Abstract
Background:
Postpartum pain contributes to increased irritability and excessive stress in the mother and consequently may inhibit successful breastfeeding, reduce a mother's ability to take care of her baby, and cause an imperfect mother-baby interaction. Evidence suggests the positive effect of ginger on reduction in uterus-associated pain. The objective of this study is to investigate the effect of ginger capsules on postpartum pain.
Materials and Methods:
The present double-blinded, randomized, placebo-controlled trial was conducted in Mahdiyeh Educational Hospital, Tehran. One hundred and twenty-eight mothers having moderate-to-severe pain following vaginal delivery were included. The participants were divided into two groups (A and B). Interventions were performed every 8 h in 24 h. In the first intervention (2 h after the delivery), Group A received 500 mg of placebo capsules (containing chickpea flour) and Group B received 500 mg of Zintoma (ginger rhizome) capsules. In the second and third interventions, Group A received 250 mg placebo capsules and Group B received 250 mg Zintoma capsules. All participants received 250 mg capsules of mefenamic acid in each intervention in addition to ginger or placebo capsules. The pain severity was measured before and half an hour, an hour, and 2 h after each intervention. Statistical analysis was performed using the SPSS software version. 22. The Chi-square, Fisher's, and t tests and the GEE model were applied to assess the pain severity.
Results:
The average pain severity was not statistically significant between the groups in the beginning of the intervention (P = 0.623). The mean score of pain significantly decreased within the duration of intervention in both groups (P < 0.001); however, the pain severity was significantly lower in the intervention group as compared to the control group at any point after the intervention (P = 0.006).
Conclusion:
Ginger can be used as an effective remedy for postpartum pain relief.
Keywords: Clinical trial, ginger, herbal medicine, pain, postpartum care
INTRODUCTION
Contractions and involution of uterine cause postpartum pain. Postpartum pain is reported to be more severe in multiparous women as compared to primiparous women.[1] This uterine originated pain is similar to dysmenorrhea and labor pain.[2] One of the most reported problems by women in the early postpartum period is pain and fatigue. This affects woman's ability to take care of herself and her infant. Untreated pain can cause postpartum depression or greater use of opioid.[3]
Breastfeeding is also associated with uterine contractions.[2] Therefore, postpartum pain may interfere with neonatal care as well.[3] Postpartum pain management can enhance woman's return to normal activities and help mother take care of her infant.[4]
After the placenta and membranes are expelled, the uterus must remain in an intense contractile state until the enlarged uterine arteries are compressed and blood clots are formed in their branches to prevent postpartum bleeding.[1] Influenced by these contractions, the chemical mediators of pain such as bradykinin, prostaglandins, serotonins, and lactic acid are released, causing postpartum pains.[2,5] Prostaglandins are lipid autacoids derived from arachidonic acid. Prostaglandins can promote inflammation.[6]
NSAIDs inhibit cyclooxygenase enzyme and therefore diminish prostaglandins production.[7] Nonsteroidal anti-inflammatory drugs are associated with gastrointestinal complications such as dyspepsia, ulcer, and gastrointestinal bleeding and may be associated with increased blood pressure, although the recent data have questioned the association between NSAIDs and hypertension.[8,9]
Ginger has been recognized as the “universal medicine” by the ancient Orientals of China. Today, ginger remains a component of more than 50% of traditional herbal remedies.[10] Ginger is composed of 6-gingerol (the main constituent), 8-gingerol, 6-shogaol, 6,10-dihydrogingerdion, 6,10-gingerdion, 6-paradol, galanal A and B, and elinoid (a minor constituent).[11] Elinoid inhibits lipooxygenase and cyclooxygenase 1, 2 enzymes, slows down or prevents the derivation of prostaglandins from arachidonic acid and inhibits leukotriene production through inhibiting 5-lipoxygenase.[12,13] Ginger is among the known safe herbs on the list of US FDA.[11] To prevent ginger's anticoagulant effect, the non-consumption of more than 4 g of dried extract and 15 g of root has been proposed.[12]
Studies have reported the pain-relieving effects of ginger on uterine origin.[14,15] Pourmaleky et al. examined the effect of ginger on postpartum pain.[16] In Pourmaleky's study, only multiparous women participated; also, pain severity was assessed only ½ h after the intervention. Studies supporting pain-relieving effects of ginger on postpartum pain are not adequate yet.
We aimed to evaluate the effect of ginger capsules on postpartum pain in primiparous and multiparous women.
MATERIALS AND METHODS
The present double-blinded, placebo-controlled trial study was designed and conducted.
Ethical considerations
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.PHNM.1396.782). Also registered in the Iranian Experimental Clinic Center with the code (IRCT201707283860N33). Written informed consent forms were obtained from those willing to take part; the participants were assured that their information would be kept confidential and that they could stop cooperating whenever they desired. Sampling lasted from July 2017 to April 2018.
Participants
A total of 128 women hospitalized at the maternity ward of Mahdiyeh Educational Hospital, Tehran, Iran, underwent investigation and intervention. Based on prior relevant literature,[17] with considering pain as main outcome, effect size is equal to one, a test power of 80%, α = 0.05,β= 0.2, S1 = 1.6, S2 = 2 and dropout rate of 25%.the sample size is obtained as 64 subjects in each group.
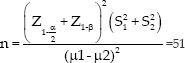
The inclusion criteria were as follows: singleton pregnancy, vaginal delivery with gestational age of 38 weeks or more, birth weight of 2500 gr or more, pain score ≥ 4 on the 10-point numerical rating scale (NRS), Iranian mothers, no addiction to narcotics, no allergies to ginger plant or ginger products and no history of common chronic diseases such as diabetes, hypertension, and cardiac diseases. We further excluded participants with serious after-delivery complications such as increased postpartum hemorrhage and temperatures higher than 39°C, symptoms of allergy to ginger during the study, no interest in continuing participation, and consumption of other narcotic analgesics during the study.
Prior to sampling, oral Zintoma capsules (containing 250 mg of ginger rhizome) were purchased from Isfahan Gol Darou Company with a license number (1228022777) and production series (02961); we also used oral capsules of 250 mg placebo in a shell similar to Zintoma in terms of color and appearance (containing chickpea flour); they were placed in close proximity to each other so that ginger aroma could percolate through all capsules. Afterward, a midwife blinded to the study placed four capsules of each Zintoma and placebo types in each packet labeled A and B, and the packets were given to the researcher. The patient, researcher, and statistical consultant were oblivious to the nature of the capsules till the end of the study.
The randomization method was performed as a classified randomization. For the homogeneity of individuals in the groups, we considered the number of deliveries (nulliparous and multiparous) and severity of primary pain (moderate: 4 ≤ NSR < 7 and severe: 7 ≤ NRS ≤ 10). In this method, the classification was primarily performed based on the above two characteristics; next, we created four categories according to the classes of two variables. In each of the four classes, the samples were randomly divided into intervention and control groups; in this regard, each class was randomized as a block permutation so that the number of samples was equal in the two groups. To this end, double blocks were first made of codes A and B. After that, according to the table of random numbers, a double block sequence was formed. Code A was allocated to patients according to their numbers, and code B was assigned according to the sequence. In each group, we considered 32 nulliparous participants (16 patients with moderate pain and 16 with severe pain) and 32 multiparous participants (16 patients with moderate pain severity and 16 with severe pain).
The researcher attended the maternity ward and surveyed the participants 2 h after vaginal delivery. The subjects first filled in the inclusion criteria form and if qualified, the researcher introduced herself, stated the objectives of her research, and invited them to participate. A written informed consent was obtained from those interested in taking part; they were further assured of confidentiality and the possibility of leaving the research. Full information about concerning postpartum pain and its nature and the benefits of ginger was given to mothers.
The pain severity was measured using a 10-point NRS. Number zero represented no pain and number 10 indicated the most severe pain. We prepared a treatment checklist completed by the researcher, including drug use form (number of capsules consumed), drug side effect registration form (includes questions for evaluation of common complications such as diarrhea, nausea, headache, etc.), patient satisfaction form (includes questions about evaluating the effect of the drug: very effective, effective, ineffective, negative, and very negative), and hemorrhage assessment form (base on an amount of the blood or clot on the sanitary pad: light, moderate, heavy, and excessive).[18] and hemoglobin evaluation form (level at admission and 6 h of delivery).
Pain severity was the primary measured variable in the outcome of the study, and the starting time of the pain relief was the secondary one.
Totally, three interventions were performed on each subject within 24 h, while pain severity was recorded before each intervention.
During the first intervention, the participants in Group A received a 250 mg capsule of mefenamic acid with two 250 mg capsules of placebo (containing a total of 500 mg of chickpea flour). The participants in Group B also received one oral capsule of mefenamic acid 250 mg and two 250 mg capsules Zintoma (containing a total of 500 mg of ginger rhizome). The second intervention was carried out 8 h after the first intervention, and the participants in group A were given a 250 mg mefenamic acid capsule with a 250 mg placebo capsule; moreover, the participants in Group B received a 250 mg mefenamic acid capsule and a 250 mg Zintoma capsule. The third intervention was conducted 8 h after the second one according to the method mentioned in the second intervention. Using a pain scale, we measured and recorded the pain severity before and ½ h, an hour, and 2 h after each intervention.
Two capsules were prescribed in the first intervention to achieve an effective dose of ginger (1 g/day).
During the sampling period, the researcher carefully supervised the participants to handle the possible complications and side effects; the participants filled in a consent form regarding the benefits and possible side effects of medication and satisfaction with treatment. The mothers were asked to report the inception of pain relief after each intervention in minutes, which was recorded by the researcher. The amount of hemorrhage was also recorded prior to each intervention base on the blood or clot on the sanitary pad. We also transcribed hemoglobin level at the time of admission and 6 h after the delivery based on hospitalization records. Of note, every mother and her newborn were in the same room at the maternity ward, and they received breastfeeding training; breastfeeding was initiated by the mother within the first 2 h of birth. The subjects were excluded from the study if they were not able to continue breastfeeding during sampling or were forbidden by the research unit doctor. If the pain continued, the senior resident doctor prescribed Diclofenac suppository and Acetaminophen pills as OCT. The researcher took note of the additional dosage of analgesics; the participants were also excluded if they took more than one additional pain killer, except during the interval between the first and second interventions.
Safety assessments
Ginger is among the known safe herbs on the list of US FDA.[11] To prevent its anticoagulant effect, a maximum 4 g of dried extract and 15 g of root have been recommended.[12]
Statistical analysis
We carried out data analysis using IBM SPSS Statistics for Windows, version 22.0 (Armonk, NY, USA: IBM Corp). Descriptive statistics for the continuous variables are presented as mean and standard deviation and for qualitative variables is presented as frequency and percent. Chi-square and Fisher's exact tests are used for comparing the nominal and ordinal variables between groups. Independent t-test was employed to compare the quantitative variables between the two groups; furthermore, the Kolmogorov–Smirnov test was utilized to specify the normality of the quantitative variables and if the normality hypothesis was not established, the relevant nonparametric test was used. We made use of the GEE-based regression analysis to compare the two groups in terms of pain severity at all measured times. The significance level was considered as 0.05.
RESULTS
Totally, 316 people were interviewed, among whom 162 women did not enter the study according to the inclusion criteria. During the study, 26 women were excluded and based on protocol design, sampling continued until achieving the desired sample size. Ultimately, the information provided by the 128 participants was investigated and analyzed statistically [Figure 1].
Figure 1.
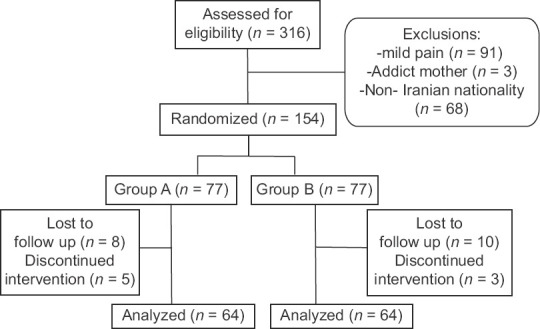
Details regarding participants
In the early surveys, the results obtained from the Mann–Whitney test and independent t-test indicated no statistical difference between the two groups in terms of demographic characteristics such as mother's age, education, job, number of pregnancies and childbirths, neonatal weight, and history of episiotomy [Table 1].
Table 1.
General characteristics of participants in intervention and control groups
| Variable | Mean±SD | P | |
|---|---|---|---|
|
| |||
| Ginger (n=64) | Placebo (n=64) | ||
| Age (years) | 26.45±4.33 | 27.94±5.38 | 0.090* |
| Gestational age (weeks) | 38.70±1.12 | 38.42±1.04 | 0.143* |
| Time of first breastfeeding (min) | 23.11±10.60 | 24.30±12.01 | 0.795** |
| Infant weigh (g) | 3336.6±415.87 | 3334.5±417.98 | 0.744* |
*Independent samples t-test; ** Mann–Whitney test. SD=Standard deviation
In this study, 86% of mothers started breastfeeding within the first ½ h following birth, and the rest (14%) had at least one breastfeeding experience during the first 2 h after delivery; thus, both groups were the same in this respect. There was no significant difference between the two groups regarding the level of administered oxytocin, the medication used for hemorrhage control, and the injected serum.
In order to characterize and compare the pain severity before and after the intervention, we used the Kolmogorov–Smirnov test to examine the normality of quantitative variables. In placebo-mefenamic acid group, 2 h after each intervention, the average severity of pain were 4.56 ± 1.47, 3.59 ± 1.37 and 2.61 ± 1.36. In the same time instances, the assessed pain score for ginger-mefenamic acid group was 3.84 ± 1.38, 2.63 ± 1.17 and 1.55 ± 1.36 showing a significant decrease compared to placebo-mefenamic acid group (P < 0.001) [Table 2]. The reduction patterns of pain intensity immediately following the administration of the first dose in ginger-mefenamic acid group showed a sharper descending slop compared to the placebo-mefenamic acid group in the following diagram [Figure 2].
Table 2.
Comparing the pain score in intervention and control groups*
| Group | Baseline 2 h after delivery | Time 1 2 h after first intervention | Time 2 2 h after second intervention | Time 3 2 h after third intervention | P**time | P**time*group | P**group |
|---|---|---|---|---|---|---|---|
| Pain | |||||||
| Intervention | 7.13±1.39 | 3.84±1.38 | 2.63±1.17 | 1.55±1.36 | <0.001 | <0.001 | 0.006 |
| Control | 7±1.48 | 4.56±1.47 | 3.59±1.37 | 2.61±1.36 | <0.001 | ||
| P*** | 0.623 | 0.005 | <0.001 | <0.001 |
*Three interventions from 2 h following of delivery every 8 h were performed; **P-values resulted GEE analysis; ***Independent sample t-test
Figure 2.
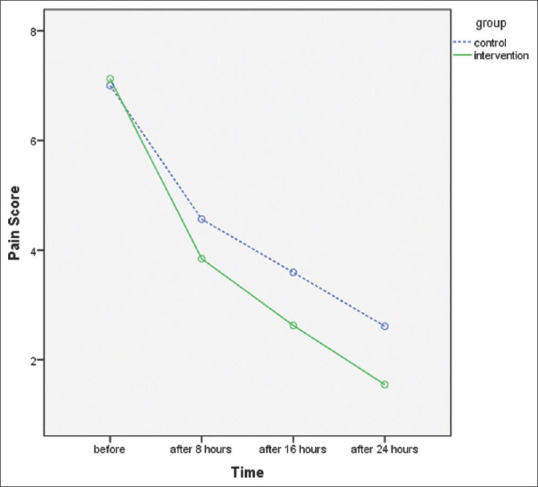
Pain scores before and after the intervention in both ginger and placebo groups
In the GEE modeling process, we considered four repeated pain score as the longitudinal response variables and group (1 = control, 2 = intervention) and time of measurement (quantitative variable) were considered as the model covariates.
The average pain severity was not statistically significant between the groups in the beginning of the intervention (P = 0.623). According to GEE analysis, the interaction between time and group was significant (P < 0.001) and the mean score of pain significantly decreased within the duration of intervention in both groups (P < 0.001); however, the results show that the pain severity was significantly lower in intervention group compared to control group at any point after the intervention [Table 2].
Amount of postpartum hemorrhage was within the normal range, and no cases of excessive postpartum hemorrhage were reported. There was no significant difference in the mean hemoglobin level in the admission time (12.29 ± 0.67 mg/dl in intervention, 12.45 ± 0.73mg/dl in control group) and 6 h after delivery (11.55 ± 0.68 mg/dl in intervention, 11.57 ± 0.75 mg/dl in control) in both groups. Satisfaction following treatment in ginger group was more than the control group (the percentage of very positive satisfaction in intervention group was 68.8% versus 21.9% in control group). Three participates in ginger and two in the placebo complained of headaches. Despite of one case of dizziness, one case of bitter taste in the mouth, and one case of overheating of the body in ginger group, there was no statistically significant differences in rate of drug side effects between two groups (P > 0.05).
DISCUSSION
In the ginger-mefenamic acid group, the average pain severity significantly decreased after each intervention compared to the placebo-Mefenamic acid group. Likewise, the onset of pain relief after each intervention was shorter in the ginger group than in the placebo group (20.31 ± 4.61 min versus 26.88 ± 3.15 min in the placebo after the first intervention). The levels of postpartum hemorrhage and medicinal side effects were not significantly different between the two groups.
Previous studies investigated the effect of ginger on pain relief and its anti-inflammatory effect on uterine disorders such as dysmenorrhea.[14,15,19] and premenstrual syndrome.[20] Only one study by Pourmaleky et al.[16] addressed postpartum pain relief among multiparous women. It is worth mentioning that the aforesaid single-blind study was only performed on multiparous women. In addition, pain severity was examined only once (half an hour after the intervention) while the current study focused on both primiparous and multiparous women, and both groups received mefenamic acid, ginger was compared with placebo, and pain severity was measured three times after each intervention. In a study on patients with osteoarthritis, administration of 1 g of ginger powder per day reduced the inflammatory agents.[21]
Pain severity was assessed half an hour, an hour, and 2 h after the intervention. Pourmaleky et al. (2013) also prescribed four 250 mg ginger capsules within the first 24 h in multiparous women and compared pain severity every hour following each intervention.[16] Ozgoli et al. and Golian[7,22] performed their evaluations an hour after each intervention. Therefore, the present study, complying with the pharmacological effects of mefenamic acid, is consistent with the existing studies on postpartum pain; furthermore, since the peak effect and effectiveness time of medicinal plants such as ginger are not definitely known, measurement and evaluation were conducted as performed for mefenamic acid.
Before the first intervention, the average pain severity was equal between ginger and placebo groups. Half an hour after the administration of the first dose, pain severity reduced in both groups; however, this reduction was significantly higher in the ginger group compared to the placebo. Evaluation of pain reduction within the 1st and 2nd h after the first dose also indicated a significant pain reduction in the ginger groups. In Pourmaleky's study, ½ h following the first intervention, postpartum pain intensity was measured using a pain scale; based on the results, the two (mefenamic acid and ginger) groups were significantly different concerning pain intensity after the first dose was administered. This means that ginger had a greater analgesic effect than mefenamic acid.[16] Seemingly, the evaluations after the first dose are in agreement with those obtained by Pourmaleky et al. In the current study, the average pain intensity before the administration of the second and third doses was lower in ginger group than in the placebo. Nonetheless, in their study (Pourmaleky et al.), the average pain intensity was not significantly different between the two groups before the second and third interventions. In the present study, upon the administration of the second and third doses, pain severity decreased in the ginger group. In this regard, the pain severity within the 2 h after the administration of the second dose in ginger group was reduced as much as 50%, and a significant difference was observed between the two groups.
It has been demonstrated that ginger contains gingerdiones and shogaols that have pharmacological properties mimicking dual-acting non-steroidal anti-inflammatory drugs (NSAIDs) in intact human leukocytes in vitro.[23] 6-Shogaol is the most potent antioxidant and anti-inflammatory properties.[24] It is known that such inhibitors have fewer side effects and are more effective than conventional NSAIDs.[25,26] On the other hand, Simarmata et al. studied the chemical components present in ginger rhizome, including phenolic compounds such as saponin, flavonoid, steroid, and triterpenoid which are converted into oleoresin; along with the anti-inflammatory g-gingerol component in ginger, these chemical components reduce inflammatory mediators such as cytokines and chemokines as activating agents of NF-kB and COX-2 enzyme; subsequently, the PGF2α biosynthesis is reduced in endometrium and an anti-inflammatory effect is induced in endometrium.[27]
The results demonstrated that the pain relief occurred over a statistically shorter time in ginger group compared to placebo group. This can be caused by combined effect of mefenamic acid and ginger, in ginger group. One major issue concerning ginger is the increased risk of hemorrhage due to the inhibition of cyclooxygenase enzyme.[1] For assessment, we used the visual estimation of postpartum hemorrhage according to the national practice guidelines on normal vaginal delivery based on the blood extent on the pad and blood indices (hemoglobin measurement upon arrival and 6 h after delivery). Measurement of serum hemoglobin amount indicated that the changes in hemoglobin concentration were similar between the two groups upon their arrival and 6 h following delivery. In this connection, Kashefi et al. studied ginger and heavy menstrual bleeding and strongly recommended the use of ginger as a highly efficient and cost-effective treatment for menstrual bleeding with very few side effects in people suffering from heavy hemorrhage.[28] In the present study, hemorrhage level was equal between the ginger and placebo groups, and increased bleeding was not detected in the ginger group.
Most women in the ginger group considered the quick effectiveness of the medication really positive in reducing pain; according to the reports of participants, one case of dizziness, one case of bitter taste in the mouth, and one case of hot flashes were observed in the ginger group. Three subjects in ginger and two in the placebo reported headaches. Shirooye et al. compared the effect of edible and local ginger on dysmenorrhea in their study. Headache, itching, burning sensation in the stomach, and reduced menstrual hemorrhage were among the side effects reported during edible ginger use.[19] Anvar Rizk reported that ginger is effective in relieving nausea, dizziness, and headache.[29] In general, based on the statistical tests in the present study, there were no significant differences between ginger and placebo groups concerning after treatment complications (P > 0.05).
Similar to other clinical trials, the present study encountered certain limitations: (1) the difference between the individuals’ tolerance thresholds in expressing the pain severity and (2) the performance of sampling in a hospital, which necessitated cautious generalization. All the same, it was hoped that the randomization would minimize the impact.
In the ginger-mefenamic acid group, the intensity of postpartum pain was reduced, and pain relief occurred after a shorter time compared with the placebo-mefenamic acid group. Hemorrhage level after delivery did not exhibit a significant difference between ginger and placebo groups.
CONCLUSION
The goal of this study was to examine pain-reliving effects of taking ginger capsules compared to placebo as an addition to mefenamic acid on the first 24 h of postpartum duration. Administration of ginger capsules lowered down the severity of postpartum pain as well as its duration compared to placebo.
The ginger and placebo group both showed similar side effects. Mothers in ginger group reported a more satisfactory treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge the Student Research Committee, Deputy of Research and Technology, the Deputy for Research, the hardworking personnel at Mahdiyeh Educational Hospital of Shahid Beheshti University of Medical Sciences, Tehran, Iran. Gol Darou Pharmaceutical Company, and all mothers who participated in this research.
REFERENCES
- 1.Cunningham FG, Kenneth SL, Bloom SL, Spong CY, Dashe JS, Hoffman, et al. Williams Obstetrics. 24th ed. New York: McGraw Hill; 2014. [Google Scholar]
- 2.Holdcroft A, Snidvongs S, Cason A, Doré CJ, Berkley KJ. Pain and uterine contractions during breast feeding in the immediate post-partum period increase with parity. Pain. 2003;104:589–96. doi: 10.1016/S0304-3959(03)00116-7. [DOI] [PubMed] [Google Scholar]
- 3.Eisenach JC, Pan PH, Smiley R, Lavand’homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94. doi: 10.1016/j.pain.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deussen AR, Ashwood P, Martis R. Analgesia for relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev. 2011;5:CD004908. doi: 10.1002/14651858.CD004908.pub2. doi:10.1002/14651858.CD004908.pub. [DOI] [PubMed] [Google Scholar]
- 5.Dox I, Melloni J, Sheld HH. Melloni's Illustrated Dictionary of Obstetrics and Gynecology. New York: Parthenon Pub Group; 2000. [Google Scholar]
- 6.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumont AS, Verma S, Dumont RJ, Hurlbert RJ. Nonsteroidal anti-inflammatory drugs and bone metabolism in spinal fusion surgery: A pharmacological quandary. J Pharmacol Toxicol Methods. 2000;43:31–9. doi: 10.1016/s1056-8719(00)00077-0. [DOI] [PubMed] [Google Scholar]
- 8.Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24:121–32. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Viteri OA, England JA, Alrais MA, Lash KA, Villegas MI, Ashimi Balogun OA, et al. Association of nonsteroidal antiinflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830–5. doi: 10.1097/AOG.0000000000002247. [DOI] [PubMed] [Google Scholar]
- 10.Leelavathi S, Hemavathy V. Effectiveness of ginger remedy on dysmenorrhea. Int J Innov Res Sci Eng Technol. 2015;4:2904–8. [Google Scholar]
- 11.Dadfar F, Hosseini E, Bahaddini A. A review of phytochemical, pharmacological and physiological properties of ginger (Zingiber officinale) J Clin Excell. 2014;3:72–86. [Google Scholar]
- 12.Herber D. PDR for Herbal Medicines. 4th ed. USA: Thomson Reterers; 2012. [Google Scholar]
- 13.Zargari A. Medicinal Plants. Tehran: University of Tehran Press; 1996. [Google Scholar]
- 14.Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J Altern Complement Med. 2009;15:129–32. doi: 10.1089/acm.2008.0311. [DOI] [PubMed] [Google Scholar]
- 15.Mozafari SH, Saei Gare Naz M, Ozgoli G. Effect of ginger on dysmenorrhea: A systematic review of clinical trials and quasi-experimental studies on world. Iran J Obstet Gynecol Infertil. 2018;21:8–21. [Google Scholar]
- 16.Pourmaleky S, Najar S, Montazery S, Haghighizadeh MH. Comparison between the effect of zintoma (Ginger) and mefenamic acid on after pain during postpartum in multioarous women. Iran J Obstet Gynecol Infertil. 2013;16:18–25. [Google Scholar]
- 17.Ozgoli G, Khodadadie A, Sheikhan Z, Jambarsang S, Mojab F, Taleb S. Comparison of efficacy between herbal capsule of anise and mefenamic acid on after- pain. J Med Plants. 2017;2:38–49. [Google Scholar]
- 18.Maternal Health Department of Ministry of Health and Treatment. National Guide to Providing Midwifery and Delivery Services in Mother-Friendly Hospitals. 3rd ed. Tehran: Iran; 2012. p. 161. [Google Scholar]
- 19.Shirooye P, Hashem Dabaghian F, Hamzeloo Moghadam M, Afrakhte M, Bioos S, Mokaberinejad R. A clinical comparative study of oral and topical ginger on severity and duration of primary dysmenorrhea. Res J Pharmacogn. 2017;4:23–32. [Google Scholar]
- 20.Khayat S, Kheirkhah M, Behboodi Moghadam Z, Fanaei H, Kasaeian A, Javadimehr M. Effect of treatment with ginger on the severity of premenstrual syndrome symptoms. ISRN Obstet Gynecol. 2014;2014:792708. doi: 10.1155/2014/792708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naderi Z, Mozaffari-Khosravi H, Dehghan A, Nadjarzadeh A, Huseini HF. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: A 12-week double-blind randomized placebo-controlled clinical trial. J Tradit Complement Med. 2016;6:199–203. doi: 10.1016/j.jtcme.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golian Tehrani SH, Mirmohammad Ali M, Soltani Moghadam A, Mehran A, Taghi Zadeh M, Baleghi M. The comparison of fennel and mefenamic acid effects on post-partum after pain. J Babol Univ Med Sci. 2015;17:7–13. [Google Scholar]
- 23.Flynn DL, Rafferty MF, Boctor AM. Inhibition of human neutrophil 5-lipoxygenase activity by gingerdione, shogaol, capsaicin and related pungent compounds. Prostaglandins Leukot Med. 1986;24:195–8. doi: 10.1016/0262-1746(86)90126-5. [DOI] [PubMed] [Google Scholar]
- 24.Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003;38:645–59. doi: 10.1016/s0223-5234(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 25.Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–9. doi: 10.1136/ard.62.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dugasani S, Pichika M, Nadarajah V, Balijepalli M, Tandra S, Korlakunta J. Comparitive antioxidant and anti- inflammatory effects of [6] - gingerol, [8] - gingerol, [10] - gingerol and [6] - shogaol. J Ethnopharmacol. 2010;127:515–20. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Simarmata M, Halim B, Ardinata D. Effects of red ginger capsule supplement in reducing PGF2 concentrations and pain intensity in primary dysmenorrhea. Earth Environ Sci. 2018;125:1–6. [Google Scholar]
- 28.Kashefi F, Khajehei M, Alavian M, Golmakani E. Effect of ginger (Zingiber officinale) on heavy menstrual bleeding: A placebo- controlled, randomized clinical trial. Phytother Res. 2014;29:114–19. doi: 10.1002/ptr.5235. [DOI] [PubMed] [Google Scholar]
- 29.Anvar Rizk S. Effect of aromatherapy abdominal massage using peppermint versus ginger oil on primary dysmenorrhea among adolescent girls. J Am Sci. 2013;9:597–605. [Google Scholar]


