Abstract
Introduction
Kligman’s formula is a topical triple combination consisting of hydroquinone, tretinoin, and topical corticosteroid. It has recently become widely popular among the general population for different purposes. Its improper use can lead to unsatisfactory results and unpleasant side effects.
Aim
This study aimed to assess the attitude, satisfaction, and complications related to topical usage of Kligman’s formula among the general population in Saudi Arabia.
Materials and methods
A cross-sectional study was conducted among the general population of Saudi Arabia. A self-administered questionnaire was distributed among the targeted population using an online survey. The questionnaire includes socio-demographic characteristics, assessment of attitude, and satisfaction in using Kligman’s formula. Data were tabulated and cleaned, and all statistical analyses were performed.
Results
A total of 292 participants met the inclusion criteria (26 males vs. 266 females) with a mean age of 26.9 (SD 7.71) years. Nearly 40% of participants showed a positive attitude in using Kligman’s formula, while 46.9% were satisfied with using it. The most common reason for using Kligman’s formula was to lighten the skin (55.8%), while skin redness was the most commonly reported adverse effect. Factors associated with increased attitude and satisfaction were using Kligman’s formula based on a doctor’s prescription and regular follow-up with a dermatologist.
Conclusion
The general population showed an improper attitude toward using Kligman's formula. However, a better attitude and satisfaction rate can be seen among those using Kligman's formula with prescription and those who regularly visit a dermatologist.
Keywords: skin lightening, acne, satisfaction, attitude, kligman’s formula
Introduction
Components of the widely known Kligman's formula are 0.1% tretinoin, 0.1% dexamethasone, 5.0% hydroquinone, and hydrophilic ointment. Kligman’s formula is an effective treatment option in treating many dermatological conditions such as melasma and postinflammatory hyperpigmentation [1]. Both postinflammatory hyperpigmentation and melasma are relatively common in Saudi Arabia since there are a high number of people in Saudi Arabia with brown skin (Fitzpatrick skin type III-V) [2,3].
Using skin-lightening products has become highly prevalent among the general population worldwide [4]. In Saudi Arabia, it has shown a prevalence of 56.2% [5]. Among many skin lightening techniques, Kligman's formula is one of the first chosen options, reflecting widespread usage. Other indications of Kligman's formula include dyspigmentation, getting rid of scars, and skin peeling/exfoliation [4,5].
Kligman's formula has been associated with many adverse events (AEs). Most common are erythema, desquamation, burning sensation, and steroid-induced telangiectasia. Less common AEs include acne/acne breakouts, hyperpigmentation, pruritus, skin atrophy, perioral dermatitis, and hypertrichosis [6-8].
This study aims to assess the attitude toward using Kligman’s formula and related outcomes among the general population in Saudi Arabia.
Materials and methods
This is a cross-sectional study conducted among the general population of Saudi Arabia to assess the attitude toward using Kligman’s formula and related outcomes. A total of 292 participants were included in the study. A self-administered questionnaire was distributed among the targeted population using an online survey. The questionnaire includes socio-demographic characteristics, assessment of attitude, and satisfaction in using Kligman’s formula. Any individuals who are using Kligman's formula or have been using Kligman's formula among Saudi populations were included in this study. Incomplete questionnaires and participants who did not use Kligman's formula were excluded. Data were tabulated and cleaned in Microsoft Excel (Microsoft Corporation, Redmond, WA).
Statistical analysis
Data management and analysis were carried out using the Statistical Package for Social Sciences (SPSS) version 26 (IBM Corp, Armonk, NY). Descriptive statistics (mean, standard deviation, frequencies, and percentages) were used to quantify continuous and categorical study variables. A chi-square test was used to compare the level of attitude and satisfaction related to study variables. A p-value of <0.05 was considered statistically significant.
Ethical approval
All procedures that involved human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards. The ethical approval was obtained from King Faisal University (reference number: KFU-REC-2021-OCT-EA00032).
Results
A total of 292 respondents have been included in the study with a mean age of 26.9 years. The majority of the respondents were female (91.1%) and Saudi nationals (97.3%). The majority were single (68.5%) and had bachelor's degrees (71.9%). Students (46.2%) and unemployed (30.5%) constituted most participants. Only 12.3% worked in the healthcare sector, and 8.6% were doctors.
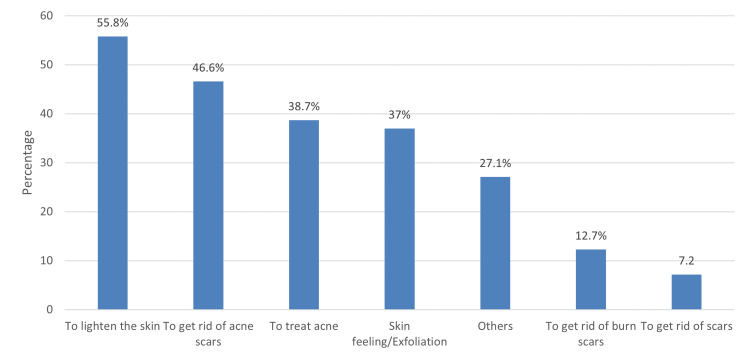
The usage of Kligman’s formula among participants is referred to different aims (Figure 1), and skin lightening represents the most common one. However, the most common sources of information for using Kligman’s formula without a doctor's prescription were electronic social network sites (68.2%), followed by friends (22.9%) and websites (20.2%). At the same time, television/radio was the least (0.4%).
Figure 1. Reasons for using Kligman's formula.
Of the participants, 38% estimated having a positive attitude in using Kligman’s formula, while 23.6% reported using Kligman’s formula according to doctor’s prescription. The prevalence of respondents who regularly visit dermatologists to guide them regarding Kligman’s formula was 17.1%. More than two-thirds (67.5%) were mixing the ingredients of Kligman’s formula by themselves. Of those self-preparing Kligman’s formula, 74.6% attempted to divide evenly without using any device. The proportion of respondents who bought a ready-made Kligman’s formula was 35.6%, while the proportion of respondents who experienced side effects due to Kligman’s formula was 50% (Table 1).
Table 1. Assessment of attitude toward the use of Kligman's formula (n = 292).
| Study variables | N (%) |
| Current attitude of using Kligman's formula | |
| Positive | 111 (38.0%) |
| Negative | 181 (62.0%) |
| Currently using Kligman's formula based on doctor’s prescription | |
| Yes | 69 (23.6%) |
| No | 223 (76.4%) |
| Do you follow up regularly with your dermatologist to see how you use Kligman's formula? | |
| Yes | 50 (17.1%) |
| No | 242 (82.9%) |
| How often do you use Kligman's formula? | |
| Daily | 114 (39.0%) |
| Every 2 days | 71 (24.3%) |
| Every 3 days | 54 (18.5%) |
| Every 2 weeks | 23 (07.9%) |
| Every month | 18 (06.2%) |
| Once in a while | 12 (04.1%) |
| Timing of use for Kligman's formula | |
| In the morning | 19 (06.5%) |
| In the evening | 235 (80.5%) |
| In the morning and evening | 38 (13.0%) |
| Duration of Kligman's formula use | |
| Less than a month | 102 (34.9%) |
| 1-2 months | 81 (27.7%) |
| 2-3 months | 46 (15.8%) |
| 3-6 months | 22 (07.5%) |
| 6-9 months | 04 (01.4%) |
| 9-12 months | 05 (01.7%) |
| More than a year | 32 (11.0%) |
| Did you collect and mix the ingredients for Kligman's formula yourself? | |
| Yes | 197 (67.5%) |
| No | 95 (32.5%) |
| How did you divide each product (what is the percentage of that product)? (n = 197) | |
| Tried to divide it evenly using the scale | 22 (11.2%) |
| Tried to divide it evenly without using the scale and without any device | 147 (74.6%) |
| Divide them differently | 28 (14.2%) |
| Do you buy Kligman's formula ready-made? | |
| Yes | 104 (35.6%) |
| No | 188 (64.4%) |
| Experience any side effects after using Kligman's formula? | |
| Yes | 146 (50.0%) |
| No | 146 (50.0%) |
| Have you noticed any sensitivity in the skin of the face when exposed to the sun? | |
| Yes | 13 (04.5%) |
| No | 279 (95.5%) |
Respondents cited face as the most common body part where Kligman’s formula was applied (92.1%), followed by hand (14.7%) and knees (14.7%). It was further observed that the most frequently used ingredients were hydroquinone cream (Hiquin®) (64.5%), followed by tretinoin cream (Acretin®) (57.4%), and adapalene cream (Differin®) (49.2%). Moreover, the most commonly mentioned side effect of Kligman’s formula was skin redness (61.6%), followed by a burning sensation (50.7%) (Figure 2).
Figure 2. Side effects after using Kligman’s formula.
The overall mean satisfaction score was 6.02 (SD 2.89) out of 10 points with 53.1%, and 46.9% were classified into dissatisfied and satisfied, respectively. We also noted that 32.5% were still using Kligman’s formula even after obtaining a satisfactory result. After stopping from using Kligman’s formula, 44.9% expressed that they did not use anything, while 21.6% used other cream types instead. However, for the changes noticed after stopping the formula, 34.2% stated recurrence of the main problem, and 29.5% expressed persistence of the therapeutic result (Table 2).
Table 2. Satisfaction and outcome after using Kligman’s formula (n = 292).
| Variables | N (%) |
| Satisfaction after using Kligman’s formula (mean ± SD) | 6.02 ± 2.89 |
| Dissatisfied (score ≤ 6) | 155 (53.1%) |
| Satisfied (score > 6) | 137 (46.9%) |
| Duration of Kligman’s formula use after reaching satisfaction | |
| Less than a month | 88 (30.1%) |
| 1-3 months | 95 (32.5%) |
| 3-6 months | 28 (09.6%) |
| 6-12 months | 09 (03.1%) |
| I was not satisfied with the result | 60 (20.5%) |
| Other | 12 (04.1%) |
| If you stopped using Kligman's formula, what did you do after stopping? | |
| I used another type of cream | 63 (21.6%) |
| I did not use anything | 131 (44.9%) |
| I went to the dermatologist to treat the side effects | 23 (07.9%) |
| I have not stopped yet | 70 (24.0%) |
| Other | 05 (01.7%) |
| What changes did you notice on your skin after you stopped using Kligman's formula? | |
| Maintaining the therapeutic result | 86 (29.5%) |
| Repetition of the main problem | 100 (34.2%) |
| Persistent side effects | 10 (03.4%) |
| Worsening side effects | 15 (05.1%) |
| I have not stopped yet | 62 (21.2%) |
| No change in the treatment | 19 (06.5%) |
When measuring the relationship of the attitude and satisfaction among participants, it was found that positive attitude in using Kligman’s formula was more common among those using Kligman’s formula based on doctor's prescription (X2 = 20.030; p < 0.001), those who had regular follow up with dermatologist (X2 = 17.290; p < 0.001), and those who bought ready-made Kligman’s formula (X2 = 5.679; p = 0.017), while negative attitude was more common among those who were using self-made Kligman’s formula (X2 = 4.119; p = 0.042).
Discussion
The present study attempted to evaluate the attitude and satisfaction in using Kligman's formula while highlighting the adverse effect of misusage. The findings of this study revealed that only 38% exhibited a positive attitude and the rest (62%) had a negative attitude about it. Of those using Kligman's formula, 26% were using it according to the doctor's advice, with 17.1% of them having a follow-up visit with the dermatologist. In India [9], a study revealed a significant increase in the awareness of the long-term use of topical steroids, including Kligman's formula, with 55.2% of the medical students using it without prescriptions. The use of topical steroids without doctor’s prescriptions had been well-discussed in most publications. For instance, Jha et al. [10] reported that 42.9% of the patients bought topical corticosteroids (TC) containing creams over the counter without prescription, among them, 20% were recommended by their friends, family members, and neighbors, and 8.5% were recommended by a beautician. These findings corroborated the report of Sendrasoa et al. [11], which indicated that the majority of respondents (61%) obtained TC from cosmetic retailers, pharmacy stores (23%), and beauticians (12%), with only 0.26% using TC based on the physician’s prescription.
Alrayyes et al. [5] noted that skin lightening products could harm the skin since users were unaware of the product's active ingredients. Incidentally, in this study, due to the inappropriate use of Kligman's formula, half of the respondents had experienced adverse effects, including 4.5% reported photosensitivity of the face when exposed to sunlight. These results are in accordance with the study of Majid [8], indicating that complaints of side effects due to the Kligman's formula were seen among 26% of the patients. In a study conducted by Sendrasoa et al. [11], 13% of the Madagascar respondents planned to seek dermatological care due to cutaneous adverse effects after using TC. However, most of them were hesitant to proceed due to costly services.
Moreover, adverse reactions resulting from using the TC or combination therapy, including Kligman’s formula, may vary according to the type of cream or containing ingredients. It includes acne, hypopigmentation, pigmentation disorder, and cutaneous atrophy [10,11]. In our study, skin redness, burning feeling, and skin peeling were the most common side effects experienced by the respondents after using Kligman’s formula. While respondents in this study indicated adverse reactions when using the formula. However, nearly three-quarters (72.9%) were using sunscreen to reduce the side effect of the mixed ingredients or to protect against the dyspigmentation of the skin.
Siadat et al. [12] pointed out that combining modified Kligman’s cream + intense pulsed light yielded better satisfaction rates than modified Kligman’s alone. They further surmised that the combination showed better efficacy and faster response in the treatment of the post-burn hyperpigmentation without experiencing side effects after the treatment. Adverse reactions due to the application of the combined formula may vary according to the conditions to be treated. Therefore, it is essential to consult a doctor before using it.
The overuse of Kligman's formula has also been noticed in this study. In our observation, about one-third (32.1%) of the respondents were still using the combination therapy even after their satisfaction. Some indicated that it is necessary to maintain therapeutic results, while some of them used another type of cream after the discontinuation of Kligman’s formula. Moreover, more than one-third (34.2%) complained of conditions recurrence, which could be the main reason for the extended usage of the combined therapy. Similarly, Dhanalakshmi et al. [9] found that students used the topical medication beyond the prescribed period. A total of 52.6% had used the medication for a maximum of one month, and the most common reasons for continuation as cited by the medical students were the treatment had no effect (30%) or skin glowed after the application (23.3%).
The limitations of the present study include the sample size and self-reported attitude and side effects. In addition, the correlations are measured using a cross-sectional design, which may not provide a well-established casualty. Nevertheless, this study could be used as a baseline to further investigate this problem in the future.
Conclusions
Kligman’s formula represents one of the therapeutic options for certain skin conditions, especially melasma and post-inflammatory hyperpigmentation. However, its application and preparation under medical advice with regular follow-up are crucial. Improper and uncontrolled prolonged formula usage could lead to different cutaneous side effects, including skin atrophy, acne, and rosacea.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Research Ethics Committee, King Faisal University issued approval KFU-REC-2021-OCT-EA00032. Having reviewed the details submitted by the applicant regarding the above-named research project, the Research Ethics Committee at King Faisal University grants its ethical approval to the protocol. Projects may be subject to an audit or any other form of monitoring by the committee at any time. The committee may request a regular report on the progress of the project to ensure that researchers are committed to the highest ethical standards. Researchers are held accountable for the storage, retention, and security of original data obtained from projects. Any substantial alterations to the project or emerging events or matters that may affect the ethical acceptability of the project must be reported immediately to the committee via email (.sa) or phone (0096615899773).
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.A new formula for depigmenting human skin. Kligman AM, Willis I. https://pubmed.ncbi.nlm.nih.gov/1119822/ Arch Dermatol. 1975;111:40–48. [PubMed] [Google Scholar]
- 2.Pattern of skin diseases in Eastern Saudi Arabia. Alakloby OM. https://pubmed.ncbi.nlm.nih.gov/16228065/ Saudi Med J. 2005;26:1607–1610. [PubMed] [Google Scholar]
- 3.Survey of acne-related post-inflammatory hyperpigmentation in the Middle East. Abanmi A, Al-Enezi M, Al Hammadi A, Galadari I, Kibbi AG, Zimmo S. http://10.1080/09546634.2018.1542807. J Dermatolog Treat. 2019;30:578–581. doi: 10.1080/09546634.2018.1542807. [DOI] [PubMed] [Google Scholar]
- 4.Skin-lightening practices behind the veil: an epidemiological study among Saudi women. Alrayyes SF, Alrayyes SF, Farooq Dar U. J Cosmet Dermatol. 2020;19:147–153. doi: 10.1111/jocd.12972. [DOI] [PubMed] [Google Scholar]
- 5.Skin-lightening patterns among female students: a cross-sectional study in Saudi Arabia. Alrayyes SF, Alrayyes SF, Farooq UD. Int J Womens Dermatol. 2019;5:246–250. doi: 10.1016/j.ijwd.2019.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hydroquinone 4%, tretinoin 0.05%, fluocinolone acetonide 0.01%: a safe and efficacious 12-month treatment for melasma. Torok HM, Jones T, Rich P, Smith S, Tschen E. https://pubmed.ncbi.nlm.nih.gov/15732437/ Cutis. 2005;75:57–62. [PubMed] [Google Scholar]
- 7.The role of triple combination topical agents in the treatment of facial melasma. Sarkar SK, Sen KG, Mostofa MK, Das AR, Saha SK, Islam MS. https://www.banglajol.info/index.php/FMCJ/article/view/34231/23086 Faridpur Med Coll J. 2017;12:68–70. [Google Scholar]
- 8.Mometasone-based triple combination therapy in melasma: is it really safe? Majid I. http://10.4103/0019-5154.74545. Indian J Dermatol. 2010;55:359–362. doi: 10.4103/0019-5154.74545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awareness and attitude of medical students over the misuse of topical steroids- prospective study in 3rd year medical students in a medical college, Tamil Nadu. Dhanalakshmi K, Pious MT, Sudarvizhi A, Jennifer G. https://doi.org/10.18231/j.ijced.2021.022 IP Indian J Clin Exp Dermatol. 2021;7:115–119. [Google Scholar]
- 10.Misuse of topical corticosteroids on the face: a cross-sectional study among dermatology outpatients. Jha AK, Sinha R, Prasad S. Indian Dermatol Online J. 2016;7:259–263. doi: 10.4103/2229-5178.185492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misuse of topical corticosteroids for cosmetic purpose in Antananarivo, Madagascar. Sendrasoa FA, Ranaivo IM, Andrianarison M, Raharolahy O, Razanakoto NH, Ramarozatovo LS, Rapelanoro Rabenja F. Biomed Res Int. 2017;2017:9637083. doi: 10.1155/2017/9637083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The comparison between modified Kligman formulation versus Kligman formulation and intense pulsed light in the treatment of the post-burn hyperpigmentation. Siadat AH, Iraji F, Bahrami R, et al. Adv Biomed Res. 2016;5:125. doi: 10.4103/2277-9175.186997. [DOI] [PMC free article] [PubMed] [Google Scholar]