Abstract
The COVID-19 pandemic has magnified the importance of clinical trials for finding a safe and effective vaccine to protect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19. Although communication about vaccines and vaccine hesitancy were challenges long before COVID-19, the twin facts of a pandemic and an “infodemic” of health information, misinformation, and disinformation have raised new challenges for vaccine-related communication and decision making. The goal of this commentary is to highlight strategies to improve communication and decision making for adults considering participation in COVID-19 vaccine clinical trials. First, I present a general conceptual model for clinical trial participation that can be applied to various vaccine and other clinical trial contexts. Next, I introduce the ASK (Assume, Seek, Know) approach for enhancing equity, inclusion, and efficiency for vaccine clinical trial recruitment: (1) assume that all patients will want to know their options, (2) seek the counsel of stakeholders, and (3) know your numbers. The ideas presented in this commentary are intended to enhance vaccine-specific clinical trial communication, decision making, and literacy, while dually offering strategies and resources that may help reduce vaccine hesitancy and increase vaccine uptake over time.
Introduction
Since March 2020, when many of the government lockdowns began, people worldwide have been emotionally, socially, and physically impacted by the COVID-19 pandemic (Cooper & Williams, 2020; Cutler & Summers, 2020). In response to the pandemic, efforts are rapidly underway to find a safe and effective vaccine (COVID-19 Prevention Network, 2020; Jeyanathan et al., 2020). In the US, for example, “Operation Warp Speed” was created to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by January 2021 (U.S. Department of Health & Human Services, 2020). Vaccines have been described as “one of the most life-saving public health interventions in history” (World Health Organization, 2018) and have helped eradicate or greatly reduce morbidity from diseases such as smallpox, polio, whooping cough, and measles (The History of Vaccines, 2018; World Health Organization, 2018). Yet, despite the many benefits of vaccines, the race to find a COVID-19 vaccine has been met with both hope and concern (O’Callaghan et al., 2020; Van Norman, 2020). On the one hand, there is hope that a vaccine will help arrest a highly transmissible virus and potentially deadly disease. On the other hand, there is concern that vaccine development has become politicized, with proposed deadlines that may give the perception that important steps in the scientific process are being skipped, which may erode public confidence in vaccines (Dyer, 2020).
Despite announcements that some COVID-19 vaccine candidates have shown promising short-term efficacy, scientists have stressed the need to continue large-scale, phase 3 placebo-controlled efficacy trials for COVID-19 vaccines (WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation, 2020). As of December 1, 2020, more than 50 COVID-19 vaccines were being explored in different phases of clinical trials (Craven, 2020; Zimmer et al., 2020). In the US, the COVID-19 Prevention Network was also exploring several phase 3 efficacy trials for COVID-19 vaccines and monoclonal antibodies (COVID-19 Prevention Network, 2020), many of which had specific recruitment goals based on demographic and occupational categories. The need to quickly recruit a diverse group of participants into COVID-19 vaccine clinical trials while simultaneously educating the public about vaccines and communicating about COVID-19 risk reduction behaviors has presented new challenges for researchers (Finset et al., 2020; Jaklevic, 2020). Moreover, trying to recruit for COVID-19 vaccine clinical trials against the backdrop of flu vaccination campaigns has further elucidated issues of vaccine hesitancy and vaccine literacy (CDC, 2020; Kreps et al., 2020). The SAGE Working Group on Vaccine Hesitancy defines vaccine hesitancy as a “delay in acceptance or refusal of vaccination despite availability of vaccination services” (MacDonald, 2015). Additionally, vaccine hesitancy can be context- or vaccine-specific, and is affected by factors including complacency, convenience, and confidence (MacDonald, 2015). Related to vaccine hesitancy, the term “vaccine literacy” falls under the larger umbrella of health literacy and is defined as “not simply knowledge about vaccines, but also developing a system with decreased complexity to communicate and offer vaccines as sine qua non of a functioning health system” (Ratzan, 2011).
In this commentary, I highlight strategies to improve communication and decision making for adults considering participation in COVID-19 vaccine clinical trials. The strategies are presented as a general conceptual model of clinical trial participation that can be applied to various clinical trial contexts (Figure 1); key components of the model are bolded in the text where described. I also introduce the ASK (Assume, Seek, Know) approach for enhancing equity, inclusion, and efficiency for vaccine clinical trial recruitment and future uptake once a vaccine is approved and available to the public.
Figure 1:
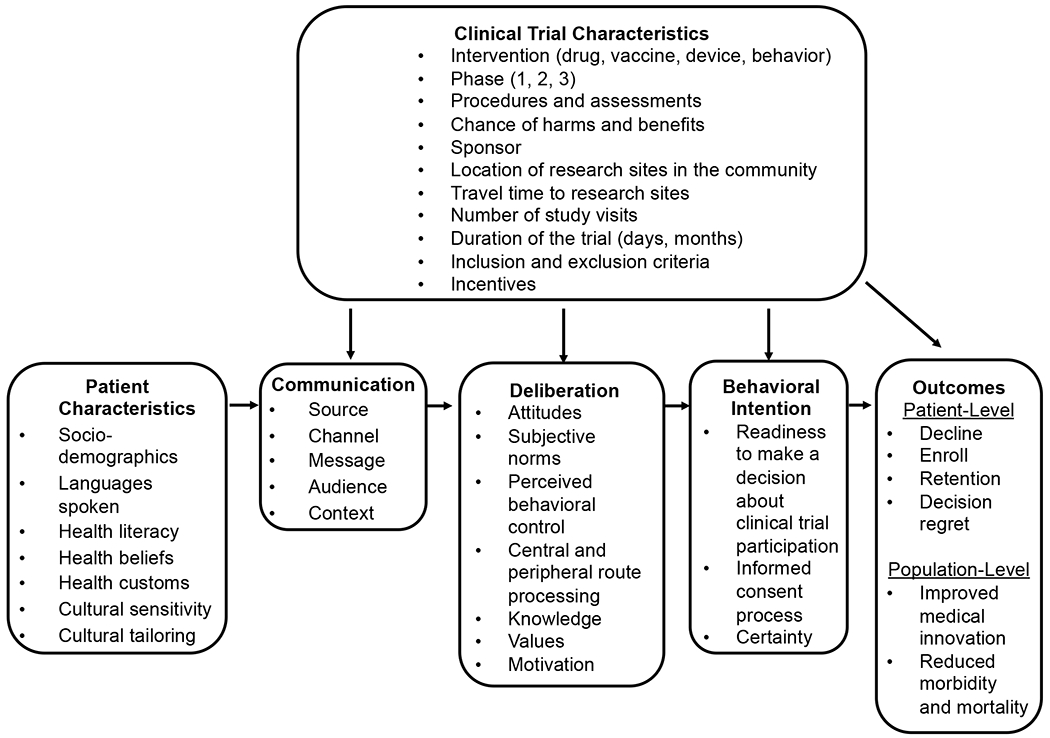
General Model of Clinical Trial Participation: Health Communication and Decision Making Considerations. This model was informed by the Theory of Planned Behavior, Elaboration Likelihood Model, and Ottawa Decision Support Framework.
General Model of Clinical Trial Participation
Patient Characteristics
An important principle in health communication is to understand your “target market” or audience. In the case of COVID-19 vaccine clinical trials, this will entail having an in-depth understanding of your patient and community population, however those populations are defined (e.g., people living within a geographic area or those with underlying medical conditions such as hypertension or diabetes). Sociodemographic factors have been associated with enrollment in vaccine and other types of clinical trials (Frew et al., 2013; Grady et al., 2017). For example, in a clinical trial conducted in Atlanta designed to evaluate a yellow fever virus vaccine, White race was the greatest predictor of enrollment despite strong interest from racially and ethnically diverse patients in the pre-screening stage (Frew et al., 2013). Given the diversity of languages spoken by potential clinical trial participants, it is important to determine which languages will be supported in a vaccine clinical trial, as this will inform the development of recruitment materials, translation of informed consent documents, and need for bilingual clinical trial staff. One study designed to characterize the prevalence of US-based clinical trials that required English fluency for trial enrollment found that this requirement increased between 2000 and 2010 from 1.7% to 9.0%, and that English fluency requirements were associated with the demographic characteristics of communities where sponsoring institutions were located (Egleston et al., 2015).
Health literacy has many implications for clinical trial participation (Burks et al., 2020; O’Leary et al., 2020; Polite et al., 2019). For example, in a qualitative study to evaluate the perceptions of women with differing health literacy levels about participating in clinical trials, Polite et al. (2019) found that women with lower health literacy levels were more likely to rely on a health care provider’s recommendation about trial participation without seeking addition health-related information. A classic definition of health literacy is “the capacity of individuals to obtain, process, and understand basic health information and services needed to make appropriate health decisions” (Ratzan & Parker, 2000.). More contemporary definitions of health literacy are included in Healthy People 2030, which sets data-driven national objectives designed to improve health and well-being across the lifespan for people living in the US (Health.gov, 2020). Personal health literacy, for example, is “the degree to which individuals have the ability to find, understand, and use information and services to inform health-related decisions and actions for themselves and others,” while organizational health literacy is “the degree to which organizations equitably enable individuals to find, understand, and use information and services to inform health-related decisions and actions for themselves and others” (Health.gov, 2020). The newer health literacy definitions were designed to (1) emphasize people’s ability to use health information rather than just understand it, (2) focus on the ability to make “well-informed” decisions rather than “appropriate” ones, (3) incorporate a public health perspective, and (4) acknowledge that organizations have a responsibility to address health literacy (Health.gov, 2020).
Although health literacy is often assessed in research contexts, health literacy advocates generally recommend against assessing health literacy in routine clinical care contexts because it can stigmatize patients (Health Literacy Out Loud, 2014). Alternatively, in the context of vaccine clinical trial communication, adhering to AHRQ’s Health Literacy Universal Precautions will likely benefit all patients considering trial participation regardless of their health literacy level (Agency for Healthcare Research and Quality, 2015a). These precautions include simplifying communication, confirming comprehension for all patients through methods such as the teach-back, and use of plain language (Agency for Healthcare Research and Quality, 2015a). Notably, plain language has been explored in clinical trial contexts (Langford et al., 2020; Schultz et al., 2017) and is broadly conceptualized as communication that an audience can understand the first time they read or hear it (PlainLanguage.gov). Moreover, the Plain Language Act of 2010 defines plain language as “writing that is clear, concise, well-organized, and follows other best practices appropriate to the subject or field and intended audience” (PlainLanguage.gov).
Given the growing levels of vaccine hesitancy among US adults (Chou & Budenz, 2020), researchers should seek to know how a person’s health beliefs (e.g., causes of disease and how best to treat them) and attitudes toward vaccines may affect vaccine-related clinical trial decision making (Daley et al., 2018). It is also important to explore what, if any, health customs may affect clinical trial communication and decision making (e.g., degree to which family members are involved in decisions about health), as these factors may influence how researchers design clinical trial communications for a target audience (Agency for Healthcare Research and Quality, 2015b). Finally, the concepts of cultural sensitivity and cultural tailoring are also considerations for developing vaccine clinical trial communications. Broadly, cultural sensitivity aims to understand the ethnic and cultural characteristics, values, norms, and experiences of a target population to inform health communication interventions (Dutta, 2007; Resnicow et al., 1999), whereas cultural tailoring entails developing messages in a way that recognizes cultural values and norms to provide meaning about a specific health behavior or problem (Kreuter et al., 2003; Resnicow et al., 1999). Culturally sensitive and culturally tailored approaches have been applied to community-based participatory research (Wallerstein et al., 2019), communication about infectious disease epidemics (Sastry & Dutta, 2017), health literacy and health promotion interventions (Tucker et al., 2019), and clinical trial education interventions (Langford et al., 2015).
Clinical Trial Characteristics
Various aspects of a specific clinical trial can affect communication, deliberation, behavioral intentions, and outcomes. For example, the type of intervention (e.g., drug, vaccine), phase of the trial (e.g., phase 1 or “first in humans” vs. phase 3 trials where there is already preliminary data), number and nature of procedures and assessments, and chance of harms and benefits have all been shown to affect clinical trial decision making and enrollment (Clark et al., 2019; Grantz et al., 2019; Houghton et al., 2020; Nipp et al., 2019). Who the sponsor is (e.g., pharmaceutical company, National Institutes of Health) may also affect protocol development, public messaging about a particular clinical trial, and patient willingness to participate in the trial. For example, in a study exploring how pregnant women make decisions about participating in vaccine and medication clinical trials, approximately 16% of participants reported they would consider participating in a vaccine trial during pregnancy (Palmer et al., 2016). In that same study, a lack of trust in pharmaceutical companies negatively affected willingness to enroll in a trial.
The notion of patient burden in clinical trials has been discussed elsewhere (Cameron et al., 2020; Getz et al., 2019), but factors such as location of research sites in the community, travel time to research sites, number of study visits, and duration of the trial contribute to patient burden or perceived “hassle” associated with being in a trial. The inclusion and exclusion criteria have implications for equity in clinical trial participation, which sociodemographic groups comprise the final sample, and generalizability of the data (Jaklevic, 2020; Kim et al., 2017; Langford et al., 2014). For example, in a systematic review and meta-analysis examining various barriers to cancer clinical trial participation, researchers found that a trial was unavailable at patients’ home institution approximately 56% of time and that, when a trial was available, approximately 22% of patients were ineligible (Unger et al., 2019). Lastly, the amount and type of incentives may positively or negatively affect clinical trial participation decisions (Detoc et al., 2019; Grady et al., 2017; Parkinson et al., 2019; VanEpps et al., 2016).
Communication: Raising Awareness and Reaching Your Audience
The Basic Communication Model is a framework developed by health communication experts that highlights five aspects of communication: source and channel, message, audience, and context (Nelson et al., 2009). It serves as the theoretical basis for the communication tools in the general conceptual model for clinical trial participation. The source refers to the person or entity who delivers messages about vaccine clinical trial participation (e.g., physician or government health organization). It is critical that selected sources are considered credible and influential by the target audience. The best channel(s) for reaching the target population will depend on the recruitment goals, but may include email or in-person conversations, social media, television, newspapers, or radio. For example, one study recruited women in Arkansas for a phase 1 clinical trial for a human papillomavirus (HPV) therapeutic vaccine through a statewide telecolposcopy network (Stratton et al., 2015), whereas another study recruited college-aged men in Pennsylvania for an HPV vaccine trial using Facebook (Raviotta et al., 2016).
Next, message(s) about vaccine clinical trials can be targeted, tailored, or framed based on the audience’s attitudes and beliefs about vaccines, and other relevant demographic or psychological factors (Evangeli et al., 2013; Hawkins et al., 2008). For example, in a study about the effects of message framing (i.e., gain vs. loss vs. control) and clinician recommendation (i.e., a simple offer vs. strong recommendation) on adult hepatitis B vaccination, researchers found no difference in vaccine uptake between gain-frame and loss-frame messages (Kasting et al., 2019). However, gain and loss–framed messages performed better overall than control messages. Ideally, messages about vaccine clinical trial opportunities will be pre-tested and iteratively revised based on feedback from the target audience. Lastly, health professionals should also be mindful of the media, health care organization, political–legal, and cultural context in which patients and the public make decisions about vaccine clinical trial participation (Street, 2003). For example, when this commentary was written, there were questions about when a COVID-19 vaccine would be available (Michaud et al., 2020), how approved vaccines would be equitably distributed (National Academies of Sciences Engineering and Medicine, 2020; Schmidt et al., 2020), and how to address medical trust among racial/ethnic minorities who may be leery about participating in COVID-19 vaccine clinical trials (Jaklevic, 2020)
Deliberation: Understanding and Supporting Decision Making
The Theory of Planned Behavior (TPB) has been used in clinical trial contexts (Peng et al., 2019; Yang et al., 2010) and its constructs are used here to help describe the clinical trial participation deliberation process (Ajzen, 1985). In TPB, attitudes, subjective norm, and perceived behavioral control precede an individual’s intention to perform a behavior or make a choice, such as whether or not to enroll in a vaccine clinical trial. Attitudes about vaccine clinical trials may be influenced by behavioral beliefs (e.g., vaccines are dangerous vs. vaccines save lives) and expected outcomes. For example, a clinical trial participant may expect personal benefits such as a protective immune response from an experimental vaccine or altruistic benefits from helping researchers find a new vaccine that will ultimately save lives and reduce morbidity (Grady et al., 2017). Subjective norm is influenced by perceptions about whether important referent others (e.g., spouse, physician, pastor) will approve or disapprove of the choice to participate in a vaccine clinical trial, as well as one’s motivation to comply with those referent others. In a study examining how, if at all, TPB constructs could predict hypothetical willingness of high-risk adolescents to participate in a phase 3 HIV vaccine trial, subjective norms were found to be the strongest predictor of willingness to participate (Giocos et al., 2008). Perceived behavioral control affects performance of a behavior and may be especially influenced by clinical trial characteristics (e.g., number of study visits and procedures required). In some cases, perceived behavioral control may be bolstered by providing patients with high-quality health information and tools to enhance knowledge, self-efficacy, trust in science, and patient–provider communication (Table 1).
Table 1:
Curated List of Vaccine, Clinical Trial, Decision Making, and Health Literacy Resources
| Organization and General Website | Specific Links of Interest |
|---|---|
|
MedlinePlus medlineplus.gov MedlinePlus is a service of the National Library of Medicine (NLM), the world’s largest medical library, which is part of the National Institutes of Health (NIH). |
Vaccines (immunizations) medlineplus.gov/ency/article/002024.htm |
|
Centers for Disease Control and Prevention (CDC) cdc.gov As the nation’s health protection agency, CDC saves lives and protects people from health threats. To accomplish its mission, CDC conducts critical science and provides health information that protects our nation against expensive and dangerous health threats, and responds when these arise. |
Vaccines & Immunizations: Basics and Common Questions cdc.gov/vaccines/vac-gen/default.htm Gateway to Health Communication cdc.gov/healthcommunication/index.html |
|
ClinicalTrials.gov ClinicalTrials.gov is an internet-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions. |
COVID-19 trials clinicaltrials.gov/ct2/results?cond=COVID-19 |
|
COVID-19 Prevention Network coronaviruspreventionnetwork.org The COVID-19 Prevention Network (CoVPN) was formed by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH) to respond to the global pandemic. The mission is to conduct Phase 3 efficacy trials for COVID-19 vaccines and monoclonal antibodies. |
Frequently Asked Questions About COVID-19 Clinical Studies coronaviruspreventionnetwork.org/clinical-studies-faq/ |
|
The Ottawa Hospital: Patient Decision Aids decisionaid.ohri.ca Mission: “To explore better ways to help patients make ‘tough’ healthcare decisions that may have: multiple options; uncertain outcomes; benefits and harms that people value differently.” |
Ottawa Personal Decision Guide decisionaid.ohri.ca/decguide.html |
|
Health Literacy Out Loud Podcasts healthliteracyoutloud.com/ “Health Literacy Out Loud (HLOL) podcasts are a lot like radio shows. You can listen in as Helen Osborne interviews those in-the-know about health literacy. You will hear why health literacy matters and learn practical ways to help.” |
Vaccine Literacy (HLOL #189) healthliteracyoutloud.com/2019/08/01/vaccine-literacy-hlol-189/ Plain Language: It’s About Smartening Up, Not Dumbing Down (HLOL #179) healthliteracyoutloud.com/?s=plain+language |
With regard to how messages are received and perceived by potential vaccine clinical trial participants, the concepts of central and peripheral route processing from the Elaboration Likelihood Model (ELM) may be helpful (Petty & Cacioppo, 1986). ELM is a theory of persuasion and has been used in vaccine-specific contexts (Frew et al., 2016; Hu et al., 2018). With central route or “deeper level” cognitive processing, potential clinical trial participants may be motivated to join a vaccine clinical trial for personal or social reasons (e.g., a parent died from COVID-19 in a nursing home). Accordingly, potential participants may be more likely to elaborate about the message and think deeply about the arguments presented (e.g., “by participating in a COVID-19 vaccine trial, you can help keep older adults in nursing homes safe in the future”). With peripheral route processing, people may be unmotivated by the message and thus have low elaboration and limited deep thinking about the content. In this scenario, peripheral cues such as culturally representative images on a flyer (e.g., seeing “someone like me”) may prompt action more so than messages designed to appeal to a greater moral or social obligation to participate in research.
Related to the notion of message processing, one study evaluated health literacy and the use of metaphors as a way to explain randomization in cancer clinical trials (Krieger et al., 2011). Medically underserved, rural women aged 50 and over were randomized to receive 1 of 3 messages: a low-literacy definition, standard metaphor, and culturally derived metaphor. Although the message strategy did not significantly affect message processing, “under conditions of low attention, participants in the culturally derived metaphor condition experienced significantly higher intentions to participate in clinical trials compared with participants in the standard metaphor condition” (Krieger et al., 2011). Another study explored the impact of patient identification and narrative transportation on intentions to participate in cancer research and found that narratives can be helpful for bolstering awareness and decision making about cancer clinical trial opportunities (Neil et al., 2019). Lastly, knowledge about vaccine clinical trials generally and vaccines specifically, trial-specific values (e.g., what harms and benefits matter most), and motivation for participating in clinical trials also influence one’s intention to participate in a vaccine trial (Grantz et al., 2019). For example, one study exploring reasons for recruitment in preventive vaccine trials found that altruism was the greatest motivation for participation, whereas fear of side effects was the greatest barrier (Detoc et al., 2019).
Behavioral Intention
In the context of vaccine clinical trials, behavioral intention can be operationalized as a person’s intention to participate in an specific vaccine clinical trial or willingness to join a general registry to learn about vaccine trials as they become available (e.g., the COVID-19 Prevention Network). Behavioral intention may include factors such as readiness to make a decision about clinical trial participation. As shown in other studies (Langford et al., 2020; Politi et al., 2016), readiness to make a decision about clinical trial participation is affected by knowledge, information-seeking ability, and clarity regarding one’s opinions about research. Another factor that affects behavioral intention is the quality of the informed consent process (Cohn et al., 2011; Mandava et al., 2012), which may affect one’s readiness to make a decision about vaccine clinical trial participation depending on how the potential participant’s questions and concerns were addressed. The quality of the informed consent process can also affect the degree to which potential participants feel certainty (i.e., less decisional conflict or uncertainty) about their decision to participate in a clinical trial (O’Connor, 1995).
Outcomes
Patient-level outcomes in vaccine clinical trials may include shorter-term outcomes such as whether or not patients decline participation or enroll in a study, retention of participants throughout the life of the study, and decision regret (Brehaut et al., 2003). Ideally, vaccine clinical trials that effectively recruit diverse participants will also result in longer-term, population-level outcomes such as improved medical innovation (e.g., a new vaccine or several new vaccines for COVID-19), and reduced morbidity and mortality from COVID-19 and other diseases.
Moving from Conceptual Model to Practice: The ASK Approach for Enhancing Trial Participation
The ASK approach is a 3-step process developed by the author as a heuristic to enhance equitable clinical trial offers and participation across sociodemographic groups. ASK stands for Assume, Seek, and Know (Table 2). This approach may be helpful for health professionals who design, implement, and evaluate clinical trial campaigns. First, assume that all patients will want to know their options, will be open to considering clinical trials, and will enroll if eligible and explicitly asked. By starting with this assumption, researchers increase the likelihood that a greater number of patients will be aware of clinical trial opportunities, thus reducing disparities in communication and clinical trial offers (Eggly et al., 2015; Wallington et al., 2012). Admittedly, making assumptions about people is typically discouraged in health care and everyday life. However, the author posits that researchers may be making assumptions about patients when determining suitability for clinical trials (Joseph & Dohan, 2009), either consciously or unconsciously, and therefore should proactively choose which assumptions will serve as the default for clinical trial communication and decision making. For example, instead of assuming that older adults or racial/ethnic minorities will not be willing to participate in vaccine-related clinical trials and avoiding the topic altogether, one can assume that older adults and minorities are indeed willing to participate and will want to know all of their health care options, including the option of joining a clinical trial (Byrne et al., 2014; Raheja et al., 2018; Wendler et al., 2006).
Table 2.
The ASK Approach to Enhancing Clinical Trial Participation
| Assume |
Assume that all patients will want to know their options and will; • Be open to considering clinical trials • Enroll if eligible and explicitly asked By starting with this assumption, researchers increase the likelihood that a greater number of patients will be aware of clinical trials opportunities and thus disparities in communication and clinical trial offers may be reduced. |
| Seek |
Seek the counsel of stakeholders. Stakeholders may include, but are not limited to: • Researchers • Patients • Caregivers • Community health workers • Community-based organizations • Faith-based organizations • Internal clinicians • External referring clinicians • Administrators • Communication and marketing professionals • Health information technology professionals • Institutional review board professionals • Pharmaceutical companies • Media partners • Policy makers This step will help ensure that researchers get the feedback needed to make adjustments to the marketing plan and successfully recruit participants. |
| Know |
Know your numbers. Key numbers to track in a clinical trial include, but are not limited to: • Number of potentially eligible patients or “pool of patients” • Number of people invited by different channels (patient portals vs. mailed letters) • Number of responses by different channels (patient portals, social media, email) • Number of interested, but not eligible patients • Number of declines by eligible patients • Number of enrolled patients Such data will help refine future efforts to recruit for similar types of clinical trials and patient populations. |
Second, seek the counsel of stakeholders. In recent years, various health professionals and organizations, including the Patient-Centered Outcomes Research Institute (PCORI), have advocated for better integration of stakeholder voices in both routine clinical care and research settings (Sharma et al., 2015). Notably, PCORI defines Engagement in Research as “the meaningful involvement of patients, caregivers, clinicians, and other healthcare stakeholders (such as training institutions, payers, industry, policy makers) throughout the research process—from topic selection through design and conduct of research to dissemination of results” (Patient-Centered Outcomes Research Institute, 2018). Depending on the context, goals, and target population for a clinical trial, stakeholders may also include communication and marketing professionals, community hospitals, federally qualified health centers, and prisons (Langford & Bateman-House, 2020). By seeking the counsel of key stakeholders throughout the clinical trial process, challenges and opportunities can be identified and addressed when there is still time to make adjustments to the recruitment plan.
Third, know your numbers. Key numbers to track in a clinical trial include the number of potentially eligible patients or “pool of patients,” number of patients invited via different channels, response rates by different channels (e.g., patient portals vs. social media advertisements), number of interested but not eligible patients, number of declines by eligible patients, and number of enrolled patients. Data dashboards are one tool to help monitor who is being screened and enrolled in clinical trials (Mattingly et al., 2015). However, one challenge of clinical trial dashboards is that they often only track final enrollment numbers. Although enrollment numbers are important data points indeed, exclusively focusing on enrollment numbers is limiting for at least three reasons. First, researchers often misjudge the number of patients who are actually eligible for their clinical trial, which in turn has negative implications for meeting recruitment goals (i.e., under-recruitment or no recruitment) (Carlisle et al., 2015). Second, the “science of recruitment” is evolving and, to date, no definitive best practices exist as to which recruitment strategies work best for different types of clinical trials, phases of clinical trials, and specific patient populations such as older adults, women, children and families, and racial/ethnic minority groups (Huang et al., 2018). Third, by understanding who is interested in joining a vaccine clinical trial, but not eligible may shed light on aspects of the protocol that are overly restrictive and thus systematically exclude certain types of patients (Carlisle et al., 2015; Langford & Bateman-House, 2020).
Example of How the ASK Approach Can Be Applied in Real-World Settings
Assume that all patients will want to know their options.
At the author’s home institution, a large and diverse group of patients were invited to join the institution’s COVID-19 vaccine registry by receiving a direct message via the patient portal (i.e., MyChart in Epic). Potentially eligible patients were identified with the help of health information technology colleagues, based on sociodemographic factors and underlying health conditions that could put them at increased risk for COVID-19. By taking a direct-to-patient approach to inviting people, we assumed that patients would want to know their options and avoided having to rely exclusively on clinicians as gatekeepers of clinical trial offers.
Seek the counsel of stakeholders.
At the author’s home institution, internal stakeholders held regular calls to discuss recruitment plans for the COVID-19 vaccine clinical trials. These internal stakeholders included clinical trial recruitment and retention experts, nurses, physicians, communications and marketing colleagues, research coordinators, and investigators with experience conducting community-based participatory research. External stakeholders included social media influencers, community-based organizations, and faith-based institutions. Further, a community advisory board (CAB) was specifically convened to advise on our COVID-19 vaccine clinical trial efforts.
Know your numbers.
At the author’s home institution, examples of numbers tracked for our COVID-19 vaccine clinical trials included (1) the total number of patients invited to join the COVID-19 vaccine registry via direct messages in the patient portal, (2) the number of people who have joined our institutional COVID-19 vaccine registry, (3) the number of people who initially learned about the general vaccine registry by various channels or outreach strategies (e.g., referred by someone, health system webpage, paid advertisement, ClinicalTrials.gov), and (4) actual versus planned enrollment in specific vaccine clinical trials. In keeping with our approach, pharmaceutical companies such as Pfizer and Moderna have made their COVID-19 vaccine trial enrollment numbers publicly available at pfizer.com/science/coronavirus/vaccineandmodernatx.com/cove-study, respectively.
Conclusion
The COVID-19 pandemic has shone a bright light on the unique challenges of rapidly recruiting participants for vaccine trials against the backdrops of vaccine-related hesitancy and misinformation. This commentary provided strategies for enhancing vaccine-specific clinical trial communication and decision making. A general model of clinical trial participation, the ASK (assume, seek, know) approach to enhancing clinical trial participation, and a curated list of resources that can be used in real-world settings were presented. Future research should explore how best to communicate vaccine clinical trial opportunities while also addressing vaccine literacy in both clinical care and public health communication contexts.
References
- Agency for Healthcare Research and Quality. (2015a). Health Literacy Universal Precautions Toolkit, 2nd Edition. Retrieved November 8 from https://www.ahrq.gov/sites/default/files/publications/files/healthlittoolkit2_4.pdf
- Agency for Healthcare Research and Quality. (2015b). Health Literacy Universal Precautions Toolkit, 2nd Edition: Consider Culture, Customs, and Beliefs=. Retrieved November 8 from https://www.ahrq.gov/health-literacy/improve/precautions/tool10.html
- Ajzen I (1985). From Intentions to Actions: A Theory of Planned Behavior. In Kuhl J & Beckmann J (Eds.), Action Control: From Cognition to Behavior (pp. 11–39). Springer Berlin; Heidelberg. 10.1007/978-3-642-69746-3_2 [DOI] [Google Scholar]
- Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, & Feldman-Stewart D (2003). Validation of a decision regret scale. Medical Decision Making, 23(4), 281–292. 10.1177/0272989X03256005 [DOI] [PubMed] [Google Scholar]
- Burks AC, Doede A, Showalter SL, & Keim-Malpass J (2020). Perceptions of Clinical Trial Participation Among Women of Varying Health Literacy Levels. Oncology Nursing Forum, 47(3), 273–280. 10.1188/20.Onf.273-280 [DOI] [PubMed] [Google Scholar]
- Byrne MM, Tannenbaum SL, Glück S, Hurley J, & Antoni M (2014). Participation in cancer clinical trials: why are patients not participating? Medical Decision Making, 34(1), 116–126. 10.1177/0272989x13497264 [DOI] [PubMed] [Google Scholar]
- Cameron D, Willoughby C, Messer D, Lux M, Aitken M, & Getz K (2020). Assessing Participation Burden in Clinical Trials: Introducing the Patient Friction Coefficient. Clinical Therapeutics, 42(8), e150–e159. 10.1016/j.clinthera.2020.06.015 [DOI] [PubMed] [Google Scholar]
- Carlisle B, Kimmelman J, Ramsay T, & MacKinnon N (2015). Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clinical Trials (London, England), 12(1), 77–83. 10.1177/1740774514558307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2020). CDC Digital Media Toolkit: 2020–21 Flu Season. Retrieved November 26 from https://www.cdc.gov/flu/resource-center/toolkit/index.htm
- Chou WS, & Budenz A (2020). Considering Emotion in COVID-19 Vaccine Communication: Addressing Vaccine Hesitancy and Fostering Vaccine Confidence. Health Commun, 35(14), 1718–1722. 10.1080/10410236.2020.1838096 [DOI] [PubMed] [Google Scholar]
- Clark LT, Watkins L, Piña IL, Elmer M, Akinboboye O, Gorham M, Jamerson B, McCullough C, Pierre C, Polis AB, Puckrein G, & Regnante JM (2019). Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Current Problems in Cardiology, 44(5), 148–172. 10.1016/j.cpcardiol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- Cohn EG, Jia H, Smith WC, Erwin K, & Larson EL (2011). Measuring the process and quality of informed consent for clinical research: development and testing. Oncology Nursing Forum, 38(4), 417–422. 10.1188/11.Onf.417-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LA, & Williams DR (2020). Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA, 324(15), 1491–1492. [DOI] [PubMed] [Google Scholar]
- COVID-19 Prevention Network. (2020). Clinical Studies. Retrieved November 29 from https://www.coronaviruspreventionnetwork.org/understanding-clinical-studies/
- Craven J (2020). COVID-19 vaccine tracker. Regulatory Affairs Professionals Society. Retrieved November 29 from https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
- Cutler DM, & Summers LH (2020). The COVID-19 Pandemic and the $16 Trillion Virus. JAMA, 324(15), 1495–1496. 10.1001/jama.2020.19759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley MF, Narwaney KJ, Shoup JA, Wagner NM, & Glanz JM (2018). Addressing Parents’ Vaccine Concerns: A Randomized Trial of a Social Media Intervention. American Journal of Preventive Medicine, 55(1), 44–54. 10.1016/j.amepre.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detoc M, Launay O, Dualé C, Mutter C, Le Huec JC, Lenzi N, Lucht F, Gagneux-Brunon A, & Botelho-Nevers E (2019). Barriers and motivations for participation in preventive vaccine clinical trials: Experience of 5 clinical research sites. Vaccine, 37(44), 6633–6639. 10.1016/j.vaccine.2019.09.048 [DOI] [PubMed] [Google Scholar]
- Dutta MJ (2007). Communicating about culture and health: Theorizing culture-centered and cultural sensitivity approaches. Communication Theory, 17(3), 304–328. [Google Scholar]
- Dyer O (2020). Covid-19: Pharma companies promise not to bow to political pressure to rush vaccine production. BMJ, 370, m3512. 10.1136/bmj.m3512 [DOI] [PubMed] [Google Scholar]
- Eggly S, Barton E, Winckles A, Penner LA, & Albrecht TL (2015). A disparity of words: racial differences in oncologist-patient communication about clinical trials. Health Expectations, 18(5), 1316–1326. 10.1111/hex.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egleston BL, Pedraza O, Wong YN, Dunbrack RL Jr., Griffin CL, Ross EA, & Beck JR (2015). Characteristics of clinical trials that require participants to be fluent in English. Clinical Trials (London, England), 12(6), 618–626. 10.1177/1740774515592881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangeli M, Kafaar Z, Kagee A, Swartz L, & Bullemor-Day P (2013). Does message framing predict willingness to participate in a hypothetical HIV vaccine trial: an application of Prospect Theory. AIDS Care, 25(7), 910–914. 10.1080/09540121.2012.748163 [DOI] [PubMed] [Google Scholar]
- Finset A, Bosworth H, Butow P, Gulbrandsen P, Hulsman RL, Pieterse AH, Street R, Tschoetschel R, & van Weert J (2020). Effective health communication - a key factor in fighting the COVID-19 pandemic. Patient Education and Counseling, 103(5), 873–876. 10.1016/j.pec.2020.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew PM, Kriss JL, Chamberlain AT, Malik F, Chung Y, Cortés M, & Omer SB (2016). A randomized trial of maternal influenza immunization decision-making: A test of persuasive messaging models. Human Vaccines & Immunotherapeutics, 12(8), 1989–1996. 10.1080/21645515.2016.1199309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew PM, Shapiro ET, Lu L, Edupuganti S, Keyserling HL, & Mulligan MJ (2013). Enrollment in YFV Vaccine Trial: An Evaluation of Recruitment Outcomes Associated with a Randomized Controlled Double-Blind Trial of a Live Attenuated Yellow Fever Vaccine. Tropical medicine & surgery, 1(2), 117–117. 10.4172/2329-9088.1000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz K, Sethuraman V, Rine J, Peña Y, Ramanathan S, & Stergiopoulos S (2019). Assessing Patient Participation Burden Based on Protocol Design Characteristics. Therapeutic innovation & regulatory science,, 2168479019867284. 10.1177/2168479019867284 [DOI] [PubMed] [Google Scholar]
- Giocos G, Kagee A, & Swartz L (2008). Predicting hypothetical willingness to participate (WTP) in a future phase III HIV vaccine trial among high-risk adolescents. AIDS and Behavior, 12(6), 842–851. 10.1007/s10461-007-9289-5 [DOI] [PubMed] [Google Scholar]
- Grady C, Bedarida G, Sinaii N, Gregorio MA, & Emanuel EJ (2017). Motivations, enrollment decisions, and socio-demographic characteristics of healthy volunteers in phase 1 research. Clinical Trials (London, England), 14(5), 526–536. 10.1177/1740774517722130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantz KH, Claudot P, Kambala M, Kouyaté M, Soumah A, Boum Y, Juan-Giner A, Jemmy JP, Cummings DAT, & Grais RF (2019). Factors influencing participation in an Ebola vaccine trial among front-line workers in Guinea. Vaccine, 37(48), 7165–7170. 10.1016/j.vaccine.2019.09.094 [DOI] [PubMed] [Google Scholar]
- Hawkins RP, Kreuter M, Resnicow K, Fishbein M, & Dijkstra A (2008). Understanding tailoring in communicating about health. Health Education Research, 23(3), 454–466. 10.1093/her/cyn004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Literacy Out Loud. (2014). Health Literacy Screening Tools (HLOL #124),. Retrieved November 8 from http://www.healthliteracyoutloud.com/?s=vital+sign
- Health.gov. (2020). Health Literacy in Healthy People. Retrieved November 8 from https://health.gov/our-work/healthy-people-2030/about-healthy-people-2030/health-literacy-healthy-people
- Houghton C, Dowling M, Meskell P, Hunter A, Gardner H, Conway A, Treweek S, Sutcliffe K, Noyes J, Devane D, Nicholas JR, & Biesty LM (2020). Factors that impact on recruitment to randomised trials in health care: a qualitative evidence synthesis. The Cochrane database of systematic reviews,, 10, Mr000045. 10.1002/14651858.MR000045.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li Q, & Chen Y (2018). Evaluation of two health education interventions to improve the varicella vaccination: a randomized controlled trial from a province in the east China. BMC Public Health, 18(1), 144. 10.1186/s12889-018-5070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GD, Bull J, Johnston McKee K, Mahon E, Harper B, & Roberts JN (2018). Clinical trials recruitment planning: A proposed framework from the Clinical Trials Transformation Initiative. Contemporary Clinical Trials, 66, 74–79. 10.1016/j.cct.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Jaklevic MC (2020). Researchers Strive to Recruit Hard-Hit Minorities Into COVID-19 Vaccine Trials. Jama, 324(9), 826–828. 10.1001/jama.2020.11244 [DOI] [PubMed] [Google Scholar]
- Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, & Xing Z (2020). Immunological considerations for COVID-19 vaccine strategies. Nature Reviews Immunology, 20(10), 615–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph G, & Dohan D (2009). Diversity of participants in clinical trials in an academic medical center: the role of the ‘Good Study Patient?’. Cancer, 115(3), 608–615. 10.1002/cncr.24028 [DOI] [PubMed] [Google Scholar]
- Kasting ML, Head KJ, Cox D, Cox AD, & Zimet GD (2019). The effects of message framing and healthcare provider recommendation on adult hepatitis B vaccination: A randomized controlled trial. Preventive Medicine, 127, 105798. 10.1016/j.ypmed.2019.105798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Bruinooge SS, Roberts S, Ison G, Lin NU, Gore L, Uldrick TS, Lichtman SM, Roach N, Beaver JA, Sridhara R, Hesketh PJ, Denicoff AM, Garrett-Mayer E, Rubin E, Multani P, Prowell TM, Schenkel C, Kozak M, Allen J, Sigal E, & Schilsky RL (2017). Broadening Eligibility Criteria to Make Clinical Trials More Representative: American Society of Clinical Oncology and Friends of Cancer Research Joint Research Statement. Journal of Clinical Oncology, 35(33), 3737–3744. 10.1200/jco.2017.73.7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps S, Prasad S, Brownstein JS, Hswen Y, Garibaldi BT, Zhang B, & Kriner DL (2020). Factors Associated With US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA network open, 3(10), e2025594. 10.1001/jamanetworkopen.2020.25594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, & Sanders-Thompson V (2003). Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health education & behavior: the official publication of the Society for Public Health Education, 30(2), 133–146. 10.1177/1090198102251021 [DOI] [PubMed] [Google Scholar]
- Krieger JL, Parrott RL, & Nussbaum JF (2011). Metaphor use and health literacy: a pilot study of strategies to explain randomization in cancer clinical trials. Journal of health communication, 16(1), 3–16. 10.1080/10810730.2010.529494 [DOI] [PubMed] [Google Scholar]
- Langford A, & Bateman-House A (2020). Clinical Trials For COVID-19: Populations Most Vulnerable To COVID-19 Must Be Included. Health Affairs Blog. Retrieved December 1 from https://www.healthaffairs.org/do/10.1377/hblog20200609.555007/full/
- Langford A, Studts JL, & Byrne MM (2020). Improving knowledge and decision readiness to participate in cancer clinical trials: Effects of a plain language decision aid for minority cancer survivors. Patient Education and Counseling. 10.1016/j.pec.2020.07.005 [DOI] [PubMed] [Google Scholar]
- Langford AT, Resnicow K, & Beasley DD (2015). Outcomes from the Body & Soul Clinical Trials Project: a university-church partnership to improve African American enrollment in a clinical trial registry. Patient Education and Counseling, 98(2), 245–250. 10.1016/j.pec.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford AT, Resnicow K, Dimond EP, Denicoff AM, Germain DS, McCaskill-Stevens W, Enos RA, Carrigan A, Wilkinson K, & Go RS (2014). Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute’s Community Cancer Centers Program. Cancer, 120(6), 877–884. 10.1002/cncr.28483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald NE (2015). Vaccine hesitancy: Definition, scope and determinants. Vaccine, 33(34), 4161–4164. 10.1016/j.vaccine.2015.04.036 [DOI] [PubMed] [Google Scholar]
- Mandava A, Pace C, Campbell B, Emanuel E, & Grady C (2012). The quality of informed consent: mapping the landscape. A review of empirical data from developing and developed countries. Journal of Medical Ethics, 38(6), 356–365. 10.1136/medethics-2011-100178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly WA, Kelley RR, Wiemken TL, Chariker JH, Peyrani P, Guinn BE, Binford LE, Buckner K, & Ramirez J (2015). Real-Time Enrollment Dashboard For Multisite Clinical Trials. Contemporary clinical trials communications, 1, 17–21. 10.1016/j.conctc.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J, Kates J, Dolan R, & Tolbert J (2020). States Are Getting Ready to Distribute COVID-19 Vaccines. What Do Their Plans Tell Us So Far? Kaiser Family Foundation. Retrieved November 25 from https://www.kff.org/coronavirus-covid-19/issue-brief/states-are-getting-ready-to-distribute-covid-19-vaccines-what-do-their-plans-tell-us-so-far/
- National Academies of Sciences Engineering and Medicine. (2020). Framework for equitable allocation of COVID-19 vaccine. National Academies Press. Retrieved December 1 from https://www.nap.edu/catalog/25917/framework-for-equitable-allocation-of-covid-19-vaccine [PubMed] [Google Scholar]
- Neil JM, Gough A, Kee F, George TJ Jr., Pufahl J, & Krieger JL (2019). The Influence of Patient Identification and Narrative Transportation on Intentions to Participate in Cancer Research. Journal of Cancer Education, 34(4), 725–734. 10.1007/s13187-018-1364-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Hesse BW, & Croyle RT (2009). Making Data Talk: Communicating Public Health Data to the Public, Policy Makers, and the Press. Oxford University Press. https://books.google.com/books?id=zwYpAQAAMAAJ [Google Scholar]
- Nipp RD, Hong K, & Paskett ED (2019). Overcoming Barriers to Clinical Trial Enrollment. American Society of Clinical Oncology educational book., 39, 105–114. 10.1200/edbk_243729 [DOI] [PubMed] [Google Scholar]
- O’Callaghan KP, Blatz AM, & Offit PA (2020). Developing a SARS-CoV-2 Vaccine at Warp Speed. JAMA, 324(5), 437–438. 10.1001/jama.2020.12190 [DOI] [PubMed] [Google Scholar]
- O’Connor AM (1995). Validation of a decisional conflict scale. Medical Decision Making, 15(1), 25–30. 10.1177/0272989x9501500105 [DOI] [PubMed] [Google Scholar]
- O’Leary C, Casey C, Webb D, Collyar D, & Pleasant A (2020). How Health Literacy Can Enhance the Design and Conduct of Clinical Trials from Consent to Conclusion. Studies in Health Technology and Informatics, 269, 275–284. 10.3233/shti200042 [DOI] [PubMed] [Google Scholar]
- Palmer S, Pudwell J, Smith GN, & Reid RL (2016). Optimizing Participation of Pregnant Women in Clinical Trials: Factors Influencing Decisions About Participation in Medication and Vaccine Trials. Journal of Obstetrics and Gynaecology Canada. Journal d’Obstétrique et Gynécologie du Canada, 38(10), 945–954. 10.1016/j.jogc.2016.04.100 [DOI] [PubMed] [Google Scholar]
- Parkinson B, Meacock R, Sutton M, Fichera E, Mills N, Shorter GW, Treweek S, Harman NL, Brown RCH, Gillies K, & Bower P (2019). Designing and using incentives to support recruitment and retention in clinical trials: a scoping review and a checklist for design. Trials, 20(1), 624. 10.1186/s13063-019-3710-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient-Centered Outcomes Research Institute. (2018). The Value of Engagement. Retrieved November 29 from https://www.pcori.org/engagement/value-engagement
- Peng W, Morgan SE, Mao B, McFarlane SJ, Occa A, Grinfeder G, & Byrne MM (2019). Ready to Make A Decision: A Model of Informational Aids to Improve Informed Participation in Clinical Trial Research. Journal of health communication, 24(12), 865–877. 10.1080/10810730.2019.1680773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty RE, & Cacioppo JT (1986). The Elaboration Likelihood Model of Persuasion. In Communication and Persuasion: Central and Peripheral Routes to Attitude Change (pp. 1–24). Springer; New York. 10.1007/978-1-4612-4964-1_1 [DOI] [Google Scholar]
- PlainLanguage.gov. What is plain language? Retrieved November 26 from https://www.plainlanguage.gov/about/definitions/
- Polite BN, Cipriano-Steffens TM, Liao C, Miller EL, Arndt NL, & Hahn EA (2019). Investigation of a multimedia, computer-based approach to improve knowledge, attitudes, self-efficacy, and receptivity to cancer clinical trials among newly diagnosed patients with diverse health literacy skills. Cancer, 125(12), 2066–2075. 10.1002/cncr.31991 [DOI] [PubMed] [Google Scholar]
- Politi MC, Kuzemchak MD, Kaphingst KA, Perkins H, Liu J, & Byrne MM (2016). Decision Aids Can Support Cancer Clinical Trials Decisions: Results of a Randomized Trial. Oncologist, 21(12), 1461–1470. 10.1634/theoncologist.2016-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheja D, Davila EP, Johnson ET, Deović R, Paine M, & Rouphael N (2018). Willingness to Participate in Vaccine-Related Clinical Trials among Older Adults. International Journal of Environmental Research and Public Health, 15(8). 10.3390/ijerph15081743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzan SC (2011). Vaccine literacy: a new shot for advancing health. Journal of health communication, 16(3), 227–229. 10.1080/10810730.2011.561726 [DOI] [PubMed] [Google Scholar]
- Ratzan SC, & Parker RM (2000.). Introduction. In National Library of Medicine current bibliographies in medicine: Health literacy, edited by Selden C, Zorn M, Ratzan S, and Parker R. NLM Pub. No. CBM 2000–1. Bethesda, MD: National Institutes of Health. In. [Google Scholar]
- Raviotta JM, Nowalk MP, Lin CJ, Huang HH, & Zimmerman RK (2016). Using Facebook™ to Recruit College-Age Men for a Human Papillomavirus Vaccine Trial. American journal of men’s health, 10(2), 110–119. 10.1177/1557988314557563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicow K, Baranowski T, Ahluwalia JS, & Braithwaite RL (1999). Cultural sensitivity in public health: defined and demystified. Ethnicity and Disease, 9(1), 10–21. [PubMed] [Google Scholar]
- Sastry S, & Dutta MJ (2017). Health communication in the time of Ebola: A culture-centered interrogation. Journal of Health Communication,, 22(sup1), 10–14. 10.1080/10810730.2016.1216205 [DOI] [PubMed] [Google Scholar]
- Schmidt H, Gostin LO, & Williams MA (2020). Is It Lawful and Ethical to Prioritize Racial Minorities for COVID-19 Vaccines? JAMA, 324(20), 2023–2024. 10.1001/jama.2020.20571 [DOI] [PubMed] [Google Scholar]
- Schultz PL, Carlisle R, Cheatham C, & O’Grady M (2017). Evaluating the Use of Plain Language in a Cancer Clinical Trial Website/App. Journal of Cancer Education, 32(4), 707–713. 10.1007/s13187-016-0994-5 [DOI] [PubMed] [Google Scholar]
- Sharma A, Angel L, & Bui Q (2015). Patient Advisory Councils: Giving Patients a Seat at the Table. Family Practice Management, 22(4), 22–27. [PubMed] [Google Scholar]
- Stratton SL, Spencer HJ, Greenfield WW, Low G, Hitt WC, Quick CM, Jeffus SK, Blackmon V, & Nakagawa M (2015). A novel use of a statewide telecolposcopy network for recruitment of participants in a Phase I clinical trial of a human papillomavirus therapeutic vaccine. Clinical Trials (London, England), 12(3), 199–204. 10.1177/1740774514566333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street RL (2003). Communication in Medical Encounters: An Ecological Perspective in The Routledge handbook of health communication. Thompson T (Ed.), Parrott R (Ed.), Dorsey A (Ed.), Miller K (Ed.). . Routledge. 10.4324/9781410607683 [DOI] [Google Scholar]
- The History of Vaccines. (2018). Disease Eradication. The College of Physicians of Philadelphia,. Retrieved November 26 from https://www.historyofvaccines.org/content/articles/disease-eradication
- Tucker CM, Kang S, Ukonu NA, Linn GS, DiSangro CS, Arthur TM, & Ralston PA (2019). A Culturally Sensitive Church-Based Health-Smart Intervention for Increasing Health Literacy and Health-Promoting Behaviors among Black Adult Churchgoers. Journal of Health Care for the Poor and Underserved, 30(1), 80–101. 10.1353/hpu.2019.0009 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services. (2020). Fact Sheet: Explaining Operation Warp Speed. https://www.hhs.gov/coronavirus/explaining-operation-warp-speed/index.html
- Unger JM, Vaidya R, Hershman DL, Minasian LM, & Fleury ME (2019). Systematic Review and Meta-Analysis of the Magnitude of Structural, Clinical, and Physician and Patient Barriers to Cancer Clinical Trial Participation. Journal of the National Cancer Institute, 111(3), 245–255. 10.1093/jnci/djy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman GA (2020). “Warp Speed” Operations in the COVID-19 Pandemic: Moving Too Quickly? JACC. Basic to translational science, 5(7), 730–734. 10.1016/j.jacbts.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEpps EM, Volpp KG, & Halpern SD (2016). A nudge toward participation: Improving clinical trial enrollment with behavioral economics. Science Translational Medicine, 8(348), 348fs313. 10.1126/scitranslmed.aaf0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerstein N, Oetzel JG, Duran B, Magarati M, Pearson C, Belone L, Davis J, DeWindt L, Kastelic S, Lucero J, Ruddock C, Sutter E, & Dutta MJ (2019). Culture-centeredness in community-based participatory research: contributions to health education intervention research. Health Education Research, 34(4), 372–388. 10.1093/her/cyz021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallington SF, Luta G, Noone AM, Caicedo L, Lopez-Class M, Sheppard V, Spencer C, & Mandelblatt J (2012). Assessing the awareness of and willingness to participate in cancer clinical trials among immigrant Latinos. Journal of Community Health, 37(2), 335–343. 10.1007/s10900-011-9450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, & Emanuel E (2006). Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine, 3(2), e19. 10.1371/journal.pmed.0030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation. (2020). Placebo-Controlled Trials of Covid-19 Vaccines — Why We Still Need Them. New England Journal of Medicine. 10.1056/NEJMp2033538 [DOI] [Google Scholar]
- World Health Organization. (2018). Vaccines: the powerful innovations bringing WHO’s mission to life every day. Retrieved November 26 from https://www.who.int/news-room/commentaries/detail/vaccines-the-powerful-innovations-bringing-who-s-mission-to-life-every-day
- Yang J, McComas K, Gay G, Leonard JP, Dannenberg AJ, & Dillon H (2010). From information processing to behavioral intentions: exploring cancer patients’ motivations for clinical trial enrollment. Patient Education and Counseling, 79(2), 231–238. 10.1016/j.pec.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Zimmer C, Corum J, & Wee S-L (2020). Coronavirus Vaccine Tracker. New York Times. Retrieved November 29 from https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html


