Abstract
Inquiry into relationships between energy metabolism and brain function requires a uniquely interdisciplinary mindset, and implementation of anti-aging lifestyle strategies based on this work also involves consistent mental and physical discipline. Dr. Mark P. Mattson embodies both of these qualities, based on the breadth and depth of his work on neurobiological responses to energetic stress, and on his own diligent practice of regular exercise and caloric restriction. Dr. Mattson created a neurotrophic niche in his own laboratory, allowing trainees to grow their skills, form new connections, and eventually migrate, forming their own labs while remaining part of the extended lab family. In this historical review, we highlight Dr. Mattson’s many contributions to understanding neurobiological responses to physical exercise and dietary restriction, with an emphasis on the mechanisms that may underlie neuroprotection in ageing and age-related disease. On the occasion of Dr. Mattson’s retirement from the National Institute on Aging, we highlight his foundational work on metabolism and neuroplasticity by reviewing the context for these findings and considering their impact on future research on the neuroscience of aging.
Keywords: Exercise, Caloric restriction, Intermittent fasting, Mitochondria, Hippocampus, Aging
1. Introduction and historical context
Aging was historically viewed as a pathological process, with widespread reports of neuronal loss and synaptic atrophy in multiple areas of the brain (Brody, 1955; Shefer, 1973). With the advent of modern stereological methods (Pakkenberg and Gundersen, 1988), unbiased quantification of neurons and synapses revealed that in the absence of age-related pathologies, neuronal number in the medial temporal lobe is unchanged, and synaptic loss is either subtle or absent (Morrison and Hof, 1997; Scheff et al, 2006). Against this background, Mark Mattson’s early work on selective neuronal vulnerability to excitotoxicity set the stage for subsequent refinement of hypotheses surrounding neuronal responses to aging (Figure 1). This work revealed greater vulnerability to glutamate-induced neuronal cell death in pyramidal neuron cultures, as opposed to primary cultures from the early postnatal rat dentate gyrus (Mattson and Kater, 1989). In addition to region-specific differences, pyramidal cells originating from a common progenitor also exhibited similar vulnerability to excitotoxicity in vitro, relative to cells arising from different progenitors (Mattson et al, 1989a). These reports set the stage for later interrogation of cell- and circuit-specific determinants of neuronal vulnerability in Huntington’s disease (Pecho-Vrieseling et al, 2014), Parkinson’s disease (Surmeier et al, 2017), and in tangle-bearing neurons in Alzheimer’s disease (Dunckley et al, 2006).
Figure 1. Mark Mattson’s contributions to understanding normal and pathological aging.
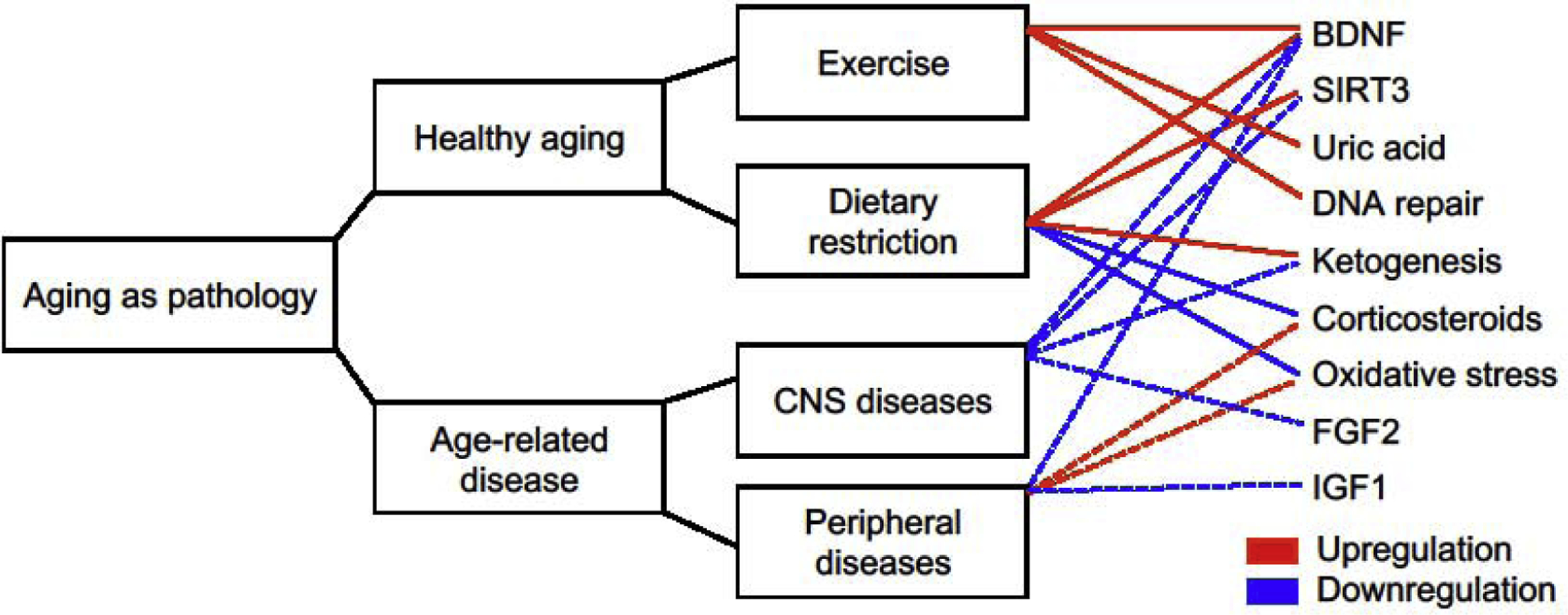
As Mark Mattson was starting his career, the field of neuroscience was reinterpreting aging as a separate condition from peripheral and neurodegenerative diseases. Dr. Mattson’s decades of work identified many bidirectional regulators of neuroplasticity and neurodegenerative disorders. This figure depicts a subset of these bidirectional relationships, which were extracted from (n=948) Pubmed citations of Mark Mattson’s work. Bidirectional relationships were defined by published research articles demonstrating opposing regulation of a molecular target or biological process using gain- or loss-of-function approaches. For this figure, published work using in vivo or in vitro models of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease were considered CNS diseases, while findings from animal models of diabetes and/or obesity were considered peripheral diseases. See Supplementary Tables 1–2 for supporting references.
Increasing recognition of heterogeneous cellular responses to aging occurred in parallel with widespread acceptance of individual differences in cognitive aging, as modeled using behaviorally categorized aged rats. In the absence of age-related pathologies, a subset of aged rats performs similarly to young animals (Zyzak et al, 1995), with similar variability reported in humans (Albert, 1997). The observation that healthy aging can be decoupled from widespread synaptic dysfunction led to broader inquiry into strategies to limit the negative impact of age-related diseases, such as diabetes and sarcopenia, on organismal aging. These areas of investigation were driven by observations that caloric restriction increases lifespan via evolutionarily conserved mechanisms in organisms ranging from yeast to mammals, including nonhuman primates (Osborne et al, 1917; Berg and Simms, 1965; Mitchell et al, 2016; Mattison et al, 2017). Early work on dietary regulation of longevity coincided with field observations on environmental complexity and behavior in nonhuman primates (Osborne et al, 1917; Yerkes, 1925), but the metabolic environment was considered separately from the physical and social environment for many years.
The first environmental enrichment studies increased environmental complexity by rearing rats as household pets (Hebb, 1949) and later, through the addition of toys, social conspecifics, and running wheels (Altman and Das, 1964; Volkmar and Greenough, 1972). In modern neuroscience, Altman and Das are most recognized for their early reports of ongoing neurogenesis in adulthood (1966). However, Altman and Das initially focused on postnatal gliogenesis, reporting increased glial proliferation in rats reared in enriched environments (Altman and Das, 1964). Running wheels were a component of the enrichment paradigm used by Altman and Das (1964), but the relative contributions of exercise, social stimulation, and environmental complexity to these effects went unresolved for thirty years, largely due to debates surrounding the proliferative capacity of the adult brain (Altman, 2011).
Initial attempts to distinguish between the effects of social, locomotor, and environmental complexity suggested that increased vascular density might underlie the impact of exercise on the cerebellum in adult rodents (Black et al, 1990). The effects of exercise were thought to be restricted to motor regions prior to the initial report from Carl Cotman’s laboratory demonstrating increases in hippocampal BDNF with voluntary wheel-running (Neeper et al, 1995). Mark Mattson’s lab would subsequently report a requirement for BDNF in the neuroprotective effects of dietary restriction (Duan et al, 2001; Bruce-Keller et al, 1999), and additive effects of exercise and caloric restriction on hippocampal dendritic spine density and BDNF expression (Stranahan et al, 2009). After the eventual acceptance of adult neurogenesis, wheel running was identified as the primary stimulus for neurogenesis among mice housed in enriched environments (van Praag et al, 1999; Kobilo et al, 2011). Social stimulation accelerated the onset of exercise-induced neurogenesis, but was not required for these effects (Stranahan et al, 2006). Adult neurogenesis declines with increasing age, and wheel running attenuated the impact of aging and improved learning and memory (van Praag et al, 2005). Although the existence of adult neurogenesis in the human brain remains debatable (Sorrells et al, 2018), exercise and dietary restriction elicit similar increases in neurogenesis in rodents (van Praag et al, 1999; Lee et al, 2002). By contrast, adult neurogenesis was impaired in nonobese diabetic rats, and in mice with genetic obesity and insulin resistance (Stranahan et al, 2008a). In the context of positive correlations between exercise and hippocampal volume in young adults (Nauer et al, in press) and aged individuals (Varma et al, 2015; Fuss et al, 2014), Dr. Mattson’s lifelong fascination with the impact of exercise and diet on brain function seems strikingly prescient, and his work in this area presents a compelling rationale for further interrogation of tissue-specific mechanisms for metabolic regulation of neuroplasticity (Figure 1).
In this review, we celebrate Mark Mattson’s research on the occasion of his retirement from the National Institute on Aging. Dr. Mattson’s work on metabolic regulation of aging and age-related disease recapitulates the developmental ontogeny of neural circuits, beginning with in vitro studies, and building towards ever-more-elegant in vivo studies of interactions between different tissues and cell types. Throughout his highly prolific career, the common thread in these studies is their focus on mechanisms for cellular vulnerability across the lifespan. These studies laid the foundation for more recent work on physical frailty and dementia risk in humans, and for broader investigation into determinants of ‘healthspan’ in addition to lifespan in aging research.
2. Regulation of intracelullar calcium in aging and AD
2.1. Calbindin-D28K and interneuronal determinants of network hyperexcitability
Mark Mattson’s early in vitro work revealed that susceptibility to glutamate-induced cytotoxicity was predicted by differential elevations in intracellular calcium (Mattson et al, 1989b; Mattson, 1990). In a series of elegant studies, Dr. Mattson identified the calcium-binding protein calbindin-D28k as a cell-autonomous determinant of vulnerability to excitotoxic cell death (Mattson et al, 1991). Overexpressing calbindin prevented the effects of presenilin-1 mutation and amyloid beta application on mitochondrial calcium handling in a neuronal cell line (Guo et al, 1998), underscoring the critical role of neuronal calcium metabolism in AD pathogenesis.
Calbindin-D28k is primarily expressed by interneurons, and in the hippocampus, calbindin-positive cells represent a significant proportion of the alveus/oriens interneuronal population with lacunosum-moleculare axon arborization (O-LM cells; Sik et al, 1995; Wouterlood et al, 2001). Although numerous interneuron classification schemes exist in the literature (Maccaferri and Lacaille, 2003), the functional importance of interneurons in regulating rhythmic firing is clearly defined (Klausberger et al, 2003). More than 20 years after his initial work on calbindin and AD-related calcium mishandling, the Mattson lab demonstrated that haploinsufficiency for the mitochondrial deacetylase protein sirtuin 3 (SIRT3) in APP/PS1 mice was associated with spontaneous epileptiform activity and age-related loss of interneurons, relative to APP/PS1 mice without the SIRT3 mutation (Cheng et al, 2020). The focus on glutamate and excitotoxicity in Dr. Mattson’s early studies laid the groundwork for subsequent work by others on interneuron dysfunction and medial temporal lobe hyperexcitability as early events in the progression from mild cognitive impairment to Alzheimer’s disease (Palop et al, 2007; Vossel et al, 2016). In the context of the broader literature on circuit dysfunction in aging and AD, the Mattson lab’s early studies of AD-related calcium dyshomeostasis have stood the test of time and will likely generate additional insights for the field moving forward.
2.2. Growth factor regulation of CNS mitochondria
During the period in neuroscience when Mark Mattson was starting his independent career, the Nobel Prize for Physiology and Medicine had recently been awarded to Rita Levi-Montalcini and Stanley Cohen for their discovery of nerve growth factor (NGF). The NGF family members glia-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) were also identified during this time and Dr. Mattson was among the first to investigate interactions between neurotrophins and AD neuropathology (Figure 1; see Supplementary Table 1). His studies of NGF and members of the fibroblast growth factor (FGF) family identified a neuroprotective role for FGF2 against beta-amyloid toxicity in primary neurons (Cheng et al, 1991; Mattson et al, 1993a; Mark et al, 1997). These early studies also identified a parallel neuroprotective role for FGF2 in models of hypoglycemia-associated tau aggregation (Cheng and Mattson, 1992a).
Dr. Mattson’s initial studies of growth factors and protection against cytotoxic stimuli in primary neurons laid the conceptual groundwork for decades of investigation into mitochondrial dysfunction and neuronal plasticity in vitro (Guo et al, 1998; Guo et al, 1999) and in vivo (Guo et al, 2008; Cheng et al, 2007). The in vivo studies carried out after his move to the National Institute on Aging in 2000 successfully scaled up the elegant mechanisms previously identified in primary neurons. Studies in leptin receptor deficient db/db mice identified JAK/STAT3-mediated mitochondrial stabilization as a determinant of cell survival after kainic acid (Guo et al, 2008). Dissociation of the anti-apoptotic mitochondrial transmembrane protein Bcl-xL also played a pivotal role in ischemia-induced cell death (Cheng et al, 2007). Conversely, intermittent fasting reduced ischemic damage and enhanced the expression of BDNF and FGF2 (Arumugam et al, 2010; Figure 1, Supplementary Table 2), consistent with the protective effects of growth factor stimulation reported in vitro (Cheng et al, 1991; Mattson et al, 1993a; Mark et al, 1997). These lines of investigation established a scalable architecture for interrogating the neuroprotective effects of exercise and diet on mitochondrial homeostasis (Cheng et al, 2016; Liu et al, 2019; see section 3.2).
2.3. Intracellular calcium, mitochondria, and membrane-associated oxidative stress
Neurons are morphologically complex and exhibit finely tuned functional specialization in different compartments. Mitochondria traffic between the soma and dendrites, and between the soma and axon terminals, allowing for compartmentalized regulation of intracellular calcium (Sheng and Cai, 2012). Mitochondrial fission and fusion are also ongoing, as is mitophagy, or targeted lysosomal degradation of dysfunctional mitochondria (Youle and van der Bliek, 2012). Given the importance of local calcium homeostasis for neuroplasticity and neuronal viability, it is logical that perturbation of mitochondrial homeostasis would have a pervasive negative impact in the CNS.
While at the University of Kentucky, Dr. Mattson’s laboratory demonstrated that overexpression of mitochondrial manganese superoxide dismutase (MnSOD) maintained mitochondrial membrane potential and protected against apoptosis in primary neurons challenged with amyloid beta (Keller et al, 1998). Superoxide anions generated during mitochondrial respiration react with the gasotransmitter nitric oxide to produce peroxynitrite and other reactive oxygen species (ROS). Amyloid beta-induced mitochondrial dysfunction was associated with oxidative modification of membrane lipids and accumulation of 4-hydroxynonenal (4-HNE), an aldehyde byproduct of lipid peroxidation (Keller et al, 1997a). The cycle of mitochondrial dysfunction and membrane-associated oxidative stress likely perpetuates neuronal hyperexcitability, as exposure to 4-HNE impairs mitochondrial respiration in synaptosome preparations (Keller et al, 1997b). Calorie restriction attenuates aging-associated lipid peroxidation (Hyun et al, 2006; Figure 1, Supplementary Table 2), consistent with a potential role for metabolic preconditioning in protection against neurodegenerative disease.
The metabolic requirements of the CNS are high and the capacity for local energy storage is very low. Given these environmental constraints, it is surprising that glial mitochondrial dynamics have not received greater attention as potential regulators of cellular and circuit function (McAvoy and Kawamata, 2019). Astroglial glutamate uptake plays an essential role in limiting glutamate ‘spillover’ from synaptic to extrasynaptic sites. Using primary astrocytes, members of the Mattson lab demonstrated that exposure to sub-apoptotic concentrations of 4-HNE reduced glutamate uptake and impaired mitochondrial function (Blanc et al, 1998). Disruption of astroglial glutamate uptake following membrane-associated oxidative stress is another potential mechanism for excitotoxicity in aging and AD, which perturbs CNS cholesterol metabolism by promoting accumulation of long-chain ceramides and HNE adducts (Cutler et al, 2004; Figure 1). Similar membrane lipid modifications were evident in the hippocampus of rats maintained on an obesogenic high-fat diet (Stranahan et al, 2011; Figure 1, Supplementary Table 1), implicating mitochondrial dysfunction and membrane-associated oxidative stress as bidirectional determinants of neuronal function.
3. Exercise: bending the curve of cognitive and metabolic aging
3.1. Physical frailty and mental fragility: sarcopenia and dementia risk in humans
Aging is accompanied by a marked decline in muscle mass known as sarcopenia, as well as a significant decline in muscle power referred to as dynapenia (Law et al., 2012). Age-related loss of muscle mass and power is associated with significant morbidity and mortality, with risk of falling as a major factor that accompanies frailty and muscle weakness (Lavin et al., 2019). In humans, cross-sectional and longitudinal studies suggest that walking speed predicts risk of age-related cognitive impairment, with slower gaits associated with greater risk (Hoogendijk et al, 2020; Ble et al, 2005).
Sarcopenia is a primary cause of frailty and slow gait, and there are a number of potential mechanisms for sarcopenia, including increased levels of inflammatory cytokines, increased oxidative stress, and mitochondrial dysfunction (Taetsch and Valdez, 2018). Each of the above processes have been linked with senescence of muscle satellite cells, impaired protein synthesis, and degradation of neuromuscular junctions (Taetsch and Valdez, 2018). A substantial body of evidence now indicates that many of these changes can be prevented or reversed with exercise (Aguirre and Villareal, 2015; Lavin et al., 2019). A key factor in the loss of muscle power with aging is a reduction in size of type II (fast-twitch) muscle fibers. Type II fiber size declines significantly with age, and loss of type II fibers accelerates with inactivity, as observed in patients with hip fracture (Kramer et al., 2017). Exercise training in older men and women significantly increases the size of type II fibers (Leenders et al., 2013). In addition, while muscle satellite cell number has been observed to decline with aging, exercise increases satellite cell number in type II fibers in aged humans and rodent models (Verdijk et al., 2009; Liu et al., 2017).
3.2. Parallel roles for exercise in maintaining synaptic stability in the brain and periphery
Stimulation of muscle satellite cells with exercise is one strategy for combating sarcopenia in older adults, but satellite cells also play a key role in maintenance of the neuromuscular junction (NMJ). Age-related loss of muscle power is associated with fragmentation of the NMJ (Taetzsch and Valdez, 2018), and evidence from animal models suggests that depleting skeletal muscle of satellite cells drives NMJ degradation (Liu et al, 2017). Gain-of-function studies also support the interaction between satellite cells and NMJs, as satellite cell-specific overexpression of Spry1, a gene implicated in satellite cell proliferation (Chakkalakal et al, 2012), rescues age-related structural degradation of NMJs (Liu et al., 2017). In addition to the interactions with satellite cells, presynaptic enrichment of mitochondria at NMJs plays a critical role in maintenance of synaptic contacts (Wang et al, 2018). Aging is associated with atrophy of NMJs, mitochondrial fragmentation, and decreased expression of mitofusin 2 in spinal motor neurons (Wang et al, 2018). Neuronal overexpression of mitofusin 2 blocked age-related muscle loss and maintained NMJ structure in aged mice, indicating that motor neuron mitochondrial function regulates sarcopenia (Wang et al, 2018).
Recent work from the Mattson lab indicates that neuronal mitochondria may also play a role in regulating synaptic stability in the CNS. The mitochondrial deacetylase SIRT3 is upregulated in the hippocampus with exercise, and whole-body ablation of SIRT3 eliminated the protective effects of exercise against excitotoxic stress (Cheng et al, 2016; Figure 1). Local hippocampal induction of SIRT3 was also required for the protective effects of intermittent fasting on hippocampal synaptic plasticity in AD mice, as determined by lentiviral knockdown in AppN-L-F transgenic mice maintained on IF for 8 months (Liu et al, 2019; Figure 1). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a transcriptional coactivator that integrates cellular stress signals and mitochondrial respiration. Consistent with the high metabolic demands associated with synaptic plasticity-associated cytoskeletal rearrangement, local knockdown of PGC-1α in the hippocampal dentate gyrus reduced dendritic spine density in vivo and eliminated BDNF-stimulated synaptogenesis in vitro (Cheng et al, 2012).
While PGC-1α has received recent attention for its role in coordinating the effects of exercise in the brain (Wrann et al, 2013; Cheng et al, 2012), an extensive literature exists surrounding PGC-1α and muscle fiber adaptations to exercise in rodents and humans (Baar et al, 2002; Pilegaard et al, 2003). Muscle-specific overexpression of PGC-1α in aged mice increases mitochondrial number and promotes the expression of genes important for NMJ function (Garcia et al., 2018). This relationship is not exclusive to the NMJ, as whole-body ablation of PGC-1α blocks hippocampal induction of fibronectin type III domain containing 5 (FNDC5), the precursor protein for the peptide hormon irisin, in mice (Wrann et al, 2013). Exercise-induced mitochondrial biogenesis is also required for synaptogenesis, as whole-body UCP2 knockout mice did not exhibit wheel running-induced increases in hippocampal synaptic and mitochondrial number (Dietrich et al, 2008). Taken together, these findings strongly implicate mitochondria as a nexus for exercise- and diet-induced synaptic regulation in the brain and periphery.
3.3. Myokines and muscle-brain crosstalk with aging and exercise
Skeletal muscle adaptations to exercise impact other organ systems via the release of muscle-derived factors, termed myokines, into circulation (Pedersen, 2019). Although hormones and cytokines have received more attention with respect to the effects of exercise on the brain, recent work indicates that myokines also participate in muscle-brain crosstalk with aging (Pedersen, 2019). The cathepsin family of lysosomal proteases regulates multiple physiologic processes, including muscle biogenesis, bone formation and turnover, and amyloidosis (Andrew et al, 2016; Reiser et al, 2010). Voluntary wheel running increased levels of cathepsin B in muscle and plasma, and these increases were required for exercise-induced neurotrophic factor expression in the hippocampus of mice (Moon et al, 2016). Similar positive correlations between circulating cathepsin B and cognition were reported after exercise training in nonhuman primates and humans, suggestive of conserved mechanisms for muscle-brain interactions (Moon et al, 2016). In addition to cathepsin B, expression of FNDC5, the gene encoding the myokine irisin, was associated with exercise-induced hippocampal neurotrophic factor expression (Wrann et al, 2013). Expression of FNDC5 is reduced in brain tissue from AD patients, and in AD model mice, intrahippocampal administration of recombinant irisin normalized cognition (Lourenco et al, 2019). Lourenco et al also reported indirect evidence that a swimming exercise paradigm co-regulates FNDC5 in the brain and periphery (2019), but the question of whether peripheral irisin drives hippocampal induction of FNDC5 following exercise has yet to be conclusively answered. Potential cellular intermediaries for such a relationship include cerebrovascular endothelial cells, pericytes, and/or astrocytes, which could respond to circulating irisin by generating alternative paracrine signals that promote FNDC5/irisin expression among neurons in the brain parenchyma. While this question is certainly important for understanding the role of FNDC5/irisin in neurobiological responses to exercise, the lack of an answer does not limit enthusiasm for further investigation of myokines and their role in regulating the CNS.
3.4. Extracellular vesicles and muscle-to-brain signaling
In addition to myokines, extracellular vesicles (EVs), including exosomes and microvesicles, have also been recognized as new players in exercise-mediated effects on cell-cell and inter-organ communication. In humans, exercise stimulates the release of EVs from skeletal muscle (Guescini et al., 2015; Whitham et al., 2018; Frühbeis et al., 2015). Transient increases in intracellular calcium are known to induce exosome secretion (Savina et al., 2003), and the release of calcium ions from the sarcoplasmic reticulum of skeletal muscle cells following motor neuron stimulation is an important mechanism driving exercise-mediated exosome release (Whitham et al. 2018). Circulating extracellular vesicles cross the blood-brain barrier and deliver their cargo to neuronal populations (Alvarez-Erviti et al. 2011; Ridder et al., 2014). While a number of studies have shown that physical activity improves executive function in the elderly (Berryman et al., 2013; Farina et al., 2016; Scherder et al., 2010), the molecular mechanisms mediating muscle-brain crosstalk with aging are not well established. Here again, Mark Mattson has been at the leading edge of work on cell-cell communication, carrying out collaborative studies of immune responses to EVs in aged humans and in patients with diabetes (Eitan et al, 2017; Freeman et al, 2018). These studies indicate that circulating EVs from aged individuals and diabetic patients plasma are internalized more readily by circulating immune cells, relative to EVs from young or nondiabetic subjects (Eitan et al, 2017; Freeman et al, 2018). Differential internalization of plasma EVs by immune cells may contribute to the pro-inflammatory systemic environment in aging, and it will be exciting to see where this line of investigation leads.
Extracellular vesicles carry both microRNA (miRNA) and full-length mRNAs that retain their biological impact on gene expression in target cells (Ying et al, 2017; Thomou et al, 2017; Pan et al, 2019). In a collaborative study, Mark Mattson reported that aging alters full-length mRNAs for pro-inflammatory cytokines in EVs from amyloid beta-stimulated macrophages (Mitsuhashi et al, 2013). Exosomes derived from different organs carry distinct signatures, with muscle-derived EVs characterized by enrichment for the transmembrane protein alpha-sarcoglycan (Guescini et al, 2015). Muscle-derived, alpha-sacroglycan positive EVs carrying the senescence-associated microRNA miR-34a are increased in the circulation with aging (Fulzele et al, 2019). This may contribute to age-related declines in multiple organ systems as the pro-survival factor Sirt1 is negatively regulated by miR-34a in cancer, non-alcoholic fatty liver disease, and age-related vascular dysfunction (Yamakuchi et al, 2008; Min et al, 2012; Guo et al, 2017).
The lipid composition of EVs also plays a role in determining their effects on target tissues. Exosomes enriched in very long chain C24:1 ceramide are elevated with age in serum of older women (Khayrullin et al., 2019), and C24:1 ceramide contributes to cell death in a variety of cell types (Hannun, 1996). Levels of very long chain C24:0 ceramide are also increased in the brains of AD patients and in CSF from patients with HIV dementia (Cutler et al, 2004; Haughey et al, 2004), with similar elevations reported in the hippocampus of rats with high-fat diet-induced elevations in serum cholesterol (Stranahan et al, 2011). Plasma EVs from patients with AD and HIV-associated neurological disorders carry distinct pathological cargoes, but have yet to be profiled with respect to lipid content (Perrotte et al, 2020; Pulliam et al, 2019; Dinkins et al, 2017). Likewise, in obesity, adipose-derived circulating EVs promote inflammation and insulin resistance, but this line of investigation focused on miRNA cargo rather than lipidomic profiles of EVs as the effector mechanism (Ying et al, 2017; Pan et al, 2019). However, given that AD, HIV dementia, and obesity are each associated with cognitive deficits and dysregulation of brain lipid metabolism (Cutler et al, 2004; Stranahan et al, 2011; Haughey et al, 2004), there may be a potential relationship between ceramide-enriched exosomes and cognitive impairment in these conditions. If these correlations were determined to be causal, modulation of exosome cargo secreted from peripheral organs and tissues may therefore serve as biomarkers and potential targets for intervention to promote brain health with aging.
3.5. Brain-to-blood efflux of extracellular vesicles in aging
Peripheral EVs have been shown to penetrate the blood-brain barrier, and emerging evidence indicates that efflux of brain-derived EVs also impacts peripheral cells and tissues (Alvarez-Erviti et al. 2011; Ridder et al., 2014; Mustapic et al., 2017). Brain-derived EVs enriched with neural cell adhesion molecule L1 have been detected in the circulation (Mustapic et al., 2017), suggesting that efflux of brain-derived EVs might influence the periphery. Neuronal-enriched extracellular vesicles (nEVs) may therefore serve as potential biomarkers for predicting cognitive decline with age and Alzheimer’s disease based on relative size and nEV cargo (Kapogiannis et al., 2019). Here again, Mark Mattson engaged in collaborative studies demonstrating that nEVs in serum from older patients with decreased walking speed have increased levels of the precursor (pro) form of brain-derived neurotrophic factor (BDNF), compared to those patients with faster walking speed (Suire et al., 2017). Given that pro-BDNF exerts distinct and opposing effects on NMJ synaptogenesis, relative to mature BDNF (Yang et al, 2009), it is possible that pro-BDNF-containing nEVs might promote age-related muscle wasting. The mechanisms modulating nEV release with age or with exercise are not well known, but future studies directed at better understanding these processes will have important implications for improving healthspan and physical function with aging.
3.6. Insulin and insulin-like growth factors in aging and exercise
Aging is accompanied by reductions in insulin sensitivity and systemic glucose metabolism (Defronzo, 1981). Even within the normal physiological range, variability in systemic glucose metabolism has been correlated with working memory, such that individuals with more efficient glycemic control perform better on cognitive tests (Rolandsson et al, 2008). Here again, Mark Mattson’s work on insulin-like growth factor (IGF) signaling advanced understanding of age-related neurobiological changes (Figure 1). After observing a pivotal role for neuronal calcium handling in response to hypoglycemia in primary neurons (Cheng et al, 1991), members of the Mattson lab determined that application of insulin or insulin-like growth factors could block hypoglycemia-associated calcium dyshomeostasis (Cheng and Mattson, 1992b). This line of investigation revealed that IGF-2 stabilized mitochondrial function without preventing ATP depletion (Mattson et al, 1993b), consistent with an increase in the dynamic range of calcium buffering under conditions of metabolic stress.
At the whole-organism level, lower circulating glucose and insulin levels reflect more efficient glycemic control. Mark Mattson’s in vitro studies were subsequently upheld by in vivo experiments demonstrating protection against excitotoxicity with dietary restriction (Anson et al, 2003). These studies also incorporated intermittent fasting (IF), using a paradigm in which food was available every other day (Anson et al, 2003). Under this feeding schedule, total caloric intake over a 48hr period was unchanged, relative to mice consuming chow ad libitum (Anson et al, 2003). The observation that IF and caloric restriction elicited similar protection against excitotoxicity and comparable metabolic improvements provided proof of principle for the concept of metabolic preconditioning (Anson et al, 2003).
Mark Mattson’s conceptual framework for metabolic preconditioning incorporated the deleterious effects of high-fat diet and the protective effects of dietary restriction and exercise. These effects were shown to be sexually dimorphic in rats, where six months of dietary restriction enhanced cognitive performance in females, but not in males (Martin et al, 2007). High-fat diet-induced obesity had no effect on cognitive performance in males or females (Martin et al, 2007), but the 14-unit T-maze task used to assess cognition recruits different structures and circuits than classical hippocampus-dependent paradigms. Specifically, the 14-unit T-maze is sensitive to both striatal and hippocampal lesions (Jucker et al, 1990; Pistell et al, 2009), while spatial memory in the water maze is unaffected by striatal lesions (Mcdonald and White, 1994; Devan et al, 1996). Given that sex differences in recruitment of hippocampal and striatal circuits during spatial memory acquisition have yet to be fully characterized, it is possible that sexually dimorphic regulation of learning and memory by diet might be circuit-specific. This possibility is indirectly supported by the convergent regulation of hippocampal gene expression in males and females maintained on a high-fat diet (Martin et al, 2008), and by the observation that middle-aged male rats maintained on high-fat diet for longer durations exhibit memory deficits in the water maze (Stranahan et al, 2008b). Conversely, aged mice housed with a running wheel from weaning exhibit improved memory in the water maze, relative to aged sedentary mice (Stranahan et al, 2010). These datasets suggest that hippocampus-dependent tasks may be more sensitive to metabolic challenges than hippocampus- and striatum-dependent paradigms, but this possibility has yet to be directly addressed.
Mark Mattson’s laboratory was among the first to use large-scale trancriptional profiling to investigate responses to energetic challenges in different brain regions. The AGEMAP (Atlas of Gene Expression in Mouse Aging Project) gene expression database included a set of anatomically comprehensive transcriptional profiling studies in males and females maintained on caloric restriction or ad libitum diets (Xu et al, 2007). The results of these studies are consistent with circuit-specific responses to energy restriction, based on more prominent transcriptional responses to aging and CR in the hippocampus and cortex, relative to striatum (Xu et al, 2007). As a followup to the AGEMAP studies, members of the Mattson lab compared transcriptional responses to water maze learning in the hippocampus of aged male mice after lifelong running (Figure 2; Stranahan et al, 2010). These studies included mice sacrificed under basal conditions, after swimming in the water maze, or after hidden platform training (Stranahan et al, 2010). Side-by-side comparison of the two studies revealed limited overlap between the genes and pathways differentially activated at baseline in the hippocampus of aged runners, relative to the transcriptional effects of caloric restriction in the hippocampus of aged mice from the AGEMAP study (Xu et al, 2007; Stranahan et al, 2010; Figure 2). The lack of overlap between the two datasets could reflect distinct metabolic programs associated with energy expenditure in runners, relative to cellular adaptations in response to reduced energy intake in mice on CR. However, the possibility of divergent transcriptional programs associated with fluctuations in caloric intake and energy expenditure remains speculative at this point. It would certainly be beneficial to revisit these themes using newer methods that capture cellular diversity, such as single-cell RNA-seq and multiplexed FISH (Ren et al, 2019; Kishi et al, 2019), but these gene array studies upheld the principles of Dr. Mattson’s early in vitro studies using large-scale in vivo approaches.
Figure 2. Distinct transcriptional programs associated with diet and exercise in the aging hippocampus.
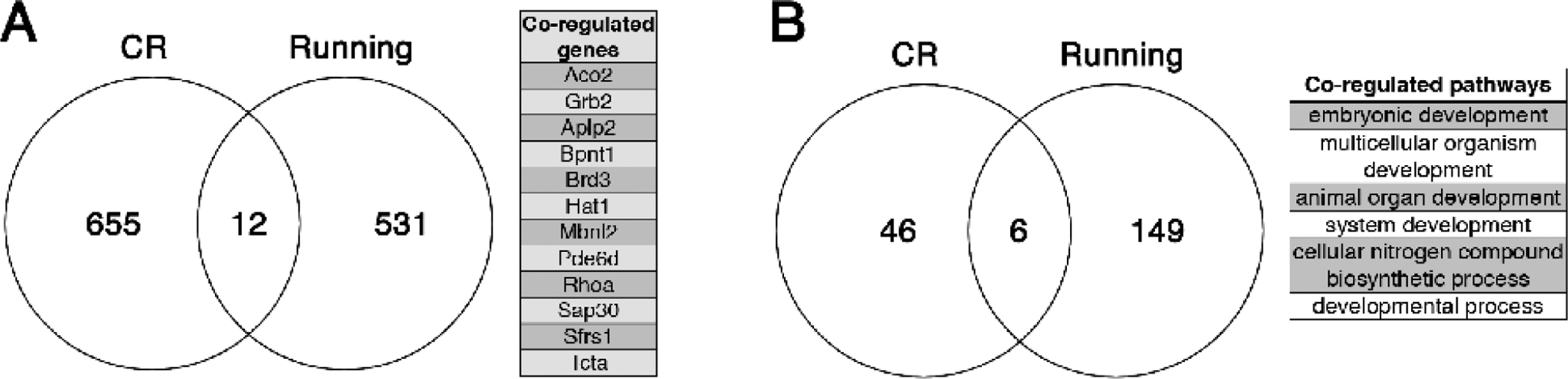
Mining the data from Mattson lab publications investigating the impact of lifelong caloric restriction (CR) or wheel running uncovered potential evidence of distinct transcriptional programs for energy restriction and expenditure in the aged hippocampus (Xu et al, 2007; Stranahan et al, 2010). Comparing hippocampal gene expression profiles following lifelong caloric restriction in 16-month-old mice (Xu et al, 2007) with those observed after lifelong wheel running in 18–20-month-old mice (Stranahan et al, 2010) revealed limited overlap at the level of individual genes (A) and Biological Processes associated with the corresponding Gene Ontology terms (B). Mark Mattson’s unique approach incorporated both breadth and depth to uncover these and other insights into metabolic regulation of brain aging.
4. From primary neurons to human studies: evolution of Mark Mattson’s research program
Not one to rest on his laurels, Mark Mattson subsequently began testing the translatability of these findings in human studies. These studies revealed improvements in glucose and lipid metabolism, but cognitive outcomes have yet to be evaluated (Catenacci et al, 2016; Harvie et al, 2013; Harvie et al, 2011). In young overweight females, six months of intermittent fasting or caloric restriction reduced circulating levels of leptin and C-reactive protein, markers of adiposity and obesity-induced inflammation, respectively (Harvie et al, 2011). Interestingly, alternate day feeding exerted more robust effects on glycemic control in humans (Harvie et al, 2011; Harvie et al, 2013), suggesting that pronounced temporal fluctuations in nutrient availability might have a greater impact than restricting overall calories.
The granularity of Dr. Mattson’s work on dietary regulation of brain function in rodents has yet to be scaled up into human studies, and questions remain surrounding the impact of caloric intake, meal timing, and nutrient composition on brain function in genetically heterogeneous species. One step toward this future direction involves studying dietary regulation of neuroplasticity in outbred rodents, such as Diversity Outbred mice (Svenson et al, 2012) and outbred rat strains (Nadon, 2005). Using outbred rodents to study dietary regulation of brain structure and function could enable subsequent identification of homologous relationships in existing genome-wide association datasets from humans, which would in turn provide a rationale for hypothesis-driven studies using human organoids or induced pluripotent stem cells.
Translation of exercise interventions in humans lags behind studies of diet due to difficulties with adherence and standardization of exercise protocols across studies (Neufer et al, 2015). However, recent work from the Mattson laboratory has identified novel pathways through which energy intake influences exercise capacity (Marosi et al, 2018). Severe, prolonged undernutrition is associated with hypoactivity and muscle wasting (Fichter et al, 1986; Felig et al, 1970), but intermittent fasting enhances exercise endurance (Marosi et al, 2018). Data on dose-response relationships between exercise and neuroplasticity are scarce, but at the lower end of the scale, significant benefits have been reported following the transition from no exercise to minimal exercise in aged rodents and in rodent models of obesity (Barrientos et al, 2011; McGee-Lawrence et al, 2017; Erion et al, 2014). The challenge for future work in this area is to identify the dynamic range for synergistic interactions between energy intake and exercise capacity, and to determine whether these interactions differ over the lifespan.
Over the course of his career, Dr. Mattson led elegant mechanistic studies in c.elegans, primary neurons, rodent models, and humans. This trajectory recapitulates brain evolution within a timeframe of decades and has identified numerous converging mechanisms for metabolic regulation of neuronal cell biology. As Mark Mattson retires from the National Institute on Aging and transitions into his new role in medical education and scientific communication at Johns Hopkins School of Medicine, we wish Mark Mattson the best in the next phase of his highly prolific scientific career.
Supplementary Material
Highlights.
Exercise and diet are critical determinants of healthy aging
Mark Mattson’s research program identified growth factors, mitochondrial biogenesis, and lipid metabolism as pivot points for maintaining brain plasticity with aging
Attentuation of age-related muscle loss may be a converging mechanism for exercise-induced extension of healthspan
Acknowledgements:
These studies were supported by grants from the National Institutes of Health to A.M.S. (R01DK110586) and M.W.H. (P01AG036675).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre LE, Villareal DT. Physical Exercise as Therapy for Frailty. Nestle Nutr Inst Workshop Ser. 2015;83:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS. The ageing brain: normal and abnormal memory. Philos Trans R Soc Lond B Biol Sci. 1997. Nov 29;352(1362):1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966. Mar;126(3):337–89. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic examination of the effects of enriched environment on the rate of glial multiplication in the adult rat brain. Nature. 1964. Dec 19;204:1161–3. [DOI] [PubMed] [Google Scholar]
- Altman J The discovery of adult mammalian neurogenesis. Published in Neurogenesis in the Adult Brain I: Neurobiology (2011) Springer. Seki T, Sawamoto K, Parent JM, and Alvarez-Buylla A (Eds). [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011. Apr;29(4):341–5. [DOI] [PubMed] [Google Scholar]
- Andrew RJ, Kellett KA, Thinakaran G, Hooper NM. A Greek Tragedy: The Growing Complexity of Alzheimer Amyloid Precursor Protein Proteolysis. J Biol Chem. 2016. Sep 9;291(37):19235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003. May 13;100(10):6216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010. Jan;67(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002. Dec;16(14):1879–86. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011. Aug 10;31(32):11578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman N, Bherer L, Nadeau S et al. (2013) Executive functions, physical fitness and mobility in well-functioning older adults. Exp Gerontol. 48(12):1402–9. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990. Jul;87(14):5568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc EM, Keller JN, Fernandez S, Mattson MP. 4-hydroxynonenal, a lipid peroxidation product, impairs glutamate transport in cortical astrocytes. Glia. 1998. Feb;22(2):149–60. [DOI] [PubMed] [Google Scholar]
- Ble A, Volpato S, Zuliani G, Guralnik JM, Bandinelli S, Lauretani F, Bartali B, Maraldi C, Fellin R, Ferrucci L. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005. Mar;53(3):410–5. [DOI] [PubMed] [Google Scholar]
- Brody H Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955. Apr;102(2):511–6. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999. Jan;45(1):8–15. [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995. Jun;15(6):4687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, Martin B, MacLean PS, Melanson EL, Troy Donahoo W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity (Silver Spring). 2016. Sep;24(9):1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012. Oct 18;490(7420):355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Arumugam TV, Liu D, Khatri RG, Mustafa K, Kwak S, Ling HP, Gonzales C, Xin O, Jo DG, Guo Z, Mark RJ, Mattson MP. Pancortin-2 interacts with WAVE1 and Bcl-xL in a mitochondria-associated protein complex that mediates ischemic neuronal death. J Neurosci. 2007. Feb 14;27(7):1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wang J, Ghena N, Zhao Q, Perone I, King TM, Veech RL, Gorospe M, Wan R, Mattson MP. SIRT3 Haploinsufficiency Aggravates Loss of GABAergic Interneurons and Neuronal Network Hyperexcitability in an Alzheimer’s Disease Model. J Neurosci. 2020. Jan 15;40(3):694–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016. Jan 12;23(1):128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. Glucose deprivation elicits neurofibrillary tangle-like antigenic changes in hippocampal neurons: prevention by NGF and bFGF. Exp Neurol. 1992. Aug;117(2):114–23. [DOI] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. IGF-I and IGF-II protect cultured hippocampal and septal neurons against calcium-mediated hypoglycemic damage. J Neurosci. 1992. Apr;12(4):1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. NGF and bFGF protect rat hippocampal and human cortical neurons against hypoglycemic damage by stabilizing calcium homeostasis. Neuron. 1991. Dec;7(6):1031–41. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004. Feb 17;101(7):2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981. Jul-Aug;4(4):493–501. [DOI] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem. 1996. Nov;66(3):305–23. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Andrews ZB, Horvath TL. Exercise-induced synaptogenesis in the hippocampus is dependent on UCP2-regulated mitochondrial adaptation. J Neurosci. 2008. Oct 15;28(42):10766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkins MB, Wang G, Bieberich E. Sphingolipid-Enriched Extracellular Vesicles and Alzheimer’s Disease: A Decade of Research. J Alzheimers Dis. 2017;60(3):757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001. Jan;76(2):619–26. [DOI] [PubMed] [Google Scholar]
- Dunckley T, Beach TG, Ramsey KE, Grover A, Mastroeni D, Walker DG, LaFleur BJ, Coon KD, Brown KM, Caselli R, Kukull W, Higdon R, McKeel D, Morris JC, Hulette C, Schmechel D, Reiman EM, Rogers J, Stephan DA. Gene expression correlates of neurofibrillary tangles in Alzheimer’s disease. Neurobiol Aging. 2006. Oct;27(10):1359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Green J, Bodogai M, Mode NA, Bæk R, Jørgensen MM, Freeman DW, Witwer KW, Zonderman AB, Biragyn A, Mattson MP, Noren Hooten N, Evans MK. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Sci Rep. 2017. May 2;7(1):1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina N, Tabet N, Rusted J. (2016) The relationship between habitual physical activity status and executive function in individuals with Alzheimer’s disease: a longitudinal, cross-lagged panel analysis. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 23:234–52. [DOI] [PubMed] [Google Scholar]
- Felig P, Pozefsky T, Marliss E, Cahill GF Jr. Alanine: key role in gluconeogenesis. Science. 1970. Feb 13;167(3920):1003–4. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976. Apr;73(4):1279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichter MM, Pirke KM, Holsboer F. Weight loss causes neuroendocrine disturbances: experimental study in healthy starving subjects. Psychiatry Res. 1986. Jan;17(1):61–72. [DOI] [PubMed] [Google Scholar]
- Freeman DW, Noren Hooten N, Eitan E, Green J, Mode NA, Bodogai M, Zhang Y, Lehrmann E, Zonderman AB, Biragyn A, Egan J, Becker KG, Mattson MP, Ejiogu N, Evans MK. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes. 2018. Nov;67(11):2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeis C, Helmig S, Tug S, Simon P, Krämer-Albers EM.(2015) Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015 Jul 2;4:28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele S, Mendhe B, Khayrullin A, Johnson M, Kaiser H, Liu Y, Isales CM, Hamrick MW. Muscle-derived miR-34a increases with age in circulating extracellular vesicles and induces senescence of bone marrow stem cells. Aging (Albany NY). 2019. Mar 25;11(6):1791–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Biedermann SV, Falfán-Melgoza C, Auer MK, Zheng L, Steinle J, Hörner F, Sartorius A, Ende G, Weber-Fahr W, Gass P. Exercise boosts hippocampal volume by preventing early age-related gray matter loss. Hippocampus. 2014. Feb;24(2):131–4. [DOI] [PubMed] [Google Scholar]
- Garcia S, Nissanka N, Marenco E, Rossi S, Peralta S, Diaz F, Rotundo R, Carvalh R, Moraes C. Overexpression of PGC-1 alpha in aging muscle enhances a subset of young-like molecular patterns. Aging Cell 2018, 17e12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992. Sep;12(9):3642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guescini M, Canonico B, Lucertini F, Maggio S, Annibalini G, Barbieri E, Luchetti F, Papa S, Stocchi V. (2015) Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS One. 2015 May 8;10(5):e0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci U S A. 1998. Mar 17;95(6):3227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Sebastian L, Sopher BL, Miller MW, Glazner GW, Ware CB, Martin GM, Mattson MP. Neurotrophic factors [activity-dependent neurotrophic factor (ADNF) and basic fibroblast growth factor (bFGF)] interrupt excitotoxic neurodegenerative cascades promoted by a PS1 mutation. Proc Natl Acad Sci U S A. 1999. Mar 30;96(7):4125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Li P, Gao L, Zhang J, Yang Z, Bledsoe G, Chang E, Chao L, Chao J. Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell. 2017. Aug;16(4):837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Jiang H, Xu X, Duan W, Mattson MP. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem. 2008. Jan 18;283(3):1754–63. [DOI] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996. Dec 13;274(5294):1855–9. [DOI] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011. May;35(5):714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP, Howell A. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013. Oct;110(8):1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004. Feb;55(2):257–67. [DOI] [PubMed] [Google Scholar]
- Hebb DO. (1949) The Organization of Behavior. New York:John Wiley and Sons. [Google Scholar]
- Hoogendijk EO, Rijnhart JJM, Skoog J, Robitaille A, van den Hout A, Ferrucci L, Huisman M, Skoog I, Piccinin AM, Hofer SM, Muniz Terrera G. Gait speed as predictor of transition into cognitive impairment: Findings from three longitudinal studies on aging. Exp Gerontol. 2020. Jan;129:110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A. 2006. Dec 26;103(52):19908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M, Kametani H, Bresnahan EL, Ingram DK. Parietal cortex lesions do not impair retention performance of rats in a 14-unit T-maze unless hippocampal damage is present. Physiol Behav. 1990. Jan;47(1):207–12. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mustapic M, Shardell MD, et al. Association of Extracellular Vesicle Biomarkers With Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2019;76(11):1340–1351. doi: 10.1001/jamaneurol.2019.2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998. Jan 15;18(2):687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997b. Oct;80(3):685–96. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997a. Jul;69(1):273–84. [DOI] [PubMed] [Google Scholar]
- Khayrullin A, Krishnan P, Martinez-Nater L, Mendhe B, Fulzele S, Liu Y, Mattison JA, Hamrick MW. 2019. Very Long-Chain C24:1 Ceramide Is Increased in Serum Extracellular Vesicles with Aging and Can Induce Senescence in Bone-Derived Mesenchymal Stem Cells. Cells. 2019 Jan 10;8(1). pii: E37. doi: 10.3390/cells8010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi JY, Lapan SW, Beliveau BJ, West ER, Zhu A, Sasaki HM, Saka SK, Wang Y, Cepko CL, Yin P. SABER amplifies FISH: enhanced multiplexed imaging of RNA and DNA in cells and tissues. Nat Methods. 2019. Jun;16(6):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003. Feb 20;421(6925):844–8. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011. Aug 30;18(9):605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer IF, Snijders T, Smeets JSJ, Leenders M, van Kranenburg J, den Hoed M, Verdijk LB, Poeze M, van Loon LJC. Extensive Type II Muscle Fiber Atrophy in Elderly Female Hip Fracture Patients. J Gerontol A Biol Sci Med Sci. 2017. Oct 1;72(10):1369–1375. [DOI] [PubMed] [Google Scholar]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002. Mar 1;22(5):1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin KM, Roberts BM, Fry CS, Moro T, Rasmussen BB, Bamman MM. The Importance of Resistance Exercise Training to Combat Neuromuscular Aging. Physiology. 2019, 34(2):112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law TD, Clark LA, Clark BC. Resistance Exercise to Prevent and Manage Sarcopenia and Dynapenia. Annu Rev Gerontol Geriatr. 2016;36(1):205–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002. Feb;80(3):539–47. [DOI] [PubMed] [Google Scholar]
- Leenders M, Verdijk LB, van der Hoeven L, van Kranenburg J, Nilwik R, van Loon LJ. Elderly men and women benefit equally from prolonged resistance-type exercise training. J Gerontol A Biol Sci Med Sci. 2013. 68(7):769–79. [DOI] [PubMed] [Google Scholar]
- Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, Tan A, Flaherty M, Miura P, Dirksen RT, Chakkalakal JV. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife. 2017;6. pii: e26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D, Saito T, Saido T, Zhu J, Wu LJ, Mattson MP. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun. 2019. Apr 23;10(1):1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco MV, Frozza RL, de Freitas GB, Zhang H, Kincheski GC, Ribeiro FC, Gonçalves RA, Clarke JR, Beckman D, Staniszewski A, Berman H, Guerra LA, Forny-Germano L, Meier S, Wilcock DM, de Souza JM, Alves-Leon S, Prado VF, Prado MAM, Abisambra JF, Tovar-Moll F, Mattos P, Arancio O, Ferreira ST, De Felice FG. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019. Jan;25(1):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Lacaille JC. Interneuron Diversity series: Hippocampal interneuron classifications--making things as simple as possible, not simpler. Trends Neurosci. 2003. Oct;26(10):564–71. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Keller JN, Kruman I, Mattson MP. Basic FGF attenuates amyloid beta-peptide-induced oxidative stress, mitochondrial dysfunction, and impairment of Na+/K+-ATPase activity in hippocampal neurons. Brain Res. 1997. May 9;756(1–2):205–14. [DOI] [PubMed] [Google Scholar]
- Martin B, Pearson M, Brenneman R, Golden E, Keselman A, Iyun T, Carlson OD, Egan JM, Becker KG, Wood W 3rd, Prabhu V, de Cabo R, Maudsley S, Mattson MP. Conserved and differential effects of dietary energy intake on the hippocampal transcriptomes of females and males. PLoS One. 2008. Jun 11;3(6):e2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007. Sep;148(9):4318–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Moehl K, Navas-Enamorado I, Mitchell SJ, Zhang Y, et al. Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation. FASEB J. 2018. Jul;32(7):3844–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017. Jan 17;8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Guthrie PB, Hayes BC, Kater SB. Roles for mitotic history in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci. 1989a. Apr;9(4):1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Kater SB. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989. Jun 19;490(1):110–25. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Murrain M, Guthrie PB, Kater SB. Fibroblast growth factor and glutamate: opposing roles in the generation and degeneration of hippocampal neuroarchitecture. J Neurosci. 1989b. Nov;9(11):3728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Rychlik B, Chu C, Christakos S. Evidence for calcium-reducing and excitoprotective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron. 1991. Jan;6(1):41–51. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Tomaselli KJ, Rydel RE. Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res. 1993a. Sep 3;621(1):35–49. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Zhang Y, Bose S. Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis, and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Exp Neurol. 1993b. May;121(1):1–13. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990. Jan;4(1):105–17. [DOI] [PubMed] [Google Scholar]
- McAvoy K, Kawamata H. Glial mitochondrial function and dysfunction in health and neurodegeneration. Mol Cell Neurosci. 2019. Dec;101:103417. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994. May;61(3):260–70. [DOI] [PubMed] [Google Scholar]
- McGee-Lawrence ME, Wenger KH, Misra S, Davis CL, Pollock NK, et al. (2017) Whole-Body Vibration Mimics the Metabolic Effects of Exercise in Male Leptin Receptor-Deficient Mice. Endocrinology. 158(5):1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012. May 2;15(5):665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Burón MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, de Cabo R. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016. Jun 14;23(6):1093–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi M, Taub DD, Kapogiannis D, Eitan E, Zukley L, Mattson MP, Ferrucci L, Schwartz JB, Goetzl EJ. Aging enhances release of exosomal cytokine mRNAs by Aβ1–42-stimulated macrophages. FASEB J. 2013. Dec;27(12):5141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016. Aug 9;24(2):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997. Oct 17;278(5337):412–9. [DOI] [PubMed] [Google Scholar]
- Mustapic M, Eitan E, Werner JK Jr, et al. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front Neurosci. 2017;11:278. Published 2017 May 22. doi: 10.3389/fnins.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadon NL. 2005. Gerontology and Age-Associated Lesions, in: Suckow M, Weisbroth S, Franklin C, Franklin CL (Eds), The Laboratory Rat, 2nd ed. Elsevier, Amsterdam, pp.761–772. [Google Scholar]
- Nauer RK, Dunne MF, Stern CE, Storer TW, Schon K. Improving fitness increases dentate gyrus/CA3 volume in the hippocampal head and enhances memory in young adults. Hippocampus. 2019. Oct 7. doi: 10.1002/hipo.23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995. Jan 12;373(6510):109. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015. Jul 7;22(1):4–11. [DOI] [PubMed] [Google Scholar]
- Osborne TB, Mendel LB, Ferry EL. The effect of retardation of growth upon the breeding period and duration of life of rats. Science. 1917. Mar 23;45(1160):294–5. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc. 1988. Apr;150(Pt 1):1–20. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007. Sep 6;55(5):697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Hui X, Hoo RLC, Ye D, Chan CYC, Feng T, Wang Y, Lam KSL, Xu A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019. Feb 1;129(2):834–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Rieker C, Fuchs S, Bleckmann D, Esposito MS, Botta P, Goldstein C, Bernhard M, Galimberti I, Müller M, Lüthi A, Arber S, Bouwmeester T, van der Putten H, Di Giorgio FP. Transneuronal propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat Neurosci. 2014. Aug;17(8):1064–72. doi: 10.1038/nn.3761. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019. Jul 15; 15(7):383–392. [DOI] [PubMed] [Google Scholar]
- Perrotte M, Haddad M, Le Page A, Frost EH, Fulöp T, Ramassamy C. Profile of pathogenic proteins in total circulating extracellular vesicles in mild cognitive impairment and during the progression of Alzheimer’s disease. Neurobiol Aging. 2020. Feb;86:102–111. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003. Feb 1;546(Pt 3):851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell PJ, Nelson CM, Miller MG, Spangler EL, Ingram DK, Devan BD. Striatal lesions interfere with acquisition of a complex maze task in rats. Behav Brain Res. 2009. Jan 30;197(1):138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J Neurovirol. 2019. Oct;25(5):702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010. Oct;120(10):3421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Isakova A, Friedmann D, Zeng J, Grutzner SM, Pun A, Zhao GQ, Kolluru SS, Wang R, Lin R, Li P, Li A, Raymond JL, Luo Q, Luo M, Quake SR, Luo L. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife. 2019. Oct 24;8. pii: e49424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder K, Keller S, Dams M et al. (2014) Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 12(6):e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolandsson O, Backeström A, Eriksson S, Hallmans G, Nilsson LG. Increased glucose levels are associated with episodic memory in nondiabetic women. Diabetes. 2008. Feb;57(2):440–3. [DOI] [PubMed] [Google Scholar]
- Savina A, Furlán M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003. May 30;278(22):20083–90. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006. Oct;27(10):1372–84. [DOI] [PubMed] [Google Scholar]
- Scherder EJ, Eggermont LH, Geuze RH, Vis J, Verkerke GJ. (2010) Quadriceps strength and executive functions in older women. Am J Phys Med Rehabil. 8:458–63. [DOI] [PubMed] [Google Scholar]
- Shefer VF. Absolute number of neurons and thickness of the cerebral cortex during aging, senile and vascular dementia, and Pick’s and Alzheimer’s diseases. Neurosci Behav Physiol. 1973. Oct-Dec;6(4):319–24. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012. Jan 5;13(2):77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995. Oct;15(10):6651–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018. Mar 15;555(7696):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Cutler RG, Button C, Telljohann R, Mattson MP. Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. J Neurochem. 2011. Aug;118(4):611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010. Nov;31(11):1937–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009. Oct;19(10):951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008a. Mar;11(3):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008b;18(11):1085–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006. Apr;9(4):526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suire CN, Eitan E, Shaffer NC, Tian Q, Studenski S, Mattson MP, Kapogiannis D. (2017) Walking speed decline in older adults is associated with elevated pro-BDNF in plasma extracellular vesicles. Exp Gerontol. 2017 Nov;98:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017. Jan 20;18(2):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics. 2012. Feb; 190(2):437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetsch T, Valdez G. NMJ maintenance and repair in aging. Curr Opinion Physiol 2018, 4:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017. Feb 23;542(7642):450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999. Mar;2(3):266–70. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005. Sep 21;25(38):8680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma VR, Chuang YF, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2015. May;25(5):605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009. 64(3):332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972. Jun 30;176(4042):1445–7. [DOI] [PubMed] [Google Scholar]
- Vossel KA, Ranasinghe KG, Beagle AJ, Mizuiri D, Honma SM, Dowling AF, Darwish SM, Van Berlo V, Barnes DE, Mantle M, Karydas AM, Coppola G, Roberson ED, Miller BL, Garcia PA, Kirsch HE, Mucke L, Nagarajan SS. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann Neurol. 2016. Dec;80(6):858–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gao J, Liu J, Siedlak SL, Torres S, Fujioka H, Huntley ML, Jiang Y, Ji H, Yan T, Harland M, Termsarasab P, Zeng S, Jiang Z, Liang J, Perry G, Hoppel C, Zhang C, Li H, Wang X. Mitofusin 2 Regulates Axonal Transport of Calpastatin to Prevent Neuromuscular Synaptic Elimination in Skeletal Muscles. Cell Metab. 2018. Sep 4;28(3):400–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018. Jan 9;27(1):237–251.e4. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Grosche J, Härtig W. Co-localization of calretinin and calbindin in distinct cells in the hippocampal formation of the rat. Brain Res. 2001. Dec 20;922(2):310–4. [DOI] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013. Nov 5;18(5):649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhan M, Duan W, Prabhu V, Brenneman R, Wood W, Firman J, Li H, Zhang P, Ibe C, Zonderman AB, Longo DL, Poosala S, Becker KG, Mattson MP. Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol. 2007;8(11):R234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008. Sep 9;105(36):13421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol. 2009. May 18;185(4):727–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM. (1925) Almost human: A study of primate intelligence and emotions. The Century Co.; First Edition. [Google Scholar]
- Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017. Oct 5;171(2):372–384.e12. [DOI] [PubMed] [Google Scholar]
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012. Aug 31;337(6098):1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyzak DR, Otto T, Eichenbaum H, Gallagher M. Cognitive decline associated with normal aging in rats: a neuropsychological approach. Learn Mem. 1995. Jan-Feb;2(1):1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


