Abstract
Background:
Preoperative evaluation needs objective measurement of the risk of anastomotic leakage (AL). This study aimed to determine if cardiovascular disease, evaluated by abdominal aortic calcification (AAC), was associated with AL after colorectal anastomoses. We conducted a retrospective case–control study on patients who underwent colorectal anastomosis between 2012 and 2016 at Reims University Hospital (France). Abdominal aortic calcification was the main variable of measurement.
Materials and Methods:
We reviewed all patients who had a left-sided colocolic or a colorectal anastomosis, all patients with AL were cases; 2 controls, or 3 when possible, without AL were randomly selected and matched by operation type, pathology, and age. For multivariate analysis, 2 logistic regression models were tested, the first one used the calcification rate as a continuous variable and the second one used the calcification rate ≥ 5% as a qualitative variable.
Results:
Forty-five cases and 116 controls were included. In univariate analysis, the calcification rate and the percentage of patients with a calcification rate ≥5% were significantly higher in cases than in control groups (4.4 ± 5.5% vs. 2.5 ± 5.2%, odds ratio [OR] =1.6 95% CI: 1.1–2.5; n = 22, 49% and n = 34.3 3%, OR = 2.8 95% CI: 1.2–6.2). In multivariate models, calcification rate as a continuous variable and calcification rate ≥5% as qualitative variable were independent significant risk factors for AL (respectively, aOR = 1.8; 95% CI: 1.1–3, P = 0.01; aOR = 3.2; 95% CI: 1.4–7.55, P < 0.01).
Conclusion:
AAC ≥5% should alert on a higher risk of AL and should lead to discussion about the decision of performing an anastomosis.
Keywords: Abdominal aortic calcification, anastomotic leakage, atherosclerosis, colorectal surgery, surgical outcomes
INTRODUCTION
Preoperative risk assessment is essential to guide decision-making and to give accurate information to patients and their families. In colorectal surgery, we need methods to predict the patient's risk of developing an anastomotic leakage (AL), as AL is the most dreaded complication, with a prevalence between 3% and 19% and a mortality rate of approximately 20%.[1,2,3] In case of suspected high-risk patients, we may choose to protect the anastomosis by a loop ileostomy, or choose to avoid the anastomosis (Hartmann procedure), or even to avoid the surgery (particularly in case of nonmalignant lesions). However, this preoperative assessment remains challenging and requires objective measurement because the current subjective clinical assessment has demonstrated a low predictive value and may underestimate the risk of AL.[4]
Cardiovascular (CV) disease is mainly due to atherosclerosis which is a build-up of fatty deposits on the inner walls of the arteries, where the presence of calcium indicates intimal atherosclerosis.[5] It may narrow the arteries including the ones of the anastomosed stumps and may cause AL, as a correct blood supply is one of the basic requirements for anastomotic healing.[6] Moreover, a link between clinical CV disease and AL has previously been demonstrated.[7]
Studies have indicated that measurement of the volume of abdominal aortic calcification (AAC) was a marker of clinical and subclinical CV disease.[8,9,10,11,12] This measure can easily be performed on preoperative computed tomography (CT) acquisitions performed before colorectal surgery.[9]
To date, only a few conflicting studies have evaluated the association between AL and extracoronary calcification from different sites, measured through various methods.[13,14,15,16,17] Knight et al. reviewed those studies but were unable to formally conclude that there is an association between AAC and AL after colorectal surgery, due to the lack of literature in this area.[18] Consequently, AAC assessment is not yet standardly performed and recommended before colorectal surgery.
We aimed to add our case–control study to the existing data by exploring the association between the volume of AAC and AL after left-sided colonic or colorectal anastomoses. We also verified the link between AAC and CV risk factors in our study population.
SUBJECTS AND METHODS
Study population
We reviewed the whole population of patients who had primary left-sided colorectal surgery between January 2012 and September 2016 from the hospital database of medical records of Reims University Hospital. Patients older than 18 years of age, who had either primary left-sided colonic or colorectal anastomoses, during elective or emergency surgery, with or without protective stoma, and for whom abdominal contrast-enhanced CT examination was available (no more than 90 days before after surgery), were included. Patients with Crohn's disease were not included in the study population.
Study design
A retrospective matched case–control study was conducted. Cases were all the patients with AL in the study population. Moreover, at least 2 or when possible 3 controls were randomly selected from the pool of controls. Controls were matched by operation (left-sided vs. colorectal anastomoses), pathology (malignant vs. benign pathology), and age ±10 years.
Data collection
Measure of AAC was conducted by a senior radiologist, blinded to postoperative outcome and to radiological reports, between January 2015 and December 2016. CT examinations were performed on a 64-row multidetector CT scanner with acquisitions during an arterial phase. Multiplanar reconstruction images of the abdominal aorta were routinely produced in the axial, sagittal, and coronal planes with 3-mm slice thickness [Figure 1]. The volume of calcification (cm3) was measured starting from the origin of the celiac trunk to the origin of the common iliac arteries. First, measurements were performed on the whole volume of the aorta, including the aortic wall, on the axial contrast-enhanced images acquired at the arterial phase. Using dedicated 3D-analysis software (Advanced Vessel Analysis Xpress, General Electric, Milwaukee, WI, USA), the surface of the aorta was measured semi-automatically on each slice. The radiologist then manually drew the outer limits of the abdominal aorta including thrombus and the arterial wall, to correct semiautomatic measurements. The volume of the selected portion of the aorta was then automatically calculated. On the maximum intensity projection images that were obtained, calcifications were automatically selected by the software and their volume was calculated.
Figure 1.
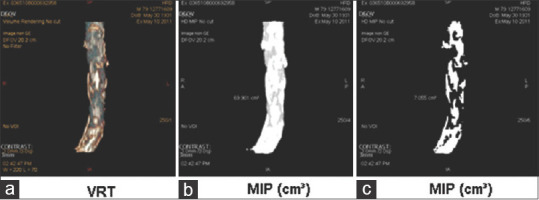
Volume of abdominal aortic calcification was measured from the origin of the celiac trunk to the origin of the common iliac arteries in a semiautomatic fashion. (a) Volume rendering technique, (b) Maximum intensity projection measurement of the entire aorta volume, (c) Measurement of the calcification volume
Other data
Data on patient characteristics, detailed in Table 1, were collected retrospectively from patient notes between January 2015 and December 2016.
Table 1.
Patient and operation characteristics in cases and controls group
| Cases (AL) (n=45), n (%) | Controls (no AL) (n=116), n (%) | P | |
|---|---|---|---|
| Gender | |||
| Male | 30 (67) | 73 (63) | 0.47 |
| Female | 15 (33) | 43 (37) | |
| Mean age (years) | 65±13 | 65±12.7 | NA |
| Nutritional status | |||
| Mean BMI | 25.8±3.4 | 25.6±6.3 | 0.76 |
| Mean preoperative albumin | 32±8.3 | 32±7.4 | 0.93 |
| Pathology | |||
| Cancer | 23 (51.1) | 69 (59.5) | NA |
| Surgery characteristics | |||
| Urgent surgery | 2 (9) | 13 (11.2) | 0.15 |
| Left sided | 10 (22.2) | 26 (22.4) | NA |
| Colorectal anastomosis | 35 (77.7) | 90 (77.5) | |
| Stapled | 28 (62.2) | 68 (58.6) | 0.57 |
| Hand sutured | 17 (37.7) | 48 (41.4) | |
| Associated procedure | 6 (13.3) | 24 (20.7) | 0.26 |
| No associated procedure | 39 (86.6) | 92 (79.3) | |
| Comorbidities | |||
| Hypertension requiring medication | 22 (48.8) | 46 (39.6) | 0.26 |
| Smoking in the past year | 14 (31.1) | 30 (25.8) | 0.41 |
| Diabetes | 9 (20) | 19 (16.3) | 0.36 |
| CV risk factor* | 31 (68.8) | 82 (70.7) | 0.92 |
| History of CV event† | 9 (20) | 20 (17.2) | 0.69 |
| AAC | |||
| Mean of AAC rate (%)‡ | 4.4±5.5 | 2.4±5.2 | 0.03 |
| AAC rate ≥5% | 22 (49) | 34 (29.3) | 0.01 |
*CV disease risk factors=Patients with current dialysis, congestive heart failure, cerebrovascular accident, transient ischemic attack, peripheral vascular disease, preoperative myocardial infarction, angina, previous percutaneous transluminal coronary angioplasty, previous ischemic colitis, hypertension requiring medication, diabetes, smoking in the past year; †History of CV disease=Cerebrovascular accident, transient ischemic attack, peripheral vascular disease, preoperative myocardial infarction, angina, previous percutaneous transluminal coronary angioplasty, or previous cardiac surgery; ‡Using LN (%+1). Continuous variables are expressed as mean±SD; categorical variables are expressed as n (%). SD=Standard deviation; AL=Anastomotic leakage; NA=Not analysed; CV=Cardiovascular; AAC=Abdominal aortic calcification; BMI=Body mass index
The criteria used to define AL were any of the following: presence of pus or enteric contents within the drains, presence of abdominal or pelvic collections in close vicinity to the anastomoses on a postoperative abdominal CT, leakage of contrast through the anastomoses after rectal opacification, or obvious anastomotic dehiscence at reoperation for postoperative peritonitis.
The primary diagnoses justifying the surgery were divided into malignant or benign lesions (diverticulitis, reversal of Hartmann's procedure, incisional hernia repair, or sigmoid volvulus). The presence of CV risk factors was defined as the presence of at least one of the following items: current dialysis, congestive heart failure, previous stroke or transient ischemic attack, peripheral vascular disease, myocardial infarction, angina, percutaneous transluminal coronary angioplasty, ischemic colitis, arterial hypertension and diabetes requiring medication, smoking during the past year before surgery. Previous history of CV disease was defined as the presence of at least one of the following events or conditions: previous stroke, transient ischemic attack, peripheral vascular disease, preoperative myocardial infarction, angina, percutaneous transluminal coronary angioplasty, or cardiac surgery.
Statistical analysis
Categorical variables are described as number (percentage) and continuous variables as mean ± standard deviation. AAC rate was calculated as AAC volume divided by aorta volume and expressed in percentage. To work on a normal distribution, for statistical analysis, AAC rate Log transformations were required (LN [AAC rate + 1]). Mean AAC rate estimation was used to dichotomize AAC rate into two categories (< mean AAC rate and ≥ mean AAC rate).
Correlation between abdominal aortic calcification and cardiovascular risk factor or disease
A univariate analysis on the whole study population was conducted to confirm the link between CV risk factors or a previous history of CV disease and AAC rate. For this analysis, continuously distributed data were assessed using the independent samples t-test under the condition of a normal distribution.
Comparison between cases and controls
Conditional univariate logistic regressions were conducted for AAC rate (as a continuous variable and as a categorial variable) and for recognized risk factors for AL. Variables associated with AL (P < 0.30) by univariate analysis were included in conditional multivariate logistic regression models to estimate adjusted odds ratios (aOR) and their 95% confidence intervals (95% CI) for AL risk. Two multivariate models were obtained. The first one was adjusted on AAC rate as a continuous variable and the second one on AAC rate as categorical value (< mean AAC rate and ≥ mean AAC rate).
Patients with missing data were excluded from the analysis of the variable for which data were missing, but they were included in all analyses of the available variable.
A P < 0.05 was considered as statistically significant. All analyses were performed using SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC, USA). The graph was designed with Excel 2007. The study was approved by the Institutional Review Board of Reims University Hospital.
RESULTS
During the study period, 327 patients underwent left-sided colocolic or colorectal surgery. Of these, 114 were not included: Forty-nine patients due to absence of anastomoses or presence of Crohn's disease and 65 due to absence of available contrast-enhanced CT scans. Moreover, 3 cases and 49 controls were excluded because they could not be matched properly. Thus, 45 cases and 116 controls were analyzed with a matched ratio of 1:2 for 19 cases and 1:3 for 26 cases [Flowchart Figure 2].
Figure 2.
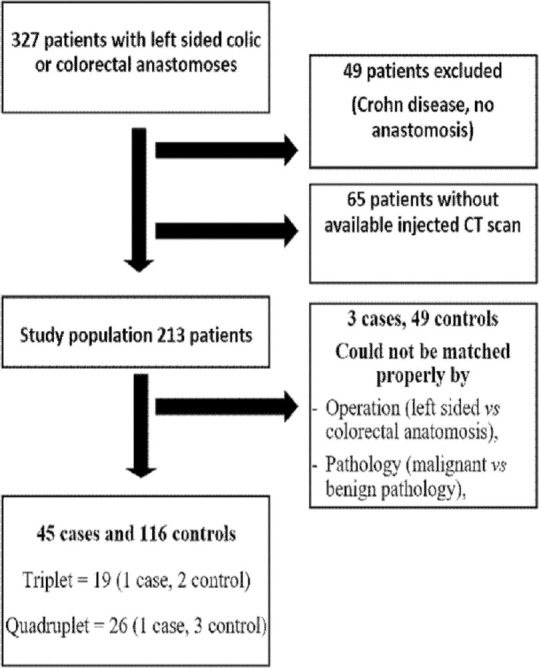
Flowchart
In the entire studied population (cases plus controls, n = 161), the mean AAC rate was 4.8 ± 5.3%. An AAC rate of 5% was used as an approximation of the mean AAC rate to categorize AAC in: AAC rate <5% and AAC rate ≥5%.
Correlation between abdominal aortic calcification rate and cardiovascular risk factors or a previous history of cardiovascular disease
A univariate analysis was conducted in the whole study population to confirm the link between CV risk factors or a previous history of CV disease and AAC rate. The mean AAC rate was significantly different in patients with or without at least one CV risk factors (3.8% ±5.7% and 1.6% ±3.7%, respectively, P < 0.01) and in patients with or without a previous history of CV disease (6% ±6%, and 2% ±4.9%, respectively, P < 0.01). Correlation analysis demonstrated that AAC rate was significantly associated with those specific conditions: age (P < 0.01), hypertension requiring medication (P < 0.01), stroke (P = 0.02), preoperative myocardial infarction (P = 0.01), previous angina (P < 0.01), and peripheral vascular disease (P = 0.02). There was no association between AAC rate and smoking (P = 0.1).
Comparison between cases and controls
Case and control groups' demographic, clinical, and operative characteristics are presented in Table 1. The mortality rates in case and control groups were, respectively, 2/46 (4.4%) and 5/116 (4.3%) (P = 0.9). In the conditional univariate analysis, AAC rate was significantly different among case and control groups (4.4% ±5.5% vs. 2.4% ±5.2%, OR = 1.6, 95% CI: 1.1–2.5, P = 0.03) [Table 1]. The number of patients with an AAC rate ≥5% was also significantly different between case and control groups (n = 22, 49% and n = 34, 29.3%, respectively, OR = 2.8, 95% CI: 1.2–6.2, P = 0.01). Variables associated with AL with a P < 0.30 by univariate analysis were AAC rate as a continuous variable, AAC rate ≥5%, hypertension requiring medication, urgent surgery, and associated procedures. These variables were included in conditional multivariate logistic regression models.
The first conditional multivariate logistic regression model was performed using AAC rate as a continuous variable, hypertension requiring medication, urgent surgery, and associated procedures. In this model, AAC rate was identified as the only significant independent risk factor for AL (aOR = 1.8, 95% CI: 1.1–2.9, P = 0.01) [Table 2].
Table 2.
Univariate logistic regression and conditional multivariate logistic regression between urgent surgery, hypertension requiring medication, associated procedures, abdominal aortic calcification rate as a continuous variable or abdominal aortic calcification rate ≥5% and risk of anastomotic leakage
| Factors | Univariate logistic regression | Multivariate model using AAC rate as a continuous variable | Multivariate model using AAC rate ≥5% | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| aOR | 95% CI | P | aOR | 95% CI | P | aOR | 95% CI | P | |
| Urgent surgery | 0.3 | 0.1-1.5 | 0.15 | 0.2 | 0.1-1.1 | 0.06 | 0.2 | 0.1-1.1 | 0.06 |
| Associated procedures | 0.6 | 0.2-1.5 | 0.26 | 0.5 | 0.2-1.5 | 0.24 | 0.6 | 0.2-1.6 | 0.27 |
| Hypertension requiring medication | 1.5 | 0.7-3 | 0.26 | 1.5 | 0.7-3.1 | 0.33 | 1.5 | 0.7-3.2 | 0.28 |
| AAC rate* | 1.6 | 1.1-2.5 | 0,03 | 1.8 | 1.1-2.9 | 0.01 | - | - | - |
| AAC rate ≥5% | 2.8 | 1.2-6.2 | 0.01 | - | - | - | 3.2 | 1.4-7.5 | <0.01 |
*Using LN (% +1). AAC=Abdominal aortic calcification; CI=Confidence interval; aOR=Adjusted odds ratio
A second conditional multivariate logistic regression model was performed using AAC rate ≥5%, hypertension requiring medication, urgent surgery, and associated procedures. In this second model, AAC rate ≥5% was identified as the only significant independent risk factor for AL (aOR = 3.2, 95% CI: 1.4–7.5, P < 0.01) [Table 2 and Figure 3].
Figure 3.

Histogram of abdominal aortic calcifications rate
DISCUSSION
Our matched case–control study highlights two results regarding a higher rate of AAC volume on abdominal aortic volume: (i) it is significantly correlated to the presence of CV risk factors and/or previous history of CV disease, as has been previously described in the literature;[8,9,10,11,12] (ii) and it is significantly correlated to an increased risk for AL, when considering either AAC volume as a continuous variable percentage or categorized with a 5% threshold. This increased risk is still present after considering confusion bias due to other AL potential risk factors such as emergency surgery, associated procedures, and hypertension requiring medication. These data suggest that the AAC rate, as a percentage of aorta volume containing calcification, should become part of an objective assessment of risk before left-sided colic or colorectal anastomosis.
In 2013, Harbaugh et al.[12] reported an association between AAC rate and postoperative infection and overall complications after major general or vascular surgery, but this large cohort provided no specific details on the occurrence of digestive AL. The association between vascular calcifications and AL has been investigated in only a few studies whose limitations were their modest cohort size, and their heterogenic methodology – visual grading or used of software, assessment of the aorta and/or iliac arteries – used to determine the calcification burden. Eveno et al. found an association between calcification burden (evaluate by the degree of circumference of AAC) and AL.[15] Boersema et al. in their case–control study found no association between aortoiliac calcium score (product of the area of calcification and its radiodensity) and AL.[14] Komen et al. found no association between the calcium score of the aortic tract and AL but found an association between the calcium score of the iliac arteries and AL.[13] Pochhammer et al. reported an association between a higher calcium score in the iliac arteries and AL.[16] Moreover, Norooz et al. reported a higher calcium score of aortic and iliac arteries in patients who develop an AL.[17] Knight et al. reviewed the literature about the relationship between arterial calcification and AL in patients undergoing esophageal and colorectal resection, but a meta-analysis could not performed due to the various methods used. The authors concluded on a possible relationship between a higher AAC burden and the development of AL in patients undergoing esophagectomy but no conclusions were possible for colorectal surgery.[18]
Quantification of AAC is not a new approach to assess the risk of CV disease. Several studies demonstrated a correlation between AAC and calcification in other fields, particularly in the coronary arteries or between AAC and clinical or subclinical CV disease.[8,9,10,11,12] Consequently, the measurement of the calcification burden should be performed on the aorta rather than on the iliac arteries. This measurement allows rapid, noninvasive, and reproducible atherosclerosis evaluation and does not require additional imaging. In our study, we used the AAC rate mainly to avoid inter-individual variation based on the length of the aorta.
A history of CV disease is not always found to be a risk factor for AL, but many CV risk factors are also described as being AL risk factors. AAC may better describe overall health rather than CV-specific health alone. Moreover, this measure may summarize and allow a quantification of the effect of several well-known factors incriminated in the occurrence of AL-smoking, high body mass index, hypertension, and hypercholesterolemia – which are also known to cause atherosclerosis and CV disease.[19]
The association between AAC and AL can fulfill some of the Hill causation criteria:[20] (1) the strength of association with high OR for AAC >5%: OR = 3.21; (2) the consistency of the association with previous studies that have described links between vascular calcifications and AL; (3) the temporality with the presence of AAC before AL; (4) the biological gradient with an increased AL risk according to an increased AAC rate – patients with 1%, 5%, 10%, or 15% of AAC saw their risk of AL increase, respectively, by 1.02 (95% CI = [1.01; 1.03]), 1.1 [1.06; 1.1], 1.2 [1.1; 1.3], 1.288 [1.2; 1.5]; (5) the plausibility with an obvious relationship between weaker tissue vascularization and difficulty for healing; (6) and the good coherence. The specificity of the relationship is the only criteria that cannot be affirmed because a lot of other AL risk factors have been described.
There are some limitations in this work that should be highlighted: (i) this is a retrospective study with a relatively small sample size (even if this the largest study published) due to limited availability of scans, and it is a single center study, (ii) the studied population included two different types of surgical procedures – left-sided resections and rectal surgery – in which morbidity is fairly different, (iii) this study did not report all recognized preoperative risk factors for AL.
CONCLUSION
AAC is potentially a current finding on preoperative CT scans. It seems to be an independent risk factor for AL in patients undergoing left-sided colonic or rectal resection followed by anastomoses. In the presence of aortic calcification, the measurement of the percentage of AAC could be added fairly simply to the cross-sectional imaging analysis. An AAC rate ≥5% should alert physician to a high AL risk and could modify their decision to perform an anastomosis or may indicate the need for a protective stoma. The assessment of patients' specific risks may also help to inform them more accurately. Nevertheless, in view of our results, the real impact of the AAC rate as a factor enabling the prediction of AL requires validation through a larger multicenter prospective study using a standardized method of AAC assessment in patients undergoing colorectal surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: A nationwide cohort study. Ann Surg. 2014;259:930–8. doi: 10.1097/SLA.0b013e3182a6f2fc. [DOI] [PubMed] [Google Scholar]
- 2.Kingham TP, Pachter HL. Colonic anastomotic leak: Risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208:269–78. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 3.McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015;102:462–79. doi: 10.1002/bjs.9697. [DOI] [PubMed] [Google Scholar]
- 4.Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis. 2009;24:569–76. doi: 10.1007/s00384-009-0658-6. [DOI] [PubMed] [Google Scholar]
- 5.Rifkin RD, Parisi AF, Folland E. Coronary calcification in the diagnosis of coronary artery disease. Am J Cardiol. 1979;44:141–44. doi: 10.1016/0002-9149(79)90263-7. [DOI] [PubMed] [Google Scholar]
- 6.Braunschmid T, Hartig N, Baumann L, Dauser B, Herbst F. Influence of multiple stapler firings used for rectal division on colorectal anastomotic leak rate. Surg Endosc. 2017;31:5318–26. doi: 10.1007/s00464-017-5611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rojas-Machado SA, Romero-Simó M, Arroyo A, Rojas-Machado A, López J, Calpena R. Prediction of anastomotic leak in colorectal cancer surgery based on a new prognostic index PROCOLE (prognostic colorectal leakage) developed from the meta-analysis of observational studies of risk factors. Int J Colorectal Dis. 2016;31 doi: 10.1007/s00384-015-2422-4. 197-31:19. [DOI] [PubMed] [Google Scholar]
- 8.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–6. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 9.Wong ND, Lopez VA, Allison M, Detrano RC, Blumenthal RS, Folsom AR, et al. Abdominal aortic calcium and multi-site atherosclerosis: The multiethnic study of atherosclerosis. Atherosclerosis. 2011;214:436–41. doi: 10.1016/j.atherosclerosis.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastos Gonçalves F, Voûte MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: A meta-analysis. Heart Br Card Soc. 2012;98:988–98. doi: 10.1136/heartjnl-2011-301464. [DOI] [PubMed] [Google Scholar]
- 11.Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, et al. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1574–9. doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbaugh CM, Terjimanian MN, Lee JS, Alawieh AZ, Kowalsky DB, Tishberg LM, et al. Abdominal aortic calcification and surgical outcomes in patients with no known cardiovascular risk factors. Ann Surg. 2013;257:774–81. doi: 10.1097/SLA.0b013e31826ddd5f. [DOI] [PubMed] [Google Scholar]
- 13.Komen N, Klitsie P, Dijk JW, Slieker J, Hermans J, Havenga K, et al. Calcium score: A new risk factor for colorectal anastomotic leakage. Am J Surg. 2011;201:759–65. doi: 10.1016/j.amjsurg.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Boersema GS, Vakalopoulos KA, Kock MC, van Ooijen PM, Havenga K, Kleinrensink GJ, et al. Is aortoiliac calcification linked to colorectal anastomotic leakage? A case-control study. Int J Surg. 2016;25:123–7. doi: 10.1016/j.ijsu.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Eveno C, Latrasse V, Gayat É, Lo Dico R, Dohan A, Pocard M. Colorectal anastomotic leakage can be predicted by abdominal aortic calcification on preoperative CT scans: A pilot study. J Visc Surg. 2016;153:253–7. doi: 10.1016/j.jviscsurg.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Pochhammer J, Tröster F, Blumenstock G, Closset J, Lang S, Weller MP, et al. Calcification of the iliac arteries: A marker for leakage risk in rectal anastomosis-a blinded clinical trial. Int J Colorectal Dis. 2018;33:163–70. doi: 10.1007/s00384-017-2949-7. [DOI] [PubMed] [Google Scholar]
- 17.Norooz MT, Moradi H, Safdarian M, Jahangiri F, Amoli HA. Does calcium score in great pelvic vessels predict colorectal anastomotic leakage? A prospective study of one hundred anastomoses. Acta Gastroenterol Belg. 2016;79:415–20. [PubMed] [Google Scholar]
- 18.Knight KA, Horgan PG, McMillan DC, Roxburgh CS, Park JH. The relationship between aortic calcification and anastomotic leak following gastrointestinal resection: A systematic review. Int J Surg. 2020;73:42–9. doi: 10.1016/j.ijsu.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Sen M, Anadol AZ, Oğuz M. Effect of hypercholesterolemia on experimental colonic anastomotic wound healing in rats. World J Gastroenterol. 2006;12:1225–8. doi: 10.3748/wjg.v12.i8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]


