summary
Endogenous peptides and structurally similar bacterial heat-stable enterotoxins (ST) bind guanylate-cyclase C (GC-C), resulting in fluid homeostasis or diarrhea, respectively. In this issue of Cell Host & Microbe, Carey et al. (2021), show how bats have evolutionarily maintained homeostatic signaling while avoiding pathogenic effects of ST.
Mammals have highly conserved mechanisms to maintain fluid homeostasis in the intestine. Guanylate cyclase-C (GC-C), a protein dimer localized to the membrane of intestinal enterocytes plays a central role by serving as a receptor for small cysteine-rich peptides produced by intestinal epithelia, guanylin and uroguanylin (Figure 1A). Binding of these ligands to the receptor domain of GC-C stimulates the conversion of GTP to cGMP by the intracellular catalytic domain. The resulting increases in cGMP activate cGMP-dependent protein kinase II (PKG) which in turn modulates the activity of cellular ion channels. This includes the sodium-hydrogen ion exchanger NHE3 and the CFTR chloride channel resulting in net export of salt and water into the intestinal lumen. These peptide hormones, in concert with their GC-C receptor, act in a paracrine fashion to maintain critical fluid efflux into the intestinal lumen.
Figure 1.
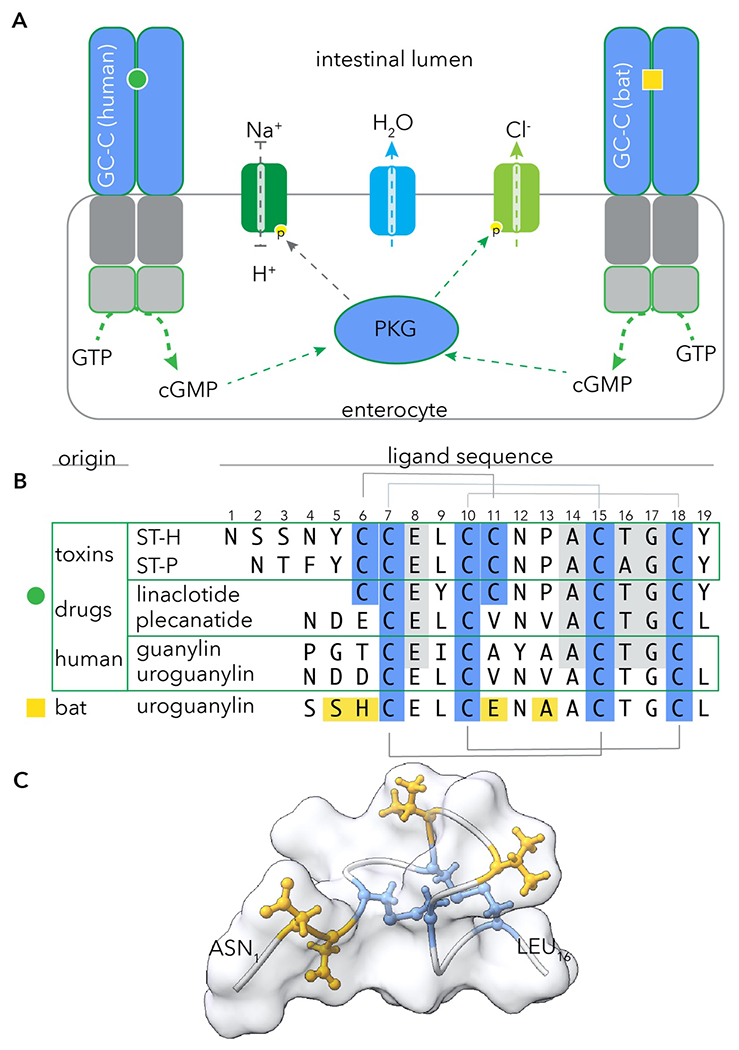
signaling through guanylate cyclase C (GC-C) maintains intestinal fluid homeostasis. A. (left) canonical GC-C-cGMP-PKG signaling pathway in which endogenous peptide ligands guanylin and uroguanylin, or heat stable toxins bind the GC-C receptor domain to signal conversion of GTP to cGMP via the intracellular catalytic domain. Upon activation by cGMP, PKG phosphorylates cellular targets including the NHE3 sodium/hydrogen ion exchanger and (CFTR), driving osmotic release of fluid into the intestinal lumen. B. Amino acid alignment of peptides including ETEC heat-stable toxins ST-H and ST-P, peptide drugs linaclotide and plecanatide, and human guanylin and uroguanylin. Cysteines are shown in blue with inter-cysteine disulfide bridges in toxins at top and in endogenous peptides at bottom. Sequence of bat uroguanylin from M. lucifugus, which preferentially activates GC-C (figure A right) from that species is shown at bottom. Amino acids in yellow correspond to mutations relative to consensus peptide. C. predicted structure of human uroguanylin (PDB 1UYA, (Marx et al., 1998)). Cysteines are depicted in blue; amino acids in yellow correspond to those altered in M. lucifugus.
Heat stable toxins (ST) are cysteine-rich short (18-19 amino acid) peptides secreted by Gram-negative enteric pathogens, particularly enterotoxigenic Escherichia coli (ETEC). They share sequence homology and overall structure with the endogenous peptide hormones, and bind GC-C with high affinity (Schulz et al., 1990) resulting in robust stimulation of the cGMP-PKG axis and pronounced secretory diarrhea (Figure 1B). Mutations in GC-C which preserve binding of peptide hormones at the expense of ST could mitigate the diarrheal effects of the toxins and offer a selective advantage.
Interestingly, in studies reported here by Carey, et al. (Carey et al., 2021), phylogenetic analysis of primate and bat GC-C sequences provides evidence for positive selection of sites confined to the ligand binding domain of the receptor, potentially selected by pressure from ST. Of note, variation in the ligand-binding domain was particularly intense in bats. These mutations in GC-C conferred a differential ability to respond to ST peptides, mitigating the impact of some while enhancing signaling by others. Remarkably, the investigators discovered that some species of bats had developed compensatory mutations in uroguanylin, offsetting the variation in GC-C and permitting continued paracrine signaling through the cGMP-PKG axis (Figure 1C). In effect, bats have evolved to change the lock to bar toxin engagement, while keeping the new keys to themselves.
Although precisely which toxins, or which pathogens, might be driving the evolutionary changes to GC-C in bats and their corresponding endogenous peptides remains unclear, the studies illustrate the potential for extraordinary co-evolution under microbial selection. An open question is whether it is actually ST-induced diarrhea per se that has driven the selection. Activation of cGMP production in the host is likely to have a multitude of effects that extend beyond modulation of ion channels to export NaCl and water. In fact, signaling through this critical “second messenger” pathway may alter transcription of multiple genes (Zhao et al., 2005), and impact other key elements of physiology, including intestinal epithelial cell proliferation (Basu et al., 2014), and stem cell maintenance vital to development of the mucosal surface architecture required for nutrient absorption (Steinbrecher and Cohen, 2011).
Elucidation of the impacts of enterotoxins on the host that may have provided the evolutionary forces underlying the positive selection of the mutations reported here is an important avenue of investigation. Enterotoxigenic Escherichia coli (ETEC) produce both heat-stable toxins (ST-H and ST-P) as well as heat-labile toxin (LT). LT also signals through a major “second messenger” cyclic nucleotide system by activation of adenylate cyclase to increase intracellular cAMP, which in turn activates protein kinase A (PKA). Like PKG, PKA modulates the activity of NHE3 and CFTR with similar fluid efflux into lumen of the small intestine. In addition, signaling through PKA can affect the transcription of thousands of genes (Zambon et al., 2005), suggesting that like ST the impact of heat-labile toxin likely extends beyond the episode of acute, typically self-limited diarrhea.
Indeed, ST-mediated signaling through GC-C has a number of other important implications for human health. Intriguingly, the proportionately low incidence of colon cancer in regions where ETEC is prevalent prompted earlier investigations of the anti-proliferative effects of ST on colonic neoplasms where GC-C signaling along the crypt-villus axis is altered in favor of epithelial proliferation (Pitari et al., 2003). The molecular mimicry of ST for endogenous peptides also underlies development of peptide drugs linaclotide and plecanatide used in treatment of irritable bowel syndrome and chronic constipation.
ETEC astoundingly account for hundreds of millions of estimated cases of diarrheal illness each year, particularly in young children in low-middle income countries that lack fundamental human needs of clean water and basic sanitation (Khalil et al., 2018). Increasingly, these pathogens have been associated with important sequelae including pathologic changes to the small intestine accompanied malnutrition and stunted growth. Malnutrition, consequently, places children at substantially increased risk of death from diarrheal disease and other infections. While the molecular pathogenesis of these sequelae has yet to be elucidated, they almost certainly are the result of repeated exposure to one or both of the toxins produced by these ubiquitous pathogens.
Bats appear to be evolving to avoid either the toxin-induced acute diarrheal illness, or perhaps subsequent intestinal pathology, while maintaining homeostatic mechanisms essential to regulation of fluid balance in the intestine. Humans will need effective toxoid vaccines (Zegeye et al., 2019) to counter the effects of these pathogens rather than waiting to evolve under their selective pressure.
Acknowledgements
Work in the Fleckenstein lab is supported by funding from the National Institute of Allergy and Infectious Diseases (NIAID) grants R01AI089894, and R01AI126887; and The Department of Veterans Affairs grant I01BX004825. Work in the Bitoun lab is supported by NIAID grant R01AI12554.
References
- Basu N, Saha S, Khan I, Ramachandra SG, and Visweswariah SS (2014). Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21. J Biol Chem 289, 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CM, Apple SE, Hilbert ZA, Kay MS and Elde NC, (2021) Diarrheal pathogens trigger rapid evolution of the Guanylate Cyclase-C signaling axis in bats. Cell Host & Microbe XX–XXX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, et al. (2018). Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990-2016. Lancet Infect Dis 18, 1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx UC, Klodt J, Meyer M, Gerlach H, Rosch P, Forssmann WG, and Adermann K (1998). One peptide, two topologies: structure and interconversion dynamics of human uroguanylin isomers. J Pept Res 52, 229–240. [DOI] [PubMed] [Google Scholar]
- Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, Hajnoczky G, Terzic A, and Waldman SA (2003). Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci U S A 100, 2695–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Green CK, Yuen PS, and Garbers DL (1990). Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 63, 941–948. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, and Cohen MB (2011). Transmembrane guanylate cyclase in intestinal pathophysiology. Curr Opin Gastroenterol 27, 139–145. [DOI] [PubMed] [Google Scholar]
- Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, and Insel PA (2005). Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci U S A 102, 8561–8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegeye ED, Govasli ML, Sommerfelt H, and Puntervoll P (2019). Development of an enterotoxigenic Escherichia coli vaccine based on the heat-stable toxin. Hum Vaccin Immunother 15, 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhuang S, Chen Y, Boss GR, and Pilz RB (2005). Cyclic GMP-dependent protein kinase regulates CCAAT enhancer-binding protein beta functions through inhibition of glycogen synthase kinase-3. J Biol Chem 280, 32683–32692. [DOI] [PubMed] [Google Scholar]


