ABSTRACT
Methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections are associated with significant morbidity and mortality. MRSA secretes a number of virulence factors and pore-forming toxins that enable tissue invasion. Prior studies have found associations between decreased toxin production and poor outcomes in invasive MRSA infection, particularly in pneumonia. In this retrospective observational cohort study of MRSA bacteremia in adult patients from 2007 to 2015, we examined whether cytotoxicity was associated with 30-day mortality. Isolates were obtained from 776 patients and screened for cytotoxicity in a human HL-60 cell model, antimicrobial susceptibility, and spa type, and clinical data were abstracted from charts. We did not find an association between low cytotoxic activity and 30-day mortality in univariate logistic regression analyses. There was a difference in distribution of the genotypes across cytotoxicity phenotypes, with spa-CC008 accounting for a larger proportion of isolates in the high cytotoxicity group. Isolates with a skin and soft tissue primary infective site had a higher median cytotoxicity. There was no association between cytotoxicity and host factors such as age or comorbidity burden. The isolates in our study came from heterogeneous primary sites of infection and were predominantly from spa-CC002 and spa-CC008 lineages, so it is possible that findings in prior studies reflect a different distribution in genotypes and clinical syndromes. Overall, in this large study of cytotoxicity of MRSA bloodstream isolates, we did not find the low cytotoxicity phenotype to be predictive of poor outcomes in MRSA bacteremia.
KEYWORDS: Staphylococcus aureus, MRSA, mortality, virulence, cytotoxicity
INTRODUCTION
Staphylococcus aureus infections are prevalent worldwide and are often accompanied by serious complications such as endocarditis and bacteremia (1, 2). S. aureus invasion of the bloodstream is associated with high mortality rates, as well as significant health care costs associated with prolonged treatment courses and hospitalizations. Infection with methicillin-resistant S. aureus (MRSA) is typically associated with higher rates of treatment failure, metastatic spread of infection, and poorer outcomes than methicillin-susceptible S. aureus (MSSA) infection, in part due to more limited antibiotic treatment options (3, 4). Developing a better understanding of the pathogenesis underlying bloodstream invasion and the bacterial attributes that allow persistence of infection and immune evasion could shed light on potential new targets for interventions.
S. aureus produces a number of toxins and virulence factors that allow it to adhere to and invade tissue (5–8). Toxin production is controlled by global regulator genes such as agr, with agr dysfunction generally associated with decreased toxin production and attenuation of phenotypic virulence (9–15). Traditionally, greater cytotoxicity has been assumed to cause greater severity of disease (16). However, recent research findings have challenged aspects of the relationship between toxin production and clinical outcomes (17, 18). This includes reports of associations between a low-cytotoxicity phenotype of MRSA isolates and complications such as bloodstream infection and increased mortality (17, 18). Additionally, dysfunction in the agr gene, a global regulator of multiple housekeeping genes and secreted toxins, has been associated with higher mortality in patients with bacteremia (19). A low-cytotoxicity phenotype and agr dysfunction have also been linked to persistence of S. aureus in the bloodstream and development of chronic infections such as osteomyelitis (17, 18, 20, 21). Based on genomic analysis of clinical isolates, it has been proposed that within-host genetic adaptation may occur to downregulate toxin production and facilitate both bacterial survival and immune evasion (18, 22, 23). This suggests that once bacteremia is established, toxin production is no longer a major driver of disease severity and clinical outcomes. As a result, targeting toxin production with the use of protein synthesis-inhibiting antibiotics may have less of a role in bacteremia.
There are also characteristic clonal patterns in toxin production and cytotoxic activity, with health care-associated MRSA clones typically demonstrating lower cytotoxic activity in phenotypical assays (17). Vancomycin-intermediate S. aureus (VISA) isolates have been found to have downregulated expression of toxins and poor cytotoxicity in vitro (17, 24, 25).
Another possible explanation for the observed association between low-cytotoxicity S. aureus strains and mortality is that, rather than representing an enhanced ability to cause invasive disease, low cytotoxicity is simply a marker of impaired host status and the tendency of such hosts to be colonized and infected with weakly cytotoxic hospital-associated clones (17). The inverse association between cytotoxicity and mortality has not consistently been supported by data (26). The variety of clinical contexts and genotypic backgrounds across which this association has been studied also makes broader conclusions about the relationship difficult.
In this study, we evaluated the cytotoxic activity of a large comprehensive collection of MRSA bloodstream isolates (27), and we described the clinical and bacterial characteristics associated with high- and low-cytotoxicity phenotypes. We then explored whether there was an association between cytotoxicity and all-cause 30-day mortality in MRSA bacteremia. We hypothesize that patients with infections with MRSA bloodstream isolates with lower cytotoxicity will have a paradoxically higher 30-day mortality.
RESULTS
Study cohort and cytotoxicity.
After excluding isolates that either did not grow or where there was greater than 30% variability in the percentage of cell killing, there were a total of 776 MRSA bacteremia episodes that were included in the analysis. The overall characteristics of the study population are shown in Table 1. The patients in the high-cytotoxicity and low-cytotoxicity cohorts were similar. The average age of the overall cohort was 62 (median, 62), and 44% were over the age of 65. Forty-three percent of isolates were from spa-CC002/ST5, and 49% were from spa-CC008/ST8. Notably, high-cytotoxicity isolates were more likely to come from a skin/soft tissue source, have a higher vancomycin MIC (measured via geometric mean), and were more often spa-CC008. Low-cytotoxicity isolates were more often agr defective.
TABLE 1.
Clinical and bacterial characteristics of MRSA bloodstream infection by cytotoxicity categorya
| Characteristic | Data for: |
P value | ||
|---|---|---|---|---|
| Total (n = 776) | High-cytotoxicity group (n = 194) | Low-cytotoxicity group (n = 582) | ||
| Avg age (median [yrs]) | 62 (62) | 63 (62) | 62 (62) | 0.62 |
| Age > 65 yrs | 341 (44) | 86 (44) | 255 (44) | 0.90 |
| Male sex | 450 (58) | 109 (56) | 341 (59) | 0.56 |
| Hospital-onset infection | 249 (32) | 58 (30) | 191 (33) | 0.45 |
| Community-onset infection | 527 (68) | 136 (70) | 391 (67) | 0.45 |
| PBS (avg [median]) | 2.7 (2.0) | 2.8 (2.0) | 2.6 (2.0) | 0.77 |
| PBS ≥ 4 | 208 (27) | 53 (27) | 155 (27) | 0.94 |
| Comorbidities | ||||
| Avg CCI (median) | 4.1 (4.0) | 3.9 (4.0) | 4.2 (4.0) | 0.35 |
| CCI ≥ 3 | 529 (68) | 128 (66) | 401 (69) | 0.45 |
| Diabetes | 81 (10) | 22 (11) | 59 (10) | 0.64 |
| Prior MI/CAD | 27 (3.5) | 5 (2.6) | 22 (3.8) | 0.43 |
| Peripheral vascular disease | 85 (11) | 23 (12) | 62 (11) | 0.64 |
| Chronic heart failure | 104 (13) | 25 (13) | 79 (14) | 0.81 |
| Chronic kidney disease | 220 (28) | 51 (26) | 169 (29) | 0.46 |
| ESRD/dialysis | 106 (14) | 23 (12) | 83 (14) | 0.40 |
| Liver disease | 64 (8.3) | 20 (10) | 44 (7.6) | 0.23 |
| Chronic pulmonary disease | 91 (12) | 21 (11) | 70 (12) | 0.65 |
| Connective tissue disease | 2 (0.26) | 0 (0) | 2 (0.34) | 0.41 |
| HIV | 73 (9.4) | 13 (6.7) | 60 (10) | 0.14 |
| Cerebrovascular disease | 8 (1.0) | 1 (0.52) | 7 (1.2) | 0.41 |
| Hemiplegia/paraplegia | 15 (1.9) | 2 (1.0) | 13 (2.2) | 0.29 |
| Malignancy | 67 (8.6) | 17 (8.7) | 50 (8.6) | 0.94 |
| Chemotherapy | 51 (6.6) | 12 (6.2) | 39 (6.7) | 0.80 |
| Infective source | ||||
| Pneumonia | 140 (18) | 32 (16) | 108 (19) | 0.52 |
| Skin and soft tissue infection | 136 (17) | 45 (23) | 91 (16) | 0.02 |
| Bone/joint | 56 (7.2) | 11 (5.7) | 45 (7.7) | 0.34 |
| Catheter related | 153 (20) | 35 (18) | 118 (20) | 0.50 |
| Device related | 8 (1.0) | 4 (2.1) | 4 (0.7) | 0.10 |
| Surgical site | 51 (6.6) | 10 (5.2) | 41 (7.0) | 0.36 |
| Endovascular | 57 (7.4) | 13 (6.7) | 44 (7.6) | 0.69 |
| Other | 12 (1.6) | 5 (2.6) | 7 (1.2) | 0.18 |
| Unknown | 163 (21) | 39 (20) | 124 (21) | 0.72 |
| Infectious disease consult | 323/508 (64) | 84/150 (56) | 239/358 (67) | 0.02 |
| MRSA treatment during hospital stay | 743 (96) | 187 (96) | 556 (96) | 0.61 |
| Duration of BSI | ||||
| ≥3 days | 248 (32) | 65 (34) | 183 (31) | 0.59 |
| ≥5 days | 136 (18) | 42 (22) | 94 (16) | 0.08 |
| ≥10 days | 36 (4.6) | 8 (4.1) | 28 (4.8) | 0.69 |
| Avg length of admission (days) | 26 | 23 | 27 | 0.10 |
| Antibiotic susceptibility | ||||
| Vancomycin MIC, geometric mean | 1.37 | 1.44 | 1.34 | 0.02 |
| Vancomycin MIC ≥ 1.5 | 332 (43) | 93 (48) | 239 (41) | 0.09 |
| Genotype by spa-CC group | ||||
| spa-CC002/ST5 | 335 (43) | 46 (24) | 289 (50) | <0.01 |
| spa-CC008/ST8 | 381 (49) | 137 (71) | 244 (42) | |
| agr defective | 243/772 (31) | 45/194 (23) | 198/578 (34) | <0.01 |
| Mortality | ||||
| 30-day mortality | 198 (26) | 45 (23) | 153 (26) | 0.39 |
Data represent number and percentage in parentheses unless otherwise specified. Abbreviations: CCI, Charlson comorbidity index; MI/CAD, myocardial infarction/coronary artery disease; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; BSI, bloodstream infection.
The most common primary foci of infection were pneumonia (18%), skin and soft tissue infection (17%), and catheter-related infection (20%) (Table 1). Twenty-one percent of the episodes were classified as “primary” bacteremia, without a clear primary source. ID consultation data were only available for 508 patients (2011 to 2015), and in 64% of these cases, an ID consultation was obtained. The 30-day mortality in this cohort was 26%.
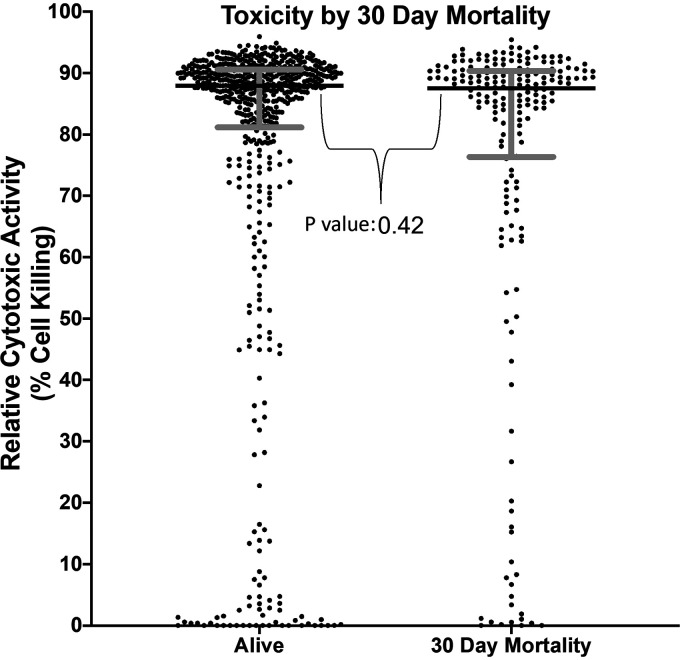
The median toxicity for all isolates was 87.9% (interquartile range [IQR], 79.50 to 90.53%). Our predefined cutoff for high cytotoxicity was >90.53% (top quartile, n = 194) cell killing. The median toxicity for the low-cytotoxic activity group (n = 582) was 85.66%. Of these low-toxicity isolates, 10.7% (n = 62) had percentage of cell killing <10. For the group with 30-day mortality, median cytotoxicity was 87.5% versus a median of 88.0% in those who survived (Fig. 1).
FIG 1.
Toxicity by 30-day mortality. Cytotoxic activity of each individual clinical isolate is indicated by filled circle for patients alive at 30 days and those with 30-day mortality. Horizontal bar for each group indicates median cytotoxicity, with gray bars indicating the interquartile range.
Genotype and cytotoxicity.
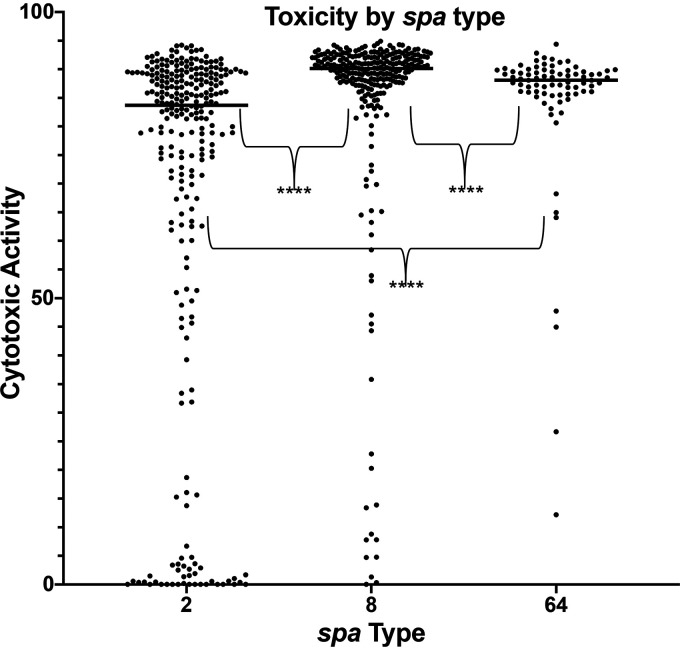
There was a significant difference in the distribution of genotypes across high- and low-cytotoxic activity phenotypes. spa-CC008 accounted for a larger proportion of isolates in the highly cytotoxic group (71% CC008 versus 24% CC002), while spa-CC002 accounted for a larger proportion of isolates in the weakly cytotoxic group (50% CC002 versus 42% CC008, P < 0.0001). The median cell killing was 83.7% for spa-CC002 isolates and 90.13% for spa-CC008 isolates (Fig. 2). agr dysfunction was more common in the low-toxicity group (34% versus 23%, P = 0.004). There was a significant difference in the vancomycin MIC distribution between the high- and low-toxicity groups measured via Mann-Whitney test (geometric mean, 1.344 versus 1.264, respectively; P = 0.02); however, there was no difference in the proportion of patients with a vancomycin MIC of ≥1.5 between the high- and low-toxicity groups.
FIG 2.
Toxicity by spa type. Cytotoxic activity of each clinical isolates from the three most common spa types from our cohort. Horizontal bar for each group indicates median cytotoxicity, with gray bars indicating the interquartile range. ****, P < 0.01.
Clinical variables and mortality.
There were no significant differences in baseline clinical characteristics between the high- and low-cytotoxicity groups (using cutoff >90.53% cell killing for high versus low), including age (average age, 62 years in low versus 63 years in high) and male gender (59% in low versus 56% in high) (Table 1). There were also no significant differences in the distribution of patients with different comorbidities across the high- and low-toxicity groups, as well as no significant difference in average Charlson score (4.2 in low versus 3.9 in high) or proportion with Charlson score ≥3 (69% in low versus 66% in high.) The average Pitt bacteremia scores (PBSs) and proportion of patients with PBS ≥4 also did not differ across the groups. There were also similar rates of community-onset (CO) and hospital-onset (HO) infections in the two groups.
Bacteremia duration was also similar across the groups, although there was a higher proportion of patients with duration ≥5 days in the high-cytotoxicity group (22% versus 16%, P = 0.08) (Table 1). Moreover, there was no difference in the median cytotoxicity of isolates causing bacteremia for greater than 10 days (86.24% killing) versus less than 3 days (87.83% killing) P value of 0.60. There was a greater proportion of skin and soft tissue infections demonstrating high cytotoxicity (16% low versus 23% high, P = 0.02). When looking at endovascular sources versus non-endovascular sources, there was no difference in median cytotoxicity (87.51% versus 87.89%, respectively). MRSA infections from isolates in the low-cytotoxic activity group had a higher rate of ID consultation (67% versus 56%, P = 0.02).
The 30-day mortality did not differ between the low- and high-cytotoxicity groups (26% versus 23%, P = 0.39) (Table 1). There was also no significant difference in longer-term 90-day mortality, although a greater proportion of patients died in the low-toxicity group (35% versus 31%, P = 0.29). There was also no association between mortality and cytotoxicity in the two most common spa types, spa-CC008 and spa-CC002. In both of these subgroups, there was no difference in median toxicity of patients with and without 30-day mortality.
Antibiotic therapy.
Ninety-six percent (743) of patients received antibiotic therapy active against MRSA during hospitalization, defined as receiving vancomycin, daptomycin, linezolid, trimethoprim-sulfamethoxazole, clindamycin, ceftaroline, or rifampin. Ninety-six percent (711) of the patients who received MRSA-directed therapy received vancomycin at some point during the treatment course. Thirty-three patients did not receive MRSA-directed therapy, 12 died within 48 h or were treated with comfort measures, 8 were considered contaminants, 8 were discharged prior to the culture result, and 5 improved during the hospitalization without treatment.
The vast majority of patients (96%) received vancomycin therapy, consistent with hospital guidelines. Antibiotic therapy was further subdivided into receipt of vancomycin alone, receipt of a bactericidal antibiotic (daptomycin and/or ceftaroline) with or without vancomycin, or receipt of a protein synthesis inhibitor (clindamycin and/or linezolid) with or without vancomycin. Five hundred seventy-four patients received vancomycin alone, 106 patients received a bactericidal antibiotic, while 40 patients received a protein synthesis inhibitor.
Predictors of 30-day mortality.
To examine predictors of all-cause 30-day mortality, we performed univariate logistic regression analyses using the clinical and microbiologic variables. In univariate analysis (Table 2), age >65 years, female sex, hospital-onset infection, Charlson score ≥3, Pitt bacteremia score ≥4, and pneumonia as the source of infection were predictive of mortality (Table 2). Community-onset infection, bone/joint infection, catheter-related infection, and receipt of a bactericidal antibiotic were inversely associated with mortality. Receipt of vancomycin alone or receipt of a protein synthesis inhibitor was not associated with mortality. In multivariate analysis, age greater than 65, diagnosis of heart failure, recent receipt of chemotherapy, Pitt bacteremia score of greater than or equal to 4, pneumonia as the primary source, and endovascular infection as the primary source were predictors of mortality. Infectious diseases consultation was the only variable inversely associated with mortality in multivariate analysis. Receipt of a bactericidal antibiotic was no longer inversely associated with mortality in multivariate analysis.
TABLE 2.
Predictors of mortality in MRSA S. aureus bacteremiaa
| Characteristic | Data for: |
Univariate OR (95% CI) | Univariate P value | Multivariate OR (95% CI) | Multivariate P value | ||
|---|---|---|---|---|---|---|---|
| Total (n = 776) | 30-day mortality (n = 198) | Survival (n = 578) | |||||
| Avg age (median) | 62 (62) | 70 (74) | 59 (58) | <0.01 | |||
| Age > 65 | 341 (44) | 122 (62) | 219 (38) | 2.63 (1.89–3.67) | <0.01 | 2.07 (1.28–3.34) | <0.01 |
| Female sex | 326 (42) | 100 (51) | 226 (39) | 1.59 (1.15–2.20) | <0.01 | 1.50 (0.95–2.36) | 0.13 |
| Hospital-onset infection | 249 (32) | 78 (39) | 171 (30) | 1.55 (1.11–2.17) | 0.01 | ||
| Community-onset infection | 527 (68) | 120 (61) | 407 (70) | 0.65 (0.46–0.90) | 0.01 | 0.72 (0.44–1.18) | 0.20 |
| Pitt Bacteremia Score, avg (med) | 2.6 (2.0) | 4.8 (4) | 1.9 (1) | <0.01 | |||
| PBS ≥ 4 | 208 (27) | 108 (55) | 100 (17) | 5.78 (4.05–8.24) | <0.01 | 4.37 (2.64–7.23) | <0.01 |
| Comorbidities | |||||||
| Avg CCI (median) | 4.1 (4.0) | 4.5 (4.0) | 4.0 (4.0) | <0.01 | |||
| CCI≥3 | 529 (68) | 160 (81) | 369 (64) | 2.38 (1.61–3.53) | <0.01 | ||
| Diabetes | 81 (10) | 14 (7) | 67 (12) | 0.58 (0.32–1.06) | 0.08 | 0.51 (0.18–1.42) | 0.20 |
| Prior MI/CAD | 27 (3.5) | 10 (5) | 17 (2.9) | 1.76 (0.79–3.90) | 0.17 | 1.98 (0.54–7.20) | 0.30 |
| Peripheral vascular disease | 85 (11) | 19 (9.6) | 66 (11) | 0.82 (0.48–1.41) | 0.48 | ||
| Chronic heart failure | 104 (13) | 35 (18) | 69 (12) | 1.58 (1.02–2.47) | 0.04 | 2.15 (1.09–4.24) | 0.03 |
| Chronic kidney disease | 220 (28) | 50 (25) | 170 (29) | 0.81 (0.56–1.17) | 0.26 | ||
| ESRD/dialysis | 106 (14) | 20 (10) | 86 (15) | 0.64 (0.38–1.08) | 0.09 | 0.90 (0.40–2.00) | 0.79 |
| Liver disease | 64 (8.3) | 18 (9.1) | 46 (8.0) | 1.16 (0.65–2.05) | 0.62 | ||
| Chronic pulmonary disease | 91 (12) | 24 (12) | 67 (12) | 1.05 (0.64–1.73) | 0.84 | ||
| Connective tissue disease | 2 (0.3) | 1 (0.5) | 1 (0.2) | 2.92 (0.18–47) | 0.45 | ||
| HIV | 73 (9.4) | 12 (6.1) | 61 (11) | 0.55 (0.29–1.04) | 0.07 | ||
| Cerebrovascular disease | 8 (1.0) | 3 (1.5) | 5 (0.9) | 1.76 (0.42–7.45) | 0.44 | ||
| Hemiplegia/paraplegia | 15 (1.9) | 0 (0) | 15 (2.6) | NA | 0.98 | ||
| Malignancy | 67 (8.6) | 21 (11) | 46 (8.0) | 1.37 (0.80–2.36) | 0.25 | ||
| Chemotherapy | 51 (6.6) | 23 (12) | 28 (4.8) | 2.58 (1.45–4.60) | <0.01 | 3.64 (1.60–8.30) | <0.01 |
| Infective source | |||||||
| Pneumonia | 140 (18) | 60 (30) | 80 (14) | 2.71 (1.84–3.97) | <0.01 | 2.14 (1.17–3.93) | 0.01 |
| Skin and soft tissue infection | 136 (17) | 27 (14) | 109 (19) | 0.68 (0.43–1.07) | 0.10 | ||
| Bone/joint | 56 (7.2) | 5 (2.5) | 51 (9) | 0.27 (0.11–0.68) | <0.01 | ||
| Catheter related | 153 (20) | 27 (14) | 126 (22) | 0.57 (0.36–0.89) | 0.01 | ||
| Device related | 8 (1.0) | 1 (0.5) | 7 (1) | 0.41 (0.05–3.39) | 0.41 | ||
| Surgical site | 51 (6.6) | 5 (0.03) | 46 (8) | 0.30 (0.12–0.77) | 0.01 | ||
| Endovascular | 57 (7.4) | 15 (7.5) | 42 (7) | 1.05 (0.57–1.93) | 0.88 | 2.63 (1.11–6.24) | 0.03 |
| Other | 12 (1.5) | 2 (1) | 10 (2) | 0.58 (0.13–2.67) | 0.48 | ||
| Unknown | 163 (21) | 56 (28) | 107 (19) | 1.74 (1.19–2.52) | <0.01 | 1.36 (0.71–2.59) | 0.35 |
| Infectious disease consult | 323/508 (64) | 66/129 (51) | 257/379 (68) | 0.50 (0.33–0.75) | <0.01 | 0.4 (0.31–0.88) | 0.02 |
| MRSA treatment during hospital stay | 743 (95) | 184 (93) | 559 (97) | 0.45 (0.22–0.91) | 0.03 | 0.44 (0.14–1.41) | 0.17 |
| Vancomycin monotherapy | 574 (74) | 154 (78) | 420 (76) | 1.32 (0.90–1.93) | 0.16 | ||
| Bactericidal therapy | 106 (14) | 17 (9) | 89 (15) | 0.52 (0.30–0.89) | 0.02 | 0.76 (0.37–1.57) | 0.46 |
| Protein synthesis inhibitor therapy | 40 (5) | 8 (4) | 32 (6) | 0.72 (0.33–1.59) | 0.41 | ||
| Duration of BSI | |||||||
| ≥3 days | 248 (32) | 59 (30) | 189 (33) | 0.87 (0.62–1.24) | 0.45 | 1.24 (0.71–2.1) | 0.45 |
| ≥5 days | 136 (17) | 33 (17) | 103 (18) | 0.92 (0.60–1.42) | 0.71 | ||
| ≥10 days | 36 (4.6) | 7 (3.5) | 29 (5) | 0.69 (0.30–1.61) | 0.39 | ||
| Vancomycin MIC ≥ 1.5 | 332 (43) | 84 (42) | 248 (43) | 0.98 (0.71–1.36) | 0.91 | ||
| Genotype by spa-CC group | |||||||
| spa-CC002/ST5 | 335 (43) | 96 (48) | 239 (41) | 1.21 (0.64–2.26) | 0.56 | ||
| spa-CC008/ST8 | 381 (49) | 87 (44) | 294 (51) | 0.89 (0.47–1.67) | 0.71 | ||
| agr functional | 529/772 (68) | 134/198 (68) | 395/574 (69) | 0.95 (0.67–1.34) | 0.77 | 1.13 (0.68–1.87) | 0.63 |
| Cytotoxic virulence | |||||||
| High cytotoxicity | 194 (25) | 45 (23) | 149 (26) | 1.18 (0.81–1.72) | 0.39 | 0.69 (0.41–1.17) | 0.17 |
| Cytotoxicity quartiles | |||||||
| Q1 | 194 (25) | 54 (27) | 140 (24) | 0.34 | |||
| Q2 versus Q1 | 194 (25) | 49 (25) | 145 (25) | 0.88 (0.56–1.38) | 0.57 | ||
| Q3 versus Q1 | 194 (25) | 50 (25) | 144 (25) | 0.90 (0.57–1.41) | 0.65 | ||
| Q4 versus Q1 | 194 (25) | 45 (23) | 149 (26) | 0.78 (0.50–1.24) | 0.30 | ||
Data represent number and percentage in parentheses unless otherwise specified. OR, odds ratio; CI, confidence interval; NA, not available.
There was no significant association between high and low cytotoxicity (using the cutoff >90% cell killing) or cytotoxicity quartile and 30-day mortality in univariate analysis (Table 2). High versus low cytotoxicity was included in the multivariate analysis a priori but did not show association with 30-day mortality. We also performed a separate analysis evaluating 30-day mortality in patients infected with isolates with 20% cell killing or less compared to patients with infections from the highest quartile. Here, we found no significant difference in 30-day mortality between these two groups (26% and 24%, respectively).
DISCUSSION
In this large cohort of patients with MRSA bacteremia, there was no significant increase in all-cause 30-day mortality for patients with BSI caused by low cytotoxicity compared with BSI from high-cytotoxicity isolates. In addition, we found no significant association between host factors such as age or comorbidity burden and cytotoxicity level; however, there was an association between site of infection (skin and soft tissue infection [SSTI] with high cytotoxicity) as well as strain type (ST5/spa-CC002 with low and ST8/spa-CC008 with high cytotoxicity) and cytotoxicity level. Additionally, we did see a trend toward a lower 30-day mortality in patients with infections caused by isolates in the highest-cytotoxicity quartile. The study of MRSA, rather than MSSA, bloodstream isolates was chosen given a more homogenous genotypic background, with significantly fewer strain types (ST) than equal numbers of MSSA isolates. Moreover, given significant differences in 30-day mortality between MRSA and MSSA bacteremia, studying the relationship between cytotoxicity and 30-day mortality in a cohort with both MRSA and MSSA isolates could introduce unknown bias.
These findings stand in contrast to prior studies that have found links between low cytotoxicity and both propensity for bloodstream infection and poor outcomes (17, 18). There are a number of differences between the current study and these prior investigations. These include patient characteristics and clinical variables (our study examined MRSA BSI isolates from a heterogeneous group of infective sites, whereas prior investigations have looked at a single or limited number of infective sources), which may contribute to differences in findings. The isolates included in this study were primarily of spa-CC002 (CC2/ST5) and spa-CC008 (CC8/ST8) lineages accounting for 92% of isolates, reflective of the molecular epidemiology of MRSA bloodstream infections in the United States, and this predominance of CC2 and CC8 genotypes mirrors that found in prior studies of cytotoxicity (17).
The CC8/ST8 lineage, most notably USA300, typically demonstrates expression of PVL and high levels of toxin production, and ST8 isolates have typically been associated with community-acquired MRSA infections. CC2/ST5 strains have traditionally been more frequently seen in hospital-onset infection; however, recent studies have suggested some blurring of these epidemiologic patterns (27, 28). Previous studies have suggested that regulation of cytotoxicity may be a strategy employed in a strain-specific manner in MRSA infection (26). While we found an association between clonal complexes and cytotoxicity, with spa-CC002 associated with low cytotoxicity and spa-CC008 associated with high cytotoxicity, there was no association between cytotoxic activity and mortality, either for the overall group or within individual strains. There was a trend toward higher mortality in patients infected with spa-CC002, the strain type associated with lower toxicity, so it is possible that the mortality association seen in other studies is due to a greater proportion of spa-CC002.
Receipt of a protein synthesis inhibiting antibiotic had no effect on 30-day mortality. This lends support to the notion that once bacteremia is established, cytotoxin production does not appear to be a major driver of clinical outcomes, particularly 30-day mortality. Downregulation of cytotoxin production, as a precursor to or a result of bloodstream invasion, may represent a bacterial adaptation for improved immune evasion and survival. Interestingly, we did see an inverse association between receipt of a bactericidal antibiotic and 30-day mortality in univariate analysis; however, this association was no longer seen multivariate analysis. Ninety-one percent of patients who received a bactericidal antibiotic had an infectious disease consultant, which was independently protective against 30-day mortality in multivariate analysis, compared to 63% in the overall cohort. This could explain the discrepancy seen between univariate and multivariate analysis. Over the 8-year study period, 28 patients who had repeat episodes of bacteremia and 4 patients who had three episodes of bacteremia were included in the study. To ensure that inclusion of these patients did not affect the outcome of the study, we performed a sensitivity analysis where only the first episode of bacteremia was included. In this analysis, only receipt of bactericidal antibiotics was no longer inversely associated with 30-day mortality (P = 0.02 to 0.08). There was no change in any other variables in the univariate or multivariate analyses.
Bacteremia is one of the most severe manifestations of MRSA infection, and detection of MRSA in the blood clearly signals infection, whereas classification of other clinical syndromes in MRSA infection can problematic due to issues such as colonization, infection at multiple sites, or difficulty assigning a primary infective site in retrospective analyses. Bloodstream infection (BSI) therefore, while resulting from a heterogeneous group of infective sites, is a clearly defined clinical entity, and all MRSA infections complicated by BSI are considered severe and are associated with high mortality. In this way, bacteremia represents an optimal model for studying the impact of cytotoxicity on outcomes. It is possible that for certain infective sites or clinical syndromes, such as pneumonia, an association exists between cytotoxicity and either bloodstream invasion or mortality, and our study of heterogeneous clinical syndromes did not capture this difference. Also, we did not look at corresponding infective site isolates; therefore, we were not able to assess whether there was a difference in cytotoxicity or downregulation between the infective site and bloodstream isolates. Indeed, toxin analysis of paired S. aureus clones from blood infection and carriage and infecting sites in individual patients with bacteremia suggests that the transition from commensalism to opportunism in S. aureus does not require full virulence in hospitalized patients (29, 30).
Although we did not find a difference in mortality between groups infected with low- versus high-cytotoxicity isolates, the presence of such low-cytotoxicity isolates in invasive infection is, in itself, interesting. Certainly, hosts with impaired immune function may be susceptible to any pathogen, including pathogens with reduced virulence, such that invasive infection with low-virulence pathogens is more a marker of host compromise. However, unlike prior studies, we did not find an association between comorbidity burden and cytotoxicity (17). While a majority of our bloodstream isolates demonstrated high cytotoxicity, it is possible that the isolates with decreased cytotoxicity developed this as a result of genetic adaptation to allow bloodstream invasion, as others have theorized (18, 20, 22).
In this study, we used a phenotypic assay that has been used in multiple prior studies to evaluate production of cytolytic toxins, particularly LukAB, by S. aureus (9, 17, 18, 31, 32). The PMN-HL60-based assay does not capture all S. aureus toxin production; however, it provides a useful laboratory phenotype that reflects overall production of pore-forming toxins. While we did not fully characterize the genetic underpinnings of the low-cytotoxicity phenotype in our isolates, we did define agr dysfunction via hemolysis assay. There was an association with low cytotoxicity and agr dysfunction, but there was not complete correlation. This suggests additional regulatory genes are involved in cytotoxicity that we did not evaluate; however, other groups have pursued this question and identified a number of potential genetic candidates (12, 14, 15, 22). Additionally, while assessing cytotoxicity, we only tested one concentration of bacterial supernatant (20%). It is possible that at lower concentrations, the cytotoxic distribution of our cohort could differ.
Despite the above limitations, this is the largest study examining the impact of cytotoxicity on mortality in MRSA bloodstream infections. While prior studies have found an association between attenuated virulence and outcomes, we did not find the low-cytotoxicity phenotype to be a predictor of mortality.
MATERIALS AND METHODS
Population and study design.
We carried out a retrospective, observational cohort study of patients ≥18 years of age with methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection (≥1 blood culture positive for MRSA) at a large academic medical center in New York City between 1 January 2007 and 31 December 2015. This included a total of 820 MRSA bacteremia isolates, with 776 being included in the final analysis. The 44 isolates that were excluded were either duplicates, from pediatric patients, did not grow in culture, or displayed greater than 30% variability in the cytotoxicity assay. These isolates were part of a prior retrospective analysis of S. aureus BSI isolates examining clinical and genotype trends over time (27). The study was approved by the Columbia University Irving Medical Center Institutional Review Board. We included only the first bloodstream infection per patient for a given year and included only infections for which there was an available isolate for cytotoxicity screening. Patients with MRSA bloodstream infection were identified through a search of the electronic medical record (EMR) system performed by the Clinical Data Warehouse Navigator.
Demographic and clinical data were extracted from the EMR through a data query by the Clinical Data Warehouse Navigator and through direct chart review by trained reviewers. Clinical data included demographic information, admission dates, comorbid conditions, infectious diseases consultation, antibiotic therapy, clinical course and outcomes, and infective source. Antibiotic data were extracted from the EMR, and patients were classified as having received MRSA treatment if they received antibiotics active against MRSA during their hospitalization. Mortality was determined through manual review of the charts, data extraction from the EMR mortality data, and search of the Social Security Administration death file. ID consultation data were not available for the 2007 to 2009 period.
Comorbidity data were obtained through a query of the EMR for International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Diseases, Tenth Revision, Clinical Modification codes corresponding to the comorbidity categories. The obtained data were used to calculate the comorbidity burden via the Charlson comorbidity index (33). Severity of illness was quantified through the Pitt bacteremia score (34), based on the highest score achieved ≤48 h prior to the index culture.
The primary infective source was determined through review of clinical data in the EMR and based on either the assessment in the treating clinicians’ notes or relevant clinical data consistent with a particular source. When the primary source of infection could not be determined, the source was classified as “unknown.” Duration of bacteremia was determined based on the number of days for which there was a positive blood culture, and cases with death on day 1 of bacteremia were excluded from duration analysis. Infections were categorized as “community onset” (CO) if a patient had an initial positive blood culture collected ≤48 h of admission and “hospital onset” (HO) if the initial positive culture was collected >48 h after admission.
Outcomes.
The primary outcome was all-cause 30-day mortality.
Antibiotic susceptibility and genotyping.
The vancomycin MIC was determined using the automated MicroScan method, based on Clinical and Laboratory Standards Institute references. For genotyping, spa type was assigned by sequencing the repeat region of the staphylococcal protein A (spa) gene and using the SpaType Ridom software (35). The multilocus sequence typing mapping database (https://spa.ridom.de/mlst.shtml), and the Based Upon Repeat Pattern (BURP) clustering algorithm within Ridom was used for assigning spa types to clonal lineages (spa-CC groups) (36, 37).
agr functionality.
Hemolytic activity of the isolates was determined by cross-streaking on sheep blood agar (SBA) with RN4220, an S. aureus strain which produces only β-hemolysin. This method was used to identify the three staphylococcal hemolysins—α, β, and δ—that are active on SBA. agr dysfunction was defined as the absence of δ-hemolysin within the beta-hemolysis zone (38). Two separate reviewers were used when results were ambiguous or hard to interpret. If there was disagreement, a third reviewer was used as a tiebreaker.
Cytotoxic activity.
The method that was used for determining cytotoxic activity has been used in prior studies of MRSA (17). MRSA isolates were inoculated into 150 μl of tryptic soy broth in 96-well round-bottomed plates. The bacterial cultures were then grown for 16 h, with orbital shaking at 225 rpm at 37°C. Dilutions of the tryptic soy broth (TSB) cultures at 1:75 were performed into Roswell Park Memorial Institute (RPMI) media supplemented with 1% Casamino Acids (RPMI+CAS) in 96-well, round-bottomed plates. We then grew 150 μl of the diluted RPMI+CAS culture for 6 h with shaking at 225 rpm at 37°C. The 96-well plates were centrifuged to pellet the bacteria. Supernatants were collected, and 20 μl of culture supernatant from each well was added to a 96-well flat-bottomed plate and stored at −80°C.
Human promyelocytic HL60 cells were then differentiated into neutrophil-like cells (PMN-HL60) using dimethyl sulfoxide at a final concentration of 1.5% (Sigma) for 72 h. We added 1.0 × 105 PMN-HL60 cells suspended in 80 μl of colorless RPMI to the thawed supernatant to achieve 20% supernatant by volume, with a final well volume of 100 μl. The PMN-HL60 cells were then incubated with the supernatant for 2 h at 37°C. After 2 h, we added 10 μl CellTiter (Promega), a colorimetric assay for determining cell viability through metabolic activity, and incubated the HL60-PMN-supernatant-CellTiter mixture for another 2 h at 37°C. Absorbance was then measured at 490 nm on a 96-well plate reader, and percentage of cell killing or viability was quantified through comparison against positive- (HL60-PMNs without supernatant, 100% viability) and negative-control (HL60-PMN cells with TritonX-100, a detergent, added to achieve 100% cell killing) wells.
We performed each assay in triplicate, which included separate assays consisting of supernatant collection from three distinct overnight cultures and the use of three independent HL-60 cultures, and we excluded isolates for which there was greater than 30% variability in the calculated percentage of cell killing. We also excluded isolates that did not grow in the overnight TSB cultures or the RPMI subculture. To define high and low cytotoxic activity in this assay, we used the cutoff of ≥90.53% cell killing, which is consistent with prior studies (17) and corresponded with our upper quartile cutoff (median, 87.9%; IQR, 79.50 to 90.53%). Median and interquartile range provide a more appropriate measure between groups, as the results of the cytotoxicity assays are not normally distributed.
Statistical analysis.
Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc. Cary, NC). We evaluated univariable relationships between clinical variables and cytotoxicity level using χ2 and Fisher’s exact for categorical variables and Student's t test or Wilcoxon rank sum tests for continuous variables when appropriate. For evaluating the relationship of clinical variables and cytotoxicity level with mortality, we used the χ2 test. The alpha value for statistical significance was defined as 0.05. For the final multivariate logistic regression model, common risk factors, including age and gender, were included, along with additional known risk factors for mortality that had a corresponding P value of less than 0.2. Cytotoxicity level, agr dysfunction, bacteremia duration, antibiotic therapy, and endovascular source were included in the model a priori.
ACKNOWLEDGMENTS
This work was supported in part by the National Institutes of Health (grants K08AI090013 to A.-C.U., 5T32AI100852-02 to E.D.A., and T32 AI100852-04 to T.H.M.) and the Columbia University Irving scholarship (to A.-C.U.). The V.J.T. lab is supported in part by the National Institutes of Health grants R01AI099394, R01AI105129, and R01AI137336.
We thank Kristen Lewis for aiding in the hemolysis assays.
A.-C.U. has received research funding from Merck, GSK, and Allergan, unrelated to the current study. V.J.T. is an inventor on patents and patent applications unrelated to the current study filed by New York University, which are currently under commercial license to Janssen Biotech Inc. Janssen Biotech Inc. provides research funding and other payments associated with the licensing agreement. All other authors have no conflict of interest to declare.
No component of this work has been presented at a scientific meeting.
A.-C.U., E.D.A., and T.H.M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. V.J.T. and D.B. helped with the design of the cytotoxicity assay, which was performed by E.D.A. and T.H.M.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Lowy FD. 2011. How Staphylococcus aureus adapts to its host. N Engl J Med 364:1987–1990. doi: 10.1056/NEJMp1100251. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki K, Lucar J, Blackshear C, Hobbs CV. 2019. Methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia: nationwide estimates of 30-day readmission, in-hospital mortality, length of stay, and cost in the United States. Clin Infect Dis 69:2112–2118. doi: 10.1093/cid/ciz123. [DOI] [PubMed] [Google Scholar]
- 4.Yaw LK, Robinson JO, Ho KM. 2014. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: an observational cohort study. Lancet Infect Dis 14:967–975. doi: 10.1016/S1473-3099(14)70876-X. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Robles T, Torres VJ. 2017. Staphylococcus aureus pore-forming toxins. Curr Top Microbiol Immunol 409:121–144. doi: 10.1007/82_2016_16. [DOI] [PubMed] [Google Scholar]
- 6.Yoong P, Torres VJ. 2013. The effects of Staphylococcus aureus leukotoxins on the host: cell lysis and beyond. Curr Opin Microbiol 16:63–69. doi: 10.1016/j.mib.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenesch F, Lina G, Henry T. 2012. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol 2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaan AN, van Strijp JAG, Torres VJ. 2017. Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat Rev Microbiol 15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakoulas G, Eliopoulos GM, Moellering RC, Wennersten C, Venkataraman L, Novick RP, Gold HS. 2002. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother 46:1492–1502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakoulas G, Moise PA, Rybak MJ. 2009. Accessory gene regulator dysfunction: an advantage for Staphylococcus aureus in health-care settings? J Infect Dis 199:1558–1559. doi: 10.1086/598607. [DOI] [PubMed] [Google Scholar]
- 11.Balasubramanian D, Ohneck EA, Chapman J, Weiss A, Kim MK, Reyes-Robles T, Zhong J, Shaw LN, Lun DS, Ueberheide B, Shopsin B, Torres VJ. 2016. Staphylococcus aureus coordinates leukocidin expression and pathogenesis by sensing metabolic fluxes via RpiRc. mBio 7:e00818-16. doi: 10.1128/mBio.00818-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson MA, Lilo S, Wasserman GA, Thoendel M, Smith A, Horswill AR, Fraser J, Novick RP, Shopsin B, Torres VJ. 2011. Staphylococcus aureus regulates the expression and production of the staphylococcal superantigen-like secreted proteins in a Rot-dependent manner. Mol Microbiol 81:659–675. doi: 10.1111/j.1365-2958.2011.07720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benson MA, Ohneck EA, Ryan C, Alonzo F, Smith H, Narechania A, Kolokotronis S-O, Satola SW, Uhlemann A-C, Sebra R, Deikus G, Shopsin B, Planet PJ, Torres VJ. 2014. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol Microbiol 93:664–681. doi: 10.1111/mmi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mootz JM, Benson MA, Heim CE, Crosby HA, Kavanaugh JS, Dunman PM, Kielian T, Torres VJ, Horswill AR. 2015. Rot is a key regulator of Staphylococcus aureus biofilm formation. Mol Microbiol 96:388–404. doi: 10.1111/mmi.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killikelly A, Benson MA, Ohneck EA, Sampson JM, Jakoncic J, Spurrier B, Torres VJ, Kong X-P. 2015. Structure-based functional characterization of repressor of toxin (Rot), a central regulator of Staphylococcus aureus virulence. J Bacteriol 197:188–200. doi: 10.1128/JB.02317-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose HR, Holzman RS, Altman DR, Smyth DS, Wasserman GA, Kafer JM, Wible M, Mendes RE, Torres VJ, Shopsin B. 2015. Cytotoxic virulence predicts mortality in nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus. J Infect Dis 211:1862–1874. doi: 10.1093/infdis/jiu554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laabei M, Uhlemann A-C, Lowy FD, Austin ED, Yokoyama M, Ouadi K, Feil E, Thorpe HA, Williams B, Perkins M, Peacock SJ, Clarke SR, Dordel J, Holden M, Votintseva AA, Bowden R, Crook DW, Young BC, Wilson DJ, Recker M, Massey RC. 2015. Evolutionary trade-offs underlie the multi-faceted virulence of Staphylococcus aureus. PLoS Biol 13:e1002229. doi: 10.1371/journal.pbio.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweizer ML, Furuno JP, Sakoulas G, Johnson JK, Harris AD, Shardell MD, McGregor JC, Thom KA, Perencevich EN. 2011. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother 55:1082–1087. doi: 10.1128/AAC.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trouillet-Assant S, Lelièvre L, Martins-Simões P, Gonzaga L, Tasse J, Valour F, Rasigade J-P, Vandenesch F, Muniz Guedes RL, Ribeiro de Vasconcelos AT, Caillon J, Lustig S, Ferry T, Jacqueline C, Loss de Morais G, Laurent F. 2016. Adaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patients. Cell Microbiol 18:1405–1414. doi: 10.1111/cmi.12582. [DOI] [PubMed] [Google Scholar]
- 21.Fowler VG, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, Stryjewski ME, Eliopoulos GM, Reller LB, Corey GR, Jones T, Lucindo N, Yeaman MR, Bayer AS. 2004. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 190:1140–1149. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Lindemann C, Young BC, Muller J, Österreich B, Ternette N, Winkler A-C, Paprotka K, Reinhardt R, Förstner KU, Allen E, Flaxman A, Yamaguchi Y, Rollier CS, van Diemen P, Blättner S, Remmele CW, Selle M, Dittrich M, Müller T, Vogel J, Ohlsen K, Crook DW, Massey R, Wilson DJ, Rudel T, Wyllie DH, Fraunholz MJ. 2016. Natural mutations in a Staphylococcus aureus virulence regulator attenuate cytotoxicity but permit bacteremia and abscess formation. Proc Natl Acad Sci USA 113:E3101-10. doi: 10.1073/pnas.1520255113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giulieri SG, Baines SL, Guerillot R, Seemann T, Gonçalves da Silva A, Schultz M, Massey RC, Holmes NE, Stinear TP, Howden BP. 2018. Genomic exploration of sequential clinical isolates reveals a distinctive molecular signature of persistent Staphylococcus aureus bacteraemia. Genome Med 10:65. doi: 10.1186/s13073-018-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron DR, Lin Y-H, Trouillet-Assant S, Tafani V, Kostoulias X, Mouhtouris E, Skinner N, Visvanathan K, Baines SL, Howden B, Monk IR, Laurent F, Stinear TP, Howden BP, Peleg AY. 2017. Vancomycin-intermediate Staphylococcus aureus isolates are attenuated for virulence when compared with susceptible progenitors. Clin Microbiol Infect 23:767–773. doi: 10.1016/j.cmi.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Howden BP, Johnson PD, Ward PB, Stinear TP, Davies JK. 2006. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 50:3039–3047. doi: 10.1128/AAC.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recker M, Laabei M, Toleman MS, Reuter S, Saunderson RB, Blane B, Török ME, Ouadi K, Stevens E, Yokoyama M, Steventon J, Thompson L, Milne G, Bayliss S, Bacon L, Peacock SJ, Massey RC. 2017. Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat Microbiol 2:1381–1388. doi: 10.1038/s41564-017-0001-x. [DOI] [PubMed] [Google Scholar]
- 27.Austin ED, Sullivan SS, Macesic N, Mehta M, Miko BA, Nematollahi S, Shi Q, Lowy FD, Uhlemann A-C. 2020. Reduced mortality of Staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007–2015. Clin Infect Dis 70:1666–1674. doi: 10.1093/cid/ciz498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David MZ, Daum RS, Bayer AS, Chambers HF, Fowler VG, Miller LG, Ostrowsky B, Baesa A, Boyle-Vavra S, Eells SJ, Garcia-Houchins S, Gialanella P, Macias-Gil R, Rude TH, Ruffin F, Sieth JJ, Volinski J, Spellberg B. 2014. Staphylococcus aureus bacteremia at 5 US academic medical centers, 2008–2011: significant geographic variation in community-onset infections. Clin Infect Dis 59:798–807. doi: 10.1093/cid/ciu410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth DS, Kafer JM, Wasserman GA, Velickovic L, Mathema B, Holzman RS, Knipe TA, Becker K, von Eiff C, Peters G, Chen L, Kreiswirth BN, Novick RP, Shopsin B. 2012. Nasal carriage as a source of agr-defective Staphylococcus aureus bacteremia. J Infect Dis 206:1168–1177. doi: 10.1093/infdis/jis483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman DR, Sullivan MJ, Chacko KI, Balasubramanian D, Pak TR, Sause WE, Kumar K, Sebra R, Deikus G, Attie O, Rose H, Lewis M, Fulmer Y, Bashir A, Kasarskis A, Schadt EE, Richardson AR, Torres VJ, Shopsin B, van Bakel H. 2018. Genome plasticity of agr-defective Staphylococcus aureus during clinical infection. Infect Immun 86:e00331-18. doi: 10.1128/IAI.00331-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DuMont AL, Yoong P, Day CJ, Alonzo F, McDonald WH, Jennings MP, Torres VJ. 2013. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci USA 110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. 2011. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Korvick JA, Bryan CS, Farber B, Beam TR, Schenfeld L, Muder RR, Weinbaum D, Lumish R, Gerding DN, Wagener MM. 1992. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother 36:2639–2644. doi: 10.1128/AAC.36.12.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam K, Torres VJ. 2019. Staphylococcus aureus secreted toxins and extracellular enzymes. Microbiol Spectr 7. doi: 10.1128/microbiolspec.GPP3-0039-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Hara FP, Suaya JA, Ray GT, Baxter R, Brown ML, Mera RM, Close NM, Thomas E, Amrine-Madsen H. 2016. spa typing and multilocus sequence typing show comparable performance in a macroepidemiologic study of Staphylococcus aureus in the United States. Microb Drug Resist 22:88–96. doi: 10.1089/mdr.2014.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald D, Vogel U. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. 2008. agr function in clinical Staphylococcus aureus isolates. Microbiology (Reading) 154:2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]