Abstract
Ehrlichia chaffeensis, the causative agent of human monocytic ehrlichiosis, is transmitted by Amblyomma americanum ticks, which are most abundant in the southern United States. Because serologic evidence suggests that residents of Connecticut are exposed to E. chaffeensis, A. americanum ticks were collected in Connecticut and Rhode Island for PCR analysis to detect E. chaffeensis DNA. Eight of 106 (7.6%) A. americanum ticks from Connecticut and 6 of 52 (11.5%) from Rhode Island contained E. chaffeensis DNA. Thus, E. chaffeensis is present in ticks in southern New England and transmission of E. chaffeensis may occur there.
Ehrlichia chaffeensis, the etiologic agent of human monocytic ehrlichiosis (HME), belongs to the Ehrlichia canis genogroup (4, 12). Because of the geographic distribution of its arthropod vector, Amblyomma americanum, HME is most frequently encountered in the southeastern United States, where this tick is most abundant (4, 13). By contrast, in southern New England and southern New York State, Ixodes scapularis ticks are also abundant and transmit the agent of human granulocytic ehrlichiosis (HGE). For this reason, most ehrlichiosis cases in southern New England and New York State are thought to be caused by the HGE agent, a member of the Ehrlichia phagocytophila genogroup, rather than E. chaffeensis. However, a recent study in Connecticut reported that approximately one-third of patients with serologic evidence of ehrlichiosis were found to have antibodies to E. chaffeensis (7). Although some of these patients could have acquired E. chaffeensis infection during travel to states with a known risk for E. chaffeensis exposure, it is conceivable that other residents may have contracted E. chaffeensis infection in Connecticut if bitten by A. americanum ticks there. In a separate study from New York State, some patients suspected of having ehrlichiosis showed seroreactivity against E. chaffeensis by indirect fluorescent antibody (IFA) staining methods (14). Because immunoblot assays could not detect antibodies to E. chaffeensis in these sera, it was thought that there was cross-reactivity in the IFA assay, rather than there being specific evidence of E. chaffeensis infections.
The geographic distribution of A. americanum, the lone star tick, has expanded northward into New York State, whereas prior to 1970, no A. americanum was reported in New York (11). These ticks have been found in Connecticut, albeit not in the same abundance as I. scapularis. By contrast, A. americanum is abundant on parts of Prudence Island, R.I. (10). Therefore, based on the serologic data indicating E. chaffeensis infections in humans, combined with the presence of A. americanum, we hypothesized that transmission of E. chaffeensis could occur in Connecticut and Rhode Island. As a first step in testing this hypothesis we used PCR amplification of DNA from A. americanum ticks, collected in Connecticut and Rhode Island, to search for E. chaffeensis DNA.
Tick collection and PCR analysis.
Adult A. americanum ticks from Prudence Island, R.I., were collected from vegetation in the fall of 1992 and were stored in 70% ethanol. In 1996, 1997, and 1998, nearly all A. americanum ticks collected were submitted by residents living mainly in coastal communities in Fairfield and New Haven Counties, Conn. Some ticks were removed while actively feeding, and others were not engorged. DNA was extracted from ticks using the QiaAmp tissue kit (Qiagen, Valencia, Calif.), following the manufacturer's instructions. Briefly, the ticks were crushed in 100 μl of lysis buffer and incubated for 1 h at 55°C and 10 min at 70°C, and the mixture was then applied to a spin column for centrifugation. After removal of cellular debris and subsequent washings, purified DNA was eluted from the column in 200 μl of Tris (10 mM, pH 8.0) and stored at −20°C until PCR amplification was performed. For PCR amplification, the primers HE1 and HE3 were used to target a portion of the 16S rRNA gene of E. chaffeensis as described previously (2, 3). In addition, the primer sets 8F and 9B and EWI and HE3 were used to assess the presence of HGE DNA and Ehrlichia ewingii DNA (3, 8). As a template for the PCR amplification, 5 μl of tick-extracted DNA was used and the reaction conditions were as described previously (2). Six of 52 (11.5%) A. americanum ticks collected on Prudence Island, R.I., contained E. chaffeensis DNA (Fig. 1). Of 106 A. americanum ticks removed from persons from Fairfield County, Conn., 8 (7.6%) contained E. chaffeensis DNA. As a control, PCR with primers specific for the agent of HGE and E. ewingii did not produce any amplification product. Aliquots of the ethanol used as a preservative were included as controls during the DNA extraction and PCR methods to exclude cross-contamination of ticks during storage. The laboratory space was never used for DNA extraction and PCR of E. chaffeensis DNA prior to this study, reducing the risk of contamination of samples. After the PCR amplification, the products from all positive samples (using HE1 and HE3 primers) were purified from the agarose gel and then sequenced using a dye-terminator sequencing reaction (Perkin-Elmer Applied Biosystems, Foster City, Calif.) and analyzed in an automated DNA sequencer (model 377; Applied Biosystems). Sequences were aligned with known sequences from GenBank. All 14 sequences were identical to the published E. chaffeensis 16S ribosomal gene sequence (1). In addition, 50 I. scapularis ticks collected from Fairfield County and 63 I. scapularis nymphal ticks from Prudence Island served as controls and were also tested for the presence of E. chaffeensis DNA. No E. chaffeensis DNA was detected in these I. scapularis ticks. All control samples from the preserving ethanol were negative in the PCR.
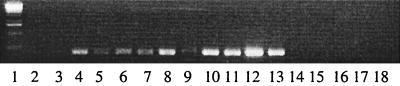
FIG. 1.
PCR amplification of the 16S gene fragment of E. chaffeensis following DNA extraction from A. americanum ticks. Lane 1, DNA size marker; lanes 2 and 3, negative controls; lane 4, positive control; lanes 5 through 13, positive samples from A. americanum ticks; lanes 14 through 18, negative samples from I. scapularis ticks.
Discussion.
Using PCR amplification procedures, we have shown that DNA of E. chaffeensis was present in 7.6 and 11.5% of the A. americanum ticks collected from Connecticut and Rhode Island, respectively. Comparison of the infection rates did not show a statistically significant difference (z = 0.522 and P = 0.602, with a 95% confidence interval of −0.136 to 0.0565). Infection rates of ticks may vary based on location and time of collection of ticks. Indeed, ticks in Rhode Island and Connecticut were collected at different times, and different collection methods were employed.
Previously, results of seroanalyses for E. chaffeensis antibodies in humans clinically suspected of having ehrlichiosis revealed possible exposure to E. chaffeensis in Connecticut (7). In addition, antibodies to E. chaffeensis have been detected in sera from white-footed mice collected as early as 1983 (6). In a previous study, hemocytes of A. americanum ticks were found to contain rickettsia-like organisms, although PCR analysis at that time could not confirm the presence of E. chaffeensis DNA (9). Differences in methods and techniques may account for the variable results between that study and the present study. Based on the combination of this previous serologic evidence and the data reported here, we suggest that transmission of E. chaffeensis, the causative agent of HME, probably occurs in southern New England.
Since 1995, ehrlichiosis has been a reportable disease in Connecticut, and testing for HGE is available to any physician in Connecticut; the testing is supported in part by the Emerging Infections Program administered jointly by the Connecticut Department of Health and Yale School of Epidemiology and Public Health. The incidence of HGE has been found to be between 24 and 57 cases per 100,000 (5). By contrast, testing for E. chaffeensis is not routinely performed and the incidence of HME is not known in the northeastern United States. Future studies that involve laboratory testing for human ehrlichiosis may need to include testing for E. chaffeensis in addition to screening for the HGE agent in order to evaluate the frequency of E. chaffeensis in southern New England and New York State. The HGE agent has been detected in white-tailed deer blood samples from widely separated sites in Connecticut (8). These animals might be harboring both ehrlichial organisms in areas where A. americanum and I. scapularis coexist. Further work on detection of E. chaffeensis and the HGE agent in deer and smaller mammals in the northeastern United States is warranted and may help clarify the natural cycle of transmission of E. chaffeensis.
Nucleotide sequence accession number.
The E. chaffeensis DNA sequence was submitted to GenBank under accession number AF305074.
Acknowledgments
We thank Bonnie Hamid for technical assistance.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Sumner J W, Dawson J E, Tzianabos T, Greene C R, Olson J G, Fishbein D B, Olsen-Rasmussen M, Holloway B P, George E H, Azad A F. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buller R S, Arens M, Hmiel S P, Paddock C D, Sumner J W, Rikhisa Y, Unver A, Gaudreault-Keener M, Manian F A, Liddell A M, Schmulewitz N, Storch G A. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 4.Dumler J S, Bakken J S. Human ehrlichioses: newly recognized infections transmitted by ticks. Annu Rev Med. 1998;49:201–213. doi: 10.1146/annurev.med.49.1.201. [DOI] [PubMed] [Google Scholar]
- 5.IJdo J W, Meek J I, Cartter M L, Magnarelli L A, Wu C, Tenuta S W, Fikrig E, Ryder R W. The emergence of another tick-borne infection in the 12-town area around Lyme, Connecticut: human granulocytic ehrlichiosis. J Infect Dis. 2000;181:1388–1393. doi: 10.1086/315389. [DOI] [PubMed] [Google Scholar]
- 6.Magnarelli L A, Anderson J F, Stafford K C, Dumler J S. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in white-footed mice. J Wildl Dis. 1997;33:466–473. doi: 10.7589/0090-3558-33.3.466. [DOI] [PubMed] [Google Scholar]
- 7.Magnarelli L A, IJdo J W, Anderson J F, Padula S J, Flavell R A, Fikrig E. Human exposure to a granulocytic ehrlichia and other tick-borne agents in Connecticut. J Clin Microbiol. 1998;36:2823–2827. doi: 10.1128/jcm.36.10.2823-2827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnarelli L A, IJdo J W, Stafford K C, Fikrig E. Infections of granulocytic ehrlichia and Borrelia burgdorferi in white-tailed deer in Connecticut. J Wildl Dis. 1999;35:266–279. doi: 10.7589/0090-3558-35.2.266. [DOI] [PubMed] [Google Scholar]
- 9.Magnarelli L A, Stafford K C, Mather T N, Yeh M T, Horn K D, Dumler J S. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–2714. doi: 10.1128/jcm.33.10.2710-2714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mather T N, Mather M E. Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J Med Entomol. 1990;27:644–650. doi: 10.1093/jmedent/27.4.646. [DOI] [PubMed] [Google Scholar]
- 11.Means R G, White D J. New distribution records of Amblyomma americanum (L.) (Acari: Ixodidae) in New York State. J Vector Ecol. 1997;22:133–145. [PubMed] [Google Scholar]
- 12.Roux V, Raoult D. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res Microbiol. 1995;146:385–396. doi: 10.1016/0923-2508(96)80284-1. [DOI] [PubMed] [Google Scholar]
- 13.Whitlock J E, Fang Q Q, Durden L A, Oliver J H. Prevalence of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from the Georgia coast and Barrier Islands. J Med Entomol. 2000;37:276–280. doi: 10.1603/0022-2585-37.2.276. [DOI] [PubMed] [Google Scholar]
- 14.Wong S J, Brady G S, Dumler J S. Serological responses to Ehrlichia equi, Ehrlichia chaffeensis, and Borrelia burgdorferi in patients from New York State. J Clin Microbiol. 1997;35:2198–2205. doi: 10.1128/jcm.35.9.2198-2205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]