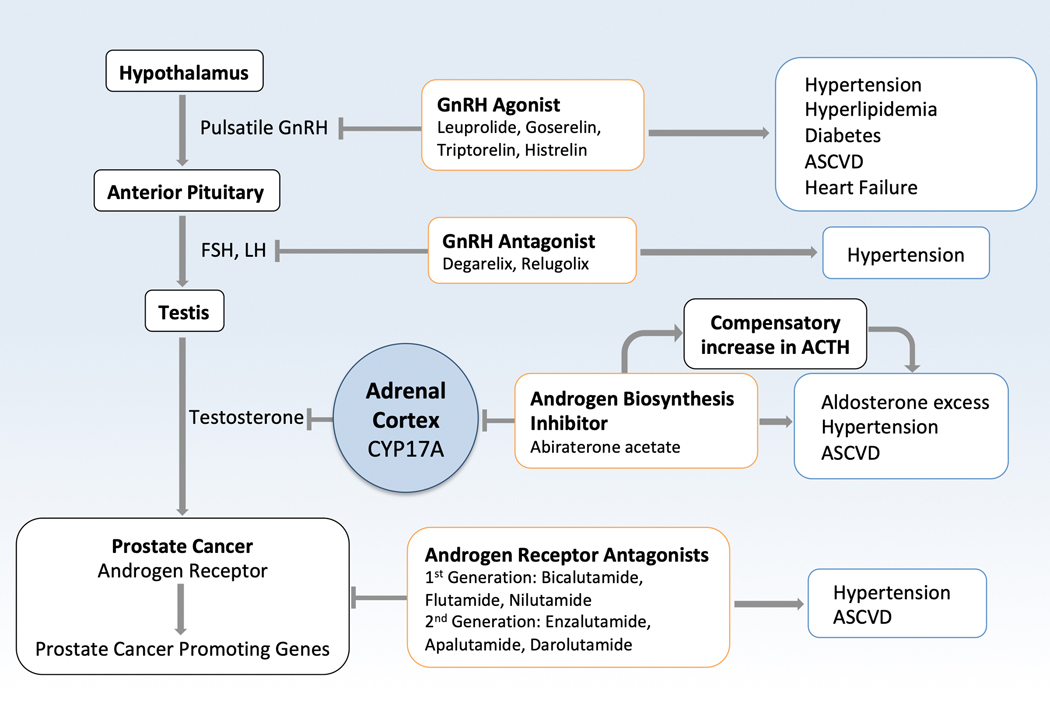
GnRH agonists, also called luteinizing hormone-releasing or LHRH agonists, are the most commonly used form of androgen deprivation therapy (Figure 5). More recently, an improved understanding of androgen-dependent ADT resistance mechanisms has led to the development of secondary androgen-receptor signaling inhibitors (ARSIs), including androgen biosynthesis inhibitors, which blunt production of extragonadal androgens, and potent androgen receptor antagonists. Abiraterone reduces the production of androgens by inhibiting CYP17A, which is expressed in adrenal and prostate tumor cells and required for androgen biosynthesis. By inhibiting CYP17A1 enzymes, abiraterone results in a compensatory increase in ACTH and potential treatment-induced symptoms of mineralocorticoid excess (hypertension, hypokalemia, fluid retention). In contemporary prostate cancer clinical management, ARSI therapies, including abiraterone, enzalutamide, apalutamide, and darolutamide, are often utilized earlier in the disease course and for prolonged durations to further attenuate androgen signaling and to enhance oncologic outcomes.120–122
Figure 5:
Androgen Deprivation Therapy and Androgen Receptor Signaling Inhibitors. GnRH agonist and GnRH agonists lead to increased risk of cardiometabolic traits of diabetes, hypertension, hyperlipidemia, and ultimately atherosclerotic cardio- and cerebrovascular disease. Abiraterone inhibits the CYP17A enzyme that leads to conversion of pregnenolone to 17-hydroxyl-pregnenalone, and then to dehydroepiandrosterone (DHEA), ultimately leading to reduced estrogen and testosterone levels. This also leads to accumulation of pregnenolone, which is ultimately converted to aldosterone, contributing to the mineralocorticoid excess seen with abiraterone. Direct androgen receptor antagonists such as enzalutamide, do not have this mineralocorticoid excess, but can still lead to hypertension. Androgen receptor signaling inhibitors also increase the risk of atherosclerotic cardio- and cerebrovascular events. GnRH-gonadotropin-releasing hormone; FSH- follicle-stimulating hormone; LH- luteinizing hormone; Gen- generation
Heart Failure Events
With GnRH agonists and ARSI therapies, there is risk of the development of HF, ischemic heart disease and the worsening of CV risk factors.123 GnRH agonists demonstrated increased risk of atherosclerotic CV events, HF, and sudden cardiac death.124 Several meta-analyses have found an increased risk of cardiac events (1.3–1.4 fold increase, incidence ~14%) with abiraterone but not with enzalutamide, while both increase the risk of hypertension significantly (2 fold increase, overall incidence ~20%).125, 126 Cardiac events were defined in these studies as ischemic heart disease, myocardial infarction, tachyarrhythmia, and HF. 127–130 A large retrospective observational analysis including >45,000 patients treated with androgen deprivation therapy noted an incidence of HF of 1–2% with enzalutamide and abiraterone. 131
In a large randomized phase 3 trial, the oral GnRH antagonist relugolix demonstrated a lower incidence of major adverse cardiovascular events (defined as non-fatal MI, non-fatal stroke, and all-cause mortality) compared to the GnRH agonist leuprolide (2.9% vs 6.2%).132 This difference was most pronounced in patients with underlying cardiovascular disease (3.6% vs 17.8%). The cardiovascular safety of these therapies is being studied in advanced prostate cancer populations. 133
Mechanisms of Cardiotoxicity
Androgen deprivation, through the use of GnRH agonists or antagonists, is associated with several detrimental cardiometabolic effects. Androgen receptor signaling in myocytes is important for maintenance of muscle mass,138 and normal levels of androgens are generally considered protective against atherosclerosis. 139 The mechanisms of atheroprotection in men with normal endogenous levels of testosterone are unclear and complex given its multi-organ, pleiotropic effects. Recent prospective evidence has indicated potential differences in CV risk between GnRH agonist and GnRH antagonist therapies. 140 While the explanatory mechanisms for this CV risk difference are unclear, hypotheses include differential effects on FSH levels post-treatment, atherosclerotic plaque instability, and the presence of GnRH receptors on cardiomyocytes and infiltrating leukocytes. Reduced estrogen synthesis with abiraterone may also play a role, as estrogen has beneficial effects on the cardiovascular system.
Animal models have demonstrated increased blood pressure with androgen supplementation.137 Enzalutamide is an androgen receptor antagonist, while abiraterone selectively inhibits the CYP17 enzyme (17α-hydroxylase and C17,20-lyase), leading to reduced estrogen and testosterone synthesis, reduced cortisol synthesis, increased adrenocorticotropic hormone (ACTH) levels, and secondary mineralocorticoid excess. Abiraterone is co-administered with prednisone in order to limit this positive feedback loop and attenuate mineralocorticoid side effects; the difference in mechanisms of action may explain why abiraterone is associated with increased risk of cardiovascular events compared to enzalutamide, though further study is warranted.
Rationale for Cardioprotective Therapies
Aggressive treatment of CV risk factors, including hypertension, is believed to be important to prevent HF in patients treated with androgen deprivation therapy. Specific therapies have not been tested. Mechanistically, mineralocorticoid receptor antagonists would appear to be appropriate for prevention of abiraterone-induced hypertension. Prospective studies suggest that eplerenone alone may be insufficient to prevent hypertension associated with mineralocorticoid excess syndrome in patients receiving abiraterone acetate,146 and combination antihypertensive therapy may be necessary. Given the association between ADT and arterial vascular events, especially in men with more comorbidities,147 standard primary and secondary prevention approaches to prevent atherosclerotic events are indicated, including statin therapy.148 Aspirin, metformin, and BB therapy have all been associated with reduced mortality in observational studies of men with prostate cancer, though it is unclear if the risk reduction is related to mitigating adverse effects of androgen deprivation therapy or prostate cancer signaling pathways.148–151
MEK/BRAF Inhibitors
Oncologic indication
Mutations in BRAF, a key gene in the mitogen-activated protein kinase (MAPK) pathway involved in cellular growth and proliferation, occur in ~50% of patients with melanoma, 2–8% of patients with non-small cell lung cancer, and ~25% of patients with anaplastic thyroid cancer152. The most common activating BRAF mutations occur at position V600 and lead to constitutive activation of BRAF. BRAF inhibitors including vemurafenib, dabrafenib, and encorafenib are ATP-competitive drugs that selectively bind to the kinase domain of mutant BRAF, rendering it incapable of binding to ATP and phosphorylating MEK, its downstream target153. Alterations in MEK can lead to bypass MAPK pathway activation and resistance to BRAF inhibitor therapy. Thus, dual inhibition of BRAF and MEK maximizes MAPK pathway inhibition and helps to prevent treatment resistance, leading to improved survival outcomes154. MEK inhibitors including trametinib, cobimetinib, and binimetinib are non-ATP competitive drugs that bind to a unique pocket adjacent to the ATP binding site, triggering conformational changes to lock MEK in an inactive state155.
Currently, three BRAK/MEK inhibitor combinations (dabrafenib/trametinib, vemurafenib/cobimetinib, and encorafenib/binimetinib) are approved for metastatic or unresectable BRAF V600-mutated melanoma; dabrafenib/trametinib is also approved for advanced non-small cell lung cancers and anaplastic thyroid cancers harboring BRAF V600E mutation156.
Heart Failure Events
CV events in patients treated with BRAF/MEK inhibitors on clinical trials included decreased LVEF and arterial hypertension157, 158. A meta-analysis including over 2300 patients found dual BRAF and MEK inhibitors were associated with a 4-fold increased risk of pulmonary embolism, 4-fold increased risk of a significant decline in LVEF, and 1.5-fold increased risk of arterial hypertension compared to BRAF inhibitor monotherapy159. In total, 8% of patients treated with dual therapy had LVEF decline, and 20% had hypertension. Another meta-analysis focused on MEK inhibitors (with or without BRAF inhibitors) noted similar relative risk of hypertension and decline in ejection fraction compared to the control arms. 160
Mechanisms of Cardiotoxicity
MAPK pathways are highly conserved across many cell types, and play a fundamental role in cancer and the CV system. There are four main MAPK subfamilies relevant to the heart- extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinases (JNK 1, 2, and 3), p38 kinase, and big MAP kinase (BMK or ERK5). In response to growth factors and other stimuli binding to receptor tyrosine kinases, a cascade of kinases phosphorylate downstream targets leading to MEK1/2 activation.161 ERK1/2 is the main downstream target of MEK and plays an important role in proliferation, survival, and cell cycle progression through phosphorylation of several targets. In the CV system, ERK1/2 is an important mediator of the hypertrophic response, and plays a role in pathologic and physiologic hypertrophy.162 It also confers cardioprotection after ischemia-reperfusion injury. 163 Neurohormonal activation via Gαq agonism leads to downstream activation of MEK/ERK signaling. 60 In response to mechanical unloading after LVAD, 164 there is decreased ERK activity, and increased endogenous ERK inhibitor. 165 In summary, MEK/ERK signaling is stimulated by CV stress and is cardioprotective.
Rationale for Cardioprotective Therapies
Neurohormonal blockade with RAAS inhibitors and BB reduce maladaptive hypertrophy signaling and have mechanistic rationale in MEK inhibitor cardiotoxicity, though have never been studied in this patient population. Carvedilol has potential benefit as it triggers a conformational change that favors inhibitory GαI signaling, leading to β-arrestin activation and promotion of pro-survival MEK/ERK and Akt signaling. 24 Other RAAS inhibitors have mechanistic rationale via downregulation of maladaptive Gαq signaling. Another agonist of pro-survival signaling is apelin. Apelin is an adipokine that is upregulated in the plasma of patients with HF. 166 Apelin binds to the apelin receptor and stimulates β-arrestin but also stimulates contractility and systemic vasodilation. 167 The increased contractility and vasodilation are potentially mediated by nitric oxide and antagonism of RAAS. 168 Apelin also exerts some of its beneficial effects through upregulation of ERK signaling.169 The apelin receptor is predominantly found in the heart; however, apelin signaling has also been implicated in cancers so its cardioprotective benefits would need to be weighed with potential reduced anti-cancer efficacy of MEK inhibitors. 170
Conclusions and Future Directions
The field of oncology continues to evolve with the development of newer targeted therapies. Critical to the continued successes of these therapies is the mitigation of treatment-related adverse events, including cardiovascular toxicities. As such, there is first an important need to advance our understanding of the basic mechanisms of toxicities. This understanding is necessary to inform and improve the design of oncologic therapeutics to increase specificity and minimize multi-organ toxicities. Many of the anti-cancer mechanisms that dampen growth, survival, and angiogenesis are cardiotoxic. Moreover, this knowledge can be translated to the development of mechanistic biomarkers to aid in cardiotoxicity risk prediction and prognosis. Second, there is an important need to advance our understanding and the development of targeted cardioprotective strategies. The hypothesized mechanisms of cardiotoxicity can be used to best inform the rationale for cardioprotective therapies, and these mechanisms must not augment oncogenic signaling cascades. Targeted cardioprotection includes the use of personalized approaches, including those involving clinical risk scores, genomics, and biomarkers to identify high cardiovascular risk individuals who may benefit the most from cardioprotection. 171 Biomarkers have played a longstanding role in prediction and prognosis both in cancer and cardiovascular medicine, and there is rationale for their use in cardio-oncology as well. Third, the application of existent and newer HF therapies, including SGLT2 inhibitors and neprilysin inhibitors, and discovery of therapies relevant to cardio-oncology to prevent and treat cancer related HF need to be clarified. There is a growing body of basic science data that support a protective cardiometabolic effect of SGLT2 inhibitors and beneficial effects of sacubitril/valsartan172 as it relates to traditional chemotherapeutics, and clinical trials investigating the use of neprilysin inhibitors (NCT03760588) with conventional chemotherapies. Their application to targeted therapies, and the application of the additional strategies in metabolic modulation and attenuating inflammation are promising avenues for additional research. However, for any of these to be realized, there is an imperative need for continued multi-disciplinary and multi-center collaboration and adequately powered clinical trials targeting the at-risk population.
Acknowledgements
We would like to acknowledge Priya Brahmbhatt and Jessica Wang for their assistance with the Figures.
Funding Sources
Dr. Ky is supported in part by R21150723 and R34HL146927.
Disclosures
VSH, LS- none. KZ- Consultant (modest)- Eidos Therapeutics. VN- Research Funding (institution): Merck, Janssen Oncology, Pfizer, Bristol Myers Squibb, TMunity Therapeutics; Consulting/Honoraria: Regeneron, Amgen, Pfizer, Janssen. DL- Research funding- Myocardial Solutions; Consultant (modest)- Roche, Astrazeneca, Lilly, Clementia, Cytokinetics, Eidos, Prothena. BK- Consultant: Roche, Cytokinetics; Honorarium: American College of Cardiology, Uptodate.
Abbreviations
- HER2
human epidermal growth factor receptor 2
- MEK
Ras/Raf/mitogen-activated-protein kinase kinase
- BRAF
v-raf murine sarcoma viral oncogene homolog B1
- LVEF
left ventricular ejection fraction
- HF
heart failure
- EGFR
epidermal growth factor receptor
- PI3K
phosphoinositide 3-kinase
- Akt
Ak transforming factor
- ERK
extracellular signal-regulated kinase
- NRG-1
Neuregulin-1
- iPSC-CMs
induced pluripotent stem cell cardiomyocytes
- mTOR
mammalian target of rapamycin
- AMPK
adenosine monophosphate-activated protein kinase
- ACE-I
Angiotensin converting enzyme inhibitors
- BB
beta-blockers
- RAAS
renin-angiotensin-aldosterone system
- LV
left ventricle
- TKIs
tyrosine kinase inhibitors
- VEGFR
vascular endothelial growth factor receptor
- PDGFR
platelet derived growth factor receptor
- FLT3
fms like tyrosine kinase 3
- ccRCC
clear cell renal cell carcinoma
- VHL
von Hippel-Lindau
- HIF
hypoxia inducible factor
- VSPI
VEGF signaling pathway inhibitors
- RAF
Raf-1
- PET
positron emission tomography
- CV
cardiovascular
- ROS
reactive oxygen species
- CCB
calcium channel blocker
- ICI
immune checkpoint inhibitors
- CTLA-4
cytotoxic T lymphocyte associated antigen 4
- PD-1
programmed death 1
- PD-L1
programmed death 1 ligand
- CAR-T
Chimeric antigen receptor T cell
- CRS
cytokine release syndrome
- UPS
ubiquitin/proteasome system
- eNOS
endothelial nitric oxide synthase
- GDMT
guideline directed medical therapy
- PI
proteasome inhibitor
- GnRH
gonadotropin-releasing hormone
- LHRH
luteinizing hormone-releasing hormone
- ADT
androgen deprivation therapy
- ARSI
androgen-receptor signaling inhibitor
- ACTH
adrenocorticotropic hormone
- MI
myocardial infarction
- FSH
follicle-stimulating hormone
- MAPK
mitogen-activated protein kinase
- ATP
adenosine triphosphate
- SGLT2
sodium-glucose cotransporter-2
References
- 1.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C and Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8. [DOI] [PubMed] [Google Scholar]
- 2.Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM, Margulies KB and Ky B. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations With Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up. Circulation. 2017;135:1397–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn VS, Lenihan DJ and Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S. Genetics of Anthracycline Cardiomyopathy in Cancer Survivors. JACC: CardioOncology. 2020;2:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macedo AVS, Hajjar LA, Lyon AR, Nascimento BR, Putzu A, Rossi L, Costa RB, Landoni G, Nogueira-Rodrigues A and Ribeiro ALP. Efficacy of Dexrazoxane in Preventing Anthracycline Cardiotoxicity in Breast Cancer. JACC: CardioOncology. 2019;1:68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leerink JM, Baat ECd, Feijen EAM, Bellersen L, Dalen ECv, Grotenhuis HB, Kapusta L, Kok WEM, Loonen J, Pal HJHvd, Pluijm SMF, Teske AJ, Mavinkurve-Groothuis AMC, Merkx R and Kremer LCM. Cardiac Disease in Childhood Cancer Survivors. JACC: CardioOncology. 2020;2:363–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padegimas A, Clasen S and Ky B. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc Med. 2020;30:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Hirji SA, Qamar A, Bajaj N, Gupta A, Zaha VG, Chandra A, Haykowsky M, Ky B, Moslehi J, Nohria A, Butler J and Pandey A. Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients With Cancer Undergoing Chemotherapy. JACC: CardioOncology. 2019;1:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, Patel A, DeCara J, Mitchell J, Harrison E, Moslehi J, Witteles R, Calabro MG, Orecchia R, de Azambuja E, Zamorano JL, Krone R, Iakobishvili Z, Carver J, Armenian S, Ky B, Cardinale D, Cipolla CM, Dent S, Jordan K and clinicalguidelines@esmo.org EGCEa. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, Murthy RK, Rodon J and Piha-Paul SA. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clinical Cancer Research. 2019;25:2033–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J and Breast Cancer International Research G. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, Wu Z and Wu S. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev. 2011;37:312–20. [DOI] [PubMed] [Google Scholar]
- 14.Yu AF, Moskowitz CS, Chuy KL, Yang J, Dang CT, Liu JE, Oeffinger KC and Steingart RM. Cardiotoxicity Surveillance and Risk of Heart Failure During HER2 Targeted Therapy. JACC: CardioOncology. 2020;2:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, Saito K, Fujisaki Y, Sugihara M, Shahidi J and Doi T. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827–836. [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. [DOI] [PubMed] [Google Scholar]
- 17.Lemmens K, Doggen K and De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–60. [DOI] [PubMed] [Google Scholar]
- 18.Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA and Lorell BH. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. 1999;100:407–12. [DOI] [PubMed] [Google Scholar]
- 19.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J Jr., Chien KR and Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. [DOI] [PubMed] [Google Scholar]
- 20.Necela BM, Axenfeld BC, Serie DJ, Kachergus JM, Perez EA, Thompson EA and Norton N. The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC-derived cardiomyocytes. Clin Transl Med. 2017;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitani T, Ong SG, Lam CK, Rhee JW, Zhang JZ, Oikonomopoulos A, Ma N, Tian L, Lee J, Telli ML, Witteles RM, Sharma A, Sayed N and Wu JC. Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in Patients With Breast Cancer. Circulation. 2019;139:2451–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan N, Shen Y, Endo Y, ElZarrad MK and Wu WJ. Trastuzumab, but Not Pertuzumab, Dysregulates HER2 Signaling to Mediate Inhibition of Autophagy and Increase in Reactive Oxygen Species Production in Human Cardiomyocytes. Mol Cancer Ther. 2016;15:1321–31. [DOI] [PubMed] [Google Scholar]
- 23.Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, Naik TJ, Prasad SV and Ardehali H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem. 2009;284:2080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Hanada K, Staus DP, Makara MA, Dahal GR, Chen Q, Ahles A, Engelhardt S and Rockman HA. Galphai is required for carvedilol-induced beta1 adrenergic receptor beta-arrestin biased signaling. Nat Commun. 2017;8:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ and Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raimondi S, Botteri E, Munzone E, Cipolla C, Rotmensz N, DeCensi A and Gandini S. Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: Systematic review and meta-analysis. Int J Cancer. 2016;139:212–9. [DOI] [PubMed] [Google Scholar]
- 27.Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, Los M, Smit WM, Nieboer P, Smorenburg CH, Mandigers CM, van der Wouw AJ, Kessels L, van der Velden AW, Ottevanger PB, Smilde T, de Boer J, van Veldhuisen DJ, Kema IP, de Vries EG and Schellens JH. Angiotensin II-Receptor Inhibition With Candesartan to Prevent Trastuzumab-Related Cardiotoxic Effects in Patients With Early Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016;2:1030–7. [DOI] [PubMed] [Google Scholar]
- 28.Blaes A, Manisty C and Barac A. How to Follow, Manage and Treat Cardiac Dysfunction in Patients With Her2+ Breast Cancer. JACC: CardioOncology. 2020;2:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, Pagano JJ, Chow K, Thompson RB, Vos LJ, Ghosh S, Oudit GY, Ezekowitz JA and Paterson DI. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol. 2017;35:870–877. [DOI] [PubMed] [Google Scholar]
- 30.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland A, Storas TH, Hagve TA, Rosjo H, Steine K, Geisler J and Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leong DP, Cosman T, Alhussein MM, Tyagi NK, Karampatos S, Barron CC, Wright D, Tandon V, Magloire P, Joseph P, Conen D, Devereaux PJ, Ellis PM, Mukherjee SD and Dhesy-Thind S. Safety of Continuing Trastuzumab Despite Mild Cardiotoxicity. JACC: CardioOncology. 2019;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odiete O, Hill MF and Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, Gannon J, Macrae CA, Griffith LG and Lee RT. An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced proneoplastic potential. Circulation. 2013;128:152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lenihan DJ, Anderson SA, Lenneman CG, Brittain E, Muldowney JAS 3rd, Mendes L, Zhao PZ, Iaci J, Frohwein S, Zolty R, Eisen A, Sawyer DB and Caggiano AO. A Phase I, Single Ascending Dose Study of Cimaglermin Alfa (Neuregulin 1beta3) in Patients With Systolic Dysfunction and Heart Failure. JACC Basic Transl Sci. 2016;1:576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R and Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. The New England journal of medicine. 2001;344:1038–42. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann JT, Haap M, Kopp HG and Lipp HP. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metab. 2009;10:470–81. [DOI] [PubMed] [Google Scholar]
- 37.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS and Investigators F. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 38.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA and Investigators A. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand K, Ensor J, Trachtenberg B and Bernicker EH. Osimertinib-Induced Cardiotoxicity. JACC: CardioOncology. 2019;1:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunimasa K, Kamada R, Oka T, Oboshi M, Kimura M, Inoue T, Tamiya M, Nishikawa T, Yasui T, Shioyama W, Nishino K, Imamura F, Kumagai T and Fujita M. Cardiac Adverse Events in EGFR-Mutated Non-Small Cell Lung Cancer Treated With Osimertinib. JACC: CardioOncology. 2020;2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SR, Brown S-AN, Kubusek JE, Mansfield AS and Duma N. Osimertinib-Induced Cardiomyopathy. JACC: Case Reports. 2020;2:641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piper-Vallillo AJ, Costa DB, Sabe MA and Asnani A. Heart Failure Associated With the Epidermal Growth Factor Receptor Inhibitor Osimertinib. JACC: CardioOncology. 2020;2:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R and Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. The New England journal of medicine. 2011;364:501–13. [DOI] [PubMed] [Google Scholar]
- 44.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G and Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. The New England journal of medicine. 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, Ghiorghiu S, Stetson D, Markovets A, Barrett JC, Thress KS and Jänne PA. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:841–849. [DOI] [PubMed] [Google Scholar]
- 46.Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, Montesinos P, Baer MR, Larson RA, Ustun C, Fabbiano F, Erba HP, Di Stasi A, Stuart R, Olin R, Kasner M, Ciceri F, Chou WC, Podoltsev N, Recher C, Yokoyama H, Hosono N, Yoon SS, Lee JH, Pardee T, Fathi AT, Liu C, Hasabou N, Liu X, Bahceci E and Levis MJ. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. The New England journal of medicine. 2019;381:1728–1740. [DOI] [PubMed] [Google Scholar]
- 47.Zhu X, Stergiopoulos K and Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol. 2009;48:9–17. [DOI] [PubMed] [Google Scholar]
- 48.Wu S, Chen JJ, Kudelka A, Lu J and Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–23. [DOI] [PubMed] [Google Scholar]
- 49.Catino AB, Hubbard RA, Chirinos JA, Townsend R, Keefe S, Haas NB, Puzanov I, Fang JC, Agarwal N, Hyman D, Smith AM, Gordon M, Plappert T, Englefield V, Narayan V, Ewer S, ElAmm C, Lenihan D and Ky B. Longitudinal Assessment of Vascular Function With Sunitinib in Patients With Metastatic Renal Cell Carcinoma. Circ Heart Fail. 2018;11:e004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T and Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghatalia P, Morgan CJ, Je Y, Nguyen PL, Trinh QD, Choueiri TK and Sonpavde G. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:228–37. [DOI] [PubMed] [Google Scholar]
- 52.Haas NB, Manola J, Ky B, Flaherty KT, Uzzo RG, Kane CJ, Jewett M, Wood L, Wood CG, Atkins MB, Dutcher JJ, Wilding G and DiPaola RS. Effects of Adjuvant Sorafenib and Sunitinib on Cardiac Function in Renal Cell Carcinoma Patients without Overt Metastases: Results from ASSURE, ECOG 2805. Clin Cancer Res. 2015;21:4048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G and Trail PA. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. [DOI] [PubMed] [Google Scholar]
- 54.Chow LQ and Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–96. [DOI] [PubMed] [Google Scholar]
- 55.Woitek F, Zentilin L, Hoffman NE, Powers JC, Ottiger I, Parikh S, Kulczycki AM, Hurst M, Ring N, Wang T, Shaikh F, Gross P, Singh H, Kolpakov MA, Linke A, Houser SR, Rizzo V, Sabri A, Madesh M, Giacca M and Recchia FA. Intracoronary Cytoprotective Gene Therapy: A Study of VEGF-B167 in a Pre-Clinical Animal Model of Dilated Cardiomyopathy. J Am Coll Cardiol. 2015;66:139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Farrell AC, Evans R, Silvola JM, Miller IS, Conroy E, Hector S, Cary M, Murray DW, Jarzabek MA, Maratha A, Alamanou M, Udupi GM, Shiels L, Pallaud C, Saraste A, Liljenback H, Jauhiainen M, Oikonen V, Ducret A, Cutler P, McAuliffe FM, Rousseau JA, Lecomte R, Gascon S, Arany Z, Ky B, Force T, Knuuti J, Gallagher WM, Roivainen A and Byrne AT. A Novel Positron Emission Tomography (PET) Approach to Monitor Cardiac Metabolic Pathway Remodeling in Response to Sunitinib Malate. PloS one. 2017;12:e0169964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truitt R, Mu A, Corbin EA, Vite A, Brandimarto J, Ky B and Margulies KB. Increased Afterload Augments Sunitinib-Induced Cardiotoxicity in an Engineered Cardiac Microtissue Model. JACC Basic Transl Sci. 2018;3:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouitbir J, Alshaikhali A, Panajatovic MV, Abegg VF, Paech F and Krahenbuhl S. Mitochondrial oxidative stress plays a critical role in the cardiotoxicity of sunitinib: Running title: Sunitinib and oxidative stress in hearts. Toxicology. 2019;426:152281. [DOI] [PubMed] [Google Scholar]
- 59.Chintalgattu V, Ai D, Langley RR, Zhang J, Bankson JA, Shih TL, Reddy AK, Coombes KR, Daher IN, Pati S, Patel SS, Pocius JS, Taffet GE, Buja LM, Entman ML and Khakoo AY. Cardiomyocyte PDGFR-beta signaling is an essential component of the mouse cardiac response to load-induced stress. J Clin Invest. 2010;120:472–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao L, Pimental DR, Amin JK, Singh K, Sawyer DB and Colucci WS. MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. 2001;33:779–87. [DOI] [PubMed] [Google Scholar]
- 61.Dey S, DeMazumder D, Sidor A, Foster DB and O’Rourke B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ Res. 2018;123:356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pinilla-Vera M, Hahn VS and Kass DA. Leveraging Signaling Pathways to Treat Heart Failure With Reduced Ejection Fraction. Circ Res. 2019;124:1618–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lankhorst S, Kappers MH, van Esch JH, Smedts FM, Sleijfer S, Mathijssen RH, Baelde HJ, Danser AH and van den Meiracker AH. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension. 2014;64:1282–9. [DOI] [PubMed] [Google Scholar]
- 64.Belcik JT, Qi Y, Kaufmann BA, Xie A, Bullens S, Morgan TK, Bagby SP, Kolumam G, Kowalski J, Oyer JA, Bunting S and Lindner JR. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J Am Coll Cardiol. 2012;60:618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waliany S, Sainani KL, Park LS, Zhang CA, Srinivas S and Witteles RM. Increase in Blood Pressure Associated With Tyrosine Kinase Inhibitors Targeting Vascular Endothelial Growth Factor. JACC: CardioOncology. 2019;1:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mir O, Coriat R, Ropert S, Cabanes L, Blanchet B, Camps S, Billemont B, Knebelmann B and Goldwasser F. Treatment of bevacizumab-induced hypertension by amlodipine. Invest New Drugs. 2012;30:702–7. [DOI] [PubMed] [Google Scholar]
- 67.Bottinor WJ, Shuey MM, Manouchehri A, Farber-Eger EH, Xu M, Nair D, Salem JE, Wang TJ and Brittain EL. Renin-Angiotensin-Aldosterone System Modulates Blood Pressure Response During Vascular Endothelial Growth Factor Receptor Inhibition. JACC CardioOncol. 2019;1:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik OP, Sabbisetti VS, Bhatt RS, Simantov R and Choueiri TK. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21:2471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Touyz RM, Lang NN, Herrmann J, van den Meiracker AH and Danser AHJ. Recent Advances in Hypertension and Cardiovascular Toxicities With Vascular Endothelial Growth Factor Inhibition. Hypertension. 2017;70:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Reynolds KL, Lyon AR, Palaskas N and Neilan TG. The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity. JACC: CardioOncology. 2021;3:35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SYS, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC and Garassino MC. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 72.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang Y-H, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB and Powles T. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 73.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G and Maziarz RT. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine. 2018;380:45–56. [DOI] [PubMed] [Google Scholar]
- 74.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J and Go WY. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. New England Journal of Medicine. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nso N, Antwi-Amoabeng D, Beutler BD, Ulanja MB, Ghuman J, Hanfy A, Nimo-Boampong J, Atanga S, Doshi R, Enoru S and Gullapalli N. Cardiac adverse events of immune checkpoint inhibitors in oncology patients: A systematic review and meta-analysis. World J Cardiol. 2020;12:584–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD and Neilan TG. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ and Johnson DB. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosh AK, Chen DH, Guha A, Mackenzie S, Walker JM and Roddie C. CAR T Cell Therapy–Related Cardiovascular Outcomes and Management. JACC: CardioOncology. 2020;2:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR and Scherrer-Crosbie M. Cardiovascular Effects of CAR T Cell Therapy: A Retrospective Study. JACC CardioOncol. 2020;2:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA and Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. [DOI] [PubMed] [Google Scholar]
- 81.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N and Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–83. [DOI] [PubMed] [Google Scholar]
- 82.Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH and Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181:2513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr., Anders RA, Sosman JAand Moslehi JJ. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A and Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matsumoto T, Sasaki N, Yamashita T, Emoto T, Kasahara K, Mizoguchi T, Hayashi T, Yodoi K, Kitano N, Saito T, Yamaguchi T and Hirata K. Overexpression of Cytotoxic T-Lymphocyte-Associated Antigen-4 Prevents Atherosclerosis in Mice. Arterioscler Thromb Vasc Biol. 2016;36:1141–51. [DOI] [PubMed] [Google Scholar]
- 86.Poels K, Leent MMTv, Boutros C, Tissot H, Roy S, Meerwaldt AE, Toner YCA, Reiche ME, Kusters PJH, Malinova T, Huveneers S, Kaufman AE, Mani V, Fayad ZA, Winther MPJd, Marabelle A, Mulder WJM, Robert C, Seijkens TTPand Lutgens E. Immune checkpoint inhibitor therapy aggravates T cell–driven plaque inflammation in atherosclerosis. JACC: CardioOncology. 2020;2:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, Mosarla RC, Lee C, Zlotoff DA, Raghu VK, Hartmann SE, Gilman HK, Gong J, Zubiri L, Sullivan RJ, Reynolds KL, Mayrhofer T, Zhang L, Hoffmann U and Neilan TG. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation. 2020;142:2299–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meng X, Zhang K, Li J, Dong M, Yang J, An G, Qin W, Gao F, Zhang C and Zhang Y. Statins induce the accumulation of regulatory T cells in atherosclerotic plaque. Mol Med. 2012;18:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asai K, Funaki C, Hayashi T, Yamada K, Naito M, Kuzuya M, Yoshida F, Yoshimine N and Kuzuya F. Dexamethasone-induced suppression of aortic atherosclerosis in cholesterol-fed rabbits. Possible mechanisms. Arterioscler Thromb. 1993;13:892–9. [DOI] [PubMed] [Google Scholar]
- 90.Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ and Kerneis M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl J Med. 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 91.Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, Lee DH, Zlotoff DA, Zhang L, Drobni ZD, Hassan MZO, Bassily E, Rhea I, Ismail-Khan R, Mulligan CP, Banerji D, Lazaryan A, Shah BD, Rokicki A, Raje N, Chavez JC, Abramson J, Locke FL and Neilan TG. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol. 2019;74:3099–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kotch C, Barrett D and Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fricker LD. Proteasome Inhibitor Drugs. Annu Rev Pharmacol Toxicol. 2020;60:457–476. [DOI] [PubMed] [Google Scholar]
- 94.Robak T, Huang H, Jin J, Zhu J, Liu T, Samoilova O, Pylypenko H, Verhoef G, Siritanaratkul N, Osmanov E, Alexeeva J, Pereira J, Drach J, Mayer J, Hong X, Okamoto R, Pei L, Rooney B, van de Velde H and Cavalli F. Bortezomib-based therapy for newly diagnosed mantle-cell lymphoma. The New England journal of medicine. 2015;372:944–53. [DOI] [PubMed] [Google Scholar]
- 95.Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, Goranova-Marinova V, Rajnics P, Suvorov A, Niesvizky R, Jakubowiak AJ, San-Miguel JF, Ludwig H, Wang M, Maisnar V, Minarik J, Bensinger WI, Mateos MV, Ben-Yehuda D, Kukreti V, Zojwalla N, Tonda ME, Yang X, Xing B, Moreau P and Palumbo A. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. The New England journal of medicine. 2015;372:142–52. [DOI] [PubMed] [Google Scholar]
- 96.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, Sandhu I, Ganly P, Baker BW, Jackson SR, Stoppa AM, Simpson DR, Gimsing P, Palumbo A, Garderet L, Cavo M, Kumar S, Touzeau C, Buadi FK, Laubach JP, Berg DT, Lin J, Di Bacco A, Hui AM, van de Velde H and Richardson PG. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. The New England journal of medicine. 2016;374:1621–34. [DOI] [PubMed] [Google Scholar]
- 97.Xiao Y, Yin J, Wei J and Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e87671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shah C, Bishnoi R, Jain A, Bejjanki H, Xiong S, Wang Y, Zou F and Moreb JS. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma. 2018;59:2557–2569. [DOI] [PubMed] [Google Scholar]
- 99.Grandin EW, Ky B, Cornell RF, Carver J and Lenihan DJ. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. Journal of cardiac failure. 2015;21:138–44. [DOI] [PubMed] [Google Scholar]
- 100.Siegel D, Martin T, Nooka A, Harvey RD, Vij R, Niesvizky R, Badros AZ, Jagannath S, McCulloch L, Rajangam K and Lonial S. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98:1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waxman AJ, Clasen S, Hwang WT, Garfall A, Vogl DT, Carver J, O’Quinn R, Cohen AD, Stadtmauer EA, Ky B and Weiss BM. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:e174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, Carver JR, Cohen AD, Engelhardt BG, Garfall AL, Goodman SA, Harrell SL, Kassim AA, Jadhav T, Jagasia M, Moslehi J, O’Quinn R, Savona MR, Slosky D, Smith A, Stadtmauer EA, Vogl DT, Waxman A and Lenihan D. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J Clin Oncol. 2019;37:1946–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Danhof S, Schreder M, Rasche L, Strifler S, Einsele H and Knop S. ‘Real-life’ experience of preapproval carfilzomib-based therapy in myeloma – analysis of cardiac toxicity and predisposing factors. European Journal of Haematology. 2016;97:25–32. [DOI] [PubMed] [Google Scholar]
- 104.Groll M, Berkers CR, Ploegh HL and Ovaa H. Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure. 2006;14:451–6. [DOI] [PubMed] [Google Scholar]
- 105.Day SM, Divald A, Wang P, Davis F, Bartolone S, Jones R and Powell SR. Impaired assembly and post-translational regulation of 26S proteasome in human end-stage heart failure. Circ Heart Fail. 2013;6:544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR and Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gavazzoni M, Lombardi CM, Vizzardi E, Gorga E, Sciatti E, Rossi L, Belotti A, Rossi G, Metra M and Raddino R. Irreversible proteasome inhibition with carfilzomib as first line therapy in patients with newly diagnosed multiple myeloma: Early in vivo cardiovascular effects. Eur J Pharmacol. 2018;838:85–90. [DOI] [PubMed] [Google Scholar]
- 108.Hasinoff BB, Patel D and Wu X. Molecular Mechanisms of the Cardiotoxicity of the Proteasomal-Targeted Drugs Bortezomib and Carfilzomib. Cardiovasc Toxicol. 2017;17:237–250. [DOI] [PubMed] [Google Scholar]
- 109.Ranek MJ, Zheng H, Huang W, Kumarapeli AR, Li J, Liu J and Wang X. Genetically induced moderate inhibition of 20S proteasomes in cardiomyocytes facilitates heart failure in mice during systolic overload. J Mol Cell Cardiol. 2015;85:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang M, Li J, Huang W, Su H, Liang Q, Tian Z, Horak KM, Molkentin JD and Wang X. Proteasome functional insufficiency activates the calcineurin-NFAT pathway in cardiomyocytes and promotes maladaptive remodelling of stressed mouse hearts. Cardiovasc Res. 2010;88:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen-Scarabelli C, Corsetti G, Pasini E, Dioguardi FS, Sahni G, Narula J, Gavazzoni M, Patel H, Saravolatz L, Knight R, Raddino R and Scarabelli TM. Spasmogenic Effects of the Proteasome Inhibitor Carfilzomib on Coronary Resistance, Vascular Tone and Reactivity. EBioMedicine. 2017;21:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Efentakis P, Doerschmann H, Witzler C, Siemer S, Nikolaou PE, Kastritis E, Stauber R, Dimopoulos MA, Wenzel P, Andreadou I and Terpos E. Investigating the Vascular Toxicity Outcomes of the Irreversible Proteasome Inhibitor Carfilzomib. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Efentakis P, Kremastiotis G, Varela A, Nikolaou PE, Papanagnou ED, Davos CH, Tsoumani M, Agrogiannis G, Konstantinidou A, Kastritis E, Kanaki Z, Iliodromitis EK, Klinakis A, Dimopoulos MA, Trougakos IP, Andreadou I and Terpos E. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood. 2019;133:710–723. [DOI] [PubMed] [Google Scholar]
- 114.Imam F, Al-Harbi NO, Al-Harbia MM, Korashy HM, Ansari MA, Sayed-Ahmed MM, Nagi MN, Iqbal M, Khalid Anwer M, Kazmi I, Afzal M and Bahashwan S. Rutin Attenuates Carfilzomib-Induced Cardiotoxicity Through Inhibition of NF-κB, Hypertrophic Gene Expression and Oxidative Stress. Cardiovasc Toxicol. 2017;17:58–66. [DOI] [PubMed] [Google Scholar]
- 115.Imam F, Al-Harbi NO, Al-Harbi MM, Ansari MA, Almutairi MM, Alshammari M, Almukhlafi TS, Ansari MN, Aljerian K and Ahmad SF. Apremilast reversed carfilzomib-induced cardiotoxicity through inhibition of oxidative stress, NF-κB and MAPK signaling in rats. Toxicol Mech Methods. 2016;26:700–708. [DOI] [PubMed] [Google Scholar]
- 116.Ranek MJ, Terpstra EJ, Li J, Kass DA and Wang X. Protein kinase g positively regulates proteasome-mediated degradation of misfolded proteins. Circulation. 2013;128:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ranek MJ, Oeing C, Sanchez-Hodge R, Kokkonen-Simon KM, Dillard D, Aslam MI, Rainer PP, Mishra S, Dunkerly-Eyring B, Holewinski RJ, Virus C, Zhang H, Mannion MM, Agrawal V, Hahn V, Lee DI, Sasaki M, Van Eyk JE, Willis MS, Page RC, Schisler JC and Kass DA. CHIP phosphorylation by protein kinase G enhances protein quality control and attenuates cardiac ischemic injury. Nat Commun. 2020;11:5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huggins C and Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. The Journal of urology. 2002;167:948–51; discussion 952. [PubMed] [Google Scholar]
- 119.Nelson WG. Commentary on Huggins and Hodges: “Studies on Prostatic Cancer”. Cancer Res. 2016;76:186–7. [DOI] [PubMed] [Google Scholar]
- 120.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, Matheson D, Millman R, Attard G, Chowdhury S, Cross WR, Gillessen S, Parker CC, Russell JM, Berthold DR, Brawley C, Adab F, Aung S, Birtle AJ, Bowen J, Brock S, Chakraborti P, Ferguson C, Gale J, Gray E, Hingorani M, Hoskin PJ, Lester JF, Malik ZI, McKinna F, McPhail N, Money-Kyrle J, O’Sullivan J, Parikh O, Protheroe A, Robinson A, Srihari NN, Thomas C, Wagstaff J, Wylie J, Zarkar A, Parmar MKB and Sydes MR. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. The New England journal of medicine. 2017;377:338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr., Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM and Scher HI. Abiraterone and increased survival in metastatic prostate cancer. The New England journal of medicine. 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, Ferreira U, Efstathiou E, Madziarska K, Kolinsky MP, Cubero DIG, Noerby B, Zohren F, Lin X, Modelska K, Sugg J, Steinberg J and Hussain M. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. The New England journal of medicine. 2020;382:2197–2206. [DOI] [PubMed] [Google Scholar]
- 123.Epstein MM, Edgren G, Rider JR, Mucci LA and Adami HO. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gandaglia G, Sun M, Popa I, Schiffmann J, Abdollah F, Trinh QD, Saad F, Graefen M, Briganti A, Montorsi F and Karakiewicz PI. The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: a population-based study. BJU Int. 2014;114:E82–E89. [DOI] [PubMed] [Google Scholar]
- 125.Moreira RB, Debiasi M, Francini E, Nuzzo PV, Velasco G, Maluf FC, Fay AP, Bellmunt J, Choueiri TK and Schutz FA. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8:84572–84578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, Muraglia A, Porcaro AB, Siracusano S, Brunelli M, Mazzarotto R, Artibani W and Tortora G. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin Genitourin Cancer. 2018;16:e645–e653. [DOI] [PubMed] [Google Scholar]
- 127.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B and Investigators P. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr., Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI and Investigators C-A-. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE and Investigators C-A-. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS and Investigators A. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- 131.Bretagne M, Lebrun-Vignes B, Pariente A, Shaffer CM, Malouf GG, Dureau P, Potey C, Funck-Brentano C, Roden DM, Moslehi JJ and Salem JE. Heart failure and atrial tachyarrhythmia on abiraterone: A pharmacovigilance study. Arch Cardiovasc Dis. 2020;113:9–21. [DOI] [PubMed] [Google Scholar]
- 132.Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, Akaza H, Bossi A, van Veenhuyzen DF, Selby B, Fan X, Kang V, Walling J, Tombal B and Investigators HS. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med. 2020;382:2187–2196. [DOI] [PubMed] [Google Scholar]
- 133.Melloni C, Slovin SF, Blemings A, Goodman SG, Evans CP, Nilsson J, Bhatt DL, Zubovskiy K, Olesen TK, Dugi K, Clarke NW, Higano CS, Roe MT and investigators obotP. Cardiovascular safety of degarelix versus leuprolide for advanced prostate cancer: The PRONOUNCE trial study design. JACC: CardioOncology. 2020;2:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A and Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–701. [DOI] [PubMed] [Google Scholar]
- 135.Laughlin GA, Barrett-Connor E and Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marin P, Holmang S, Gustafsson C, Jonsson L, Kvist H, Elander A, Eldh J, Sjostrom L, Holm G and Bjorntorp P. Androgen treatment of abdominally obese men. Obes Res. 1993;1:245–51. [DOI] [PubMed] [Google Scholar]
- 137.Davis DD, Ruiz AL, Yanes LL, Iliescu R, Yuan K, Moulana M, Racusen LC and Reckelhoff JF. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity but increases blood pressure. Hypertension. 2012;59:726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P and Vanderschueren D. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009;150:3558–66. [DOI] [PubMed] [Google Scholar]
- 139.Boese AC, Kim SC, Yin KJ, Lee JP and Hamblin MH. Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am J Physiol Heart Circ Physiol. 2017;313:H524–H545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Margel D, Peer A, Ber Y, Shavit-Grievink L, Tabachnik T, Sela S, Witberg G, Baniel J, Kedar D, Duivenvoorden WCM, Rosenbaum E and Pinthus JH. Cardiovascular Morbidity in a Randomized Trial Comparing GnRH Agonist and GnRH Antagonist among Patients with Advanced Prostate Cancer and Preexisting Cardiovascular Disease. J Urol. 2019;202:1199–1208. [DOI] [PubMed] [Google Scholar]
- 141.Monticone S, D’Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F and Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. [DOI] [PubMed] [Google Scholar]
- 142.Moss ME and Jaffe IZ. Mineralocorticoid Receptors in the Pathophysiology of Vascular Inflammation and Atherosclerosis. Front Endocrinol (Lausanne). 2015;6:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schafer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Luscher TF, Verrey F and Matter CM. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J. 2013;34:3515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, Funder JW and McMahon EG. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003;93:69–76. [DOI] [PubMed] [Google Scholar]
- 145.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J and Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 146.Pia A, Vignani F, Attard G, Tucci M, Bironzo P, Scagliotti G, Arlt W, Terzolo M and Berruti A. Strategies for managing ACTH dependent mineralocorticoid excess induced by abiraterone. Cancer Treat Rev. 2013;39:966–73. [DOI] [PubMed] [Google Scholar]
- 147.Keating NL, O’Malley AJ and Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. [DOI] [PubMed] [Google Scholar]
- 148.Hamilton RJ, Ding K, Crook JM, O’Callaghan CJ, Higano CS, Dearnaley DP, Horwitz EM, Goldenberg SL, Gospodarowicz MK and Klotz L. The Association Between Statin Use and Outcomes in Patients Initiating Androgen Deprivation Therapy. Eur Urol. 2020. [DOI] [PubMed] [Google Scholar]
- 149.Jacobs EJ, Newton CC, Stevens VL, Campbell PT, Freedland SJ and Gapstur SM. Daily aspirin use and prostate cancer-specific mortality in a large cohort of men with nonmetastatic prostate cancer. J Clin Oncol. 2014;32:3716–22. [DOI] [PubMed] [Google Scholar]
- 150.Richards KA, Liou JI, Cryns VL, Downs TM, Abel EJ and Jarrard DF. Metformin Use is Associated with Improved Survival for Patients with Advanced Prostate Cancer on Androgen Deprivation Therapy. J Urol. 2018;200:1256–1263. [DOI] [PubMed] [Google Scholar]
- 151.Grytli HH, Fagerland MW, Fossa SD and Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635–41. [DOI] [PubMed] [Google Scholar]
- 152.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR and Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. [DOI] [PubMed] [Google Scholar]
- 153.Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K and Hirth P. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov. 2012;11:873–86. [DOI] [PubMed] [Google Scholar]
- 154.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JBAG, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin A-M, Le N, Patel K and Flaherty K. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. New England Journal of Medicine. 2014;371:1877–1888. [DOI] [PubMed] [Google Scholar]
- 155.Ohren JF, Chen H, Pavlovsky A, Whitehead C, Zhang E, Kuffa P, Yan C, McConnell P, Spessard C, Banotai C, Mueller WT, Delaney A, Omer C, Sebolt-Leopold J, Dudley DT, Leung IK, Flamme C, Warmus J, Kaufman M, Barrett S, Tecle H and Hasemann CA. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004;11:1192–7. [DOI] [PubMed] [Google Scholar]
- 156.Subbiah V, Baik C and Kirkwood JM. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends in Cancer. 2020;6:797–810. [DOI] [PubMed] [Google Scholar]
- 157.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA 3rd, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K and Weber J. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine. 2012;367:1694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R, Chiarion-Sileni V, Dutriaux C, de Groot JWB, Yamazaki N, Loquai C, Moutouh-de Parseval LA, Pickard MD, Sandor V, Robert C and Flaherty KT. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. [DOI] [PubMed] [Google Scholar]
- 159.Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T and Totzeck M. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Network Open. 2019;2:e198890–e198890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abdel-Rahman O, ElHalawani H and Ahmed H. Risk of Selected Cardiovascular Toxicities in Patients With Cancer Treated With MEK Inhibitors: A Comparative Systematic Review and Meta-Analysis. J Glob Oncol. 2015;1:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rose BA, Force T and Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN and Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, Pages G, De Windt LJ, Doevendans PA and Molkentin JD. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–41. [DOI] [PubMed] [Google Scholar]
- 164.Flesch M, Margulies KB, Mochmann HC, Engel D, Sivasubramanian N and Mann DL. Differential regulation of mitogen-activated protein kinases in the failing human heart in response to mechanical unloading. Circulation. 2001;104:2273–6. [DOI] [PubMed] [Google Scholar]
- 165.Huebert RC, Li Q, Adhikari N, Charles NJ, Han X, Ezzat MK, Grindle S, Park S, Ormaza S, Fermin D, Miller LW and Hall JL. Identification and regulation of Sprouty1, a negative inhibitor of the ERK cascade, in the human heart. Physiol Genomics. 2004;18:284–9. [DOI] [PubMed] [Google Scholar]
- 166.Chong KS, Gardner RS, Morton JJ, Ashley EA and McDonagh TA. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur J Heart Fail. 2006;8:355–60. [DOI] [PubMed] [Google Scholar]
- 167.Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan AD and Newby DE. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121:1818–27. [DOI] [PubMed] [Google Scholar]
- 168.Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, Anderson JP, Tsao PS, Lenardo MJ, Ashley EA and Quertermous T. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008;118:3343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wysocka MB, Pietraszek-Gremplewicz K and Nowak D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Front Physiol. 2018;9:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Picault FX, Chaves-Almagro C, Projetti F, Prats H, Masri B and Audigier Y. Tumour co-expression of apelin and its receptor is the basis of an autocrine loop involved in the growth of colon adenocarcinomas. Eur J Cancer. 2014;50:663–74. [DOI] [PubMed] [Google Scholar]
- 171.Chaix M-A, Parmar N, Kinnear C, Lafreniere-Roula M, Akinrinade O, Yao R, Miron A, Lam E, Meng G, Christie A, Manickaraj AK, Marjerrison S, Dillenburg R, Bassal M, Lougheed J, Zelcer S, Rosenberg H, Hodgson D, Sender L, Kantor P, Manlhiot C, Ellis J, Mertens L, Nathan PC and Mital S. Machine learning identifies clinical and genetic factors associated with anthracycline cardiotoxicity in pediatric cancer survivors. JACC: CardioOncology. 2020;2:690–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boutagy NE, Feher A, Pfau D, Liu Z, Guerrera NM, Freeburg LA, Womack SJ, Hoenes AC, Zeiss C, Young LH, Spinale FG and Sinusas AJ. Dual angiotensin receptor-neprilysin inhibition with sacubitril/valsartan attenuates systolic dysfunction in experimental doxorubicin-induced cardiotoxicity. JACC: CardioOncology. 2020;2:774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]