Abstract
Background.
Biliary rhabdomyosarcoma (RMS) is the most common biliary tumor in children. The biliary tract is classified as a favorable primary site. Therefore, patients with localized biliary RMS were included in two consecutive low-risk studies, D9602 and ARST0331, by the Children’s Oncology Group (COG). The outcome for these patients treated with low-risk therapy has not been reported.
Procedure.
Patients with biliary RMS enrolled on COG low-risk trials D9602 or ARST0331 were analyzed. All patients received systemic chemotherapy and those with Group II (microscopic residual) or Group III (macroscopic residual) disease received 36–50.4 Gy adjuvant radiotherapy (RT). Delayed primary excision (DPE) was allowed on both studies.
Results.
Seventeen patients with biliary RMS were treated on D9602 (n = 7) or ARST0331 (n = 10). Median age was 3.5 years (range 1.7 to 10.3). Ten (59%) patients had tumors > 5 cm and 14 (82%) had Group III disease. Fifteen (88%) patients received RT. The five-year event-free survival (EFS) and overall survival (OS) were 70.6% [95% confidence interval (CI): 46.9%−94.3%] and 76.5% (95% CI: 54.6%−98.4]), respectively. The majority of patients (80%) who received RT did not have disease recurrence while both patients who did not receive RT had local relapse. Five (36%) of 14 patients with Group III disease underwent DPE; two experienced a local relapse. In the nine patients without DPE, two developed local relapse.
Conclusions.
Patients with localized biliary RMS treated on low-risk studies had suboptimal outcomes. These patients may benefit from therapy on intermediate-risk studies.
Keywords: Biliary rhabdomyosarcoma, chemotherapy, radiotherapy, surgery
Introduction
RMS is the most common pediatric soft-tissue sarcoma with an incidence of 0.4 cases per 100,000 children per year in the United States [1]. First recognized in 1875, biliary RMS comprises approximately 1% of all RMS and is the most common biliary tumor in children [2, 3]. Biliary RMS occurs in young patients with a median age of 3.4 years, and 90% of patients present before five years of age with jaundice accompanied by abdominal distention, choluria, acholic stools, nausea, vomiting, or fever that may be mistaken for a choledochal cyst or infectious hepatitis [4–7]. The majority of cases are of embryonal histology [4, 8]. Biliary RMS typically arises in the common bile duct but may originate anywhere along the biliary tree [5, 6, 9]. Regional and distant metastases have been reported in 30–40% of cases [5, 7].
Prior to 1965 and the use of multimodal therapies, outcomes were extremely poor with fatality in 83% of children with biliary RMS [7]. Intergroup Rhabdomyosarcoma Study (IRS) protocols I and II demonstrated that multimodal therapy with surgery, chemotherapy, and RT impacts survival [5]. Furthermore, reports in the late 1990s suggested that chemotherapy alone could lead to a complete pathological response [10, 11].
An analysis of 25 patients with biliary RMS treated per IRS I-IV from 1972 to 1998 demonstrated that gross total resection was not possible in the majority of patients [4]. Thirteen (93%) of the 14 patients with Group III disease had no evidence of disease (NED) at follow-up, and postoperative complications were more common in patients who underwent upfront radical surgery. Furthermore, the majority of patients with Group III disease who underwent DPE for residual imaging abnormalities had no viable tumor removed on pathology. Based on these results, surgery was only recommended to establish a diagnosis and the extent of local-regional disease. Finally, the 5-year OS for patients with local-regional disease was 78% (95% CI: 58–97%); all of the disease-related deaths occurred in patients treated on IRS I. After IRS IV, these findings led to re-classification of the biliary tract as a favorable primary site in the IRS staging system. Patients with localized biliary RMS subsequently received treatment on low-risk studies [12–15]. Thus, utilizing a retrospective analysis of COG low-risk clinical trials D9602 and ARST0331, we sought to describe the outcomes of patients with localized biliary RMS treated with reduced chemotherapy and RT on these low-risk studies.
Methods
Treatment of Patients on D9602 and ARST0331
From 1997 to 2004, patients with localized, embryonal biliary RMS were eligible for enrollment on D9602 [15]. Embryonal histology included botryoid and spindled variants. Patients with Group I (completely resected) or Group II disease without nodal involvement were assigned to Subgroup A and received vincristine and dactinomycin (VA) for 45 weeks. Patients with Group II disease with nodal involvement or Group III disease were assigned to Subgroup B and received vincristine, dactinomycin, and cyclophosphamide (VAC) for 45 weeks, with a total cumulative cyclophosphamide dose of 28.6 g/m2. Those with Group I disease did not receive RT while those with Group II disease received RT at week 3. Meanwhile, at week 12 those with Group III disease received either RT or DPE followed by RT. RT doses were given as follows: 36 Gy for Group II without nodal involvement, 41.4 Gy for Group II with nodal involvement, and 50.4 Gy for Group III. Patients with initial nodal involvement regardless of disease status after DPE received 41.4 Gy. Otherwise, those with negative margins or microscopic residual disease after DPE received 36 Gy, and those with gross residual disease after DPE received 50.4 Gy. Proton RT and intensity-modulated RT were allowed.
From 2004 to 2010, patients with localized, embryonal biliary RMS were eligible for enrollment on ARST0331 [16, 17]. Embryonal histology included botryoid and spindled variants. All patients enrolled on ARST0331 initially received four cycles of VAC, with a total cumulative cyclophosphamide dose of 4.8 g/m2. Patients with Group I or II biliary RMS then received four cycles of VA (Subset 1); meanwhile, patients with Group III disease received 12 cycles of VA (Subset 2). Patients with Group II disease received RT at Week 13 and those with Group III disease received either RT or DPE followed by RT. RT dosing guidelines were the same as those on D9602.
Patients and Tumors
All patients with biliary RMS, defined as arising from the intrahepatic or extrahepatic biliary tree, gallbladder, cystic duct, or ampulla of Vater, treated on either D9602 or ARST0331 were included for analysis. Clinical variables were analyzed including gender, age, stage, group, and whether patients received RT or DPE. Diagnoses were established by central pathologic review. All RT underwent centralized quality assurance review.
Statistical Analysis
Local failure was defined as progression or relapse at the primary site as a first event (with or without concurrent regional and/or distant failure). Regional failure was defined as recurrence in tissue adjacent to the primary site or regional lymph nodes, and distant failure was defined as the appearance of distant metastases. EFS was defined as the time from study enrollment to disease recurrence, second malignancy, or death, whichever occurred first. OS was defined as the time from enrollment to death from any cause. The Kaplan-Meier method was used to estimate survival distributions and the Peto-Peto method was used to estimate the standard error of the Kaplan-Meier estimate [18, 19]. Patient follow-up was current through December 31, 2018.
Results
Clinical Characteristics
Clinical characteristics are listed in Table 1. Seventeen patients with biliary RMS were treated on D9602 (n = 7) or ARST0331 (n = 10). Nine (53%) patients were male. The median age at diagnosis was 3.5 years (range 1.7 to 10.3). Ten tumors (59%) were > 5 cm in size. Fourteen (82%) patients had Group III, two patients (12%) had Group IIA, and one (6%) patient had Group I disease. Four patients (24%) had regional nodal involvement by exam or imaging, two (12%) by pathologic evaluation. All tumors had embryonal histology including eight (47%) botryoid and one (6%) spindled variant.
TABLE 1.
Clinical Characteristics
| Characteristic | N (%) |
|---|---|
|
| |
| Gender | |
| Male | 9 (52.9%) |
| Female | 8 (47.1%) |
|
| |
| Race | |
| Caucasian | 12 (70.6%) |
| African American | 2 (11.8%) |
| Asian | 1 (5.8%) |
| Unknown | 2 (11.8%) |
|
| |
| Ethnicity | |
| Hispanic or Latino | 4 (23.5%) |
| Not Hispanic or Latino | 13 (76.5%) |
|
| |
| Age, Years | |
| ≤ 2 | 3 (17.7%) |
| 2–10 | 13 (76.5%) |
| > 10 | 1 (5.8%) |
|
| |
| Study | |
| D9602 | 7 (41.2%) |
| ARST0331 | 10 (58.8%) |
|
| |
| Histology | |
| Embryonal | 8 (47.1%) |
| Botryoid | 8 (47.1%) |
| Spindled | 1 (5.8%) |
|
| |
| Tumor Size | |
| ≤ 5 cm | 6 (35.3%) |
| > 5 cm | 10 (58.8%) |
| Unknown | 1 (5.9%) |
|
| |
| Regional Nodal Involvement | |
| Clinical or Imaging Evidence | |
| N0 (No nodal involvement) | 12 (70.6%) |
| N1 (Nodal involvement) | 4 (23.5%) |
| Not Evaluated/Unknown | 1 (5.9%) |
| Pathological Involvement | |
| No | 4 (23.5%) |
| Yes | 2 (11.8%) |
| Not Evaluated/Unknown | 11 (64.7%) |
|
| |
| Group | |
| I (Localized, completely resected) | 1 (5.9%) |
| II A (Grossly resected, microscopic residual) | 2 (11.8%) |
| III (Incompletely resected, gross residual) | 14 (82.3%) |
|
| |
| Radiotherapy | |
| Yes | 15 (88.2%) |
| No | 2 (11.8%) |
|
| |
| Delayed primary resection | |
| Yes | 6 (42.9%) |
| No | 8 (57.1%) |
RT
Fifteen (88%) patients received RT, seven with a boost. Of those, 11 (65%) received RT according to protocol guidelines based on central review. Two patients had minor RT deviations from protocol and two had insufficient RT data for central review. Two patients did not receive RT: one according to protocol guidelines for Group I disease and the other came off study at Week 12 after developing postoperative intestinal necrosis after a sub-total DPE. Both patients who were less than two years of age received RT according to protocol guidelines.
Surgery
Upfront complete resection was attempted in six patients and resulted in one patient with Group I disease; the type of surgery performed in this patient is unknown. Two patients underwent hepatic lobectomy and five patients underwent bile duct excision with Roux-en-y reconstruction. Two of these surgeries resulted in patients with Group II disease (one treated on D9602) and three resulted in patients with Group III disease (one treated on D9602). All of these patients later received RT. Five (36%) of the 14 patients with Group III disease underwent DPE. One patient had no tumor identified on exploration and no tissue was excised; RT data was not submitted for central review for this patient. One patient had tissue resected, but no disease was found on pathology. This patient received protocol-directed, reduced RT at 36 Gy. Two patients, one whose DPE was delayed until week 24 after RT to avoid a possible hepatic lobectomy, had tissue resected with pathology demonstrating viable tumor with extensive necrosis; however, both patients had gross residual disease left after DPE and received RT at 50.4 Gy. Finally, one patient had a partial resection with pathology showing predominantly viable disease and gross residual disease after DPE. This patient developed postoperative intestinal necrosis, came off study, and did not receive RT.
Outcomes
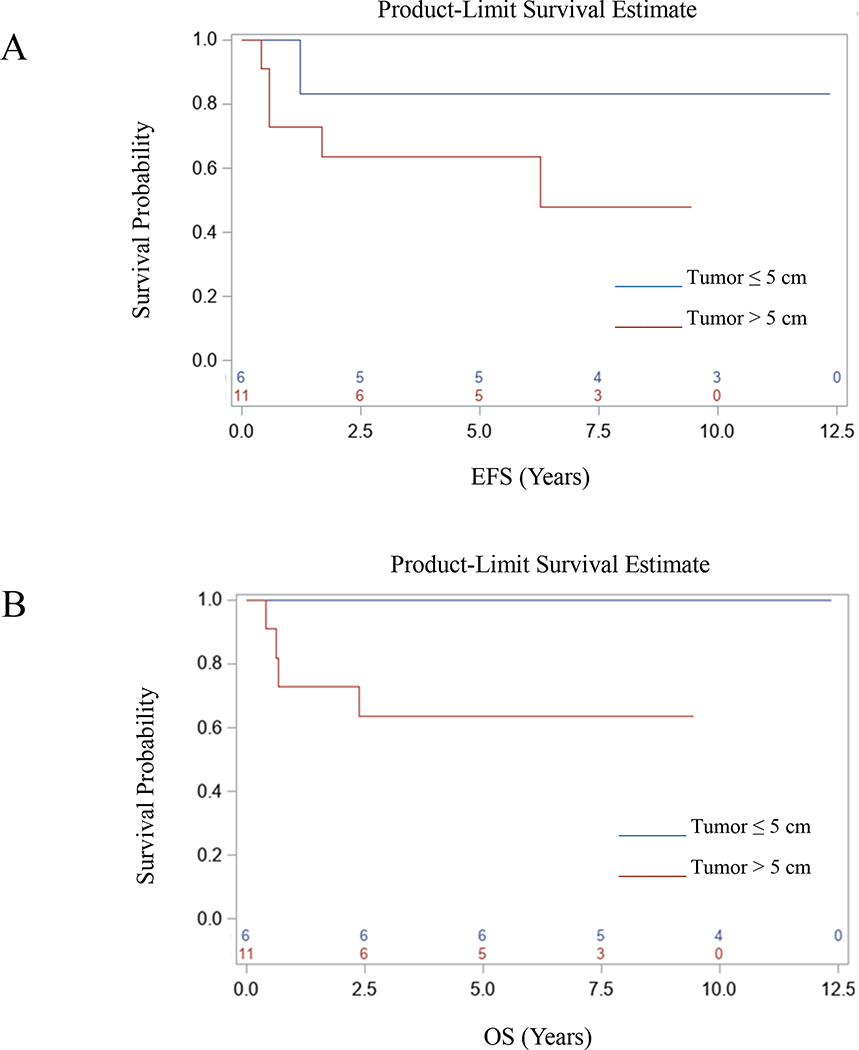
A summary of treatment and outcome for each patient is provided in Table 2. Of the 15 patients who received RT, three died. One patient (Patient 3) with Group II disease died from severe sinusoidal obstruction syndrome (SOS) and sepsis that developed one week after beginning RT. The other two patients died of disease (DOD); one of whom had Group III disease after an attempt at upfront resection. Twelve of the 15 patients were alive, 11 with NED (one of whom was Patient 5 with Group III disease who developed moderate SOS after receiving week 25 of therapy and came off study) and one was alive with disease (AWD). The two patients who did not receive RT relapsed: one DOD and the other had NED at last follow-up. Of the 14 patients with Group III disease, three DOD, and one was AWD. Of the five patients who underwent DPE, one patient (Patient 14) suffered post-operative intestinal necrosis and ultimately DOD; three had NED, and another was AWD at last follow-up. In the nine patients without DPE, two DOD. Seven patients were treated on D9602; one died within 6 months of starting treatment from severe SOS and sepsis that developed one week after RT, two DOD, and one was AWD. Ten patients were treated on ARST0331, and one DOD. For the entire cohort, the five-year EFS and OS were 70.6% (95% CI: 46.9–94.3%) and 76.5% (95% CI: 54.6–98.4%), respectively (Figs. 1A and 1B). There was a trend toward poorer EFS and OS for patients with tumors > 5 cm in size compared to patients with tumors ≤ 5 cm in size (Figs. 2A and 2B).
TABLE 2.
Summary of Treatment and Outcome
| Patient | Age (Yrs.) | Gender | Histology | TNM Stage | Clinical Group | Largest Tumor Diameter (cm) | Study | RT (Gy) | RT Boost (Yes/No) | DPE (Yes/No) | Relapse (Yes/No) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.1 | F | Botryoid | T1N0M0 | I* | 2.5 | ARST0331 | NA | NA | N | Y | NED |
| 2 | 1.9 | F | Embryonal | T2N0M0 | IIA* | 8.8 | ARST0331 | 36 | N | N | N | NED |
| 3 | 1.7 | M | Botryoid | T1N0M0 | IIA* | 7 | D9602 | 37.8 | Y | N | N | DOC |
| 4 | 2.7 | M | Botryoid | T2N0M0 | III | 5.5 | ARST0331 | 50.4 | N | N | N | NED |
| 5 | 2.6 | M | Botryoid | T1N1M0 | III* | 8.4 | ARST0331 | 50.4 | Y | N | N | NED |
| 6 | 2.9 | F | Embryonal | T1N0M0 | III | 3.2 | ARST0331 | 50.4 | Y | N | N | NED |
| 7 | 2.7 | M | Embryonal | T1NXM0 | III | 3.2 | ARST0331 | 50.4 | N | N | N | NED |
| 8 | 3.7 | F | Embryonal | T2N1M0 | III | 6.2 | ARST0331 | 50.4 | N | N | N | NED |
| 9 | 2.2 | M | Embryonal | T1N0M0 | III | 8.4 | ARST0331 | Unevaluable | Unevaluable | N | N | NED |
| 10 | 2.1 | M | Botryoid | T1N1M0 | III* | 2.6 | ARST0331 | 50.4 | Y | N | N | NED |
| 11 | 2 | F | Botryoid | T1N0M0 | III | 4 | D9602 | Unevaluable | Unevaluable | Y | N | NED |
| 12 | 3.3 | F | Botryoid | T1N0M0 | III | 8 | D9602 | 36 | N | Y | N | NED |
| 13 | 6.3 | M | Embryonal | T1N0M0 | III | 14 | D9602 | 50.4 | N | Y | N | NED |
| 14 | 2.8 | F | Embryonal | T1N0M0 | III | 8.8 | ARST0331 | NA | NA | Y | Y | DOD |
| 15 | 4.7 | F | Botryoid | T2N0M0 | III* | Unknown | D9602 | 45 | Y | N | Y | DOD |
| 16 | 10.3 | M | Embryonal | T1N0M0 | III | 8.1 | D9602 | 50.4 | Y | N | Y | DOD |
| 17 | 6.1 | M | Spindled | T2N1M0 | III | 2.9 | D9602 | 50.4 | N | Y | Y | AWD |
Abbreviations:
upfront primary excision attempted
RT, radiotherapy; NA, no radiotherapy received; DPE, delayed primary excision; NED, alive with no evidence of disease; DOC, died of complications; DOD, died of disease; AWD, alive with disease
FIGURE 1.
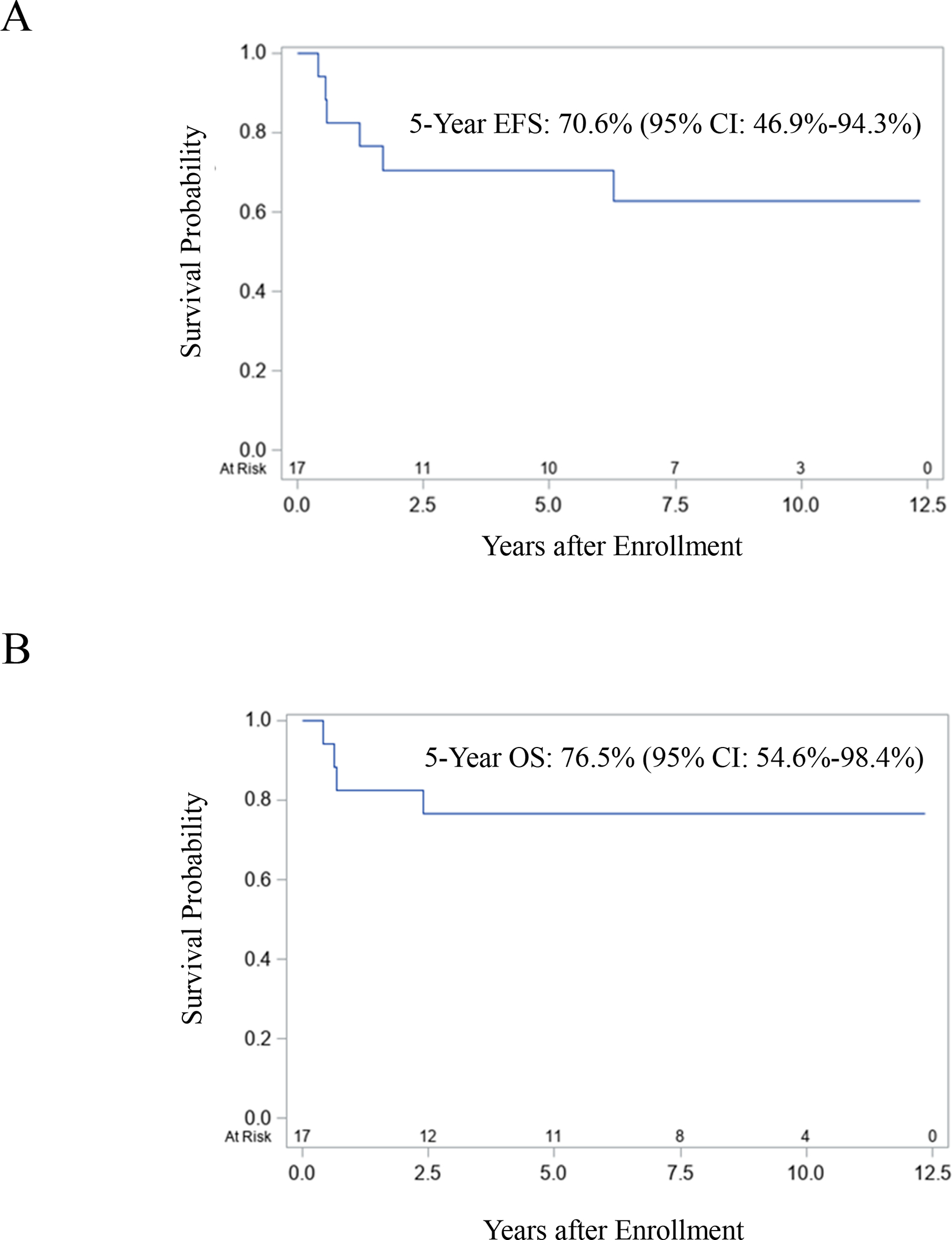
Event-free and overall survival.
FIGURE 2.
Event-free and overall survival based on tumor size.
Recurrences
Five patients experienced a recurrence. One patient with Group I disease had local recurrence but was salvaged with subsequent therapy. Four patients with Group III disease experienced relapse: two local-only relapses, one local + distant relapse, and one local + regional + distant relapse. Three patients who received RT experienced an event: one local-only relapse, one local + distant relapse, and one local + regional + distant relapse. All three patients received photon RT without deviation from protocol; two received a RT boost. The two patients with minor RT deviations from protocol did not develop disease recurrence. Twelve of 15 patients who received RT did not have local recurrence (80% local control) while both patients who did not receive RT had a local recurrence (0% local control). Five (36%) of 14 patients with Group III disease underwent DPE; two (40%) experienced a local relapse. The patient who underwent a complete resection by DPE and subsequently received reduced RT did not experience a local relapse. In the nine patients without DPE, two (22%) developed local relapse.
Discussion
Consistent with prior studies, our analysis demonstrates patients with biliary RMS are typically less than 10 years of age with Group III, embryonal disease [4, 8, 20]. IRS IV was the first study to incorporate staging in addition to grouping as part of RMS risk stratification, and at the time, the biliary tract was considered an unfavorable site for staging [13, 21]. Outcomes from IRS I-IV for patients with biliary RMS demonstrated that only those treated on IRS I died of disease and that patient outcomes significantly improved with intensified chemotherapy, which led to a switch of the biliary tract to a favorable site [4, 14–17]. Patients with localized, embryonal biliary RMS were then included in subsequent low-risk studies. D9602 and ARST0331 were non-inferiority, low-risk clinical trials with the aim of reducing therapy while maintaining failure-free survival (FFS) at approximately 85% [15–17]. We demonstrated that the survival rate for patients with localized biliary RMS treated on D9602/ARST0331 was suboptimal.
The five-year EFS for patients with biliary RMS in the current analysis [70.6% (95% CI: 46.9–94.3%)] is similar to the historical three-year FFS on IRS III-IV (77%) and lower than the five-year FFS rates for both low-risk subgroups treated on D9602, 89% (95% CI: 84%−92%) for Subgroup A and 85% (95% CI: 74%−91%) for Subgroup B, respectively [13, 15]. Moreover, the five-year EFS is also lower compared to the three-year FFS for Subset 1 patients [89% (95% CI: 85%−92%)] and comparable to the inferior three-year FFS for Subset 2 patients treated on ARST0331 [70% (95% CI: 57%−80%)]. Taken together, these findings suggest that the biliary tract should revert to an unfavorable site and support the current strategy of classifying patients with Group III biliary RMS as having intermediate-risk disease, defined by a 3-year FFS rate of 55–76% [22].
The similar outcome to IRS IV in the current series was achieved with less therapy. The two patients in our series with Group II disease without regional lymph node involvement received less RT (36 Gy) compared to IRS IV (41.4 Gy) and the patient treated on D9602 did not receive cyclophosphamide whereas they would have received 26.4 g/m2 on IRS IV [12, 13, 15–17, 21]. Neither of these patients experienced disease recurrence. Additionally, the 10 patients treated on ARST0331 received a much lower cumulative dose of cyclophosphamide compared to IRS IV (4.8 g/m2 vs. 26.4 g/m2), and only two patients, one of whom had Group III disease, experienced a recurrence [12, 13 16, 17]. Meanwhile, despite a higher dose of cyclophosphamide compared to IRS-IV (26.4 g/m2), outcomes for patients treated on D9602 (28.6 g/m2) were no better than outcomes for patients treated on ARST0331 (4.8 g/m2) as three of the six patients with Group III disease treated on D9602 experienced disease recurrence. Given our small sample size and inter-trial differences, it is difficult to draw strong conclusions regarding the ideal chemotherapy dosing for patients with biliary RMS.
Patients with Group III biliary RMS are eligible for COG intermediate-risk study protocol ARST1431 [NCT02567435]. On ARST1431, patients with biliary RMS will receive VAC in addition to vincristine and irinotecan (VI), with or without temsirolimus, as well as six cycles of maintenance therapy with cyclophosphamide and vinorelbine. The total cumulative dose of cyclophosphamide therapy on ARST1431 (12.6 g/m2) will be greater than what they received on ARST0331 (4.8 g/m2) but less than on D9602 (28.6 g/m2) [NCT02567435, 15, 17]. Six of 14 patients (43%) with biliary RMS treated on IRS III and IV had tumors greater than 5 cm; no patients relapsed [4]. In our current series, 59% of patients had tumors greater than 5 cm with a trend toward poorer outcomes. Larger tumor size may explain the worse outcomes in our series compared to prior cohorts. Patients with tumors > 5 cm will now receive RT to 59.4 Gy on ARST1431. It remains to be seen if the addition of VI with or without temsirolimus, maintenance therapy, and higher RT dosing will improve outcomes for these patients.
SOS is a potentially life-threatening condition characterized by painful hepatomegaly, jaundice, weight gain, and ascites [23]. VAC therapy has been associated with SOS, and an analysis of patients treated on IRS IV demonstrated a 1.2% incidence of SOS that was attributed to the dose-escalation of cyclophosphamide from prior IRS studies [24, 25]. COG intermediate-risk study, D9803, determined the greatest risk factor for development of SOS after VAC therapy was age with patients less than 36 months having the greatest risk [26]. In addition to VAC, patients with non-biliary RMS treated with VA with or without RT on D9602 also developed SOS [27]. RT itself is also a risk factor for the development of SOS [28]. Both patients in our analysis who developed moderate to severe SOS had Group III disease and were less than 36 months of age. One was treated with VA and RT per protocol on D9602 and died early from SOS. The other was treated with VAC/VA with RT on ARST0331, recovered from SOS, and had NED at follow-up. Given that the majority of the patients in our analysis were less than 36 months of age (65%) and assigned to receive RT (94%), it is important to consider the potential risk for SOS in treating patients with biliary RMS. Newer RT techniques such as intensity modulated proton therapy may aid in reducing this risk [29].
While aggressive surgery and liver transplantation have been used to achieve local control in biliary RMS [6, 9, 30–35], our data support the use of chemotherapy and RT without aggressive upfront or delayed surgery for patients with Group III disease. Our findings are supported by a report on 10 patients with biliary RMS from three consecutive Italian studies [20]. Two patients in these studies who received a liver transplant died; one died from progressive disease and the other from liver transplant complications. Meanwhile, in the five patients who were alive with a complete response at the end of therapy, surgery was not radical and was preceded or followed by chemotherapy and RT with no serious sequelae. Additionally, a German cooperative group report on 12 patients with localized, biliary RMS recommended DPE alone for patients with resectable Group III embryonal disease and DPE followed by RT for patients with Group III alveolar disease or incompletely resectable Group III embryonal disease [8]. Their report demonstrated a five-year EFS of 37% (95% CI: 17–57%); however, direct comparison to our series is difficult as their study included alveolar histology. Given the high rate of local control seen in patients who received RT in our study, we recommend RT for all patients with Group III disease. Additionally, our data and a series from IRS I-IV recommend against the use of DPE since there is no apparent benefit to local control, only one patient received reduced RT as a result of their DPE, and DPE led to severe surgical complications in one patient [4].
In conclusion, our study demonstrated that low-risk therapy resulted in suboptimal outcomes, as defined for low-risk patients, for patients with localized biliary RMS. Additionally, aggressive upfront surgery and DPE do not appear beneficial, and RT cannot be safely eliminated for patients with Group III biliary RMS. Overall, these patients may benefit from therapy on intermediate-risk trials.
Acknowledgements
Funding was provided by: COG Grants U10CA098543, U10CA098413, U10CA180899, and U10CA180886, and St. Baldrick’s Foundation.
Abbreviations:
- AWD
Alive with disease
- CI
Confidence interval
- COG
Children’s Oncology Group
- DOD
Died of disease
- DPE
Delayed primary excision
- EFS
Event-free survival
- FFS
Failure-free survival
- IRS
Intergroup Rhabdomyosarcoma Study
- NED
No evidence of disease
- OS
Overall survival
- RMS
Rhabdomyosarcoma
- RT
Radiotherapy
- SOS
Sinusoidal obstruction syndrome
- VA
Vincristine and dactinomycin
- VAC
Vincristine, dactinomycin, and cyclophosphamide
- VI
Vincristine, irinotecan
Footnotes
Conflict of Interest
The authors declare no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability Statement
Research data are not shared.
References
- 1.Perez EA, Kassira N, Cheung MC, et al. Rhabdomyosarcoma in children: a SEER population based study. J Surg Res. 2011;170(2):e243–e251. 10.1016/j.jss.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Wilks S, Moxon W: Lectures on Pathological Anatomy. 2nd ed. London, England, Longmans, Green, & Co, 1875, p. 462–467. [Google Scholar]
- 3.Chung EM, Lattin GE Jr, Cube R, et al. From the archives of the AFIP: Pediatric liver masses: radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics. 2011;31(2):483–507. 10.1148/rg.312105201. [DOI] [PubMed] [Google Scholar]
- 4.Spunt SL, Lobe TE, Pappo AS, et al. Aggressive surgery is unwarranted for biliary tract rhabdomyosarcoma. J Pediatr Surg. 2000;35(2):309–316. 10.1016/s0022-3468(00)90030-7. [DOI] [PubMed] [Google Scholar]
- 5.Ruymann FB, Raney RB Jr, Crist WM, et al. Rhabdomyosarcoma of the biliary tree in childhood. A report from the Intergroup Rhabdomyosarcoma Study. Cancer. 1985;56(3):575–581. . [DOI] [PubMed] [Google Scholar]
- 6.Davis GL, Kissane JM, Ishak KG. Embryonal rhabdomyosarcoma (sarcoma botryoides) of the biliary tree. Report of five cases and a review of the literature. Cancer. 1969;24(2):333–342. . [DOI] [PubMed] [Google Scholar]
- 7.Lack EE, Perez-Atayde AR, Schuster SR. Botryoid rhabdomyosarcoma of the biliary tract. Am J Surg Pathol. 1981;5(7):643–652. 10.1097/00000478-198110000-000067. [DOI] [PubMed] [Google Scholar]
- 8.Urla C, Warmann SW, Sparber-Sauer M, et al. Treatment and outcome of the patients with rhabdomyosarcoma of the biliary tree: Experience of the Cooperative Weichteilsarkom Studiengruppe (CWS). BMC Cancer. 2019;19(1):945. Published 2019 Oct 14. 10.1186/s12885-019-6172-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akers DR, Needham ME. Sarcoma botryoides (rhabdomyosarcoma) of the bile ducts with survival. J Pediatr Surg. 1971;6(4):474–479. 10.1016/s0022-3468(71)80011-84. [DOI] [PubMed] [Google Scholar]
- 10.Sanz N, de Mingo L, Florez F, et al. Rhabdomyosarcoma of the biliary tree. Pediatr Surg Int. 1997;12(2/3):200–201. [PubMed] [Google Scholar]
- 11.Pollono DG, Tomarchio S, Berghoff R, et al. Rhabdomyosarcoma of extrahepatic biliary tree: initial treatment with chemotherapy and conservative surgery. Med Pediatr Oncol. 1998;30(5):290–293. . [DOI] [PubMed] [Google Scholar]
- 12.Baker KS, Anderson JR, Link MP, et al. Benefit of intensified therapy for patients with local or regional embryonal rhabdomyosarcoma: results from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2000;18(12):2427–2434. 10.1200/JCO.2000.18.12.2427. [DOI] [PubMed] [Google Scholar]
- 13.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 14.Meza JL, Anderson J, Pappo AS, Meyer WH; Children’s Oncology Group. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children’s Oncology Group. J Clin Oncol. 2006;24(24):3844–3851. 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 15.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2011;29(10):1312–1318. 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walterhouse DO, Pappo AS, Meza JL, et al. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2014;32(31):3547–3552. 10.1200/JCO.2014.55.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walterhouse DO, Pappo AS, Meza JL, et al. Reduction of cyclophosphamide dose for patients with subset 2 low-risk rhabdomyosarcoma is associated with an increased risk of recurrence: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Cancer. 2017;123(12):2368–2375. 10.1002/cncr.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958; 53: 457–481. 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 19.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Statis Soc A. 1972;135(2):185–207. 10.2307/2344317. [DOI] [Google Scholar]
- 20.Perruccio K, Cecinati V, Scagnellato A, et al. Biliary tract rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Associazione Italiana Ematologia Oncologia Pediatrica. Tumori. 2018;104(3):232–237. 10.5301/tj.5000692. [DOI] [PubMed] [Google Scholar]
- 21.Breneman J, Meza J, Donaldson SS, et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: a report from the Children’s Oncology Group D9602 study. Int J Radiat Oncol Biol Phys. 2012;83(2):720–726. 10.1016/j.ijrobp.2011.06.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raney RB, Anderson JR, Barr FG, et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 2001. May;23(4):215–20. 10.1097/00043426-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014;4(4):332–346. 10.1016/j.jceh.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanwar VS, Albuquerque ML, Ribeiro RC, Kauffman WM, Furman WL. Veno-occlusive disease of the liver after chemotherapy for rhabdomyosarcoma: case report with a review of the literature. Med Pediatr Oncol. 1995;24(5):334–340. 10.1002/mpo.2950240513. [DOI] [PubMed] [Google Scholar]
- 25.Ortega JA, Donaldson SS, Ivy SP, Pappo A, Maurer HM. Venoocclusive disease of the liver after chemotherapy with vincristine, actinomycin D, and cyclophosphamide for the treatment of rhabdomyosarcoma. A report of the Intergroup Rhabdomyosarcoma Study Group. Childrens Cancer Group, the Pediatric Oncology Group, and the Pediatric Intergroup Statistical Center. Cancer. 1997;79(12):2435–2439. . [DOI] [PubMed] [Google Scholar]
- 26.Arndt C, Hawkins D, Anderson JR, Breitfeld P, Womer R, Meyer W. Age is a risk factor for chemotherapy-induced hepatopathy with vincristine, dactinomycin, and cyclophosphamide. J Clin Oncol. 2004;22(10):1894–1901. 10.1200/JCO.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 27.Sulis ML, Bessmertny O, Granowetter L, Weiner M, Kelly KM. Veno-occlusive disease in pediatric patients receiving actinomycin D and vincristine only for the treatment of rhabdomyosarcoma. J Pediatr Hematol Oncol. 2004;26(12):843–846. [PubMed] [Google Scholar]
- 28.Pater L, Turpin B, Mascia A. Pencil Beam Scanning Proton Therapy for Rhabdomyosarcoma of the Biliary Tract. Cureus. 2017;9(10):e1747. Published 2017 Oct 5. 10.7759/cureus.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koay EJ, Owen D, Das P. Radiation-Induced Liver Disease and Modern Radiotherapy. Semin Radiat Oncol. 2018;28(4):321–331. 10.1016/j.semradonc.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Chaudhary S, Kumar M, et al. Rhabdomyosarcoma of biliary tract- a diagnostic dilemma. Indian J Surg Oncol. 2012;3(4):314–316. 10.1007/s13193-012-0186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caty MG, Oldham KT, Prochownik EV. Embryonal rhabdomyosarcoma of the ampulla of Vater with long-term survival following pancreaticoduodenectomy. J Pediatr Surg. 1990;25(12):1256–1258. 10.1016/0022-3468(90)90523-c. [DOI] [PubMed] [Google Scholar]
- 32.Kebudi R, Görgun O, Ayan I, et al. Rhabdomyosarcoma of the biliary tree. Pediatr Int. 2003;45(4):469–471. 10.1046/j.1442-200x.2003.01763.x. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-F LA, Haase GM, Koep LJ, et al. Rhabdomyosarcoma of the biliary tree: the case for aggressive surgery. J Pediatr Surg. 1982;17(5):508–511. 10.1016/s0022-3468(82)80099-78. [DOI] [PubMed] [Google Scholar]
- 34.Paganelli M, Beaunoyer M, Samson Y, et al. A child with unresectable biliary rhabdomyosarcoma: 48-month disease-free survival after liver transplantation. Pediatr Transplant. 2014;18(5):E146–E151. 10.1111/petr.12279. [DOI] [PubMed] [Google Scholar]
- 35.Shen CH, Dong KR, Tao YF, et al. Liver Transplantation for Biliary Rhabdomyosarcoma With Liver Metastasis: Report of One Case. Transplant Proc. 2017;49(1):185–187. 10.1016/j.transproceed.2016.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.