Abstract
Staphylococcus epidermidis is a commensal bacterium in humans. To persist in the bacterial flora of the host, some bacteria produce antibacterial factors such as the antimicrobial peptides known as bacteriocins. In this study, we tried to isolate bacteriocin-producing S. epidermidis strains. Among 150 S. epidermidis isolates from the oral cavities of 287 volunteers, we detected two bacteriocin-producing strains, KSE56 and KSE650. Complete genome sequences of the two strains confirmed that they carried the epidermin-harboring plasmid pEpi56 and the nukacin IVK45-like-harboring plasmid pNuk650. The amino acid sequence of epidermin from KSE56 was identical to the previously reported sequence, but the epidermin synthesis-related genes were partially different. The prepeptide amino acid sequences of nukacin KSE650 and nukacin IVK45 showed one mismatch, but both mature peptides were entirely similar. pNuk650 was larger and had an additional seven ORFs compared to pIVK45. We then investigated the antibacterial activity of the two strains against several skin and oral bacteria and found their different activity patterns. In conclusion, we report the complete sequences of 2 plasmids coding for bacteriocins from S. epidermidis, which were partially different from those previously reported. Furthermore, this is the first report to show the complete sequence of an epidermin-carrying plasmid, pEpi56.
Introduction
Staphylococci are classified into two groups, Staphylococcus aureus and coagulase -negative staphylococci (CoNS) due to their clinical importance. CoNS are abundant colonizers on the skin and are considered to contribute to the maintenance of skin integrity and homeostasis [1–3]. CoNS assist in immune activity to prevent pathogen colonization by inducing antimicrobial peptides from the epithelium, by direct production of antibacterial factors such as phenol-soluble modulins (PSMs) and bacteriocins [4–6]. Therefore, the colonization of CoNS provides several benefits to the host. However, CoNS are commonly isolated in clinical cultures and considered to be major nosocomial pathogens in humans [7, 8]. CoNS are often isolated from blood and indwelling medical implants such as intravascular catheters and urinary catheters, leading to opportunistic infectious diseases. In addition, most clinical isolates of Staphylococcus epidermidis carry the genes encoding for antibiotic resistance and biofilm formation, which significantly challenge current antibiotic therapy [9, 10].
In the oral cavity, oral bacterial flora is composed of a great diversity of bacterial species. Many oral indigenous bacteria, including oral streptococci, are known to produce antimicrobial factors such as bacteriocins and hydrogen peroxide [11–15]. Bacteriocins exhibit a wide range of antimicrobial activity against the bacterial species that make up the oral flora [16]. Therefore, bacteriocins are thought to be involved in the exclusion and symbiosis of other bacteria in the oral cavity. S. epidermidis is also found in oral cavity [17, 18]. Some S. epidermidis are known to produce antimicrobial peptides known as bacteriocins, including epidermin [19–21], Pep5 [21–23], epilancin K7 [21, 24], epilancin 15X [25, 26], epicidin 280 [27] and Nukacin IVK-45 [28]. These bacteriocins are known to be lantibiotics containing specific amino acids such as lanthionine, β-methyllanthionine, and dehydrated amino acids [11–13]. However, there are no reports about the bacteriocin produced by S. epidermidis isolated from the oral cavity.
So far, there have been many reports on bacteriocins produced by oral isolates of streptococcal species [11–13, 16] but very few reports on other oral bacterial species. To understand the meaning of bacteriocins for bacterial flora formation, more information about bacteriocins produced by many oral bacterial species is required. In this study, we focused on the bacteriocins of oral-derived S. epidermidis to understand the antibacterial activity against oral and skin bacteria. We examined 150 S. epidermidis strains isolated from the oral cavity and investigated their bacteriocin-producing activity. As a result, we found two strains that produced epidermin and nukacin IVK45. We performed the complete-genome analysis of these two strains and identified the plasmids harboring the epidermin or nukacin IVK45-like bacteriocin gene clusters. The nucleotide sequences of these plasmids were not entirely similar to the previously reported sequences. Additionally, we evaluated the antibacterial activity of these two bacteriocins against the skin and oral commensal bacteria.
Materials and methods
Bacterial strains and growth conditions
S. epidermidis clinical isolates were grown in trypticase soy broth (TSB) (Becton, Dickinson and Company [BD], Franklin Lakes, NJ, USA) at 37°C. The Staphylococcus aureus MW2 strain and braRS-inactivated mutant were obtained previously [29]. Other bacteria used in this study are listed in Table 1. Staphylococcal strains and Micrococcus luteus were grown in TSB at 37°C and 30°C, respectively. Streptococcal strains were grown in TSB at 37°C with 5% CO2. Cutibacterium acnes was grown on sheep blood agar at 37°C anaerobically. Corynebacterium and Rothia mucilanginosa were grown at 37°C in R medium and BHI (BD) aerobically, respectively. The composition of R medium is as follows: 1g of bacto peptone (BD), 0.5g of yeast extract (BD), 0.5g of malt extract (BD), 0.5g of casamino acids (BD), 0.2g of beef extract (BD), 0.2g of glycerol, 5mg of Tween 80, 0.1g of MgSO4 in 100 ml distilled water. When necessary, tetracycline (5 μg/ml) was added to the medium.
Table 1. Strains used in this study.
| Strains | Character | origin |
|---|---|---|
| Staphylococcus epidermidis | ||
| KSE1 | Wild type | This study |
| KSE3 | Wild type | This study |
| KSE56 | Wild type | This study |
| KSE650 | Wild type | This study |
| KSE56- | KSE56 plasmid deleted | This study |
| KSE650- | KSE650 plasmid deleted | This study |
| Staphylococcus warneri ISK-1 | Wild type | |
| Staphylococcus hominis JCM31912 | Wild type | Riken BRC 1 |
| Staphylococcus haemolyticus JCM2416 | Wild type | Riken BRC 1 |
| Staphylococcus capitis subsp. capitis JCM2420 | Wild type | Riken BRC 1 |
| Staphylococcus simulans JCM2424 | Wild type | Riken BRC 1 |
| Cutibacterium acnes JCM6425 | Wild type | Riken BRC 1 |
| Corynebacterium accolens JCM8331 | Wild type | Riken BRC 1 |
| Corynebacterium pseudodiphtheriticum JCM1320 | Wild type | Riken BRC 1 |
| Rothia mucilaginosa JCM10910 | Wild type | Riken BRC 1 |
| Micrococcus luteus JCM1464 | Wild type | Riken BRC 1 |
| Streptococcus mutans UA159 | Wild type | [30] |
| Streptococcus sanguinis GTC217 | Wild type | Gifu University |
| Streptococcus salivarius GTC215 | Wild type | Gifu University |
| Streptococcus gordonii JCM12995 | Wild type | Riken BRC 1 |
| Staphylococcus aureus | ||
| COL | Wild type | [31] |
| RN4220 | NCTS8325 derivative | [32] |
| MW2 | clinical strain, methicillin-resistant (mecA+) | [33] |
| ΔTCS16 | braRS inactivation in MW2, Tcr2 | [29] |
1. Japan Collection of Microorganisms.
2. Tetracycline resistance.
Isolation of Staphylococcus epidermidis from the oral cavity
S. epidermidis strains were isolated from the oral cavities of 287 volunteers. Saliva collected from the oral cavity was plated on No.110 medium (Eiken Chemical Co. Ltd, Tokyo, Japan) and incubated for 2 days at 37°C. The strains were picked from a single white colony on the agar and further investigated by PCR with specific primers for S. epidermidis (forward primer: GGCAAATTTGTGGGTCAAGA, reverse primer: TGGCTAATGGTTTGTCACCA). Isolated S. epidermidis strains were replated on TSB containing 2% agar (TSA) medium. The isolated strains were then replated again on TSA to pick up a single colony and finally, S. epidermidis confirmed by PCR was used in this study. Clinical isolates were designated as KSE strains. Saliva collection and S. epidermidis isolation were approved by the Ethical Committee of the Kagoshima University Graduate School of Medical and Dental Sciences (No. 701) and the Ethical Committee for Epidemiology of Hiroshima University (E-1998). Written informed consent was obtained from all participants. All methods were performed in accordance with the approved guidelines and regulations.
Screening of bacteriocin-producing S. epidermidis
To investigate bacteriocin production among S. epidermidis strains, we performed a direct assay using S. aureus MW2 braRS knockout mutant as an indicator strain because this mutant showed increased susceptibility to several bacteriocins [34]. Overnight cultures of S. epidermidis strains were spotted on a TSA plate and cultured at 37°C for 24 h. Then, 3.5 ml of prewarmed half-strength TSB soft agar (1%) containing braRS knockout mutant cells (107 cells/ml) were poured over the TSA plate. The plates were incubated at 37°C for 24 h. The strains which showed the growth inhibition zones surrounding S. epidermidis strain were picked up. The strains were reconfirmed for bacteriocin production by the direct assay again.
Complete genome sequences of bacteriocin-producing S. epidermidis strains
To perform whole-genome sequencing of S. epidermidis strains, DNA was extracted from each strain. S. epidermidis cells grown overnight in 5 ml TSB were collected and then suspended in 0.5 ml of CS buffer (100 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10 mM EDTA) containing lysostaphin (Sigma-Aldrich, St. Louis, MO, USA) (final concentration: 50 μg/ml) and RNase (Nippon Gene, Tokyo, Japan) (final: 20 μg/ml). After incubation at 37°C for 1 h, proteinase K (Nacalai Tesque, Kyoto, Japan) (final: 150 μg/ml) and SDS (final 1%) were added, followed by incubation at 55°C for 5 h. After treatment with phenol followed by phenol-chloroform, DNA was precipitated by ethanol. Whole-genome sequencing (WGS) of S. epidermidis strains was performed using the Illumina MiSeq sequencing platform, followed by annotation with Rapid Annotation using Subsystem Technology (RAST) version 2.0 [35]. After confirming the presence of bacteriocin genes using WGS, long-read sequencing by MinION (Oxford Nanopore Technologies, UK) was carried out to determine the complete sequences of the chromosomes and plasmids of these strains. Hybrid assembly of Illumina short reads and MinION long reads was performed with Unicycler v0.4.8. The complete sequences of plasmids harboring bacteriocin genes were selected, including epidermin-carrying plasmid pEpi56 and nukacin-carrying plasmid pNuk650. Each plasmid was compared with publicly available plasmids or gene clusters, including the epiY’-epiP gene cluster (X62386), epiG-epiT’’ gene cluster (U77778), and pIVK45 (accession number KP702950).
Accession numbers
The complete plasmids carrying epidermin (pEpi56) and nukacin (pNuk650) have been deposited in the NCBI database under accession numbers OK031036 and OK031035, respectively.
Identification of epidermin and nukacin KSE650 produced by S. epidermidis
To identify the bacteriocin, we purified the bacteriocin from two S. epidermidis strains. Overnight cultures (500 ml) of S. epidermidis KSE56 and KSE650 were centrifuged at 4,000 x g for 15 min. Macro-Prep cationic resin (1.5 ml) (Bio rad, USA) was added to the supernatant and stirred for 12 h. The resin was collected into an open column, then washed three times with 10 ml of 25 mM ammonium acetate (pH 7.5). To elute the bacteriocin, the resin was treated with 500 μl of 5% acetic acid. This elution was repeated 10 times. After each fraction was evaporated completely, the samples were dissolved in 50 μl of distilled water. Each solution was tested for antibacterial activity against M. luteus. Overnight cultures of M. luteus (100 μl) were inoculated on TSA plates. Then, 5 μl of each solution was spotted on TSA. After overnight incubation at 37°C, growth inhibition was observed. Samples with antibacterial activity were subjected to HPLC chromatography using an Octadecyl C18 column. After equilibrating the column with 0.1% TFA water, the sample was injected. Thereafter, a linear gradient of 0 to 60% acetonitrile for 30 min was applied to the column. Each peak was fractionated, and the samples were evaporated, then dissolved with 50 μl of distilled water. Subsequently, the antibacterial activity of each fraction was tested with the method above. ESI-MS analysis was performed by LTQ Orbitrap XL (Thermo Fisher Scientific, USA).
Isolation of the strain curing bacteriocin-encoded plasmid
Plasmid deletion in KSE56 and KSE650 was performed with the method described elsewhere [36]. Overnight cultures of KSE56 or KSE650 were inoculated into 5 ml of fresh TSB and incubated at 37°C with shaking. When the OD660 reached 0.5, acriflavine was added at a concentration of 25 μg/ml. After incubation for 12 h, the culture was diluted and plated on TSA. After 24 h of incubation at 37°C, colonies were picked, replated on TSA and then incubated at 37°C for 24 h. Next, 0.75% soft agar (3.5 ml) containing Bacillus coagulans (200 μl of overnight culture) was poured on that plate and incubated at 37°C for 24 h. The strains with no inhibitory zone were picked. Finally, PCR was performed using specific primers for S. epidermidis-specific genes and bacteriocin genes coding for nukacin KSE650 or epidermin.
Susceptibility tests
Two methods were used for the evaluation of bacteriocins. A direct assay was performed with a previously described method [34]. An overnight culture of the bacteriocin-producing strain was spotted on a TSA plate and cultured at 37°C for 24 h. Then, 3.5 ml of prewarmed half-strength TSB soft agar (1%) containing indicator bacterial cells (107 cells/ml) was poured over the TSA plate. The plates were incubated at 37°C for 16 h. The diameters of the growth inhibition zones surrounding the bacteriocin-producing strains were measured in three directions. Three independent experiments were performed, and the average diameter was calculated.
Another method was to evaluate the minimal antibacterial dose of purified bacteriocins. Purified epidermin and nukacin KSE650 were adjusted to 0.5 mg/ml. The bacteriocin solution underwent 2-fold serial dilutions (2-fold to 128-fold dilution). Then, 3.5 ml of prewarmed half-strength TSB soft agar (1%) containing bacterial cells (107 cells/ml) was poured over the TSA plate. Thereafter, 2 μl of the bacteriocin solutions with serial dilution (1 μg to 0.03 μg) were spotted on the plate. After the incubation at 37°C for 16 h, the minimum antibacterial dose for the growth inhibition zones was determined.
Co-culture of S. epidermidis with M. luteus
For analysis of the proportion of each bacterium (S. epidermidis and M. luteus) in co-culture by qPCR, we first set up the method for the calculation of bacterial cell number by qPCR. A single overnight culture of the bacterium was first adjusted to OD660 = 1.0, and then a 10-fold serial dilution was performed in 500 μl of lysis buffer. After heating at 95°C for 15 min, samples were centrifuged at 15,000 x rpm for 10 min. Using the supernatant, qPCR was performed with the respective specific primers. For S. epidermidis, the forward and reverse primers used were GGCAAATTTGTGGGTCAAGA and TGGCTAATGGTTTGTCACCA, respectively. For M. luteus, the forward and reverse primers were GGGTTGCGATACTGTGAGGT and TTCGGGTGTTACCGACTTTC, respectively. Finally, the linear relationship between bacterial cell number and cut off value (Ct value) was constructed in each bacterium. Overnight cultures of S. epidermidis KSE1 (no bacteriocin production), KSE56, KSE650 and M. luteus were adjusted to OD660 = 1.0, and the bacterial culture was diluted to 10-fold. Next, 100 μl of S. epidermidis culture and M. luteus were mixed thoroughly. A small portion (20 μl) of mixed culture was spotted on TSA. After overnight incubation at 37°C, the bacterial colonies growing on agar plates were scraped and suspended in 500 μl of lysis buffer. After heating at 95°C for 15 min, the bacterial suspension was centrifuged at 15,000 x rpm for 10 min and the culture supernatant was stocked as the template for quantitative PCR (qPCR). qPCR was performed using appropriate specific primers to determine the cell number of each bacterium in the co-culture samples. Finally, the proportion of 2 bacterial species was determined. Three independent experiments were performed. Post hoc multiple comparisons were made using Tukey’s test.
Results
Isolation of S. epidermidis that produced bacteriocin
From 287 volunteers, 150 S. epidermidis strains (52.3%) were isolated from the oral cavity. Among 150 S. epidermidis strains, 2 strains showing a clear inhibitory zone against the S. aureus MW2 braRS inactivated mutant were identified by the direct method (Fig 1).
Fig 1. Direct assay of bacteriocin-producing S. epidermidis against braRS-inactivated S. aureus.
The antibacterial activity of bacteriocin-producing S. epidermidis was evaluated by the direct assay using S. aureus MW2 braRS-inactivated mutant.
Nucleotide sequence of epidermin-encoding plasmid
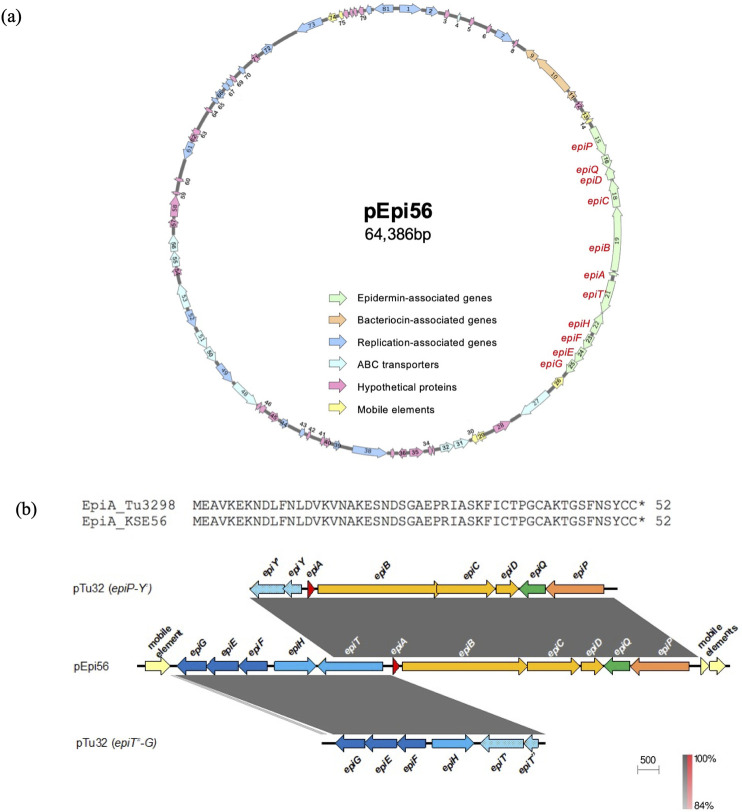
The size of the entire plasmid, pEpi56, is 64,386 bp, with 81 ORFs (Fig 2A and Table 2). The plasmid contains epidermin synthesis genes (epiA coding for epidermin KSE56, modification genes epiBCD, processing genes epiP, export genes epiHT, immunity genes epiGEF, and regulatory gene epiQ), replication-related genes, and other genes including the genes coding for hypothetical proteins (Table 2). Compared with epidermin-related genes in the Tü3298 strain [19] epiT, which codes for an exporter, was intactin pEpi56, while a gene disrupted into two fragments (epiT’ and epiT” or epiY and epiY’) was found in the Tü3298 strain (Figs 2B and S1). The nucleotide sequence of epiA in KSE56 showed 2 mismatches with that of the Tü3298 strain (S2 Fig). However, the amino acid sequence of epidermin KSE56 showed 100% identity with that in the Tü3298 strain.
Fig 2. Gene map of the epidermin-carrying plasmid in KSE56.
(a) Epidermin-encoding plasmid from KSE56 (pEpi56). ORFs are shown as arrows, indicating the orientation of transcription. The arrow numbers indicate the ORF number displayed in Table 2. Colors indicate the classification of gene function. (b) Bacteriocin-coding region (KSE56 epidermin). The bacteriocin-coding region from pEpi56 was compared with pTu32 epiP-Y’ (accession number X62386) and pTu32 epiT"-G (accession number U77778). Striped blue arrows indicate truncated epiT.
Table 2. Genes in pEpi56.
| No. | Location (bp) | Size (aa)a | Translation signalb | Homologue as determined by BLAST and/or FASTA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Description(s) | Identity (%) | Overlap (aa)c | Accession no. | Note | ||||
| 1 | 190–1191 | 333 | GAGGTTTTTTATTATG | S. epidermidis | replication initiator protein A | 99 | 333/338 | WP_002498716.1 | |
| 2 | 1423–1983 | 186 | AAGGAGTAATAAAAATG | S. epidermidis | TIGR00730 family Rossman fold protein | 99 | 186/186 | WP_158171994.1 | |
| 3 | 2300–2515 | 71 | - | S. epidermidis | hypothetical protein | 67 | 48/78 | MBM0824966.1 | |
| 4 | 2889–3014 | 41 | GGAGAATAATTAATAAACCCGTTACAAAATAAGCAATATCTATAAGTTTTTTAAAAATTAAAAATTCTAAAATATGTAAGTATG | S. epidermidis SK135 | ATP-binding cassette domain-containing protein | 100 | 41/41 | EFA87131.1 | |
| 5 | 3507–3695 | 62 | GAGTTAGACCAATAAATTGAAACGAAAAAACAATTGTTG | S. epidermidis | hypothetical protein | 100 | 62/62 | MBC8789835.1 | |
| 6 | 4346–4513 | 55 | GGAGGCATTTGTCATG | S. epidermidis | hypothetical protein | 100 | 55/55 | WP_002498713.1 | |
| 7 | 4819–5685 | 288 | GGAGTGATATATATG | S. epidermidis | RepB family plasmid replication initiator protein | 99 | 287/288 | WP_203085279.1 | |
| 8 | 5791–5934 | 47 | GGAGACATAAAAAGTTATG | S. epidermidis | hypothetical protein | 100 | 47/47 | WP_002498711.1 | |
| 9 | 6397–7026 | 209 | GAGTAATCATG | S. epidermidis | ABC transporter, ATP-binding protein | 100 | 209/209 | EJD97739.1 | |
| 10 | 7029–9071 | 680 | AGGTATTTATACATATG | S. epidermidis NIHLM040 | bacteriocin-associated integral membrane protein | 100 | 680/680 | EJD97738.1 | |
| 11 | 9165–9557 | 130 | GGAGGATTAAGTTGATG | S. epidermidis NIHLM040 | bacteriocin, lactococcin 972 family | 100 | 130/130 | EJD97736.1 | |
| 12 | 9743–10105 | 120 | GAGAATTATACAAAAATG | S. epidermidis | DUF3139 domain-containing protein | 100 | 120/120 | WP_002498706.1 | |
| 13 | 10304–10669 | 121 | GAGGGACATACATTAGATATTTGGTTG | S. epidermidis NIHLM040 | IS431mec, transposase | 100 | 121/121 | EJD97734.1 | |
| 14 | 10732–10884 | 50 | GGAGTCTTCTGTATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 50/50 | EJD97733.1 | |
| 15 | 11171–12556 | 461 | GAGGTGCTATATG | S. epidermidis NIHLM040 | putative epidermin leader peptide-processing serine protease EpiP | 100 | 461/461 | EJD97732.1 | epiP |
| 16 | 12567–13184 | 205 | GGAATAAAATG | S. epidermidis | winged helix family transcriptional regulator | 100 | 205/205 | MBM0752529.1 | epiQ |
| 17 | 13181–13726 | 181 | GGAGGAATAAGATATG | S. epidermidis NIHLM040 | epidermin decarboxylase | 100 | 181/181 | EJD97730.1 | epiD |
| 18 | 13742–14992 | 416 | GGATGGTTGTG | S. epidermidis NIHLM040 | putative epidermin biosynthesis protein EpiC | 100 | 416/416 | EJD97729.1 | epiC |
| 19 | 14985–17945 | 986 | GAGGTGAAATAGAATTG | S. epidermidis NIHLM040 | thiopeptide-type bacteriocin biosynthesis domain protein | 100 | 986/986 | EJD97728.1 | epiB |
| 20 | 18011–18169 | 52 | AGGAGTGTTTAAAATG | S. epidermidis NIHLM040 | lantibiotic epidermin | 100 | 52/52 | EJD97726.1 | epiA |
| 21 | 18419–19969 | 516 | GGACTAATATTGAGTTTG | S. epidermidis | ABC transporter ATP-binding protein/permease | 100 | 516/516 | WP_002498696.1 | epiT’ |
| 22 | 19985–20977 | 330 | GAGATAAGGGAGATATATG | S. epidermidis | YdcF family protein | 100 | 330/330 | WP_032605946.1 | epiH |
| 23 | 21136–21831 | 231 | GGAGGAATAATTCTTG | S. epidermidis | lantibiotic protection ABC transporter ATP-binding protein | 100 | 231/231 | WP_002498693.1 | epiF |
| 24 | 21833–22597 | 254 | GGAAATAATATG | S. epidermidis | lantibiotic immunity ABC transporter MutE/EpiE family permease subunit | 100 | 254/254 | WP_002498692.1 | epiE |
| 25 | 22587–23279 | 230 | GGAATATAAATG | S. epidermidis | epidermin immunity protein F | 100 | 230/230 | WP_002498691.1 | epiG |
| 26 | 23432–24034 | 200 | GAGGTGGAAATCAATG | S. epidermidis NIHLM040 | putative transposon DNA-invertase Bin3 | 100 | 200/200 | EJD97719.1 | |
| 27 | 24455–26071 | 538 | GGAGGAAGAAAAATG | S. epidermidis NIHLM040 | ABC transporter, ATP-binding protein | 100 | 538/538 | EJD97718.1 | |
| 28 | 26621–27463 | 280 | GGAGCATTAATTATG | S. epidermidis | hypothetical protein | 100 | 280/280 | WP_002498688.1 | |
| 29 | 27952–28383 | 143 | AAGGAGTCTTCTGTATG | S. epidermidis NIHLM040 | IS431mec, transposase family protein | 100 | 143/143 | EJD97715.1 | |
| 30 | 28376–28627 | 83 | AGGCACCTTCAACGAAGGTAGCAATG | S. epidermidis NIHLM040 | IS431mec, transposase family protein | 100 | 83/83 | EJD97714.1 | |
| 31 | 28733–29455 | 240 | GGAGTGTAAGCTTTG | S. epidermidis | peptide ABC transporter permease | 100 | 240/240 | WP_002498749.1 | |
| 32 | 29472–30107 | 211 | GGAGCTGTAAACATTG | S. epidermidis NIHLM040 | ABC transporter, ATP-binding protein | 100 | 211/211 | EJD97793.1 | |
| 33 | 30389–30484 | 31 | GGAGAGATTAAATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 31/31 | EJD97792.1 | |
| 34 | 30495–30665 | 56 | AGGTTAATTTTATG | S. epidermidis | hypothetical protein | 100 | 56/56 | TID00490.1 | |
| 35 | 30897–31535 | 212 | AGGTTCAAGATGAAAACAAAGAAATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 212/212 | EJD97791.1 | |
| 36 | 31698–32063 | 121 | GAGGAGAGAACTTTTAAAATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 121/121 | EJD97790.1 | |
| 37 | 32230–32406 | 58 | GGAGTGATTTAATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 58/58 | EJD97789.1 | |
| 38 | 32573–34183 | 536 | GGAAGGATTATTATG | S. epidermidis | DNA mismatch repair protein MutS | 100 | 536/536 | WP_002498743.1 | |
| 39 | 34762–35058 | 98 | GGATTGAATG | S. epidermidis | replication initiation protein | 100 | 98/98 | MBF2337202.1 | |
| 40 | 35232–35510 | 92 | GGAGAGATTAAATG | S. epidermidis | hypothetical protein | 100 | 92/92 | WP_002498740.1 | |
| 41 | 35521–35691 | 56 | GGATTTTATG | S. epidermidis | hypothetical protein | 100 | 56/56 | WP_099800689.1 | |
| 42 | 36232–36369 | 45 | GGAG ACATAAGAAGGTATG | S. epidermidis | hypothetical protein | 100 | 45/45 | MBM6015004.1 | |
| 43 | 36517–36732 | 71 | GGAAATGACACATCTTAAATCGACATATTCCAAAAATATGTTTAGAATACTGGTTACATG | S. epidermidis | hypothetical protein | 100 | 71/71 | WP_002498738.1 | |
| 44 | 37358–37726 | 122 | GAGACGTCTATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 122/122 | EJD97781.1 | |
| 45 | 37880–38335 | 151 | - | S. epidermidis | putative plasmid recombination enzyme | 100 | 151/151 | TID00443.1 | |
| 46 | 38651–38905 | 84 | GGAGTTCCTTTAAATG | S. epidermidis | hypothetical protein | 100 | 84/84 | EJD97779.1 | |
| 47 | 38927–39067 | 46 | GGAAGATGAAATAGTCCTAATG | S. epidermidis | hypothetical protein | 100 | 46/46 | WP_151520775.1 | |
| 48 | 39102–40481 | 459 | GGAGGTATGATAGATG | S. epidermidis NIHLM040 | drug resistance MFS transporter, drug:H+ antiporter-2 family | 100 | 459/459 | EJD97777.1 | |
| 49 | 40630–41637 | 335 | GGAGCGATGGAAATG | S. epidermidis | tryptophan—tRNA ligase | 100 | 335/335 | WP_002498732.1 | |
| 50 | 41862–42590 | 242 | AAGGAGAATAAACAATG | S. epidermidis NIHLM040 | ABC transporter permease | 100 | 242/242 | EJD97775.1 | |
| 51 | 42594–43457 | 287 | AAGGAGAATAAAATG | S. epidermidis NIHLM040 | ABC transporter, ATP-binding protein | 100 | 287/287 | EJD97774.1 | |
| 52 | 43704–44525 | 273 | GGAGGATTTTATG | S. epidermidis NIHLM040 | transcriptional regulator, LysR family | 100 | 273/273 | EJD97773.1 | |
| 53 | 44678–45817 | 379 | GAGGATGGGATAATAATG | S. epidermidis NIHLM040 | MFS transporter | 100 | 379/379 | EJD97772.1 | |
| 54 | 46236–46613 | 125 | GGAAAAGAGTAAATG | S. epidermidis NIHLM040 | hypothetical protein | 96 | 125/125 | EJE04311.1 | |
| 55 | 46649–47338 | 229 | GGAGACGATAATGTG | S. epidermidis NIHLM040 | ABC transporter, ATP-binding protein | 100 | 229/229 | EJD97770.1 | |
| 56 | 47346–48107 | 253 | GGAGGAATGAAGCAATTATG | S. epidermidis | ABC transporter permease | 99 | 253/253 | WP_002503830.1 | |
| 57 | 48465–48857 | 130 | - | S. epidermidis NIHLM040 | hypothetical protein | 100 | 130/130 | EJD97768.1 | |
| 58 | 48948–49919 | 323 | GGAGAAATTATG | S. epidermidis | DUF418 domain-containing protein | 99 | 323/323 | WP_095694513.1 | |
| 59 | 49974–50108 | 44 | GGAAGGATTG | S. epidermidis | hypothetical protein | 100 | 44/44 | EFA87101.1 | |
| 60 | 50567–50722 | 51 | - | S. epidermidis | hypothetical protein | 100 | 51/51 | MBC2926404.1 | |
| 61 | 51633–52454 | 273 | AGGTGTGATTTAAATG | S. epidermidis | relaxase MobL | 99 | 273/273 | WP_161382396.1 | |
| 62 | 52466–52849 | 127 | GGAGGAATAAAATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 127/127 | EJD97765.1 | |
| 63 | 52851–53129 | 92 | GGAATGATTTTTTTG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 92/92 | EJD97764.1 | |
| 64 | 54078–54224 | 48 | S. epidermidis | hypothetical protein | 100 | 48/48 | WP_002456268.1 | ||
| 65 | 54621–54800 | 59 | GGAGGCTTATACATG | S. epidermidis NIHLM040 | CsbD family protein | 100 | 59/59 | EJD97762.1 | |
| 66 | 54833–55231 | 132 | GAGGTGTTTGTATATG | S. epidermidis | YolD-like family protein | 100 | 132/132 | WP_002498728.1 | |
| 67 | 55394–55651 | 85 | - | S. epidermidis NIHLM040 | prevent-host-death family protein | 100 | 85/85 | EJD97760.1 | |
| 68 | 55651–55917 | 88 | - | S. epidermidis NIHLM040 | addiction module toxin, Txe/YoeB family | 100 | 88/88 | EJD97759.1 | |
| 69 | 55934–56104 | 56 | GGAGGACTCGTTAATG | S. epidermidis | hypothetical protein | 100 | 56/56 | KAB2267008.1 | |
| 70 | 56465–56689 | 74 | S. epidermidis | putative glycoside hydrolase | 100 | 74/74 | QRX38739.1 | ||
| 71 | 57190–57546 | 118 | GGAGGTTGTATGTATG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 118/118 | EJD97756.1 | |
| 72 | 57860–58408 | 182 | - | S. epidermidis NIHLM040 | putative resolvase | 100 | 182/182 | EJD97755.1 | |
| 73 | 59658–60926 | 422 | GGAGAATTTAATAATG | S. epidermidis | penicillin-binding protein PBP4 | 99 | 422/422 | WP_002498725.1 | |
| 74 | 61202–61603 | 133 | - | S. epidermidis | transposase DNA-binding domain protein | 100 | 133/133 | TID00494.1 | |
| 75 | 61744–61926 | 60 | GAGTCGTTTAGATG | S. epidermidis | transposase | 98 | 60/60 | WP_203079065.1 | |
| 76 | 61958–62188 | 76 | GAGGTGTATTGACATG | S. epidermidis NIHLM040 | hypothetical protein | 99 | 76/76 | EJD97751.1 | |
| 77 | 62255–62407 | 50 | GGAGGAATTAAATTG | S. epidermidis NIHLM040 | hypothetical protein | 100 | 50/50 | EJD97750.1 | |
| 78 | 62434–62595 | 53 | GGAGGCGGGAAATTG | S. epidermidis | BH0509 family protein | 100 | 53/53 | EJD97749.1 | |
| 79 | 62670–62909 | 79 | GGAGGAAGATAATG | S. epidermidis | hypothetical protein | 100 | 79/79 | WP_002498719.1 | |
| 80 | 63024–63272 | 82 | GGAGGTATCAAGGTTATG | S. epidermidis | CopG family transcriptional regulator | 100 | 82/82 | MBM0752797.1 | |
| 81 | 63390–64280 | 296 | - | S. epidermidis | ParA family protein | 100 | 268/296 | WP_002498717.1 | |
a aa, amino acids.
b Bold letters indicate start codons. Underlines indicate putative ribosome binding sites complementary to the 3’ end of the 16s rRNA.
c Overlap is indicated as the number of overlapping amino acids/total number of amino acids.
Nucleotide sequence of nukacin-encoding plasmid
The size of the entire plasmid, pNuk650, was 26,160 bp, with 29 open reading frames (ORFs). The plasmid contained nukacin KSE650 synthesis genes (nukA coding for prepeptide nukacin KSE650, posttranslational modification enzyme genes nukM, processing and secretion transporter genes nukT, and immunity protein genes nukFEGH), replication-related genes, and other genes including genes coding for hypothetical proteins (Fig 3A and Table 3). Compared to the plasmid pIVK45 (21,840 bp), which carried the gene coding for nukacin IVK45 [28] pNuk650 was larger with a higher number of ORFs (Fig 3A). The amino acid sequence of nukacin KSE650 showed similarity to nukacin IVK45 with one mismatch at the 4th position, but displayed lower similarity to nukacin ISK-1 with 10 mismatches [36, 37] (Fig 3B). The mature peptide of nukacin KSE650 showed a perfect match with nukacin IVK45 and 5 mismatches with nukacin ISK-1.
Fig 3. Nukacin-carrying plasmids and amino acid sequences of nukacin.
(a) Nukacin-encoding plasmid from KSE650 (pNuk650) and the comparison with pIVK45. (b) Amino acid alignment of nukacin ISK-1, nukacin 3299, nukacin KQU131, nukacin IVK45 and nukacin KSE650.
Table 3. Genes in pNuk650.
| No. | Location (bp) | Size (aa)a | Translation signalb | Homologue as determined by BLAST and/or FASTA | ||||
|---|---|---|---|---|---|---|---|---|
| Source | Description(s) | Identity (%) | Overlap (aa)c | Accession no. | ||||
| 1 | 413–541 | 42 | GGAAAAGATATCCATG | S. epidermidis | RepB (pAQZ2) | 83 | 42/42 | AZL87916 |
| 2 | 680–850 | 56 | - | S. epidermidis | replication protein | 91 | 56/56 | WP_194376762 |
| 3 | 976–1911 | 311 | GGAAGAGGTTTATATTATG | S. epidermidis | replication initiator protein A | 100 | 311/311 | WP_194378689 |
| 4 | 2467–3261 | 264 | AGGAGGTATTATTTTG | S. epidermidis | ParA family protein | 100 | 264/264 | WP_172686110 |
| 5 | 3258–3467 | 69 | GAGGGTGTGTG | S. epidermidis | plasmid replication associated protein, putative transcriptional regulator | 98 | 66/69 | AKQ51589 |
| 6 | 3821–3994 | 57 | AGGGGGTATTATAATG | S. epidermidis (pIVK45) | NukA | 98 | 57/57 | AKQ51579 |
| 7 | 4068–4250 | 60 | AGGTACGCGTTTTTAAATTGTATATATG | S. epidermidis | transposase family protein | 92 | 38/60 | MBV5159007 |
| 8 | 4256–4393 | 45 | GAGACCATG | S. epidermidis | hypothetical protein | 100 | 45/45 | WP_194378692 |
| 9 | 4605–4844 | 79 | - | S. epidermidis | transposase | 100 | 74/79 | WP_172686114 |
| 10 | 5583–6326 | 247 | GAGTGAATTATATG | S. epidermidis | LytTR family transcriptional regulator DNA-binding domain-containing protein | 100 | 247/247 | WP_194378694 |
| 11 | 6570–9323 | 917 | AGGAGAGGTTGTTATATATG | S. epidermidis (pIVK45) | NukM | 100 | 917/917 | AKQ51580 |
| 12 | 9345–11429 | 694 | AGGTGAATACAATTG | S. epidermidis (pIVK45) | NukT | 99 | 694/694 | KP702950 |
| 13 | 11442–12350 | 302 | AGGAGGTTCAATTTATG | NukF | 99 | 302/302 | AKQ51583 | |
| 14 | 12351–13103 | 250 | GGAAAGGAATATTTATAAATG | S. epidermidis (pIVK45) | NukE | 99 | 250/250 | AKQ51582 |
| 15 | 13100–13837 | 245 | AAGGAGAGATTTATCTTG | S. epidermidis (pIVK45) | NukG | 88 | 245/245 | AKQ51591 |
| 16 | 13844–14122 | 92 | GAGGATTAATAACTAATG | S. epidermidis (pIVK45) | NukH | 100 | 92/92 | AKQ51584 |
| 17 | 14444–14623 | 59 | - | S. epidermidis | replication initiator protein A, partial | 88 | 59/272 | WP_064595943 |
| 18 | 14790–14930 | 46 | GGATAACAAAATAACATCAACACAATGTCACGATTTCATAATATAGCATG | S. epidermidis | hypothetical protein | 98 | 46/46 | WP_172686106 |
| 19 | 15014–15157 | 47 | GGAATGATAAATTCAACTTTTTCTTTCCGATCATTAATAAAATAAATG | no significant similarity found | ||||
| 20 | 15425–16423 | 332 | TAAGGTGTCGAATCTAAATAAAACTGGGGGCTTTTTTATG | S. epidermidis | protein rep | 98 | 332/332 | WP_145461985 |
| 21 | 17100–17483 | 127 | AGGGGTTTTTTTATG | S. epidermidis IS-K | bacterial transcription activator, effector-binding domain protein | 99 | 127/127 | EID36019 |
| 22 | 17957–18664 | 235 | GAGAGGTGTTTTTTTATGTCTGGTGAAACAGTAGTATATAGAAATG | S. epidermidis | RepB family plasmid replication initiator protein | 100 | 235/235 | WP_194378685 |
| 23 | 18712–19323 | 203 | AGGAGTAGTTTATG | S. epidermidis | helix-turn-helix domain-containing protein | 99 | 203/203 | WP_194378686 |
| 24 | 19890–20699 | 269 | GGAGAGAAATATATATTG | S. epidermidis | CPBP family intramembrane metalloprotease | 100 | 269/269 | WP_168429436 |
| 25 | 20725–21039 | 104 | GAGGTGTAAAAAATG | S. epidermidis | helix-turn-helix domain-containing protein | 99 | 104/104 | WP_002455864 |
| 26 | 21312–22928 | 538 | AGGATTATTATG | S. epidermidis | MutS family DNA mismatch repair protein | 99 | 538/538 | WP_194378687 |
| 27 | 23374–25119 | 581 | AGGTGAAGTTAAAAGTG | S. epidermidis | AIPR family protein | 100 | 581/581 | WP_194378688 |
| 28 | 25145–25853 | 202 | GGAATCAATG | S. epidermidis (pIVK45) | Sin recombinase | 100 | 202/202 | AKQ51586 |
| 29 | 25976–26077 | 33 | AAGGAGGAATACTATG | S. epidermidis | NAD-dependent epimerase/dehydratase family protein | 100 | 33/33 | WP_172686124 |
a aa, amino acids.
b Bold letters indicate start codons. Underlines indicate putative ribosome binding sites complementary to the 3’ end of the 16s rRNA.
c Overlap is indicated as the number of overlapping amino acids/total number of amino acid.
Identification of epidermin KSE56 and nukacin KSE650
Epidermin KSE56 and nukacin KSE650 were purified from the culture supernatant of KSE56 and KSE650, respectively. After applying the sample purified by Macro Prep resin to Octadecyl C18 column, peak fractions in both samples were collected and each peak fraction was checked for the antibacterial activity against M. luteus. In both samples, one peak fraction showed a strong antibacterial activity (Fig 4A). Using ESI-MS analysis, the molecular masses of purified epidermin KSE56 and nukacin KSE650 were found to be 2163.97 Da and 2938.36 Da, respectively (Fig 4B). The mass of these peptides corresponded to calculated mass of epidermin (2163.95 Da) and nukacin KSE650 (2938.33 Da).
Fig 4. Purification of epidermin and nukacin KSE650 by reverse phase-HPLC and mass determination by ESI-MS.
(a) RP-HPLC chromatogram of epidermin and nukacin KSE650. The arrow shows the peak corresponding to epidermin (upper) or nukacin KSE650 (lower). (b) Mass determination of epidermin (upper) or nukacin KSE650 (lower) by ESI-MS. Several isotopic peaks in each mass/charge (m/z) state.
Antibacterial activity of epidermin KSE56 and nukacin KSE650 against several skin and oral commensal bacteria
In this study, S. epidermidis strains were isolated from the oral cavity. S. epidermidis is also known as a commensal bacterium. Therefore, we investigated the antibacterial activity of the two bacteriocins against oral and skin commensal bacterial species.
We first performed a direct assay using KSE56, KSE650 and plasmid-deleted strains. The plasmid-deleted strains showed no inhibitory zone against S. hominis, while the wild-type strains, KSE56 and KSE650, displayed inhibitory zones (Fig 5).
Fig 5. Antibacterial activity of KSE56, KSE650, and their plasmid-deleted strains.

Direct assays were performed using KSE56, KSE650, and their plasmid-deleted strains. S. hominis was used as an indicator strain.
Afterwards, we performed a direct assay using KSE56 and KSE650 as bacteriocin-producing strains (Table 4). The epidermin-producing strain, KSE56, showed a strong antibacterial activity (>20 mm diameter inhibitory zone) against M. luteus, and an activity (>5 mm diameter) against R. mucilaginosa, C. pseudodiphtheriticum, S. haemolyticus, S. captis, S. hominis, S. simulans, and S. saprophyticus. KSE56 also showed an antibacterial activity against S. epidermidis without bacteriocin production (KSE1, 10, 12, 16), plasmid-curing KSE56 and plasmid-curing KSE650. The inhibitory zone was not observed in S. epidermidis KSE56, S. epidermidis KSE650, C. accolens, S. warneri ISK-1, and S. aureus strains. Regarding oral streptococci, KSE56 showed a strong activity against S. salivarius and S. gordonii, and modest activity against S. mutans and S. sanguinis.
Table 4. Antibacterial activity of KSE56 and KSE650 against various bacterial species.
| Indicator strains | Halo size (mm) | ||
|---|---|---|---|
| KSE56 | KSE650 | S. warneri | |
| Corynebacterium pseudodiphtheriticum JCM1320 | 10.0±0.8 | 10.7±0.5 | 11.7±0.5 |
| Corynebacterium accolens JCM8331 | - | - | 11.3±0.5 |
| Micrococcus luteus JCM1464 | 31.7±1.2 | 27.0±0 | 33.0±0 |
| Rothia mucilaginosa JCM10910 | 8.7±0.5 | 8.0±0 | 13.0±0 |
| Cutibacterium acnes JCM6425 | 15.0±0.8 | - | - |
| Staphylococcus haemolyticus JCM2416 | 11.7±0.6 | 13.3±0.5 | 16.0±0.8 |
| Staphylococcus capitis JCM2420 | 11.3±0.6 | 27.3±0.5 | 17.3±0.5 |
| Staphylococcus simulans JCM2424 | 13.7±0.6 | 28.7±0.5 | 22.7±0.5 |
| Staphylococcus saprophyticus JCM20595 | 13.0±0 | 12.3±0.5 | 13.3±0.5 |
| Staphylococcus hominis JCM31912 | 15.3±0.6 | 16.3±0.5 | 21.7±0.5 |
| Staphylococcus epidermidis KSE1 | 12.3±0.5 | 7.0±0.8 | N.D.2 |
| Staphylococcus epidermidis KSE10 | 12.0±0 | 7.3±0.5 | N.D. |
| Staphylococcus epidermidis KSE12 | 17.0±0.8 | 9.7±0.5 | N.D. |
| Staphylococcus epidermidis KSE16 | 14.3±0.5 | 8.7±0.5 | N.D. |
| Staphylococcus epidermidis KSE56 | - | - | - |
| Staphylococcus epidermidis KSE650 | - | - | - |
| Staphylococcus epidermidis KSE56 plasmid-deleted | 20.3±0.5 | 11.3±0.5 | N.D. |
| Staphylococcus epidermidis KSE650 plasmid-deleted | 11.0±0 | 11.7±0.5 | N.D. |
| Staphylococcus warneri ISK-1 | - | - | - |
| Staphylococcus aureus MW2 | - | - | 11.3±0.5 |
| Staphylococcus aureus COL | - | - | 11.0±0 |
| Staphylococcus aureus RN4220 (MSSA) | - | - | 10.7±0.5 |
| Streptococcus mutans UA159 | 15.0±0.8 | - | - |
| Streptococcus sanguinis GTC217 | 12.0±0 | - | 10.3±0.9 |
| Streptococcus salivarius GTC215 | 27.7±0.5 | 12.3±0.5 | 18.3±0.5 |
| Streptococcus gordonii JCM12995 | 29.0±0 | 17.0±0 | 23.0±0 |
"-" and "N.D." represent "no inhibitory zone" and "Not determined", respectively.
The nukacin KSE650-producing strain KSE650, showed strong antibacterial activity (>20 mm diameter) against M. luteus, S. captis, and S. simulans, and an activity (>5 mm diameter) against C. pseudodiphtheriticum, R. mucilaginosa, S. haemolyticus, S. hominis, and S. saprophyticus. KSE650 also showed an antibacterial activity against S. epidermidis without bacteriocin production (KSE1, 10, 12, 16), plasmid-curing KSE56 and plasmid-curing KSE650. The inhibitory zone was not observed in S. epidermidis KSE56, S. epidermidis KSE650, C. accolens, S. warneri ISK-1, and S. aureus strains. Regarding oral streptococci, KSE650 showed activity against S. salivarius and S. gordonii, and no activity against S. mutans and S. sanguinis. Compared to the nukacin ISK-1-producing S. warneri strain, S. warneri showed stronger activity against commensal and oral bacteria except for S. capitis and S. simulans. Notably, S. warneri ISK-1 showed activity against the S. aureus strain.
We also checked the antibacterial activity using purified epidermin and nukacin KSE650 (Table 5). The antibacterial pattern against each bacterium was similar to the results of the direct assay.
Table 5. Minimum antibacterial dose of purified epidermin and nukacin KSE650.
| Indicator strains | Minimum antibacterial dose (μg) | |
|---|---|---|
| Epidermin | Nukacin KSE650 | |
| Corynebacterium pseudodiphtheriticum JCM1320 | 2 | 2 |
| Corynebacterium accolens JCM8331 | > 2 | > 2 |
| Micrococcus luteus JCM1464 | < 0.03 | < 0.03 |
| Rothia mucilaginosa JCM10910 | 0.5 | 1 |
| Staphylococcus haemolyticus JCM2416 | 0.125 | 0.25 |
| Staphylococcus capitis JCM2420 | 0.125 | < 0.03 |
| Staphylococcus simulans JCM2424 | 0.125 | < 0.03 |
| Staphylococcus saprophyticus JCM20595 | 0.5 | 0.25 |
| Staphylococcus hominis JCM31912 | 0.06 | 0.25 |
| Staphylococcus epidermidis KSE1 | 0.5 | 1 |
| Staphylococcus aureus MW2 | 2 | 2 |
| Streptococcus mutans UA159 | 1 | > 2 |
| Streptococcus sanguinis GTC217 | 0.5 | 1 |
| Streptococcus salivarius GTC215 | 0.06 | 0.25 |
| Streptococcus gordonii JCM12995 | 0.06 | 0.125 |
Co-culture of S. epidermidis with M. luteus
Co-cultures of S. epidermidis KSE1 (bacteriocin negative), KSE56, and KSE650 with M. luteus JCM1464 were analyzed. M. luteus was utilized as an indicator bacterium in co-culture assay because in the direct method, KSE56 and KSE650 showed a significant antibacterial effect against M. luteus. In co-culture with M. luteus, the proportion of S. epidermids KSE1 was 46.2%, while the proportions of KSE56 and KSE650 were 70.4% and 79.8%, respectively (Fig 6).
Fig 6. The proportion of S. epidermidis KSE1, KSE56, and KSE650 in co-culture with M. luteus.

Co-culture assays were performed according to the method described in the Materials and methods. Post hoc multiple comparisons were made using Tukey’s test.
Discussion
In this study, we tried to isolate S. epidermidis strains that produced bacteriocin. We used the S. aureus MW2 braRS-inactivated mutant as the indicator strain for screening. We previously reported that BraRS was involved in resistance to several bacteriocins including nisin A, nukacin ISK-1 and bacitracin [34]; therefore, a braRS-inactivated mutant increased susceptibility to these bacteriocins. Nisin A and nukacin ISK-1 are lantibiotics that act against lipid II molecules, which are responsible for cell wall biosynthesis, and subsequently, form a pour complex [38]. In addition, it was reported that many gram-positive bacteria, including staphylococci, streptococci, bacilli, lactococci and enterococci, produced lantibiotics that bind to lipid II [12, 19–27, 39, 40] Therefore, the braRS-inactivated mutant is a good indicator strain to screen lipid II-binding lantibiotics. Finally, we identified 2 strains that produce epidermin and nukacin IVK45-like bacteriocins. Whole genome analysis of the 2 strains revealed that both genes were located on the plasmids (S2A and 4 Figs).
Epidermin was first identified in the S. epidermidis Tü3298 strain [19, 41]. In the Tü3298 strain, epidermin is located on the plasmid, pTu32. Recently, the whole genome sequence of the Tü3298 strain was determined [42], but the entire plasmid sequence of pEpi56 was not reported. Therefore, our study is the first to report the complete nucleotide sequence of epidermin harboring plasmids. Additionally, the epidermin-producing strain identified in this study was the second strain, following the Tü3298 strain. The nucleotide sequence of the epiA coding epidermin showed 2 mismatches between the two strains, but the amino acid sequence was similar. When the epidermin synthesis genes were compared between the 2 strains, epiT showed a significant difference (Fig 2B). epiT in KSE56 was intact, while this gene in Tu3298 was disrupted into 2 genes, epiT’ and epiT” in Tü3298.
EpiT is involved in the secretion of the peptide. In previous reports that demonstrated the antibacterial activity of epidermin in Tü3298 [19–21], epidermin was correctly modified and secreted externally. However, Peschel A et al reported that the introduction of intact gdmT, encoding the secretion protein for gallidermin, which was close to epidermin in Tü3298, increased the production of epidermin in culture supernatant [43]. Therefore, the secretion activity of epiT’/T” is considered to be partial, while the intact epiT gene in KSE56 may be responsible for full secretion of the epidermin peptide.
Nukacin IVK-1 was first identified in S. warneri [37]. Since then, nukacin ISK-1 like bacteriocins have been identified in S. epidermidis [28], S. hominis [44], and S. simulans [45]. The amino acid sequence of KSE650 shows a high similarity with that of IVK45 by only one mismatch in the entire peptide, and 100% match with the mature peptide. Comparison of the plasmid between the two strains showed that KSE650 was larger than Tü3298, but the composition and the order of nukacin-related genes were identical (Fig 2A). The larger size of pNuk650 was due to the insertion of an approximately 8 kbp fragment, which was detected in pNuk650 but not in pIVK45 (Fig 3A, red arrows).
The antibacterial activity of these peptides against skin and oral commensal bacteria (oral streptococci) showed different patterns. In particular, the epidermin-producing strain (KSE56) had antibacterial activity against oral streptococci, while nukacin-producing strains had less activity. Interestingly, comparing nukacin ISK-1 and nukacin KSE650 suggested that 5 amino acid differences (Fig 7) were responsible for the different activities against several bacteria used in this study. Previously, it was reported that the structure of ring A in nukacin ISK-1 binds to the pyrophosphate moiety of lipid II, the precursor for cell wall peptidoglycan biosynthesis, and ring C was also associated with the binding of the isoprene chain [46]. Since lipid II molecules are widely conserved among gram positive bacteria, the different antibacterial activities between nukacin ISK-1 and nukacin KSE650 are influenced by the other molecules specific to each bacterial species. Furthermore, it is noteworthy that epidermin and nukacin KSE650 showed no inhibitory zone against S. epidermidis KSE650 and KSE56, respectively, while epidermin and nukacin KSE650 showed an activity against plasmid-curing KSE650 and plasmid-curing KSE56, respectively (Table 4). Although the immunity factors for epidermin and nukacin KSE650 were EpiFEG and NukFEG/NukH, respectively, which could be found in a respective plasmid, our results indicate that these immunity factors showed a cross-resistance to another bacteriocin. We previously reported that BraRS and ApsRS, TCSs, are involved in resistance to nisin A and nukacin ISK-1 [34]. Since S. epidermidis also possesses TCSs with similarity to BraRS and ApsRS, S. epidermidis TCSs may be involved in the resistance to epidermin and nukacin KSE650.
Fig 7. Structure of nukacin ISK-1 and nukacin KSE650.

The mature peptide sequences of nukacin ISK-1 and nukacin KSE650 are shown. The deduced calculated mass of mature nukacin KSE650 is consistent with that observed by ESI-MS. The structure is identical to that of nukacin ISK-1, except for the residues indicated by gray circles. Dhb, Ala-S-Ala, and Abu-S-Ala indicate dehydrobutyrine, lanthionine, and 3-methyllanthionine respectively.
In conclusion, we determined the complete sequence of two plasmids encoding epidermin and nukacin KSE650 in S. epidermidis isolated from the oral cavity. S. epidermidis is the major commensal bacterium in human skin and the oral cavity. Based on our findings of the direct assay and co-culture assay, it is speculated that bacteriocins produced by S. epidermidis affect the bacterial composition of the host flora, including the skin, nasal and oral flora. However, in this study, we focused on the isolation of lantibiotic-producing strains using a braRS-inactivated strain as the indicator. Therefore, it is possible that S. epidermidis also produces other types of bacteriocins. Further studies are required to demonstrate the influence of S. epidermidis bacteriocins on the formation of bacterial flora.
Supporting information
(PDF)
Comparison of nucleotide (A) and amino acid sequences (B) of epiA between the KSE56 and Tü3298 strains.
(PDF)
Acknowledgments
We thank Dr. Tomoko Amimoto, the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University for the measurement of ESI-MS analysis.
Data Availability
All complete plasmid files are available from the NCBI database (accession numbers OK031036 and OK031035).
Funding Statement
Research Program on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED) under grant number 21fk0108604j0001. Grant in Aid for Scientific Research (C) (Grant No: 21K09858A) from the Ministry of Education, Culture, Sports, Sciences, and Technology of Japan.
References
- 1.Parlet CP, Brown MM, Horswill AR. Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends Microbiol. 2019;27: 497–507. doi: 10.1016/j.tim.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324: 1190–1192. doi: 10.1126/science.1171700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16: 143–155. doi: 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TH, Park MD, Otto M. Host Response to Staphylococcus epidermidis Colonization and Infections. Front Cell Infect Microbiol. 2017;7: 90. doi: 10.3389/fcimb.2017.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130: 2211–2221. doi: 10.1038/jid.2010.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scharschmidt TC, Vasquez KS, Truong H-A, Gearty S V, Pauli ML, Nosbaum A, et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity. 2015;43: 1011–1021. doi: 10.1016/j.immuni.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers KL, Fey PD, Rupp ME. Coagulase-negative staphylococcal infections. Infect Dis Clin North Am. 2009;23: 73–98. doi: 10.1016/j.idc.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 8.Uçkay I, Pittet D, Vaudaux P, Sax H, Lew D, Waldvogel F. Foreign body infections due to Staphylococcus epidermidis. Ann Med. 2009;41: 109–119. doi: 10.1080/07853890802337045 [DOI] [PubMed] [Google Scholar]
- 9.Otto M. Staphylococcus epidermidis—the “accidental” pathogen. Nat Rev Microbiol. 2009;7: 555–567. doi: 10.1038/nrmicro2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miragaia M, Couto I, de Lencastre H. Genetic diversity among methicillin-resistant Staphylococcus epidermidis (MRSE). Microb Drug Resist. 2005;11: 83–93. doi: 10.1089/mdr.2005.11.83 [DOI] [PubMed] [Google Scholar]
- 11.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3: 777–788. doi: 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- 12.Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59: 171–200. doi: 10.1128/mr.59.2.171-200.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissen-Meyer J, Nes IF. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol. 1997;167: 67–77. [PubMed] [Google Scholar]
- 14.Ryan CS, Kleinberg I. Bacteria in human mouths involved in the production and utilization of hydrogen peroxide. Arch Oral Biol. 1995;40: 753–763. doi: 10.1016/0003-9969(95)00029-o [DOI] [PubMed] [Google Scholar]
- 15.García-Mendoza A, Liébana J, Castillo AM, de la Higuera A, Piédrola G. Evaluation of the capacity of oral streptococci to produce hydrogen peroxide. J Med Microbiol. 1993;39: 434–439. doi: 10.1099/00222615-39-6-434 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe A, Kawada-Matsuo M, Le MN-T, Hisatsune J, Oogai Y, Nakano Y, et al. Comprehensive analysis of bacteriocins in Streptococcus mutans. Sci Rep. 2021;11: 12963. doi: 10.1038/s41598-021-92370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira AR, Hiller NL, Powell E, Kim LH-J, Spirk T, Modesto A, et al. Profiling microorganisms in whole saliva of children with and without dental caries. Clin Exp Dent Res. 2019;5: 438–446. doi: 10.1002/cre2.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McManus BA, Daly B, Polyzois I, Wilson P, Brennan GI, Fleming TE, et al. Comparative Microbiological and Whole-Genome Analysis of Staphylococcus aureus Populations in the Oro-Nasal Cavities, Skin and Diabetic Foot Ulcers of Patients With Type 2 Diabetes Reveals a Possible Oro-Nasal Reservoir for Ulcer Infection. Front Microbiol. 2020;11: 748. doi: 10.3389/fmicb.2020.00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allgaier H, Jung G, Werner RG, Schneider U, Zähner H. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur J Biochem. 1986;160: 9–22. doi: 10.1111/j.1432-1033.1986.tb09933.x [DOI] [PubMed] [Google Scholar]
- 20.Schnell N, Entian KD, Schneider U, Götz F, Zähner H, Kellner R, et al. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature. 1988;333: 276–278. doi: 10.1038/333276a0 [DOI] [PubMed] [Google Scholar]
- 21.Bierbaum G, Götz F, Peschel A, Kupke T, van de Kamp M, Sahl HG. The biosynthesis of the lantibiotics epidermin, gallidermin, Pep5 and epilancin K7. Antonie Van Leeuwenhoek. 1996;69: 119–127. doi: 10.1007/BF00399417 [DOI] [PubMed] [Google Scholar]
- 22.Kaletta C, Entian KD, Kellner R, Jung G, Reis M, Sahl HG. Pep5, a new lantibiotic: structural gene isolation and prepeptide sequence. Arch Microbiol. 1989;152: 16–19. doi: 10.1007/BF00447005 [DOI] [PubMed] [Google Scholar]
- 23.Weil HP, Beck-Sickinger AG, Metzger J, Stevanovic S, Jung G, Josten M, et al. Biosynthesis of the lantibiotic Pep5. Isolation and characterization of a prepeptide containing dehydroamino acids. Eur J Biochem. 1990;194: 217–223. doi: 10.1111/j.1432-1033.1990.tb19446.x [DOI] [PubMed] [Google Scholar]
- 24.van de Kamp M, Horstink LM, van den Hooven HW, Konings RN, Hilbers CW, Frey A, et al. Sequence analysis by NMR spectroscopy of the peptide lantibiotic epilancin K7 from Staphylococcus epidermidis K7. Eur J Biochem. 1995;227: 757–771. doi: 10.1111/j.1432-1033.1995.tb20199.x [DOI] [PubMed] [Google Scholar]
- 25.Ekkelenkamp MB, Hanssen M, Danny Hsu S-T, de Jong A, Milatovic D, Verhoef J, et al. Isolation and structural characterization of epilancin 15X, a novel lantibiotic from a clinical strain of Staphylococcus epidermidis. FEBS Lett. 2005;579: 1917–1922. doi: 10.1016/j.febslet.2005.01.083 [DOI] [PubMed] [Google Scholar]
- 26.Velásquez JE, Zhang X, van der Donk WA. Biosynthesis of the antimicrobial peptide epilancin 15X and its N-terminal lactate. Chem Biol. 2011;18: 857–867. doi: 10.1016/j.chembiol.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidrich C, Pag U, Josten M, Metzger J, Jack RW, Bierbaum G, et al. Isolation, characterization, and heterologous expression of the novel lantibiotic epicidin 280 and analysis of its biosynthetic gene cluster. Appl Environ Microbiol. 1998;64: 3140–3146. doi: 10.1128/AEM.64.9.3140-3146.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janek D, Zipperer A, Kulik A, Krismer B, Peschel A. High Frequency and Diversity of Antimicrobial Activities Produced by Nasal Staphylococcus Strains against Bacterial Competitors. PLoS Pathog. 2016;12: e1005812. doi: 10.1371/journal.ppat.1005812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo M, Kato F, Oogai Y, Kawai T, Sugai M, Komatsuzawa H. Distinct two-component systems in methicillin-resistant Staphylococcus aureus can change the susceptibility to antimicrobial agents. The Journal of antimicrobial chemotherapy. 2010. pp. 1536–1537. doi: 10.1093/jac/dkq141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murchison HH, Barrett JF, Cardineau GA, Curtiss R 3rd. Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect Immun. 1986;54: 273–282. doi: 10.1128/iai.54.2.273-282.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kornblum J, Hartman BJ, Novick RP, Tomasz A. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol. 1986;5: 714–718. doi: 10.1007/BF02013311 [DOI] [PubMed] [Google Scholar]
- 32.Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305: 709–712. doi: 10.1038/305709a0 [DOI] [PubMed] [Google Scholar]
- 33.Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999;48: 707–710. [PubMed] [Google Scholar]
- 34.Kawada-Matsuo M, Yoshida Y, Zendo T, Nagao J, Oogai Y, Nakamura Y, et al. Three Distinct Two-Component Systems Are Involved in Resistance to the Class I Bacteriocins, Nukacin ISK-1 and Nisin A, in Staphylococcus aureus. Otto M, editor. PLoS One. 2013;8: e69455. doi: 10.1371/journal.pone.0069455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014;42: D206–14. doi: 10.1093/nar/gkt1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aso Y, Koga H, Sashihara T, Nagao J-I, Kanemasa Y, Nakayama J, et al. Description of complete DNA sequence of two plasmids from the nukacin ISK-1 producer, Staphylococcus warneri ISK-1. Plasmid. 2005;53: 164–178. doi: 10.1016/j.plasmid.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 37.Aso Y, Sashihara T, Nagao J-I, Kanemasa Y, Koga H, Hashimoto T, et al. Characterization of a gene cluster of Staphylococcus warneri ISK-1 encoding the biosynthesis of and immunity to the lantibiotic, nukacin ISK-1. Biosci Biotechnol Biochem. 2004;68: 1663–1671. doi: 10.1271/bbb.68.1663 [DOI] [PubMed] [Google Scholar]
- 38.Islam MR, Nagao J-I, Zendo T, Sonomoto K. Antimicrobial mechanism of lantibiotics. Biochem Soc Trans. 2012;40: 1528–1533. doi: 10.1042/BST20120190 [DOI] [PubMed] [Google Scholar]
- 39.Vogel V, Spellerberg B. Bacteriocin Production by Beta-Hemolytic Streptococci. Pathog (Basel, Switzerland). 2021;10. doi: 10.3390/pathogens10070867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbosa J, Caetano T, Mendo S. Class I and Class II Lanthipeptides Produced by Bacillus spp. J Nat Prod. 2015;78: 2850–2866. doi: 10.1021/np500424y [DOI] [PubMed] [Google Scholar]
- 41.Schnell N, Engelke G, Augustin J, Rosenstein R, Ungermann V, Götz F, et al. Analysis of genes involved in the biosynthesis of lantibiotic epidermin. Eur J Biochem. 1992;204: 57–68. doi: 10.1111/j.1432-1033.1992.tb16605.x [DOI] [PubMed] [Google Scholar]
- 42.Moran JC, Horsburgh MJ. Whole-Genome Sequence of Staphylococcus epidermidis Tü3298. Genome Announc. 2016;4. doi: 10.1128/genomeA.00112-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peschel A, Schnell N, Hille M, Entian KD, Götz F. Secretion of the lantibiotics epidermin and gallidermin: sequence analysis of the genes gdmT and gdmH, their influence on epidermin production and their regulation by EpiQ. Mol Gen Genet. 1997;254: 312–318. doi: 10.1007/s004380050421 [DOI] [PubMed] [Google Scholar]
- 44.Wilaipun P, Zendo T, Okuda K, Nakayama J, Sonomoto K. Identification of the nukacin KQU-131, a new type-A(II) lantibiotic produced by Staphylococcus hominis KQU-131 isolated from Thai fermented fish product (Pla-ra). Biosci Biotechnol Biochem. 2008;72: 2232–2235. doi: 10.1271/bbb.80239 [DOI] [PubMed] [Google Scholar]
- 45.Ceotto H, Holo H, da Costa KFS, Nascimento J dos S, Salehian Z, Nes IF, et al. Nukacin 3299, a lantibiotic produced by Staphylococcus simulans 3299 identical to nukacin ISK-1. Vet Microbiol. 2010;146: 124–131. doi: 10.1016/j.vetmic.2010.04.032 [DOI] [PubMed] [Google Scholar]
- 46.Fujinami D, Mahin A-A, Elsayed KM, Islam MR, Nagao J-I, Roy U, et al. The lantibiotic nukacin ISK-1 exists in an equilibrium between active and inactive lipid-II binding states. Commun Biol. 2018;1: 150. doi: 10.1038/s42003-018-0150-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Comparison of nucleotide (A) and amino acid sequences (B) of epiA between the KSE56 and Tü3298 strains.
(PDF)
Data Availability Statement
All complete plasmid files are available from the NCBI database (accession numbers OK031036 and OK031035).