Abstract
Background
Women with obesity and infertility are counseled to lose weight prior to conception and infertility treatment to improve pregnancy rates and birth outcomes, although confirmatory evidence from randomized trials is lacking. We assessed whether a preconception intensive lifestyle intervention with acute weight loss is superior to a weight neutral intervention at achieving a healthy live birth.
Methods and findings
In this open-label, randomized controlled study (FIT-PLESE), 379 women with obesity (BMI ≥ 30 kg/m2) and unexplained infertility were randomly assigned in a 1:1 ratio to 2 preconception lifestyle modification groups lasting 16 weeks, between July 2015 and July 2018 (final follow-up September 2019) followed by infertility therapy. The primary outcome was the healthy live birth (term infant of normal weight without major anomalies) incidence. This was conducted at 9 academic health centers across the United States. The intensive group underwent increased physical activity and weight loss (target 7%) through meal replacements and medication (Orlistat) compared to a standard group with increased physical activity alone without weight loss. This was followed by standardized empiric infertility treatment consisting of 3 cycles of ovarian stimulation/intrauterine insemination. Outcomes of any resulting pregnancy were tracked. Among 191 women randomized to standard lifestyle group, 40 dropped out of the study before conception; among 188 women randomized to intensive lifestyle group, 31 dropped out of the study before conception. All the randomized women were included in the intent-to-treat analysis for primary outcome of a healthy live birth. There were no significant differences in the incidence of healthy live births [standard 29/191(15.2%), intensive 23/188(12.2%), rate ratio 0.81 (0.48 to 1.34), P = 0.40]. Intensive had significant weight loss compared to standard (−6.6 ± 5.4% versus −0.3 ± 3.2%, P < 0.001). There were improvements in metabolic health, including a marked decrease in incidence of the metabolic syndrome (baseline to 16 weeks: standard: 53.6% to 49.4%, intensive 52.8% to 32.2%, P = 0.003). Gastrointestinal side effects were significantly more common in intensive. There was a higher, but nonsignificant, first trimester pregnancy loss in the intensive group (33.3% versus 23.7% in standard, 95% rate ratio 1.40, 95% confidence interval [CI]: 0.79 to 2.50). The main limitations of the study are the limited power of the study to detect rare complications and the design difficulty in finding an adequate time matched control intervention, as the standard exercise intervention may have potentially been helpful or harmful.
Conclusions
A preconception intensive lifestyle intervention for weight loss did not improve fertility or birth outcomes compared to an exercise intervention without targeted weight loss. Improvement in metabolic health may not translate into improved female fecundity.
Trial registration
ClinicalTrials.gov NCT02432209.
Richard Legro and colleagues investigate the impact of a preconception weight loss intervention on healthy live birth rates in women with obesity and unexplained infertility.
Author summary
Why was this study done?
Obesity in women is associated with an increased time to pregnancy, pregnancy loss, and many pregnancy complications.
Consequently, women who are obese and infertile are recommended to lose weight prior to conception to increase their chance for a healthy baby.
There are few data from prospective trials to support this recommendation.
What did the researchers do and find?
In this study, we conducted an open-label, randomized controlled trial examining the effects of an intensive preconception intervention designed to lose weight acutely compared to a standard intervention designed to maintain weight consisting of exercise alone for 16 weeks prior to initiating routine infertility treatment in women with unexplained infertility in the US.
We found that our intensive intervention resulted in about 7% weight loss in the intensive group versus no significant weight loss in the standard group.
After the preconception intervention, both groups received 3 cycles of routine infertility treatment for both groups. There were no significant differences in cumulative pregnancy or live birth rates between groups.
The small number of pregnancies reduced the statistical power for examining differences in the rate of pregnancy complications between groups.
What do these findings mean?
Our study supports other studies that have found no improved live birth or healthy live birth rates in women who were obese or overweight who participated in a preconception weight loss intervention prior to starting infertility therapy.
Further research will be needed to investigate potential harms and benefits of weight loss interventions that did not reach statistical significance in the present trial.
There is not strong evidence to recommend weight loss prior to conception in women who are obese with unexplained infertility.
Introduction
Weight loss in women with obesity prior to pregnancy is thought to improve not only the chance for pregnancy but also a healthy live birth. Epidemiological evidence overwhelmingly supports a strong association between obesity and infertility [1], pregnancy loss [2–4], and an increased rate of maternal and fetal complications of pregnancy [5–7]. Consequently, experts, major medical societies, and public health programs have endorsed or mandated weight loss in women with obesity before initiating infertility therapy [8–10]. Data from case series [11], prospective randomized trials of preconception interventions in women with obesity [12,13], or from registry studies of women who have conceived after bariatric surgery [14,15] are less supportive of benefit. Some studies identified potential harms such as pregnancy loss after dietary caloric restriction [11,12] or increased rates of preterm delivery and/or small for gestational age (SGA) babies after bariatric surgery [15].
We designed a trial to test 2 preconception lifestyle interventions, one (intensive) with a multifocal approach of caloric restriction, weight loss medication, and increased physical activity to achieve clinically meaningful weight loss (7% target) and the second intervention (standard) primarily to increase activity without targeted weight loss [16]. The rationale is that previous preconception intervention studies have randomized patients to either immediate infertility treatment or intensive lifestyle intervention followed by infertility treatment [12,13], creating a lack of equipoise and a bias toward success with immediate infertility treatment. Increased physical activity alone was chosen as a comparator given the extensive literature summarizing this as an effective complementary therapy to infertility treatment [17]. Our hypothesis was that the intensive intervention was more likely to achieve a healthy term normal weight infant than standard intervention for women with obesity and unexplained infertility.
Methods
Study design and participants
The FIT-PLESE trial was a multicenter randomized controlled parallel group trial of 2 types of preconception lifestyle modification, one designed to lose weight and one to keep weight constant in which participants were randomly allocated in a 1:1 ratio. The FIT-PLESE protocol (S1 Protocol), designed by the steering committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Reproductive Medicine Network, was based on previous studies of preinfertility treatment weight loss [18] and treatments of unexplained infertility [19] to create a 2 phase study, the first phase of lifestyle intervention and a standardized second phase of infertility treatment (ClinicalTrials.gov: NCT02432209). It was approved before study initiation by both an NICHD appointed advisory board and a data and safety monitoring board, which provided oversight. A single Institutional Review Board (IRB) at the University of Pennsylvania approved the study with administrative review by each site’s IRB. All participants (women and their male partners) gave written informed consent at 9 study sites across the US. Enrollment began in July 2015 and finished in July 2018.
A total of 379 women (ages 18 to 40 years) whose body mass index (BMI) was ≥30 kg/m2 were randomized. They were in good health, had a history of ≥1 year of infertility, and had regular ovulation (defined as ≥9 spontaneous menses per year) with normal ovarian reserve. A normal uterine cavity and at least 1 open fallopian tube were confirmed by sonohysterography, hysterosalpingography, a combined hysteroscopy and laparoscopy, or evidence of an unassisted intrauterine pregnancy within the immediate 3 years [19,20]. The male partner had at least 5 million total motile sperm in the ejaculate within 1 year of study initiation. Additionally, the couple agreed to comply with intercourse instructions and collection of semen for insemination.
Randomization and masking
A SAS procedure (PROC PLAN) was used to generate the random allocation sequence. Randomization was stratified by study site and female BMI at baseline ≥40 with random block sizes of 2, 4, and 6. The random sequence was imported to a data management system owned and administrated by an investigative drug service company (Almac’s WebEZ system) and blinded to study investigators. The statisticians at the DCC generated the random allocation sequence. The investigators or nurse coordinators enrolled participants and assigned participants to intervention. Interventions were known to the investigator and patients.
Procedures
This clinical trial compared 2 types of 16-week lifestyle modifications: one intensive, focusing on weight loss through increasing physical activity, caloric restriction, and anti-obesity medication and the other less intensive (standard), focused on increasing physical activity alone. In the second phase, both groups received 3 cycles of empiric ovarian stimulation with clomiphene citrate (CC) combined with intrauterine insemination.
The lifestyle modification interventions were adapted from gold standard interventions for obesity treatment [21] and used in other infertility studies [18]. The interventions were implemented in a manner that could be replicated in clinical practice. Both groups had identical on-site in person study visits (monthly during the first phase). Wireless devices were used to monitor physical activity (Fitbit Activity Monitors) and weight (Fitbit Aria Scale), and data were automatically uploaded to a central website accessible to study personnel. Women with infertility have been shown to be very compliant with tracking physical activity using Fitbit Activity Monitors [22].
The intensive group received nutritional counseling and meal replacement products (3 meals/day) to promote portion control and energy restriction (Nutrisystem) and a gastric lipase inhibitor (Orlistat, to reduce fat absorption) with a target of 7% weight loss. In addition, patients were instructed to consume 2 servings of fruit, 3 servings of vegetables, and 2 servings of low-fat dairy per day. This diet provided approximately 1,100 kcal/day with the following macronutrient profile: 30% calories from protein, 45% calories from carbohydrate; and 25% calories from fat. An additional 100 calories could be consumed as desired. This meal plan, totaling approximately 1,200 kcal/d, was consistent with that used in the Look AHEAD study [23]. Orlistat was initiated at a dose of 60 mg per meal at lunch and dinner. The morning dose was avoided due to the low-fat content of breakfast meal replacements. Patients in this group were also given a daily multivitamin supplement to be taken at least 2 hours before or after Orlistat to ensure adequate vitamin status.
Both groups received identical physical activity interventions. Patients used a Fitbit physical activity tracker during the screening phase to establish the mean number of steps over a 7-day period at baseline. They were instructed to increase steps by 500 steps a day per week until the upper limit of 10,000 steps a day was reached and then to maintain this rate throughout the study. Patients in the standard lifestyle group did not receive any dietary instruction or weight loss medication. An algorithm was created to ensure the weekly step increase in both groups (500 step/week up to but not exceeding 10,000 steps per week) and to avoid excessive weight loss. Weekly, weight and steps were monitored remotely by study coordinators by the Fitbit database and a twice monthly teleconference with a nutritionist (Dr. Kris-Etherton) and a psychologist (Dr. Sarwer) to troubleshoot management of noncompliant patients. Patients had intercourse ad lib during this intervention, and no contraception was prescribed. Meal replacements and Orlistat were discontinued after a positive pregnancy test.
Patients who did not conceive naturally during first phase received up to 3 cycles of ovarian stimulation/intrauterine insemination in the second phase. Both groups were advised to maintain weight and activity during this treatment period. CC was administered at a dose of 100 mg/d for 5 days starting on cycle Day 3 (±2 days).
The patients were monitored by transvaginal ultrasound after completing clomiphene. Visits were individualized based on follicular development until criteria for human chorionic gonadotropin (hCG) administration (at a dose of 10,000 U) were met [19]. The cycle was canceled if a leading follicle did not reach a mean diameter of 18 mm after 18 days, endogenous LH surge happened, or when more than 4 follicles developed (mean diameter >18 mm) to avoid the risk of ovarian hyperstimulation and/or high-order multiple gestations [19]. Doses were adjusted accordingly after cycle cancelation. One insemination was performed ≤44 hours after hCG administration. For inseminations, each site utilized its own standard semen preparation method and catheter [24].
A serum quantitative hCG pregnancy test was performed after menses, any positive home pregnancy test, or 2 weeks after insemination if a participant had no menses. Levels were followed for an appropriate rise. Transvaginal ultrasound documented the location of the pregnancy and number of implantation sites and was repeated to document fetal cardiac activity. Follow-up during pregnancy was then arranged with the treating obstetricians. Pregnancy outcomes were documented by review of maternal and neonatal records. Any patients who withdrew from the study without conception before the end of cycle 3 in Phase II were defined as dropouts.
Outcomes
The primary outcome was a healthy birth outcome defined as defined as a live birth of an infant born at ≥37 weeks, with a birth weight between 2,500 and 4,000 g and without a major congenital anomaly.
Secondary outcomes included live birth (birth after 20 weeks) rate, time to pregnancy, pregnancy loss rate, multiple pregnancy rate, and pregnancy complication rate including development of gestational hypertension and diabetes, birth weight, and neonatal complication rate.
Statistical analysis
Estimates used for the power calculation are based on our experience from previous trial [18,19]. We chose this narrower definition of a healthy birth outcome rather than live birth per se, as a healthy child is the patient, and provider-desired outcome and similar criteria have been used in previous trials [12,13]. We anticipated the proportion to be 0.25 in the standard lifestyle intervention arm and 0.40 in the intensive lifestyle modification arm. A sample size of 152 per treatment arm provided 80% power to detect a 0.15 absolute difference in the proportions of healthy births using a 2-sided test with a significance level of 0.05. The sample size was inflated to a total of 380 participants to allow for a 20% dropouts. The 20% dropout rate was used based on our experience in previous multicenter infertility trials [18–20,25].
All data entry, data management, and analyses were performed at the data coordinating center (DCC; Collaborative Center for Statistics in Science at Yale University).
The primary analyses used an intent-to-treat principle, wherein all randomized patients were analyzed according to their randomized treatment assignment, regardless of the actual treatment they received, protocol violations, or dropouts for the primary outcome. A chi-squared test (or Fisher exact test if any frequency count was <5) was used for testing differences between the 2 treatment groups for categorical variables and a Wilcoxon rank sum test used for continuous comparisons. All hypothesis tests were 2 sided, and all analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina, US) or R (open source). Statistical significance was defined as a 2-sided P value of less than 0.05. Cox proportional hazards models and the Kaplan–Meier method were applied to compare time to pregnancy and time to live birth in the treatment groups (S1 Fig). This study is reported as per the Consolidated Standards of Reporting Trials (CONSORT) guideline (S1 Checklist).
Results
Characteristics of the patients
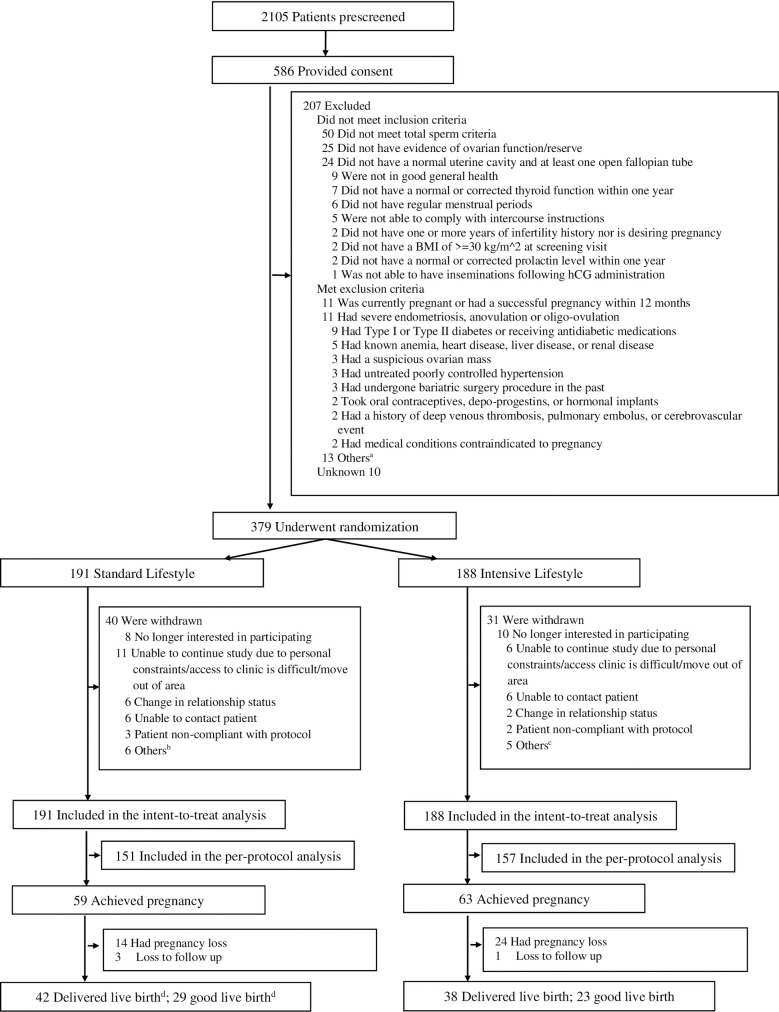
A total of 379 women were randomized to 1 of 2 treatment groups (Fig 1). The 2 groups were well matched at baseline (Table 1). A total of 40 of 191(20.9%) women withdrew from the standard lifestyle group and 31 of 188 (16.5%) from the intensive group (P = 0.267). There were no significant differences between groups for reasons of study withdrawal (S1–S8 Tables). The last patient was delivered in September of 2019.
Fig 1. Flowchart: Enrollment and outcomes of the trial.
aOne had a history of or suspected cervical/endometrial/or breast carcinoma; 1 had known Cushing disease, known or suspected adrenal or ovarian secreting tumors, or a history of gout; 1 had a history of alcohol abuse; 1 had an allergy, known hypersensitivity, or contraindication to the treatment medications; 1 had a presence of severe, untreated psychiatric illness; 1 had medical conditions that would be contraindicated to Orlistat, 1 had contraindication to study requirements including diet recommendation and activity requirements; 1 currently participating in lifestyle intervention program; and 1 in a period of acute weight loss or lost more than 5% body weight within the last 6 months. One participant or her male partner had previous sterilization procedures; 1 participant or her partner legally married to someone else; 1 using donated semen; and 1 have had pelvic radiation. bOne borderline HgA1C, 1 diagnosed with pulmonary embolism; 1 no response to Clomid; 1 patient developed a large complex cyst; 1 persistent cyst unable to start CC/IUI cycle; and 1 did not want to return for end of study visit. cOne patient had persistent ovarian cyst greater than 3 cm in size; 1 patient refused to see mental health provider regarding suicidal ideation; 1 PI discontinued patient from study; 1 patient’s spouse did not have adequate amount of sperm for IUI; and 1 husband did not want to proceed. dIncluding 1 patient who withdrew the study due to the borderline HgA1C and later achieved pregnancy and delivered live twin babies. BMI, body mass index; CC/IUI, clomiphene citrate/intrauterine insemination; hCG, human chorionic gonadotropin; HgA1C, hemoglobin A1c; PI, principal investigator.
Table 1. Baseline characteristics of the patients.
| Standard lifestyle | Intensive lifestyle | |
|---|---|---|
| Demographic | ||
| Age (y) | 191 | 188 |
| 32.4 ± 4.0 | 32.1 ± 4.5 | |
| 32.0 (30.0 to 35.0) | 32.0 (29.0 to 35.0) | |
| Level of education | ||
| High school graduate or less | 19/191 (9.9%) | 20/188 (10.6%) |
| College graduate or some college | 134/191 (70.2%) | 138/188 (73.4%) |
| Graduate degree | 38/191 (19.9%) | 30/188 (16.0%) |
| Ethnicity | ||
| Not Hispanic or Latino | 177/191 (92.7%) | 179/188 (95.2%) |
| Hispanic or Latino | 9/191 (4.7%) | 3/188 (1.6%) |
| Unknown | 5/191 (2.6%) | 6/188 (3.2%) |
| Race | ||
| White | 140/191 (73.3%) | 126/188 (67.0%) |
| Black | 37/191 (19.4%) | 45/188 (23.9%) |
| Asian | 3/191 (1.6%) | 4/188 (2.1%) |
| American Indian or Alaska Native | 2/191 (1.0%) | 2/188 (1.1%) |
| Native Hawaiian or other Pacific Islander | 0/191 (0.0%) | 0/188 (0.0%) |
| Unknown | 4/191 (2.1%) | 5/188 (2.7%) |
| Mixed race | 5/191 (2.6%) | 6/188 (3.2%) |
| How long has the patient been attempting conception (months)? | 188 | 187 |
| 39.1 ± 33.0 | 38.7 ± 28.6 | |
| 25.0 (16.5 to 48.0) | 24.0 (18.0 to 48.0) | |
| Prior live birth | 70/191 (36.6%) | 57/187 (30.5%) |
| Current smoker | 16/191 (8.4%) | 16/188 (8.5%) |
| Weight (kg) | 191 | 187 |
| 107.4 ± 20.8 | 108.4 ± 22.7 | |
| 106.3 (91.8 to 119.9) | 105.0 (92.9 to 120.3) | |
| BMI at baseline (kg/m2) | 191 | 188 |
| 39.4 ± 6.9 | 39.2 ± 7.0 | |
| 38.1 (33.9 to 44.3) | 37.8 (33.6 to 43.4) | |
| Waist circumference at baseline (cm) | 191 | 188 |
| 115.3 ± 15.6 | 115.6 ± 15.6 | |
| 114.0 (103.5 to 125.0) | 113.0 (104.1 to 123.5) | |
| Systolic blood pressure (mm Hg) | 189 | 187 |
| 123.3 ± 11.4 | 123.7 ± 12.6 | |
| 122.0 (116.0 to 131.0) | 124.0 (115.0 to 131.0) | |
| Diastolic blood pressure (mm Hg) | 190 | 187 |
| 79.8 ± 10.6 | 80.0 ± 9.9 | |
| 80.0 (74.0 to 87.0) | 81.0 (74.0 to 86.0) | |
| Average steps per day at baseline (steps) | 189 | 187 |
| 6,945 ± 2,770 | 6,723 ± 2,501 | |
| 6,635 (4,845 to 8,708) | 6,260 (4,869 to 8,091) | |
| Standard lifestyle | Intensive lifestyle | |
| Biochemical | ||
| Total testosterone (ng/dL) | 189 | 185 |
| 23.3 ± 15.2 | 26.1 ± 17.4 | |
| 18.5 (12.1 to 30.8) | 20.7 (13.3 to 33.1) | |
| SHBG (nmol/L) | 190 | 185 |
| 35.8 ± 18.9 | 39.0 ± 19.9 | |
| 32.9 (23.6 to 43.4) | 34.3 (25.9 to 48.4) | |
| Fasting glucose (mg/dL) | 190 | 185 |
| 95.4 ± 14.3 | 94.0 ± 13.6 | |
| 92.4 (84.7 to 104.2) | 91.8 (85.3 to 99.3) | |
| Fasting insulin (uIU/mL) | 188 | 185 |
| 17.9 ± 20.7 | 19.3 ± 16.9 | |
| 14.0 (9.1 to 19.8) | 14.7 (9.5 to 22.8) | |
| Leptin (ng/mL) | 190 | 185 |
| 77.9 ± 49.7 | 79.9 ± 49.8 | |
| 62.2 (44.6 to 85.7) | 66.1 (48.5 to 86.2) | |
| HbA1c (%) | 172 | 169 |
| 5.5 ± 0.4 | 5.4 ± 0.4 | |
| 5.4 (5.2 to 5.7) | 5.4 (5.2 to 5.6) | |
| Adiponectin (ng/mL) | 190 | 185 |
| 12,517 ± 8,323.9 | 11,409 ± 6,969.8 | |
| 10,518 (6,909.4 to 15,634) | 9,746.6 (6,536.0 to 13,963) | |
| hs-CRP (mg/L) | 189 | 185 |
| 7.5 ± 6.4 | 9.9 ± 13.7 | |
| 5.7 (2.8 to 10.1) | 6.2 (2.9 to 13.1) | |
| Total cholesterol (mg/dL) | 183 | 176 |
| 186.8 ± 35.0 | 187.3 ± 33.9 | |
| 183.0 (161.0 to 210.0) | 183.5 (165.5 to 210.0) | |
| Triglycerides (mg/dL) | 183 | 176 |
| 128.8 ± 62.5 | 130.0 ± 59.2 | |
| 118.0 (83.0 to 158.0) | 117.0 (90.5 to 157.0) | |
| HDL cholesterol (mg/dL) | 183 | 176 |
| 40.3 ± 9.0 | 40.1 ± 8.4 | |
| 39.0 (34.0 to 46.0) | 39.5 (33.5 to 45.0) | |
| Integrated biometric and biochemical | ||
| Metabolic syndrome | 98/183 (53.6%) | 93/176 (52.8%) |
| Questionnaire | ||
| Total score of fertility QoLa | 188 | 184 |
| 75.7 ± 13.1 | 78.0 ± 12.6 | |
| 77.1 (66.7 to 85.9) | 80.7 (69.8 to 87.5) | |
| Male partner | ||
| Age (y), maleb | 191 | 187 |
| 34.9 ± 5.8 | 33.5 ± 5.3 | |
| 35.0 (30.0 to 39.0) | 33.0 (30.0 to 37.0) | |
| BMI (kg/m2), male | 191 | 188 |
| 32.8 ± 7.5 | 32.5 ± 7.7 | |
| 32.3 (26.9 to 37.2) | 30.9 (27.5 to 36.0) | |
| Total motile sperm (million) | 191 | 188 |
| 86.4 ± 154.6 | 77.7 ± 85.3 | |
| 44.5 (21.6 to 105.3) | 46.9 (21.5 to 94.7) | |
Continuous variables are shown as n (top), mean ± SD (middle), and median (interquartile range) (bottom). Categorical variables are shown as no./total n (%).
aScore ranges from 0 to 100, with higher scores indicating better quality of life.
bP = 0.025 for the comparison between the 2 groups.
BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; QoL, Quality of Life; SHBG, sex hormone–binding globulin.
Effects of preconception intervention
Both groups significantly increased their step counts compared to baseline; there was no signficant difference between groups. Weight loss was significantly greater in the intensive group and approached our target of 7% (−6.6 ± 5.4%) compared to the standard group (P < 0.001), which did not experience significant weight loss (Table 2). A significantly greater proportion achieved a 5% and 10% weight loss in the intensive group than in the standard group (P < 0.001). Compared to the standard, the intensive lifestyle group experienced significant improvements in multiple metabolic and reproductive parameters, both biometric (decreased blood pressure and waist circumference) and biochemical (decreased total testosterone, insulin, glycohemoglobin, leptin, hsCRP, and triglycerides and increased sex hormone–binding globulin and adiponectin levels). The incidence of the metabolic syndrome decreased significantly in the intensive group compared to the standard group (P = 0.003). There were no significant differences between groups in quality of life measures and multiple other measures (S1–S8 Tables).
Table 2. Effect of 16-week lifestyle intervention programs on biometric and biochemical parameters.
| Standard lifestyle | Intensive lifestyle | P valueb | |
|---|---|---|---|
| Biometric | |||
| Change in average steps per day (steps) | 187 | 185 | |
| 1,819 ± 2,844 | 2,022 ± 2,794 | ||
| 1,732 (61 to 3,840) | 2,108 (140 to 4,245) | 0.430 | |
| Absolute change BMI (kg/m2) | 184 | 180 | |
| −0.1 ± 1.3 | −2.6 ± 2.1 | ||
| −0.1 (−0.8 to 0.6) | −2.3 (−4.2 to −1.0) | <0.001 | |
| Absolute change in weight (kg) | 184 | 180 | |
| −0.3 ± 3.4 | −7.3 ± 6.0 | ||
| −0.3 (−2.2 to 1.5) | −6.4 (−11.4 to −2.9) | <0.001 | |
| Percentage of weight (%) | 184 | 180 | |
| −0.3 ± 3.2 | −6.6 ± 5.4 | ||
| −0.3 (−2.0 to 1.5) | −6.2 (−10.3 to −2.9) | <0.001 | |
| Weight loss by 5% or more | 12/184 (6.5%) | 107/180 (59.4%) | <0.001 |
| Weight loss by 10% or more | 2/184 (1.1%) | 48/180 (26.7%) | <0.001 |
| Absolute change in systolic blood pressure | 182 | 180 | |
| −1.1 ± 12.7 | −3.1 ± 11.6 | ||
| 0.0 (−9.0 to 8.0) | −4.5 (−10.5 to 5.0) | 0.064 | |
| Absolute change in diastolic blood pressure | 183 | 180 | |
| 0.3 ± 10.8 | −1.8 ± 10.3 | ||
| 1.0 (−6.0 to 5.0) | −2.0 (−8.5 to 4.0) | 0.012 | |
| Absolute change in waist circumference (cm) | 184 | 180 | |
| −0.8 ± 6.8 | −7.7 ± 8.4 | ||
| −1.0 (−5.0 to 3.0) | −7.0 (−12.0 to −2.3) | <0.001 | |
| Biochemical | |||
| Total testosterone (ng/dL) | 156 | 146 | |
| 1.1 ± 14.0 | −3.6 ± 13.1 | ||
| 0.0 (−7.9 to 9.1) | −3.5 (−11.3 to 2.5) | 0.002 | |
| SHBG (nmol/L) | 159 | 149 | |
| 1.5 ± 14.1 | 4.6 ± 14.2 | ||
| 0.6 (−5.3 to 6.3) | 2.5 (−3.6 to 13.4) | 0.018 | |
| Fasting insulin (uIU/mL) | 158 | 149 | |
| −1.2 ± 22.1 | −4.1 ± 15.0 | ||
| 0.0 (−3.9 to 3.7) | −3.7 (−7.6 to 0.3) | <0.001 | |
| Leptin (ng/mL) | 160 | 149 | |
| −5.1 ± 37.6 | −29.0 ± 43.0 | ||
| −2.4 (−20.0 to 13.5) | −21.1 (−38.2 to −6.4) | <0.001 | |
| 133 | 125 | ||
| HgbA1c (%) | 0.09 ± 0.33 | −0.03 ± 0.25 | |
| 0.10 (−0.10 to 0.20) | 0.00 (−0.20 to 0.10) | 0.005 | |
| Adiponectin (ng/mL) | 160 | 149 | |
| −324.6 ± 7,402.0 | 1,900.0 ± 6,333.8 | ||
| 114.7 (−2,389 to 2,813.2) | 1,555.0 (−500.5 to 4,751.0) | 0.001 | |
| hs-CRP (mg/L) | 159 | 149 | |
| 0.4 ± 5.2 | −2.3 ± 14.1 | ||
| 0.3 (−1.6 to 2.1) | −1.1 (−2.9 to 0.6) | <0.001 | |
| Standard lifestyle | Intensive lifestyle | P value | |
| Triglycerides (mg/dL) | 152 | 136 | |
| −2.1 ± 50.3 | −15.9 ± 48.9 | ||
| −1.0 (−25.0 to 18.5) | −15.0 (−35.0 to 3.5) | 0.005 | |
| Integrated biochemical and biometric | |||
| Prevalence of the metabolic syndrome | 78/158 (49.4%) | 46/143 (32.2%) | 0.003 |
| Questionnaire | |||
| Total score of fertility QoLa | 161 | 146 | |
| −0.8 ± 9.0 | −1.6 ± 10.2 | ||
| 0.0 (−6.3 to 5.2) | −0.4 (−7.3 to 5.2) | 0.670 | |
Continuous variables are shown as n (top), mean ± SD (middle), and median (interquartile range) (bottom). Categorical variables are shown as no./total n (%) at the end of the intervention. Data reported are the change from baseline reported in Table 1.
aScore ranges from 0 to 100, with higher scores indicating better quality of life.
bP values were calculated using chi-squared or Fisher exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; QoL, Quality of Life; SHBG, sex hormone–binding globulin.
Healthy live births and secondary outcomes
The rate of having a healthy live birth was not significantly different between groups (S1 Fig, Table 3). Similarly, there was no significant difference in the rates of live births, multiple pregnancies, or the time to live birth. Pregnancy loss was greater in the intensive group, although not statistically significant. Duration of pregnancy, rate of cesarean section, and birth weight in grams were similar between the 2 groups. Per protocol analysis, likewise found no signficant differences (S1–S8 Tables). There were no significant differences in the pregnancy rates by time of conception during the study (S1–S8 Tables, S5 Fig). A post hoc BMI tertile analysis of the groups did not find any significant subgroup benefit of either lifestyle intervention on the primary outcome (S2 Fig) or having a live birth (S3 Fig). Results were similar with no statistically significant interaction when the patients were stratified by male partner age (S8 Table).
Table 3. Pregnancy outcomes according to intervention groups.
| Standard lifestyle | Intensive lifestyle | Rate ratio in intensive lifestyle group (95% CI) | P valuea | |
|---|---|---|---|---|
| Good live birth | 29/191 (15.2%) | 23/188 (12.2%) | 0.81 (0.48 to 1.34) | 0.404 |
| Live birth | 42/191 (22.0%) | 38/188 (20.2%) | 0.92 (0.62 to 1.36) | 0.672 |
| Singleton live birth | 39/42 (92.9%) | 32/38 (84.2%) | 0.91 (0.77 to 1.07) | 0.296 |
| Twin live birth | 3/42 (7.1%) | 6/38 (15.8%) | 2.21 (0.59 to 8.23) | 0.296 |
| Birth weight, grams | N = 42 | N = 36 | ||
| 3,105.9 ± 794.4 | 3,198.9 ± 711.7 | |||
| 3,189.3 (2,636.5 to 3,671.3) | 3,217.7 (2,802.4 to 3,642.9) | 0.952 | ||
| Low birth weight (<2,500 g) | 8/42 (19.0%) | 5/36 (13.9%) | 0.73 (0.26 to 2.03) | 0.542 |
| High birth weight (>4,000 g) | 5/42 (11.9%) | 4/36 (11.1%) | 0.93 (0.27 to 3.22) | 1.000 |
| Duration of pregnancy, weeks | N = 42 | N = 36 | ||
| 37.8 ± 2.6 | 38.2 ± 1.7 | |||
| 38.8 (37.0 to 39.0) | 38.3 (37.0 to 39.5) | 0.984 | ||
| Method of delivery | ||||
| Vaginal birth | 17/40 (42.5%) | 17/36 (47.2%) | 1.11 (0.67 to 1.83) | 0.679 |
| Cesarean section | 23/40 (57.5%) | 19/36 (52.8%) | 0.92 (0.61 to 1.38) | 0.679 |
| Conception | 59/191 (30.9%) | 63/188 (33.5%) | 1.08 (0.81 to 1.45) | 0.585 |
| Time to conception, days | N = 52 | N = 59 | ||
| 158.1 ± 80.1 | 148.6 ± 62.4 | |||
| 163.0 (98.5 to 214.0) | 160.0 (102.0 to 190.0) | 0.564 | ||
| Clinical pregnancy | 47/191 (24.6%) | 52/188 (27.7%) | 1.12 (0.80 to 1.58) | 0.499 |
| Pregnancy | 45/191 (23.6%) | 48/188 (25.5%) | 1.08 (0.76 to 1.54) | 0.656 |
| Singleton pregnancy | 42/45 (93.3%) | 41/48 (85.4%) | 0.92 (0.80 to 1.05) | 0.319 |
| Twin pregnancy | 3/45 (6.7%) | 7/48 (14.6%) | 2.19 (0.60 to 7.95) | 0.319 |
| Sex ratio at birth (boys: girls) | 0.88 (21:24) | 0.76 (19:25) | 0.87 (0.38 to 2.00) | 0.741 |
| Pregnancy loss among women who conceived | 14/59 (23.7%) | 24/63 (38.1%) | 1.61 (0.92 to 2.80) | 0.087 |
| Loss in first trimester | 14/59 (23.7%) | 21/63 (33.3%) | 1.40 (0.79 to 2.50) | 0.241 |
| Biochemical | 6/59 (10.2%) | 7/63 (11.1%) | 1.09 (0.39 to 3.06) | 0.866 |
| Miscarriage | 3/59 (5.1%) | 10/63 (15.9%) | 3.12 (0.90 to 10.79) | 0.077 |
| Ectopic pregnancy | 4/59 (6.8%) | 2/63 (3.2%) | 0.47 (0.09 to 2.46) | 0.428 |
| Pregnancy of unknown location | 1/59 (1.7%) | 2/63 (3.2%) | 1.87 (0.17 to 20.12) | 1.000 |
| Standard lifestyle | Intensive lifestyle | Rate ratio in intensive lifestyle group (95% CI) | P value a | |
| Loss in second or third trimester | 0/59 (0.0%) | 3/63 (4.8%) | NA | 0.245 |
Live birth was defined by the delivery of a live-born infant. Good live birth was defined by the delivery of a live birth of an infant born at ≥37 weeks, with a birth weight between 2,500 and 4,000 g and without a major congenital anomaly. Conception was defined as having a rising serum level of hCG for 2 consecutive tests. Clinical pregnancy was defined by the observation of gestational sac on ultrasound. Pregnancy was defined by observation of fetal heart motion on ultrasonography. Miscarriage was defined as the loss of a clinical pregnancy.
aP values were calculated with the use of the chi-squared test or Fisher exact test for categorical data and the Wilcoxon rank sum test for continuous data.
CI, confidence interval; hCG, human chorionic gonadotropin; NA, not applicable.
Adverse events and pregnancy and neonatal complications
Four serious adverse events occurred in the standard group and 3 in the intensive group with no clear relationship to the interventions (Table 4). Gastrointestinal adverse events, specifically diarrhea, oily stools or discharge, and flatulence, were significantly more common in the intensive group (consistent with acknowledged side effects of Orlistat). While we were not able to demonstrate a statistically significant benefit to preconception weight loss on later perinatal complications, most major pregnancy (i.e., preterm labor, premature rupture of membranes [PROM], preeclampsia, and gestational diabetes) and neonatal (intrauterine growth restriction [IUGR] and neonatal intensive care unit [NICU] admission) morbidities had nonsignificant rate improvements in the intervention group (Table 4). The list of adverse events occurring at a frequency ≥2% is in S5 Table (S1–S8 Tables).
Table 4. Serious adverse events (all) and adverse events (with more than 2% of patients experiencing them) between the intervention groups.
| Standard lifestyle | Intensive lifestyle | P valuea | |
|---|---|---|---|
| Before conception | |||
| Serious adverse | |||
| Hospitalization | 0/191 | 2/188 (1.1%) | 0.245 |
| Pelvic pain | 0/191 | 1/188 (0.5%) | 0.496 |
| Appendicitis | 1/191 (0.5%) | 0/188 | 1.000 |
| Pneumonia | 1/191 (0.5%) | 0/188 | 1.000 |
| Pulmonary embolism | 1/191 (0.5%) | 0/188 | 1.000 |
| Complex cyst resulting in surgical intervention | 1/191 (0.5%) | 0/188 | 1.000 |
| Other adverse events | |||
| Constipation | 7/191 (3.7%) | 23/188 (12.2%) | 0.002 |
| Diarrhea | 9/191 (4.7%) | 35/188 (18.6%) | <0.001 |
| Fever | 0/191 | 5/188 (2.7%) | 0.029 |
| Flatulence | 2/191 (1.0%) | 33/188 (17.6%) | <0.001 |
| Mood swings | 9/191 (4.7%) | 2/188 (1.1%) | 0.062 |
| Nausea/vomiting | 24/191 (12.6%) | 41/188 (21.8%) | 0.017 |
| Oily stools/discharge | 0/191 | 43/188 (22.9%) | <0.001 |
| After conception | |||
| Serious adverse events—mother | |||
| Hospitalization during first trimester | 0/59 | 1/63 (1.6%) | 1.000 |
| Ectopic pregnancy | 2/59 (3.4%) | 1/63 (1.6%) | 0.610 |
| Pregnancy of unknown location | 3/59 (3.4%) | 3/63 (4.8%) | 1.000 |
| Marginal placenta previa | 0/59 | 1/63 (1.6%) | 1.000 |
| Placenta previa and preterm birth | 1/59 (1.7%) | 0/63 | 0.484 |
| Hospitalization | 1/59 (1.7%) | 2/63 (3.2%) | 1.000 |
| Other adverse events—mother | |||
| Preterm labor | 6/59 (10.2%) | 2/63 (3.2%) | 0.154 |
| Preeclampsia/eclampsia | 7/59 (11.9%) | 4/63 (6.3%) | 0.352 |
| Gestational diabetes | 10/59 (16.9%) | 6/63 (9.5%) | 0.225 |
| Incompetent cervix | 0/59 | 2/63 (3.2%) | 0.496 |
| PROM | 4/59 (6.8%) | 2/63 (3.2%) | 0.428 |
| Other complication | 3/59 (5.1%) | 4/63 (6.3%) | 1.000 |
| Placental abnormalities | 4/59 (6.8%) | 5/63 (7.9%) | 1.000 |
| Postpartum infection | 2/59 (3.4%) | 0/63 | 0.232 |
| Postpartum hemorrhage | 1/59 (1.7%) | 0/63 | 0.484 |
| Other postpartum complication(s) | 1/59 (1.7%) | 3/63 (4.8%) | 0.620 |
| Serious adverse events—fetus/infant | |||
| Hospitalization—infant | 2/42 (4.8%) | 1/38 (2.6%) | 1.000 |
| Myelomeningocele | 0/42 | 1/38 (2.6%) | 0.475 |
| Neonatal death | 0/42 | 0/38 | |
| Stillbirth | 0/42 | 0/38 | |
| Other adverse events—fetus/infant | |||
| Standard lifestyle | Intensive lifestyle | P value | |
| IUGR | 4/42 (9.5%) | 1/38 (2.6%) | 0.362 |
aP value was calculated using chi-squared or Fisher exact test.
IUGR, intrauterine growth restriction; PROM, premature rupture of membranes.
Discussion
In women with both obesity and unexplained infertility, an intensive preconception lifestyle intervention with an average weight loss of 7% did not improve the rate of having a healthy live birth or any live birth compared to an activity based intervention that was weight neutral. Likewise, there was no improvement in pregnancy rates, time to pregnancy, or birth weight. These results were unexpected despite improved cardiometabolic indicators after weight loss. These findings support that weight loss per se and improved cardiometabolic health obtained through preconception intervention do not guarantee improved pregnancy outcomes.
Our results are similar to 2 recent high-quality clinical trials of preconception weight loss interventions for women with obesity prior to infertility treatments. A trial conducted in the Netherlands found a significantly lower probability of live birth in infertile women treated with a 6-month preconception lifestyle intervention compared with proceeding immediately to infertility treatment [12]. A trial conducted in Sweden, which evaluated a 12-week preconception intervention, albeit with a more aggressive caloric restriction prior to in vitro fertilization (IVF), found no benefit for weight loss on the live birth rate compared to those patients who did not lose weight and immediately underwent IVF [13].
Despite similar outcomes, our trial offered several novel strengths. First, our trial only included women with unexplained infertility with the assumption that obesity per se is a significant infertility factor and did not include patients with other known infertility factors such as tubal disease or anovulation. Second, unlike our trial, neither of the 2 trials referenced provided a comparator treatment. The nonintervention groups went immediately to infertility treatment, thus skewing the nonintervention group toward a shorter time to pregnancy and the benefit of a longer period to receive infertility treatment. We sought moderate weight loss utilizing a multifocal approach with treatments transferable to the clinic and wireless digital devices to achieve and monitor compliance. Our patients achieved an average weight loss intermediate between the Dutch and Swedish studies. Differences to note are that the Dutch women lost weight over a comparatively longer period (only 37% achieved the targeted ≥5% weight loss), and the Swedish women lost excessive weight over a comparatively short time period (55% achieved a ≥10% weight loss). Nevertheless, there was a similar lack of benefit.
There are potential concerns with preconception lifestyle interventions that small trials, such as ours and the others, may be underpowered to detect. However, each of the studies including ours found more pregnancy loss in the weight loss intervention groups (albeit not significant for any individual trial). Pooling the results did indicate more miscarriages (relative risk 1.79, 95% confidence interval [CI]: 1.20 to 2.67). Pooled results are in S6 Table (63/620 or 10.0% in the weight loss group versus 35/628 or 5.6% in the nonweight loss groups) (S6 Table). Our results indicate that these losses tended to occur after implantation and ultrasound visualization of the gestational sac (S4 Fig) and were more likely to occur in later rather than earlier cycles of infertility treatment (P = 0.049) (S1–S8 Tables). It is possible that in our studies and others, vitamin or micronutrient deficiency, including decreased long-chain polyunsaturated fatty acid absorption, may have contributed to pregnancy loss. While we did not assess dietary intake and composition throughout the study, participants in the intensive intervention group received a multivitamin supplement during the intervention phase as commonly done with the use of Orlistat, and both groups were prescribed a prenatal vitamin upon randomization. We also chose our meal replacements as they were of high quality and nutritionally balanced.
Another strength of our study is that we collected not only pregnancy outcomes, but also all maternal and neonatal complications after conception. Future studies, including individual patient data meta-analyses, tracking these outcomes will better illuminate the effects of preconception weight loss. The best available epidemiologic evidence of the mixed effects of significant preconception weight loss on pregnancy morbidities comes from Swedish national registry reports, where women who underwent bariatric surgery had a lower rate of gestational diabetes, but significantly higher rates of spontaneous preterm delivery and SGA babies compared to women without obesity [15]. The weight loss mechanism differs after different types of bariatric surgery, and more significant weight loss usually occurs after surgery compared to our intervention with a corresponding greater chance for malabsorption.
The present investigation was underpowered to address these other perinatal outcomes. Further studies and the use of individual patient data meta-analysis may be necessary to achieve the necessary numbers required to see differences in rare but severe morbidities related to obesity and pregnancy. In our study, birth weight (which did not differ between the lifestyle groups) may be the best integrated marker of perinatal health [26,27]. Our findings may only be specific to women with unexplained infertility as opposed to other disorders such as anovulation due to polycystic ovary syndrome [18]. Our live birth rates were also significantly less than we expected, presumably due to both the severity of obesity in our patient population and the comparative ineffectiveness of our frontline infertility therapies.
There are many avenues for future research. Other interventions of varying duration and/or intensity prior to conception may yield more favorable outcomes. A period of weight stabilization and maintenance after a weight loss intervention prior to commencing infertility therapy is worth exploring. However, this must be balanced against the desire of the couple to have a baby as soon as possible and their unwillingness to delay meaningful treatment. Recruitment and retention into such a trial with prolonged participation and delayed treatment may be difficult. Developing better comparators for weight loss interventions, beyond exercise or observation (which the couple may interpret as doing nothing), is another option. Alternate trial designs and comparators must provide equipoise to all participants.
Our findings directly impact current standards of clinical care, where women who are obese with unexplained infertility are to our knowledge routinely counseled to lose weight prior to initiation of infertility treatment. Presently, there is no Level I evidence to support the recommendation that preconception weight loss in women with obesity and unexplained infertility prior to treatment leads to either a higher chance of a healthy live birth or a shorter time to pregnancy.
In conclusion, we have demonstrated that we can achieve significant weight loss and improvement in cardiometabolic health through an intensive lifestyle intervention in women who are obese with unexplained infertility in a reasonably short time period of 16 weeks. This does not translate to a shortened time to pregnancy, an improved live birth, or healthy live birth rate. Improved weight and female cardiometabolic health may not equal improved fecundity.
Supporting information
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Good live birth rate is shown according to treatment group in panel A, and live birth rate is shown in panel B.
(TIF)
Good live birth rates are shown according to treatment group and maternal BMI (the weight in kilograms divided by the square of the height in meters) in panels A, B, and C. BMI, body mass index.
(TIF)
Live birth rates are shown according to treatment group and maternal BMI (the weight in kilograms divided by the square of the height in meters) in panels A, B, and C. BMI, body mass index.
(TIF)
Pregnancy loss rates are shown according to treatment groups.
(TIF)
P value was for the testing of the difference across the study phase and treatment cycles using the generalized linear model.
(TIF)
CONSORT, Consolidated Standards of Reporting Trials.
(DOC)
Acknowledgments
We thank the participants and the site personnel who assisted with the trial: Penn State: William Dodson, MD, Stephanie Estes, MD, Allen R. Kunselman, Patsy Rawa, Jamie Ober, RN, and Heidi Watts, RN; Penn State College of Health and Human Development: Jennifer Fleming, PhD, RD; University of Oklahoma: Michelle R. Starkey-Scruggs RN, LaTasha B. Craig, MD, Heather R. Burks, MD, Kisha Y. Turner, RN, and Christy Zornes; Augusta University: S. Brakta, J. Thiesen, L. Layman, L. Gavrilova-Jordan, L. Ogdon, and C. Laserna; University of Pennsylvania: Karen Lecks, CRNP and Bridget McKinney, MBE; Wayne State University: A. Awonuga, L. Cedo, K. Collins, E. Puscheck, and M. Yoscovits; University of North Carolina: Steve L. Young, MD, PhD and R. Matthew Coward, MD; University of California San Francisco: Rebecca Wong; University of Utah: Kathryn Szczotka; Yale University: Fangbai Sun, Tracey Thomas, Heatherly Carlson, Donna DelBasso, and Lilian Sakai; Advisory Board: David S. Guzick, MD, PhD (Chair), Amy M. Branum, PhD, Michael A. Thomas, MD, J. Bruce Redmon, MD, and Marlene B. Goldman, ScD; Data and Safety Monitoring Board: Frank R. Witter, MD (Chair), PonJola Coney, MD, Stacey A. Missmer, ScD, The Reverend Phillip Carlyle Cato, PhD, DD, Lurdes Y.T. Inoue, PhD, and Robert E. Brannigan, MD.
Disclaimers
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or NIH.
Abbreviations
- BMI
body mass index
- CC
clomiphene citrate
- CI
confidence interval
- CONSORT
Consolidated Standards of Reporting Trials
- DCC
data coordinating center
- hCG
human chorionic gonadotropin
- IRB
Institutional Review Board
- IUGR
intrauterine growth restriction
- IVF
in vitro fertilization
- NICHD
National Institute of Child Health and Human Development
- NICU
neonatal intensive care unit
- PROM
premature rupture of membranes
- SGA
small for gestational age
Data Availability
Anonymised data will be made available in the National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH) at: https://dash.nichd.nih.gov. Data will be shared with other researchers who submit a proper request to the Reproductive Medicine Network Steering Committee. Information about data and specimen sharing can be found at: https://publichealth.yale.edu/c2s2/rmn/resource/ Requests should be directed to rmn.rada@mailman.yale.edu Data will be de-identified.
Funding Statement
Funding for this project was provided by the National Institutes of Health (NIH)/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants U10 HD38992 (to R.S.L.), U10HD077680 (to K.R.H.), U10 HD39005 (to M.P.D.); U10HD077844 (to A. Z. S.), U10HD055925 (to H.Z.) U10 HD27049 (to C.C.), U54-HD29834 and R24-HD102061 (to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core of the Specialized Cooperative Centers Program in Reproduction and Infertility Research); The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR002014 (to Penn State University) and UL1 TR001863 (to Yale University). Nutrisystem (Nutrisystem, Fort Washington, PA 19034) provided the study organizers with discounted coupons to purchase monthly food allotments in the standardized group. Fitbit (FitBit, San Francisco, CA 9410) provided the study organizers with discounted Fitbits for activity monitoring. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a european multicenter study on infertility and subfecundity. J Epidemiol. 2000. Jun 1;151(11):1072–1079. doi: 10.1093/oxfordjournals.aje.a010150 [DOI] [PubMed] [Google Scholar]
- 2.Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3. doi: 10.1093/humrep/17.12.3220 [DOI] [PubMed] [Google Scholar]
- 3.Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90(3):714–26. doi: 10.1016/j.fertnstert.2007.07.1290 [DOI] [PubMed] [Google Scholar]
- 4.Wang JX, Davies MJ, Norman RJ. Obesity increases the risk of spontaneous abortion during infertility treatment. Obes Res. 2002;10(6):551–4. doi: 10.1038/oby.2002.74 [DOI] [PubMed] [Google Scholar]
- 5.Garbaciak JA Jr, Richter M, Miller S, Barton JJ. Maternal weight and pregnancy complications. J Obstet Gynecol. 1985;152(2):238–45. doi: 10.1016/s0002-9378(85)80029-6 [DOI] [PubMed] [Google Scholar]
- 6.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9(2):140–50. doi: 10.1111/j.1467-789X.2007.00464.x [DOI] [PubMed] [Google Scholar]
- 7.Sarwer DB, Allison KC, Gibbons LM, Markowitz JT, Nelson DB. Pregnancy and obesity: a review and agenda for future research. J Womens Health (Larchmt). 2006;15(6):720–33. doi: 10.1089/jwh.2006.15.720 [DOI] [PubMed] [Google Scholar]
- 8.Balen AH, Anderson RA. Policy, Practice Committee of the BFS. Impact of obesity on female reproductive health: British Fertility Society. Policy and Practice Guidelines. Hum Fertil (Camb). 2007;10(4):195–206. doi: 10.1080/14647270701731290 [DOI] [PubMed] [Google Scholar]
- 9.Johnson NP, Stewart AW, Falkiner J, Farquhar CM, Milsom S, Singh V-P, et al. PCOSMIC: a multi-centre randomized trial in women with PolyCystic Ovary Syndrome evaluating Metformin for Infertility with Clomiphene. Hum Reprod. 2010;25(7):1675–83. doi: 10.1093/humrep/deq100 [DOI] [PubMed] [Google Scholar]
- 10.Farquhar CM, Gillett WR. Prioritising for fertility treatments—should a high BMI exclude treatment? BJOG. 2006;113(10):1107–9. doi: 10.1111/j.1471-0528.2006.00994.x [DOI] [PubMed] [Google Scholar]
- 11.Tsagareli V, Noakes M, Norman RJ. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertil Steril. 2006;86(1):227–9. doi: 10.1016/j.fertnstert.2005.12.041 [DOI] [PubMed] [Google Scholar]
- 12.Mutsaerts MA, van Oers AM, Groen H, Burggraaf JM, Kuchenbecker WK, Perquin DA, et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N Engl J Med. 2016;374(20):1942–53. doi: 10.1056/NEJMoa1505297 [DOI] [PubMed] [Google Scholar]
- 13.Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlstrom PO, et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod. 2017;32(8):1621–30. doi: 10.1093/humrep/dex235 [DOI] [PubMed] [Google Scholar]
- 14.Roos N, Neovius M, Cnattingius S, Trolle LY, Saaf M, Granath F, et al. Perinatal outcomes after bariatric surgery: nationwide population based matched cohort study. BMJ. 2013;347:f6460. doi: 10.1136/bmj.f6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson K, Cnattingius S, Naslund I, Roos N, Trolle LY, Granath F, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372(9):814–24. doi: 10.1056/NEJMoa1405789 [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mena GP, Mielke GI, Brown WJ. The effect of physical activity on reproductive health outcomes in young women: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(5):541–63. doi: 10.1093/humupd/dmz013 [DOI] [PubMed] [Google Scholar]
- 18.Legro RS, Dodson WC, Kris-Etherton PM, Kunselman AR, Stetter CM, Williams NI, et al. Randomized Controlled Trial of Preconception Interventions in Infertile Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2015;100(11):4048–58. doi: 10.1210/jc.2015-2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, et al. Letrozole, Gonadotropin, or Clomiphene for Unexplained Infertility. N Engl J Med. 2015;373(13):1230–40. doi: 10.1056/NEJMoa1414827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–29. doi: 10.1056/NEJMoa1313517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–79. doi: 10.1056/NEJMoa1109220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mumford SL, Johnstone E, Kim K, Ahmad A, Salmon S, Summers K, et al. A Prospective Cohort Study to Evaluate the Impact of Diet, Exercise, and Lifestyle on Fertility: Design and Baseline Characteristics. Am J Epidemiol. 2020;189(11):1254–65. doi: 10.1093/aje/kwaa073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Look AHEAD Research Group, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obes Res. 2006;14(5):737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen KR, Peck JD, Coward RM, Wild RA, Trussell JC, Krawetz SA, et al. Intrauterine insemination performance characteristics and post-processing total motile sperm count in relation to live birth for couples with unexplained infertility in a randomised, multicentre clinical trial. Hum Reprod. 2020;35(6):1296–305. doi: 10.1093/humrep/deaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–66. doi: 10.1056/NEJMoa063971 [DOI] [PubMed] [Google Scholar]
- 26.Wilcox AJ, Russell IT. Birthweight and perinatal mortality: I. On the frequency distribution of birthweight. Int J Epidemiol. 1983;12(3):314–8. doi: 10.1093/ije/12.3.314 [DOI] [PubMed] [Google Scholar]
- 27.Wilcox AJ, Russell IT. Birthweight and perinatal mortality: II. On weight-specific mortality. Int J Epidemiol. 1983;12(3):319–25. doi: 10.1093/ije/12.3.319 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Good live birth rate is shown according to treatment group in panel A, and live birth rate is shown in panel B.
(TIF)
Good live birth rates are shown according to treatment group and maternal BMI (the weight in kilograms divided by the square of the height in meters) in panels A, B, and C. BMI, body mass index.
(TIF)
Live birth rates are shown according to treatment group and maternal BMI (the weight in kilograms divided by the square of the height in meters) in panels A, B, and C. BMI, body mass index.
(TIF)
Pregnancy loss rates are shown according to treatment groups.
(TIF)
P value was for the testing of the difference across the study phase and treatment cycles using the generalized linear model.
(TIF)
CONSORT, Consolidated Standards of Reporting Trials.
(DOC)
Data Availability Statement
Anonymised data will be made available in the National Institute of Child Health and Human Development (NICHD) Data and Specimen Hub (DASH) at: https://dash.nichd.nih.gov. Data will be shared with other researchers who submit a proper request to the Reproductive Medicine Network Steering Committee. Information about data and specimen sharing can be found at: https://publichealth.yale.edu/c2s2/rmn/resource/ Requests should be directed to rmn.rada@mailman.yale.edu Data will be de-identified.