Abstract
Background
People admitted to intensive care units and those with chronic health care problems often require long‐term vascular access. Central venous access devices (CVADs) are used for administering intravenous medications and blood sampling. CVADs are covered with a dressing and secured with an adhesive or adhesive tape to protect them from infection and reduce movement. Dressings are changed when they become soiled with blood or start to come away from the skin. Repeated removal and application of dressings can cause damage to the skin. The skin is an important barrier that protects the body against infection. Less frequent dressing changes may reduce skin damage, but it is unclear whether this practice affects the frequency of catheter‐related infections.
Objectives
To assess the effect of the frequency of CVAD dressing changes on the incidence of catheter‐related infections and other outcomes including pain and skin damage.
Search methods
In June 2015 we searched: The Cochrane Wounds Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE and EBSCO CINAHL. We also searched clinical trials registries for registered trials. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
All randomised controlled trials (RCTs) evaluating the effect of the frequency of CVAD dressing changes on the incidence of catheter‐related infections on all patients in any healthcare setting.
Data collection and analysis
We used standard Cochrane review methodology. Two review authors independently assessed studies for inclusion, performed risk of bias assessment and data extraction. We undertook meta‐analysis where appropriate or otherwise synthesised data descriptively when heterogeneous.
Main results
We included five RCTs (2277 participants) that compared different frequencies of CVAD dressing changes. The studies were all conducted in Europe and published between 1995 and 2009. Participants were recruited from the intensive care and cancer care departments of one children's and four adult hospitals. The studies used a variety of transparent dressings and compared a longer interval between dressing changes (5 to15 days; intervention) with a shorter interval between changes (2 to 5 days; control). In each study participants were followed up until the CVAD was removed or until discharge from ICU or hospital.
Confirmed catheter‐related bloodstream infection (CRBSI)
One trial randomised 995 people receiving central venous catheters to a longer or shorter interval between dressing changes and measured CRBSI. It is unclear whether there is a difference in the risk of CRBSI between people having long or short intervals between dressing changes (RR 1.42, 95% confidence interval (CI) 0.40 to 4.98) (low quality evidence).
Suspected catheter‐related bloodstream infection
Two trials randomised a total of 151 participants to longer or shorter dressing intervals and measured suspected CRBSI. It is unclear whether there is a difference in the risk of suspected CRBSI between people having long or short intervals between dressing changes (RR 0.70, 95% CI 0.23 to 2.10) (low quality evidence).
All cause mortality
Three trials randomised a total of 896 participants to longer or shorter dressing intervals and measured all cause mortality. It is unclear whether there is a difference in the risk of death from any cause between people having long or short intervals between dressing changes (RR 1.06, 95% CI 0.90 to 1.25) (low quality evidence).
Catheter‐site infection
Two trials randomised a total of 371 participants to longer or shorter dressing intervals and measured catheter‐site infection. It is unclear whether there is a difference in risk of catheter‐site infection between people having long or short intervals between dressing changes (RR 1.07, 95% CI 0.71 to 1.63) (low quality evidence).
Skin damage
One small trial (112 children) and three trials (1475 adults) measured skin damage. There was very low quality evidence for the effect of long intervals between dressing changes on skin damage compared with short intervals (children: RR of scoring ≥ 2 on the skin damage scale 0.33, 95% CI 0.16 to 0.68; data for adults not pooled).
Pain
Two studies involving 193 participants measured pain. It is unclear if there is a difference between long and short interval dressing changes on pain during dressing removal (RR 0.80, 95% CI 0.46 to 1.38) (low quality evidence).
Authors' conclusions
The best available evidence is currently inconclusive regarding whether longer intervals between CVAD dressing changes are associated with more or less catheter‐related infection, mortality or pain than shorter intervals.
Plain language summary
How often should dressings on central venous access devices (CVADs) be changed to reduce catheter‐related infection?
Background A central venous access device (CVAD, also known as a central venous catheter) is a hollow tube that is placed in a large vein with the tip sitting near the heart. CVADs allow medications, fluids and blood products to be given straight into the bloodstream and allow blood samples to be taken for analysis. One of the negative consequences of a CVAD can be an infection of the blood stream which is called catheter‐related bloodstream infection or CRBSI which can be serious and even life‐threatening. Some CVADs can stay in place for weeks, months or years. Most patients admitted to an intensive care unit will have a CVAD inserted and patients with poor veins or requiring long‐term treatment will be offered a CVAD. Dressings are placed over the insertion site of the catheter where it enters the vein, usually in the chest, neck or arm, to protect the surrounding skin. Dressings help prevent infections from starting and they stop the CVAD from moving around. Dressings are changed when they become dirty or they start to fall off. Frequent dressing changes can cause damage to the surrounding skin, so patients may experience pain or skin damage when the dressing is removed. Changing the dressing frequently is also expensive.
We wanted to see if there were any advantages or disadvantages to longer or shorter time intervals between CVAD dressing changes. Some hospitals or healthcare facilities recommend changing dressings every few days, while others keep dressings in place for longer.
Review question We reviewed the available evidence about the effect of different time intervals between dressing changes for CVADs and whether they had an effect on the risk of CRBSI and other complications. We found five studies that provided information for our review.
Study characteristics The five studies that were included in the review were published between 1995 and 2009 and involved a total of 2277 participants. Four countries were represented (two studies from France and one each from Italy, Sweden, and the Czech Republic). One study involved children and the remaining four trials included only adults. Four of the studies included cancer patients and one included patients in an intensive care unit.
We classified the time intervals between dressing changes as short (2 ‐ 5 days) in the more frequently changed dressings group and long (5‐15 days) in the less frequently changed group. All studies used transparent dressings made of synthetic materials and two studies used gauze (a fabric dressing that does not stick to the skin) secured with tape when skin was damaged. CVAD dressings were monitored on a daily basis in all trials and participants were followed up at least until the CVAD was removed or until discharge. In one study, the manufacturer provided one of the products, but had no influence in the design or how the results were analysed and reported.
Key results The current evidence leaves us uncertain whether the frequency of dressing changes for CVADs influences risk of CRBSI or death. Of particular interest to patients are problems that may be associated with the dressing themselves, such as pain when they are removed and the skin damage that the dressing may cause. We found no clear evidence that pain, which was assessed daily, was affected by the frequency of dressing changes.
Quality of evidence The quality of the evidence was very low or low. We downgraded quality because of small and few studies, poor study designs and differences in results between the studies. Better designed studies are still needed to show whether longer interval or shorter intervals between dressing changes are more effective in preventing catheter related infections, mortality, skin damage, dressing removal pain, quality of life and cost.
This plain language summary is up‐to‐date as of 10 June 2015.
Summary of findings
Summary of findings for the main comparison. Longer intervals (5‐15 days) (intervention) versus shorter intervals (2‐5 days) (control) between dressing changes for preventing catheter‐related infection in people with central venous access devices.
| Patient or population: patients with a central venous access device Setting: Hospital or community settings in Europe Intervention: longer intervals between dressing changes (5 ‐ 15 days) (intervention) Comparison: shorter intervals between dressing changes (2 ‐ 5 days) (control) | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality of the evidence (GRADE) | What happens | ||
| Without longer interval (5 ‐ 15 days) | With longer interval (5 ‐ 15 days) | Difference | ||||
| Catheter‐related blood stream infection (CRBSI) assessed with: as defined by CDC (2002) follow up: median 11 days № of participants: 995 (1 RCT) | RR 1.42 (0.40 to 4.98) | Study population | ⊕⊕⊝⊝ LOW 1 2 | Longer intervals between dressing changes may have little or no effect on catheter‐related blood stream infection | ||
| 8 per 1000 | 12 per 1000 (3 to 41) | 4 more per 1000 (5 fewer to 33 more) | ||||
| All‐cause mortality assessed with: unclear follow up: range 48 hours after discharge from ICU to 120 days № of participants: 896 (3 RCTs) | RR 1.06 (0.90 to 1.25) | Study population | ⊕⊕⊝⊝ LOW 3 4 | Longer intervals between dressing changes probably have little or no effect on death from any cause | ||
| 354 per 1000 | 375 per 1000 (318 to 442) | 21 more per 1000 (35 fewer to 88 more) | ||||
| Skin damage
№ of participants: 1587
(4 RCTs) Follow up: unclear |
Not estimable | Skin damage was reported in four studies. Two provided data but their results were not combined due to inconsistency of size and direction of the effects. One study in children found less skin damage in the longer interval group (8/56) compared with the shorter interval group (24/56). Rates of skin damage in one study in adults were similar (7/39 in longer interval versus 6/42 in shorter interval).9 | ⊕⊝⊝⊝ VERY LOW 5 6 7 | It is uncertain whether longer (compared with shorter) intervals between dressing changes reduce skin damage | ||
| Pain
№ of participants: 193
(2 RCTs) Follow up: unclear |
RR 0.80 (0.46 to 1.38) | Study population | ⊕⊕⊝⊝ LOW 1 7 8 | It is uncertain whether longer (compared with shorter) intervals between dressing changes affect pain on dressing removal | ||
| 347 per 1000 | 278 per 1000 (160 to 479) | 69 fewer per 1000 (187 fewer to 132 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for risk of bias due to lack of blinding of participants and personnel and for a probable unit of analysis error (individual participants randomised but numbers of infections reported)
2 Downgraded for serious imprecision: result consistent with a reduction in CRBSI or an almost 5 fold increase
3 Downgraded for risk of bias due to lack of blinding of participants and personnel
4 Downgraded for imprecision: result consistent with a 10% reduction in mortality or a 25% increase
5 Downgraded twice for serious risk of bias: risk of performance bias due to lack of blinding of participants and personnel; different dressings were used in response to skin damage
6 Downgraded for inconsistency: experimental and control groups were different between studies and frequency of dressing changes overlapped between longer and shorter groups
7 Downgraded for imprecision
8 Downgraded for risk of bias: blinding of outcome assessment not described
9 Data from two additional RCTs could not be extracted and used within the analysis. One study presented toxicity on a 5‐point scale and reported no differences between groups. We are unable to use the data from the fourth study due to the 2 x 2 factorial design.
Background
See Appendix 1 for glossary of terms.
Description of the condition
Central venous access devices (CVADs), also commonly called central venous catheters, are inserted when a patient requires venous access over an extended period of time. These devices are commonly used in patients admitted to intensive care units, for patients with oncological and haematological malignancies and other chronic health problems. CVADs are used to administer intravenous drugs including chemotherapy and immunosuppression, fluids, blood products, total parenteral nutrition, and for blood sampling.
The external portion of the CVAD can be partially tunnelled under the skin or non‐tunnelled. Non‐tunnelled catheters are those where the insertion site is directly above the entry into the vein (CNSA 2007); they are for short‐term use and can be sited using the jugular, subclavian or femoral veins (Hayden 2005). Peripherally inserted central catheters (PICCs) are also non‐tunnelled and are inserted into the central circulation usually from a peripheral vein in the upper arm ‐ they can remain in place for months (Gabriel 2005; Hayden 2005; RNAO 2005). Tunnelled CVADs are surgically implanted with a section of the catheter positioned in a subcutaneous tunnel between the entry site, which heals over, to the vein and the skin exit site (CNSA 2007), and are typically placed into the superior vena cava.
CVADs are covered with a dressing and secured with a separate securement device or skin adhesive, such as tape or transparent adhesive film, to prevent infection and movement (Hunt 1997; Wilson 2006 and Elkabir 2001; Rippon 2007, respectively). Newer products are available that combine both the dressing and securement function. The repeated application and removal of adhesives or adhesive tapes and dressings from the same site can cause damage to the skin by skin stripping, that is the removal of the superficial stratum corneum, which can cause development of inflammatory skin reactions, oedema and soreness (Cutting 2008).The entry and exit sites of CVADs are inspected visually daily for signs of infection, and this may require removal of the dressing. Dressings are replaced if they become loose, soiled or wet. Frequent dressing changes can impact upon the skin integrity surrounding the CVAD entry and exit sites. If skin integrity is compromised, rates of catheter‐related infection (CRI) including CRBSI may be affected.
Description of the intervention
The intervention of interest in this review is the frequency of dressing changes. Adhesives or adhesive tapes are designed to bond to the skin under a variety of conditions, such as flexure, changing temperatures, in the presence of perspiration and external moisture, but should also be easy to peel off in order to ensure minimal discomfort and trauma (Karwoski 2004). Choice of dressings and frequency of changes depends upon clinical practice protocols, and patient and clinician preferences (CNSA 2007; Gillies 2003; O'Grady 2011). The general consensus is for gauze dressings to be changed every 48 hours (CNSA 2007; Hadaway 2003; O'Grady 2011; Rosenthal 2003; RNAO 2005), and transparent semi‐permeable dressings every seven days, or earlier if the integrity of the dressings is compromised or there is blood underneath the dressing (Camp‐Sorrell 2004; CNSA 2007; Loveday 2014; Hadaway 2003; INS 2011; IVNNZ 2012; O'Grady 2011; Rosenthal 2003; RNAO 2005).
How the intervention might work
CVADs are commonly used in patients admitted to intensive care and those diagnosed with chronic diseases and cancer. These patients are often immunocompromised and healing processes are diminished due to their disease or treatment (Cutting 2008; Lotti 1998). The skin provides protection as a barrier to infection (Tortura 2000), so maintaining skin integrity is particularly important for these patients. Chemotherapy and radiation regimens can cause adverse skin changes (DeSpain 1992; Glean 2001; Hopewell 1990). Other patients at particular risk of skin damage are older adults, babies and young children who, by nature of their age, have fragile skin (Hollingworth 2009), and patients with disease‐related factors associated with dermatological changes (Cutting 2008). Constantly removing adhesives or adhesive tapes to change dressings may further aggravate already damaged skin (Hollingworth 2009). Thus, reducing the frequency of dressing changes may reduce skin damage, pain, costs, incidence of skin colonization and the potential for CRIs. A theoretical risk exists that transparent dressings increase surface humidity, which may lead to increased microbial colonisation at the catheter site and so increase the risk of CRI (Wille 1993). Therefore, prolonging the interval between dressing changes may increase infection due to increased skin colonisation underneath the dressing.
Why it is important to do this review
There is a lack of clear evidence concerning the optimal frequency of dressing changes for CVADs. Clinical guidelines have influenced a general consensus around the timing of dressing changes for CVADs, but the guidelines themselves are based on limited evidence. For example, recommendations in the Centers for Disease Control and Prevention (CDC) guidelines suggest only tunnelled CVADs with well‐healed sites might not require dressings (O'Grady 2011), but there is no recommendation about frequency of changes before sites are healed. Patients diagnosed with cancer are particularly vulnerable to skin damage because of the treatment they receive (Cutting 2008; Lotti 1998). Extending the time between dressing changes may reduce the damage and also reduce the associated costs. However, it remains unclear whether prolonging the time between changes results in other complications, such as an increased risk of bloodstream infection. We will examine the existing research to determine how frequently dressings that are used to protect CVADs should be changed. We are primarily interested in the incidence of CRI, but will also consider outcomes such as pain and skin damage.
Objectives
To assess the effect of the frequency of CVAD dressing changes on the incidence of CRIs and other outcomes including pain and skin damage.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) evaluating the effect of the frequency of CVAD dressing changes on the incidence of CRIs. We excluded studies comparing different dressing products and studies where the frequency of dressing change was not the only systematic difference between treatment arms as we required an explicit focus upon the frequency of changing the same type of dressing. Cluster‐randomised controlled trials, quasi‐randomised trials and cross‐over trials were not included in order to minimise potential bias in accordance with Reeves 2011.
Types of participants
Participants of any age requiring a CVAD in any healthcare or community setting.
Types of interventions
Trials comparing any frequency of changing the same type of dressings for the securement of a CVAD.
Types of outcome measures
Primary outcomes
Incidence of confirmed catheter‐related bloodstream infection (CRBSI) defined as bacteraemia or fungaemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (i.e. fever, chills, and/or hypotension), and no apparent source for the bloodstream infection except the catheter. One of the following should be present for a positive diagnosis: a positive semi‐quantitative (> 15 colony forming units (CFU)/catheter segment) or quantitative (> 10³ CFU/catheter segment) culture from a catheter segment in which the same organism (species and antibiogram) is isolated from the catheter segment and peripheral blood (CDC 2002).
Incidence of suspected CRBSI, as described by the trial investigator.
All‐cause mortality.
Secondary outcomes
Incidence of catheter entry and exit site infection, as described by the trial investigator.
Skin damage, using an assessment tool (such as the Eastern Cooperative Oncology Group Common Toxicity Criteria for Skin (ECOG 2007; see Appendix 2).
Pain, using any validated measure or scale described by the trial investigator.
Quality of life, using any validated measure or scale described by the trial investigator.
Cost.
Search methods for identification of studies
Electronic searches
In June 2015 we searched the following electronic databases to identify reports of relevant randomised clinical trials:
The Cochrane Wounds Specialised Register (searched 11 June 2015);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 6);
Ovid MEDLINE (1946 to 10 June 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 10 June 2015);
Ovid EMBASE (1974 to 10 June 2015);
EBSCO CINAHL (1982 to 11 June 2015).
The search strategies used can be found in Appendix 3. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL search with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN 2011). There were no restrictions with respect to language, date of publication or study setting.
In July 2014 we searched the following clinical trials registers:
Australian and New Zealand Clinical Trials Register (www.anzctr.org.au)
ClinicalTrials.gov (www.clinicaltrials.gov/)
Current Controlled Trials (www.controlled‐trials.com/)
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/)
European Union Clinical Trials Register (www.clinicaltrialsregister.eu/)
Searching other resources
We searched reference lists of all retrieved and relevant publications identified by these strategies for further studies not identified by the methods outlined above.
Data collection and analysis
Selection of studies
Two review authors (NG and JW) acting independently located potentially eligible studies by screening titles and abstracts from the search. We obtained full copies of potentially eligible studies and acting independently, decided on inclusion based on the predefined inclusion and exclusion criteria. We have listed the excluded studies with reasons for their exclusion (Characteristics of excluded studies). Disagreements were resolved by discussion among the review authors.
Data extraction and management
We extracted data from eligible studies using a data extraction sheet. This summary contained baseline characteristics of study and control group participants and included the number of participants, age, gender, disease, treatment, reason for insertion of CVAD, method of insertion, profession of inserter (doctor, radiographer or nurse), anatomical location of insertion, type of CVAD, number of lumens on the CVAD, dwell time of the CVAD, dressing protocol, deviation from planned dressing day and reason, number of dressing changes during dwell time of the CVAD, known allergies to dressings, skin complexion and known history of or current positive blood cultures. We extracted the criteria for patient inclusion and exclusion, a description of the intervention and the number of patients randomised to each intervention. We recorded the healthcare settings in which the interventions were performed. In addition, we extracted the duration of follow‐up and numbers lost to follow‐up as well as outcomes.
When more than one publication arose from a study, we extracted data from all relevant publications, but did not duplicate data in analyses. Two review authors (NG and JW) extracted all data independently. Disagreements were resolved by discussion. If this had not resulted in consensus, the third review author's opinion would have been decisive (RC).
Assessment of risk of bias in included studies
Two review authors (NG and JW) independently assessed each eligible study using the Cochrane tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (for example, extreme baseline imbalance; see Appendix 4 for details of criteria on which the judgements were based). We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study. Disagreements were discussed in a consensus meeting.
The assessment of risk of bias is presented using a 'Risk of bias' summary figure, which includes all the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the confidence the reader may give to the results of the particular studies.
Measures of treatment effect
Event rates for dichotomous outcomes are presented as risk ratios (RR) with 95% confidence intervals (CI). There were no continuous outcomes or time to event outcomes.
Unit of analysis issues
The unit of analysis was individual patients with a CVAD in situ. All five studies included in the review randomised the patients and not their CVAD, but three studies presented some results per CVAD or per dressing and we contacted the authors to obtain the results per patient (Engervall 1995; Rasero 2000; Timsit 2009). Timsit 2009 was the only paper to present CRBSI, our primary outcome. A decision was made to present the data per catheter rather than per patient for this one outcome in the absence of any other data. Cross‐over and cluster‐randomised trials were not included.
Dealing with missing data
If data were missing from the published trial reports, we made attempts to contact the study authors to complete the information necessary for the analysis and 'Risk of bias' assessment. We did not impute data if the missing data were not obtained after several attempts to contact the author. If no further information was provided we used an available case analysis. We addressed the potential impact of missing data on the findings of the review in the Discussion.
Assessment of heterogeneity
We included trials in a meta‐analysis if the study population and the interventions studied were sufficiently similar. We assessed statistical heterogeneity using the I² statistic (Higgins 2003), which examines the percentage of total variance across studies due to heterogeneity rather than chance. Values of I² under 25% indicate a low level of heterogeneity and justify use of a fixed‐effect model for meta‐analysis. Values of I² between 25% and 75% are considered moderate and a random‐effects model can be used. Values of I² higher than 75% indicate high levels of heterogeneity and pooling should not be undertaken.
Assessment of reporting biases
We reported each outcome separately. We were not able to use funnel plots to assess reporting biases, as an insufficient number of studies was included.
Data synthesis
If the studies were sufficiently similar we pooled them using a fixed‐effect model for values of I² under 25%. In the event of moderate heterogeneity we employed a random‐effects model. If the studies were statistically heterogenous (I² ≥ 75%) we produced a qualitative summary (O'Rourke 1989).
'Summary of findings' tables
We have presented the main results of the review in 'Summary of findings' (SoF) tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The SoF tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence with regard to the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). The following outcomes, which we believe to be the most important both clinically and to the consumer, are presented in the SoF tables:
CRBSI;
all‐cause mortality;
skin damage;
pain.
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analyses. We planned to investigate heterogeneity using sensitivity analysis (see below).
Sensitivity analysis
Too few studies were included in the meta‐analyses to conduct a sensitivity analysis. We were not able to explore the effect of concealment of allocation (adequate versus not reported, unclear or not undertaken).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
In this review comparisons were grouped by longer duration between dressing changes versus shorter duration between dressing changes with the shorter duration treated as the control group as this is considered standard practice by the trial authors.
Results of the search
The electronic search identified 471 titles. Of these, 453 were excluded by an examination of the titles and abstracts: two were duplicates; 136 were excluded because they did not contain information about CVADs; and 315 compared different dressings or were on other topics. The remaining 18 full texts were retrieved and reviewed. Of these, 10 did not meet the inclusion criteria and were excluded (see Characteristics of excluded studies). Five published RCTs met the inclusion criteria (Benhamou 2002; Engervall 1995; Rasero 2000; Timsit 2009; Vokurka 2009), and three were supplementary references to included papers (see Criteria for considering studies for this review and Characteristics of included studies and Figure 1).
1.
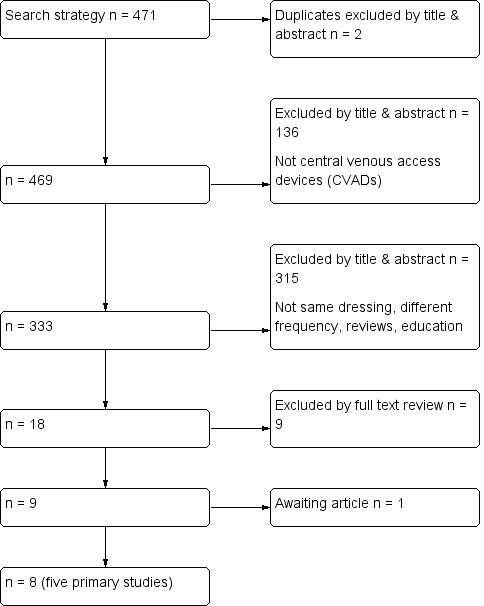
Flow diagram of included and excluded studies
A search of the clinical trials registers did not identify any additional trials. Only one study had been pre‐registered (Timsit 2009).
The reference lists of all retrieved and relevant publications were searched. One study was considered to be relevant but was not available through library resources (Fessard 1994). Attempts to contact the author and locate the journal are continuing.
Included studies
Types of patients
The five trials involved a total of 2277 participants, with the totals in individual trials ranging from 32 to 1653. One study involved children (Benhamou 2002), and the remaining four included adults only (Engervall 1995; Rasero 2000; Timsit 2009; Vokurka 2009). Two studies were set in a bone marrow transplant unit (Benhamou 2002; Rasero 2000), two studies consisted of patients undergoing treatment for a haematological malignancy (Engervall 1995; Vokurka 2009), and one study recruited patients receiving treatment in intensive care (Timsit 2009). Four countries were represented (two studies from France and one each from Italy, Sweden, and the Czech Republic). All studies were conducted in acute in‐patient settings. Patients were excluded from the studies if their skin was already damaged (Benhamou 2002; Rasero 2000; Vokurka 2009); were having treatment that would make them more susceptible to skin damage, such as the chemotherapeutic drug busulphan‐thiotepa (Benhamou 2002), or radiation to the chest (Vokurka 2009); or if they had allergies to polyurethane dressings (Rasero 2000; Timsit 2009; Vokurka 2009), chlorhexidine (Timsit 2009), or disinfectant (Vokurka 2009).
Types of interventions
Time frames for dressing changes varied between 2 and15 days. One study planned to compare 15‐day and 4‐day dressing changes for tunnelled catheters (Benhamou 2002). CVAD dressings were monitored on a daily basis in all trials and patients were followed up until the CVAD was removed or until discharge as a minimum. However, in this study there were a large number of protocol violations, that is, dressings were changed on days other than the day indicated in the protocol. In the 15‐day group, only 67 (17%) of the 365 dressing were changed on day 15 and, in the 4‐day group, 516 (76%) of the 678 dressings were changed on the correct day. This meant that dressing changes in the 15‐day group were actually changed, on average, every eight days and, in the 4‐day group, every four days. Reasons for the protocol violations included soiled and dislodged dressings and problems with the catheter that required the dressing to be removed. Two studies compared once versus twice‐weekly dressing changes for tunnelled CVADs (Engervall 1995; Timsit 2009), two studies compared once versus twice‐weekly dressing changes for non‐tunnelled CVADs (Timsit 2009; Vokurka 2009), and one study compared 5‐day versus 10‐day dressing changes for tunnelled CVADs and 2‐day versus 5‐day dressing changes for non‐tunnelled CVADs (Rasero 2000). Again however, reflecting the reality of pragmatic research in clinical settings, many of the dressings were not changed according to the group schedule. In the tunnelled CVAD 10‐day group 9.6% were not changed on the correct day, while in the tunnelled CVAD 5‐day group the proportion was 8.0%; in addition 6.8% of non‐tunnelled CVCs in the 5‐day group were not changed as scheduled and in the non‐tunnelled CVC 2‐day group the rate was 12.5%.
The dressings were applied under controlled conditions in all groups. Three studies stated that nurses were responsible for the dressing changes (Benhamou 2002; Engervall 1995; Rasero 2000). Four studies used Tegaderm (3M, St Paul, USA) dressings (Benhamou 2002; Engervall 1995; Rasero 2000; Timsit 2009), and one study used Bioclusive (Johnson and Johnson, New Jersey, USA; Vokurka 2009). One study used chlorhexidine gluconate‐impregnated sponges (Biopatch, Ethicon, New Jersey, USA) around the entry or exit site of the CVAD under the dressings (Timsit 2009).
Three studies used the same dressings throughout the period of observation. Two studies used different dressings that depended upon skin damage. One study used a Tegaderm (3M) covering a sterile gauze for grade 0 to 1 skin damage (48/56; 85% in the 15‐day group and 32/56; 57% in the 4‐day group), sterile gauze with Mefix for grade 2 to 3 skin damage (7/56; 13% in the 15‐day group and 23/56; 41% in the 4‐day group) and sterile gauze with tape for grade 4 skin damage (1/56; 2% in both the 15‐ and 4‐day groups; Benhamou 2002). The other study used a Tegaderm (3M) for undamaged skin or an exit site with mild erythema, but if the exit site had extensive erythema or other signs of local infection then the dressings were changed daily using a gauze dressing moistened with 10% ethanol with aluminium acetotartrate 10% until the erythema had disappeared, at which point the patient was returned to the allocated group (Engervall 1995). Patients in the once‐weekly group had more extra dressings due to erythema compared to the twice‐weekly group (3%; 0 to 91% once‐weekly group; 0%; 0 to 17% twice‐weekly group; P value 0.08 expressed as extra dressings days per CVAD days).
Skin decontamination varied between the groups. Two studies used the same antiseptic solution to clean the skin before the insertion of the CVAD and at dressing changes; one study used an alcohol‐based povidone‐iodine solution (Timsit 2009); and one used povidone‐iodine solution; whether the antiseptic was alcohol‐based was unclear (Vokurka 2009). One study used a 0.5% alcohol based chlorhexidine solution during insertion and at dressing changes, but changed to aqueous‐based povidone‐iodine if the skin became damaged (Benhamou 2002). One study did not describe skin decontamination that occurred prior to CVAD insertion but used 70% ethanol at dressing changes (Engervall 1995). One study did not mention which antimicrobial solution was used for skin decontamination (Rasero 2000).
Types of outcomes
Only one trial used a standard definition for confirmed CRBSI (Timsit 2009), two trials reported blood culture results (Benhamou 2002; Vokurka 2009), and one study reported blood culture and CVAD‐tip culture results separately (Engervall 1995). Blood cultures were performed on clinical suspicion of infection or determined by a temperature threshold stipulated by each study author. Three studies provided information about suspected CRBSI but these studies used different definitions (Benhamou 2002; Engervall 1995; Timsit 2009): Benhamou 2002 did not provide a definition; Engervall 1995 defined suspected CRBSI as not responding to antibiotics; and Timsit 2009 had an investigator blinded to the study group review the patient's case including the medical chart in order to perform an independent blinded review. In all five studies, catheter‐site infection was defined by skin colonisation and additionally in three studies by local signs of catheter‐site infection such as the presence of inflammation, erythema, tenderness, swelling or discharge (Benhamou 2002; Engervall 1995; Timsit 2009). Two studies measured pain: Benhamou 2002 used categories of none, moderate or severe; and Vokurka 2009 used a visual analogue score ranging from 0 to 10 (0: no pain, 5: moderate pain, 10: severe pain). No study measured quality of life. Two studies measured cost (Rasero 2000; Timsit 2009).
In the Timsit 2009 trial, a 2 x 2 factorial design was used, in which participants were randomised to a 3‐ or 7‐day dressing change and to a dressing alone or with chlorhexidine gluconate‐impregnated sponge (CHGIS). They also combined arterial and central catheters in their analysis. The authors were contacted and provided information based on central catheters only and reported separately for the CHGIS and non‐CHGIS groups. For our analysis we have included only the non‐CHGIS group, to maintain consistency with other trials.
Excluded studies
The Table of Characteristics of excluded studies specifies our reasons for excluding 10 studies. One was a systematic review (Zitella 2003); three studies compared different dressings (Davidson 1986; Hagerstrom 1994; Lucas 1996); one was a study protocol (Bystricka 2004); one was a letter to the editor commenting on a study of dressings (Dickerson 1989); in three studies the frequency of dressing change was not the only systematic difference between treatment groups (Powell 1985; Samsoondar 1985; Young 1988); and one was a cluster RCT (Ishizuka 2011).
Risk of bias in included studies
See the 'Risk of bias' tables in the Characteristics of included studies section and Figure 2; Figure 3; Table 2 and Table 3.
2.
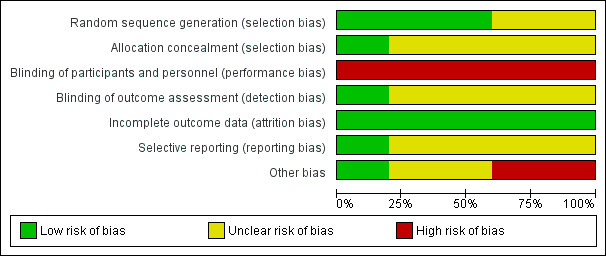
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
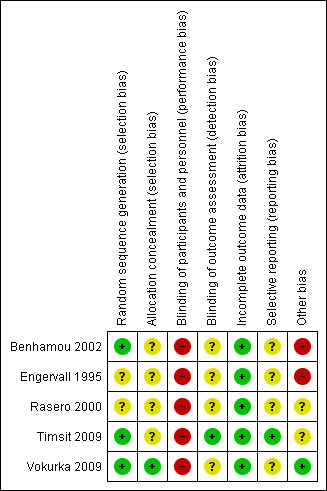
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
1. Blinding of participants and personnel (performance bias).
| Benhamou 2002 | Engervall 1995 | Rasero 2000 | Timsit 2009 | Vokurka 2009 | |
| CRBSI | Not applicable | Not applicable | Not applicable | High risk | Not applicable |
| Suspected CRBSI | High risk | High risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | High risk | High risk | High risk | High risk | High risk |
| Skin damage | High risk | Not applicable | High risk | High risk | High risk |
| Pain | HIgh risk | Not applicable | Not applicable | Not applicable | High risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | High risk | Not applicable | Not applicable |
2. Blinding of outcome assessment (detection bias).
| Benhamou 2002 | Engervall 1995 | Rasero 2000 | Timsit 2009 | Vokurka 2009 | |
| CRBSI | Not applicable | Not applicable | Not applicable | Low risk | Not applicable |
| Suspected CRBSI | Unclear risk | Unclear risk | Not applicable | Not applicable | Not applicable |
| All‐cause mortality | Low risk | Low risk | Not applicable | Low risk | Not applicable |
| Catheter‐site infection | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Skin damage | Unclear risk | Not applicable | Unclear risk | Unclear risk | Unclear risk |
| Pain | Unclear risk | Not applicable | Not applicable | Not applicable | Unclear risk |
| Quality of life | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Cost | Not applicable | Not applicable | Unclear risk | Not applicable | Not applicable |
Allocation
Random sequence generation
Three studies used computer‐generated lists to generate the allocation sequence (Benhamou 2002; Timsit 2009; Vokurka 2009). One study used manually mixed envelopes (Engervall 1995). One study did not describe how the random sequence was generated (Rasero 2000).
Allocation concealment
Vokurka 2009 used computer software to conceal the allocation of trial patients into individual groups. We contacted the trialists of Engervall 1995 who stated that they had used randomisation envelopes. The other three studies did not describe how the allocation was concealed (Benhamou 2002; Rasero 2000; Timsit 2009).
Blinding
Blinding of participants and personnel
None of the studies was able to blind participants or staff involved in direct care from identifying the allocated intervention due to the nature of the intervention.
Blinding of outcome assessment
Timsit 2009 mentioned blinding of outcome assessment, stating that staff involved in analysing catheter cultures and reviewing CRBSI were blinded to the study groups. It was unclear in the remaining four trials whether outcome assessors were blinded (Benhamou 2002; Engervall 1995; Rasero 2000; Vokurka 2009).
Incomplete outcome data
A flow chart was provided by Timsit 2009 that included the numbers of patients screened, excluded, randomised to each group and withdrawals and reasons for exclusions from the per‐protocol analysis. Four studies accounted for all randomised participants and their withdrawal from each group (Benhamou 2002; Engervall 1995; Rasero 2000; Vokurka 2009). Two studies reported sample size calculations and used an intention‐to‐treat analysis (Benhamou 2002; Timsit 2009). Two studies presented results per patient (Benhamou 2002; Vokurka 2009). Two studies presented results per catheter and per patient (Engervall 1995; Timsit 2009). One study presented results per dressing and per patient (Rasero 2000). Overall, reported attrition rates were low and well balanced. There was a proportionally higher attrition rate in one arm of the Timsit 2009 trial, but losses were marginal and unlikely to have had an impact on outcomes.
Four studies monitored the CVAD sites closely and dressings were changed if they were loose or soiled (Benhamou 2002; Rasero 2000; Timsit 2009; Vokurka 2009). This meant that approximately one‐third of the participants had additional dressing changes that constituted protocol violations. These violations were reported in the results.
Selective reporting
A study protocol was available for one study (Timsit 2009). All other authors provided results for outcomes mentioned in their published methods section (Benhamou 2002; Engervall 1995; Rasero 2000; Vokurka 2009).
Other potential sources of bias
Benhamou 2002 and Engervall 1995 varied their dressing protocol according to the grade of skin damage. The interim analysis in the Engervall 1995 study showed no statistical significance for the rates of the primary outcome between groups, so the study was stopped and the secondary outcomes analysed. Rasero 2000 did not present baseline data. In one study, the manufacturer provided one of the products but they had no influence in the design or how the results were analysed and reported (Timsit 2009).
Effects of interventions
See: Table 1
Primary outcomes
Confirmed catheter‐related bloodstream infection (995 central venous catheters)
Only one study (Timsit 2009), that had uncertain risk of bias for allocation concealment, reported confirmed CRBSI as per our protocol. There was no clear evidence of a difference between groups for this outcome (RR 1.42; 95% CI 0.40 to 4.98; Analysis 1.1). LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision): (Table 1).
1.1. Analysis.
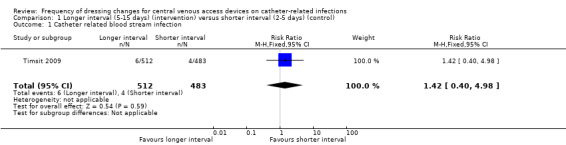
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 1 Catheter related blood stream infection.
Suspected catheter‐related bloodstream infection (151 participants)
We were able to extract data from two studies that reported suspected CRBSI (Benhamou 2002; Engervall 1995). Benhamou 2002 stated that no CVADs were removed due to suspicion of CRBSI. In the Engervall 1995 trial 6/20 (30%) of CVADs were removed in the once‐weekly group and 4/19 (21%) in the twice‐weekly group due to suspected CRBSI (RR 0.70; 95% CI 0.23 to 2.10; Analysis 1.2). Both studies were at uncertain risk of bias for allocation concealment, blinding of outcome assessment and selective reporting. There was no clear evidence of a difference between the groups for this outcome. LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision).
1.2. Analysis.
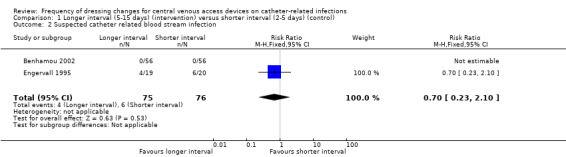
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 2 Suspected catheter related blood stream infection.
All‐cause mortality (896 participants)
Three studies at uncertain risk of bias, included information about all‐cause mortality (Benhamou 2002; Engervall 1995; Timsit 2009). It was possible to combine the data from all these studies; the studies were homogenous so the fixed‐effect model was used for data synthesis (I² = 0%). There was no clear difference in all‐cause mortality between longer (5‐15 days) and shorter (2‐5 days) time intervals between dressing changes (RR 1.06; 95% CI 0.90 to 1.25; Analysis 1.3). LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision): (Table 1).
1.3. Analysis.
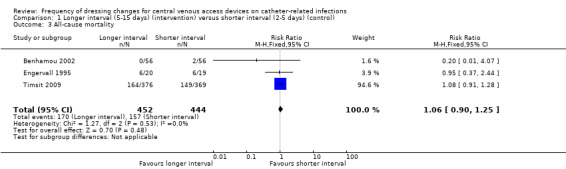
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 3 All‐cause mortality.
Secondary outcomes
Catheter‐site infection (371 participants)
All five studies reported catheter‐site infection but in a variety of different ways. Benhamou 2002 and Rasero 2000 reported the proportions of participants developing a catheter‐site infection. Engervall 1995 reported the rate of exit site infections per 100 CVAD days; Vokurka 2009 reported positive skin swabs and Timsit 2009 reported rates of skin colonisation, Data from the two studies (Benhamou 2002; Rasero 2000) that reported risk of catheter‐site infection in a similar way were pooled using a fixed effect model (I2 = 0%). There was no clear evidence of a difference in the risk of catheter‐site infection rate between longer (5‐15 days) and shorter (2‐5 days) time intervals between dressing changes (RR 1.07; 95% CI 0.71 to 1.63; Analysis 1.4). LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision).
1.4. Analysis.
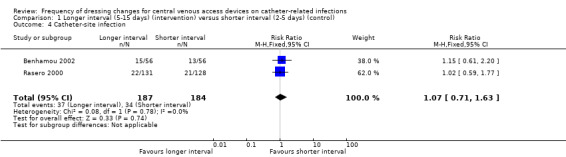
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 4 Catheter‐site infection.
Engervall 1995 reported 1.6 exit site infections per 100 CVAD days (median, range 0 to 13.3) in the longer interval (less frequent) group compared with 0 per 100 CVAD days (median, range 0 to 9.1) in the short interval (more frequent) group. Vokurka 2009 reported 13 positive skin swabs across both treatment groups but did not report by group. We contacted the trialists of Timsit 2009 but they were unable to provide per patient data. The Timsit 2009 study reported catheter‐site infection rates per catheter rather than by patient in their published paper.
Consequently it remains unclear whether longer or shorter intervals between dressing changes for CVADs influences the risk of catheter‐site infection.
Skin damage (1587 participants)
Skin damage was reported in four studies (Benhamou 2002; Rasero 2000; Timsit 2009; Vokurka 2009). Data from two trials were included in the forest plot (Benhamou 2002; Vokurka 2009). Results were highly heterogenous (I² = 78%), probably due to different scales being used to assess skin damage and dissimilar time frames for assessment, so we did not pool the data. One of these trials (Benhamou 2002) included only children and showed that fewer participants in the longer interval group (8/56) scored grade ≥ 2 on the skin damage scale compared with 24/56 in the shorter interval group (RR 0.33; 95% CI 0.16 to 0.68; P value 0.012; Analysis 1.5). There was no clear evidence of a difference in rates of skin damage between long and short intervals in adult patients (Vokurka 2009) (RR 1.26; 95% CI 0.46 to 3.41; Analysis 1.5. VERY LOW QUALITY EVIDENCE (downgraded for risk of bias, imprecision and heterogeneity).
1.5. Analysis.
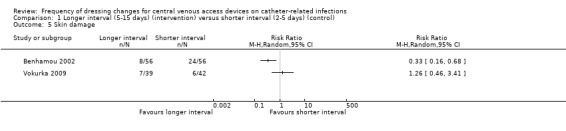
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 5 Skin damage.
Two trials could not be included in the skin damage forest plot. In the Rasero 2000 trial toxicity was graded on a 5‐point scale, but there were no reported differences between groups. We are unable to use the data from the Timsit 2009 trial due to the 2 x 2 factorial design.
Pain
Pain was assessed on a daily basis in two studies (Benhamou 2002; Vokurka 2009). The maximum intensity of pain reported was analysed. When data from the two studies were combined there was no clear evidence of a difference in pain however this comparison is underpowered (RR 0.80; 95% CI 0.46 to 1.38; Analysis 1.6; Benhamou 2002; Vokurka 2009). This was rated as LOW QUALITY EVIDENCE (downgraded for risk of bias and imprecision): (Table 1). The pain classification systems used are detailed in the Characteristics of included studies. The pain data were dichotomised on the basis of a judgement that any pain experienced and reported by the patient was clinically significant.
1.6. Analysis.
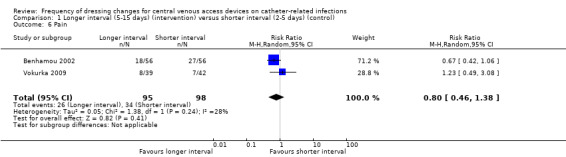
Comparison 1 Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control), Outcome 6 Pain.
Quality of life
None of the studies reported quality of life.
Cost
Rasero 2000 reported the costs of nursing time and dressings and stated that less frequent dressing changes would reduce costs by 400% in the tunnelled CVAD group and by 50% in the non‐tunnelled CVAD group when compared to the standard practice of changing dressings every second day. The monetary figures presented in the text and the table were different. Several attempts have been made to contact the authors for clarification but without success.
Discussion
Summary of main results
This systematic review included five RCTs (2277 participants) at unclear or high risk of bias. We assessed the effects of prolonging the frequency of dressing changes for CVADs on the incidence of confirmed CRBSI, suspected CRBSI, all‐cause mortality, CVAD entry and exit site infection, skin damage, pain, quality of life and cost. All studies used transparent polyurethane dressings, which are often favoured over gauze dressings because they allow the catheter site to be monitored visually for signs of infection without removal of the dressing. The longer intervals of dressing changes ranged from 5 to 15 days and the shorter intervals from 2 to 5 days. It was not possible to obtain data that would facilitate analysis at the level of the patient rather than the catheter from two of the trial authors. Rasero 2000 presented data for every dressing change and Timsit 2009 reported data per catheter. One of the authors who was contacted for additional information no longer had the data in an accessible form due to technological advances (Engervall 1995). Most published literature in this field was ineligible for this review as it compared the effect of different dressings on CRIs rather than different frequencies of dressing change within the context of a constant dressing type.
From the available data, we can draw no conclusions about the incidence of confirmed or suspected CRBSI associated with different intervals of dressing frequency. We used the CDC definition of confirmed CRBSI (CDC 2002), which requires the CVAD to be removed so that the tip can be quantitatively or semi‐quantitatively cultured. Clinically this definition is impractical as it requires the removal of the CVAD. Mermel 2009 offers a more practical definition of two blood samples drawn (one from a catheter hub and the other from a peripheral vein) that, when cultured, meet CRBSI criteria for quantitative blood cultures or differential time to positivity which would enable CVADs to remain in place until the results of the blood cultures become available.
Similarily, neither benefits or harms of the intervention could be demonstrated for all‐cause mortality, CVAD entry and exit site infection, pain, quality of life and cost. Most comparisons are underpowered and therefore clinically important effects cannot be excluded.
As highlighted in the Included studies section, each study used a variety of antimicrobial solutions for skin decontamination. The recent guidelines recommend using a > 0.5% chlorhexidine skin preparation with 70% alcohol for skin decontamination or a 1% to 2% tincture of iodine or povidone‐iodine for sensitive skin (Loveday 2014; INS 2011; IVNNZ 2012; O'Grady 2011). At the time of these studies this preparation was not available. When patients' skin damage worsened in Benhamou 2002, Tegaderm (3M) was no longer used and it is unclear how frequently the Mefix tape or sterile gauze and tape dressing were changed. Skin damage grade ≥ 2 occurred more frequently in the 4‐day group, which may have an effect upon the rates of skin infection.
It was not possible to provide an overall estimate of the effect of changing dressings less frequently on skin damage. Data from two small studies of limited quality reported contradictory results; one trial favoured shorter times between dressing changes (two dressing changes per week; Vokurka 2009), and the other favoured longer times (up to 15 days; Benhamou 2002). In addition, the Benhamou 2002 study was powered to detect a 30% improvement in the rate of grade ≥ 2 skin damage in the 15‐day group, but only 17% of dressings in this group remained intact for this length of time. In the Benhamou 2002 study, on average, the longer interval dressings were in place for 8 days with no adverse events occurring in either trial. Consequently, this raises the possibility of replacing dressings only when clinically indicated, especially in the paediatric and neonatal population where skin is fragile. Patients receiving radiotherapy, or those with existing sensitivities, may also benefit from extending the time between dressing changes.
Overall completeness and applicability of evidence
The primary and secondary outcomes of clinical interest included confirmed and suspected CRBSI, all‐cause mortality, catheter‐site infection, skin damage, pain, quality of life and cost, but these were poorly reported and many results could not be extracted for this review. These outcomes should be included in any future clinical trials involving frequency of dressing changes.
The five studies included in this review were undertaken in acute care settings in Europe. CVADs are usually placed in patients requiring intensive care, treatment for malignancies and other patients requiring long‐term treatment. Four of the studies recruited participants with haematological malignancies or those undergoing a bone marrow transplant. This population is immunocompromised due to their underlying disease or treatment, hence these results may not be easily applied to patients with chronic health problems or those being cared for in other settings.
Dressings and products for decontamination continue to evolve, with new products constantly being developed and marketed. So, although all of the studies in this review used transparent dressings, older studies may have used products that are no longer available. Other reviews have been published comparing different dressings.
The final limitation to the completeness and generalisability of results is that all of the studies compared changing the frequency of transparent polyurethane dressings only. Consequently, studies comparing the frequency of changing other types of dressings, such as gauze and tape, may provide different results. Only one trial used chlorhexidine impregnated sponges, which are now commonly used, as part of the dressing regimen, but we could not extract these data.
Quality of the evidence
Limitations in study design and implementation
Risk of bias was assessed according to six components: sequence generation; allocation concealment; blinding; selective outcome reporting, incomplete follow‐up and other potential biases. The risk of bias was difficult to assess due to poor reporting in most of the studies (Figure 2; Figure 3). Only three studies provided sufficient information to assess how the randomisation sequence was generated; and two study authors we contacted described the method used for allocation concealment. It would not be possible for the participants and personnel to be blinded to the frequency of dressing changes, but only one study blinded outcome assessments.
Two of the studies comprised 81% (2163/2675) of the total participants (Benhamou 2002; Timsit 2009). These two studies calculated the required sample size, used random‐number generation to allocate the sequence and used an intention‐to‐treat analysis. However, neither was rated as being at low risk of bias for allocation concealment.
Protocol deviations were common in the treatment and control arms. Dressings were changed early as they were soiled or not intact. This issue reflects the reality of pragmatic research in clinical settings and the importance of visual inspections of the dressing to improve care and maintenance of the CVAD.
Indirectness of evidence
This review was limited by a lack of uniformity in the experimental and control groups. The frequency of the dressing changes overlapped at the outer limits of the longer (5 to 15 days ) and shorter (2 to 5 days) intervals between dressings. Confirmed CRBSI was reported in only one trial (Timsit 2009). These limitations restrict confident decision making regarding the effect of frequency of dressing changes on CRIs.
Unexplained heterogeneity or inconsistency of results
All‐cause mortality and catheter‐site infection were the only outcomes that could be pooled using fixed‐effect model for meta‐analysis. Pain was pooled using a random‐effects model for meta‐analysis. It was not possible to pool the skin damage results due to heterogeneity. Heterogeneity was generally due to differences in populations and different scales and definitions that were used for the various outcomes.
Imprecision of results
There was serious imprecision in all the results, even when meta‐analysis was undertaken, with wide confidence intervals due to the small sample sizes. Consequently, results reflect a lack of evidence of a difference rather than evidence of no difference (between CVAD dressing change intervals). Further research is therefore very likely to have an important impact on the confidence of the estimates of effect for all of the measured outcomes.
Publication bias
Lack of information about most of the important clinical outcomes could suggest selective outcome reporting, but we were unable to confirm this as only one study was registered with a trials registry.
Potential biases in the review process
The authors are confident that all studies meeting the inclusion criteria were selected. The reference lists were handsearched and only one additional title was found (Fessard 1994). The full paper was requested from the author and from the journal, but to date our requests remain unanswered. Clearly described procedures were followed to prevent potential biases in the review process. The methods used are transparent and reproducible. One of the authors (CR) has given lectures for 3M and received an unrestricted research Grant from Centurion. No products from these companies were included in this review.
Agreements and disagreements with other studies or reviews
Zitella 2003 reviewed the literature concerning CVAD care for patients undergoing bone marrow transplantation: only the Engervall 1995 and Rasero 2000 studies were included in both that review and ours. The Benhamou 2002 study was published in the month that the Zitella 2003 review was accepted for publication and the other studies in our review were published after 2003. With regard to frequency of dressing changes, Zitella 2003 concluded firstly that the Engervall 1995 study showed more positive catheter tip cultures in the once‐weekly group, but the study was limited by a small sample size, and secondly that the Rasero 2000 study showed no significant difference in skin colonisation between the four groups. Overall, the authors concluded that colonisation is a imperfect measure for CRBSI.
One of the excluded studies allocated participants to routine (every 72 hours) and non‐routine (until removal of the CVAD) intervals between dressing changes based upon the ward they were admitted to (cluster randomisation; Ishizuka 2011). There was a significant inter‐group difference in the duration of catheter dwell time (routine group 9.1 ± 0.5 days and non‐routine group 11.9 ± 0.7 days). There was a no significance in CRBSI between groups (13/241 in the routine group and 10/266 in the non‐routine group). Kaplan‐Meier analysis and the log rank test revealed a significant difference in the period from insertion to the development of CRBSI between the groups favouring the routine interval between dressing changes (P value 0.026).
The Timsit 2009 RCT was subjected to a secondary analysis in a later publication,Timsit 2012, which reported data on 1419 patients (3275 combined arterial catheters and CVADs) who had their dressings replaced on the allocated third or seventh day versus those with dressings replaced before any of the scheduled days. They found that early dressing disruption (replacement) occurred for 67% of scheduled dressings and was significantly associated with increased skin colonisation, CRBSI and major CRI (CRBSI or suspected CRBSI). For subclavian CVADs alone (n = 547), it was reported that both percentage of dressings disrupted (P value 0.0043), and disruption of the final dressing (P value 0.0004) were significantly associated with greater levels of skin colonisation at CVAD removal. Those authors concluded that disruption of dressings was common and an important risk factor for infection.
The wound and skin adhesive literature recognises that multiple factors influence the degree of adhesion of the same product to different people's skin (Rippon 2007). It is also acknowledged that trauma caused by repeated removal and application of adhesives or adhesive tapes causes an erythematous reaction that affects the barrier function of the skin (Cutting 2008; Hollingworth 2009). Compromised barrier function becomes important when bacterial overgrowth has been associated with occlusive dressings (Dykes 2007), such as the polyurethane dressings commonly used to secure CVADs. However, whether polyurethane dressings are more likely than other adhesive products to cause skin stripping remains unclear (Cutting 2008; Dykes 2001). It is also unclear whether there is an association between skin stripping and an increased incidence of infection. However, damaged skin provides a potential entry point for infection, so it makes sense to prevent the skin damage occurring. The notion of preventing skin damage to avoid CRI is supported by an infection control practice guideline, which recommends not shaving insertion sites, to avoid micro‐abrasions that may encourage bacterial colonisation (Wilson 2006).
The counter argument to the skin stripping theory is that organisms originating from patients’ own skin are likely to be the ones that cause many CRIs (Casey 2010; Elliott 1998; Gillies 2003; Maki 1997; Mermel 2000); these may be capable of migrating from the skin surface along the outside of the catheter to cause infection irrespective of skin damage (Wilson 2006). If skin around the catheter site is disinfected regularly then colonisation and CRBSI should be reduced. However, catheter‐site infections cannot be relied upon to identify or predict CRBSIs (Safdar 2002), and can exist independently of a systemic infection (Walshe 2002). Moreover, efforts to maintain skin integrity may assist in reducing CRBSIs. While this may be true, the proportion of positive skin cultures around the exit site has been found to be higher in the presence of erythema when compared with healthy skin (Engervall 1995).
Authors' conclusions
Implications for practice.
There are insufficient data to draw a conclusion regarding whether the frequency of dressing changes influences CRBSI, suspected CRBSI, all‐cause mortality, catheter‐site infection, skin damage in adults, pain, quality of life or cost in people with central venous access devices (CVAD). Although one small study suggested that longer intervals between changes may lower the risk of skin damage in children, this was very low quality evidence. In the absence of clear evidence of an increased risk associated with extending the time between dressing changes, it is reasonable to base decisions on patient preference and cost. CVAD sites should be inspected on a daily basis to ensure dressings are clean and intact with no signs of localised infection. Clinically indicated dressing changes should occur if the dressing is soiled or not intact.
Implications for research.
Future primary research on the frequency of CVAD dressing replacement should report confirmed CRBSI, suspected CRBSI and all‐cause mortality data. Researchers should use standardised definitions and measures and use per patient rather than per CVAD or per dressing data to facilitate inclusion in future systematic reviews and meta‐analyses.To improve quality, future studies should calculate sample sizes and report allocation concealment.
The link between skin colonisation and the incidence of CRBSI raises an important question that should continue to be investigated in future research; future research should report matched positive skin and blood culture results obtained from individual patients.
Economic analysis under the guidance of a health economist offers comprehensive information about the costs and savings to healthcare organisations and should be considered in future trials. Engaging the views of patients and clinicians would be helpful, as the frequency of dressing changes for CVADs is often guided by patient tolerance.
Acknowledgements
The authors would like to acknowledge the contribution of peer referees: Mieke Flour, Kurinchi Gurasamy, Gill Worthy, Anneke Andriessen, Dayanithee Chetty, Donna Gillies and Gemma Villanueva and copy‐editors Jenny Bellorini and Elizabeth Royle.
Appendices
Appendix 1. Glossary
Asymptomatic: Without symptoms, even though the condition is present. Catheter‐related infection (CRI): A general term to describe any infection associated with a catheter and may included skin infection, catheter colonisation or blood stream infection.
Catheter‐related blood stream infection (CRBSI): see primary outcome for definition.
Central venous access device (CVAD): A venous catheter for placing in a vein that leads directly to the heart. Central venous catheter (CVC): A venous catheter for placing in a vein that leads directly to the heart. This term is used interchangeably with CVAD. Dermatitis: A general term used to describe inflammation of the skin. Differential time to positivity: The growth of microbes from a blood sample drawn from a catheter hub at least 2 hours before microbial growth is detected in a blood sample obtained from a peripheral vein. Dwell time: Number of hours or days that a device remains in the patient. Erythema: Redness or inflammation of the skin or mucous membranes that is the result of dilatation and congestion of superficial capillaries. Exfoliative dermatitis: Any inflammatory skin disorder characterised by excessive peeling or shedding of skin. Macule: A small flat blemish or discolouration that is level with the surface of the skin. Quantitative blood cultures: A colony count of microbes grown from blood obtained through the catheter hub that is at least three‐fold greater than the colony count from blood obtained from a peripheral vein. Quantitative culture: Culture of the catheter segment requires either flushing the segment with microbe‐sustaining broth, or vortexing, or sonication in broth, followed by serial dilutions and surface plating on blood agar. Papular: A small superficial elevation of the skin. Pruritis: The symptom of itching, an uncomfortable sensation leading to the urge to itch. Semi‐quantitative culture: Catheter segment is rolled across surface of an agar plate and colony forming units (CFU) are counted after overnight incubation. Skin integrity: A description of whether or not skin is intact. Symptomatic: Causing symptoms or side effects. Ulcerative dermatitis: A skin disorder associated with bacterial growth often initiated by self‐trauma (scratching) due to a possible allergic response. Vena cava: The superior vena cava is the large vein that returns blood to the heart from the head, neck and both upper limbs. The inferior vena cava returns blood to the heart from the lower part of the body. Vesicular: Pertaining to a blister‐like condition.
Appendix 2. ECOG Common toxicity criteria for skin
Stage 0: None or no change. Stage 1: Scattered macular or papular eruption or erythema that is asymptomatic. Stage 2: Scattered macular or papular eruption or erythema associated with pruritus or other associated symptoms. Stage 3: Generalised symptomatic macular, papular or vesicular eruption. Stage 4: Exfoliative dermatitis or ulcerating dermatitis.
Appendix 3. Search strategies
The Cochrane Wounds Specialised Register
(catheter* and dressing*) AND (INREGISTER)
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library)
#1 MeSH descriptor: [Catheterization, Central Venous] explode all trees #2 MeSH descriptor: [Catheters, Indwelling] explode all trees #3 "central venous access":ti,ab,kw #4 (central next venous next catheter*):ti,ab,kw #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Bandages] explode all trees #7 MeSH descriptor: [Hydrogels] explode all trees #8 MeSH descriptor: [Alginates] explode all trees #9 MeSH descriptor: [Silver] explode all trees #10 MeSH descriptor: [Silver Sulfadiazine] explode all trees #11 MeSH descriptor: [Honey] explode all trees #12 (dressing* or hydrocolloid* or alginate* or hydrogel* or "foam" or "bead" or "film" or "films" or tulle or gauze or non‐adherent or "non adherent" or silver or honey or matrix):ti,ab,kw #13 #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 #5 and #13
Ovid MEDLINE
1 exp Catheterization, Central Venous/ 2 exp Catheters, Indwelling/ 3 central venous access.tw. 4 central venous catheter*.tw. 5 or/1‐4 6 exp Bandages/ 7 exp Hydrogels/ 8 exp Alginates/ 9 exp Silver/ 10 exp Honey/ 11 exp Sulfadiazine Silver/ 12 (dressing* or hydrocolloid* or alginate* or hydrogel* or foam*1 or bead*1 or film*1 or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix).tw. 13 or/6‐12 14 5 and 13 15 randomized controlled trial.pt. 16 controlled clinical trial.pt. 17 randomized.ab. 18 placebo.ab. 19 clinical trials as topic.sh. 20 randomly.ab. 21 trial.ti. 22 or/15‐21 23 exp animals/ not humans.sh. 24 22 not 23 25 14 and 24
Ovid EMBASE
1 exp central venous catheter/ 2 exp indwelling catheter/ 3 central venous access.tw. 4 central venous catheter*.tw. 5 or/1‐4 6 exp wound dressing/ 7 exp hydrogel/ 8 exp alginic acid/ 9 exp silver/ 10 exp sulfadiazine silver/ 11 exp honey/ 12 (dressing* or hydrocolloid* or alginate* or hydrogel* or foam*1 or bead*1 or film*1 or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix).tw. 13 or/6‐12 14 5 and 13 15 Randomized controlled trials/ 16 Single‐Blind Method/ 17 Double‐Blind Method/ 18 Crossover Procedure/ 19 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. 20 (doubl$ adj blind$).ti,ab. 21 (singl$ adj blind$).ti,ab. 22 or/15‐21 23 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 24 human/ or human cell/ 25 and/23‐24 26 23 not 25 27 22 not 26 28 14 and 27
EBSCO CINAHL
S26 S13 AND S25 S25 S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S24 MH "Quantitative Studies" S23 TI placebo* or AB placebo* S22 MH "Placebos" S21 TI random* allocat* or AB random* allocat* S20 MH "Random Assignment" S19 TI randomi?ed control* trial* or AB randomi?ed control* trial* S18 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S17 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S16 TI clinic* N1 trial* or AB clinic* N1 trial* S15 PT Clinical trial S14 MH "Clinical Trials+" S13 S11 AND S12 S12 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 S11 S1 OR S2 OR S3 S10 TI ( dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix) or AB ( dressing* or hydrocolloid* or alginate* or hydrogel* or foam or bead or film or films or tulle or gauze or non‐adherent or non adherent or silver or honey or matrix ) S9 (MH "Honey") S8 (MH "Silver Sulfadiazine") S7 (MH "Silver") S6 (MH "Alginates") S5 (MH "Hydrogel Dressings") S4 (MH "Bandages and Dressings+") S3 TI central venous access or AB central venous access S2 (MH "Vascular Access Devices+") S1 (MH "Catheterization, Central Venous+")
Appendix 4. 'Risk of bias' table judgement criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process available to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information available to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following.
Insufficient information available to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk was enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes was enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information available to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Longer interval (5‐15 days) (intervention) versus shorter interval (2‐5 days) (control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter related blood stream infection | 1 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.40, 4.98] |
| 2 Suspected catheter related blood stream infection | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.23, 2.10] |
| 3 All‐cause mortality | 3 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| 4 Catheter‐site infection | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.63] |
| 5 Skin damage | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Pain | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.46, 1.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benhamou 2002.
| Methods |
Study design: Single centre RCT Sample size calculation: Yes ITT analysis: Yes Ethics and informed consent: Kremlin‐Bicetre, France Registration number and name of registry: Not stated |
|
| Participants |
Population: Children with a malignancy, who were candidates for high dose chemotherapy and autologous or allogeneic bone marrow transplantation Setting: Paediatric Bone Marrow Transplantation unit at the Gustave Roussy Institute, Villejuif, France Number: A total of 113 patients were randomised, 57 in the 15‐day group and 56 in the 4‐day group. There was 1 post‐randomisation exclusion, results were reported for 112 participants (56 in each group) Age: 15‐day group: median 5 years, range 1‐22 years. 4‐day group: median 7 years, range 2‐19 years Gender (male:female): 15‐day group: 33:23. 4‐day group: 25:31 Skin complexion: 15‐day group: white 43/56; 'mat' 10/56; black 3/56. 4‐day group: white 47/56, 'mat' 6/56, black 3/56 Known allergies to dressings: Not stated Known history of current BSI: Not stated Inclusion criteria: Children with a malignancy, who were candidates for high dose chemotherapy and autologous or allogeneic bone marrow transplant. A qualitative culture of the skin at the catheter entry site was performed before randomisation: only children with a negative culture for Staphyloccus epidermis were eligible Exclusion criteria: Children were only included once in the trial. Those treated with the busulfan‐thiotepa conditioning regimen and those who already had grade ≥ 2 cutaneous toxicity at the catheter dressing site were not eligible |
|
| Interventions |
Aim: To compare the efficacy of 2 catheter dressing change frequencies (15‐days versus 4‐days) Intervention: Dressing changed every 15 days Control: Dressing changed every 4 days Dressing protocol in both groups: "Three types of dressings were used according to cutaneous toxicity; the adhesive transparent oxygen‐permeable type (Tegaderm) for grade 0 and 1 (48/56; 85% in 15 day group and 32/56; 57% in the 4 day group); the Mefix type for grade 2 and 3 (7/56; 13% in the 15 day group and 23/56; 41% in the 4 day group); and the sterile gauze and tape (American style) dressing (Surgifix, Smith & Nephew, Hull or Velpeau) for grade 4 (1/56; 2% in both the 15 and 4 day groups). Dressings were changed by the nurse in charge of the patient, under sterile conditions: the dressing was cautiously unstuck, the skin was cleaned with a sterile gauze and Hibidil from the catheter entry point towards the periphery. A sterile gauze was then applied under the dressing. The dressing had to cover the catheter entry point as well as the catheter hub, and the upper limit of the extension line, whatever the dressing type." Duration of follow‐up: Daily surveillance of the dressing and its periphery began on the day of randomisation and was continued throughout hospitalisation Numbers lost to follow‐up: 1 child relapsed in the 15‐day arm before HDC Reason for CVAD insertion: HDC for autologous and allogeneic BMT Method of CVAD insertion: "Catheters were all inserted (subclavian site) in the operating room under strict aseptic conditions. Physicians wore a cap, a mask, sterile gloves and a gown. The insertion site was first qualitatively cultured and then prepared with 0.5% alcoholic chlorhexidine (Hibidil). The catheters were then inserted cutaneously using the Seldinger technique, and tunnelled subcutaneously up to 10 cm on average in order to allow rapid removal of the material if severe infectious complications were suspected. In the absence of catheter‐related adverse events, the device was left in place until the patient was discharged from the bone marrow transplant unit." Anatomical location of CVAD: Subclavian site Profession of CVAD inserter: Physician Type of CVAD: Silastic catheters (Vygon) Number of CVAD lumens: Single Dwell time of CVAD: Not stated Study dates: July 1990‐April 1993 |
|
| Outcomes |
Primary outcomes CRBSI: Not included Suspected CRBSI: Blood cultures were taken in the event of fever above 38.5°C and/or signs of local infection All‐cause mortality: Reported mortality with causes Secondary outcomes Catheter‐site infection: Bacteriological samples were taken from skin around the catheter entry point, using plastic agar‐coated slides (Unipath SA, Dardilly, France). All colonies appearing within 48 h of incubation (37°C) were identified by the usual qualitative bacteriological procedures. Catheter entry site cultures were taken in the event of fever above 38.5°C and/or signs of local infection. Skin damage: Skin toxicity at the catheter dressing site and its periphery. Skin toxicity classified as grade 0: healthy skin; grade 1: slightly inflamed skin; grade 2: minor cutaneous lesions, dressing difficult to remove; grade 3: lesions reaching periphery of the dressing; grade 4: cutaneous lesions to such and extent that the usual dressing could no longer be used Pain: Pain during and between dressing changes. Local pain (classified as none, moderate or severe) during the dressing change and between dressing changes Quality of life: Not included Cost: Not included Other outcomes reported in the trial None Inter‐rater reliability: As dressing changes were performed by many different nurses, the skin toxicity grading scale was tested during the 6 months preceding the trial so that the different nursing teams could familiarise themselves with its use Time points: Daily surveillance of the dressing and its periphery began on the day of randomisation and was continued throughout hospitalisation. Whenever the dressing was changed, the grade of skin toxicity was recorded |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Evidence: "Computer‐generated list was used to allocate patients" Comment: Adequate generation of the randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk |
Evidence: Not stated in the trial report Comment: Unable to judge |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Evidence: Not stated in the trial report Comment: Not possible to blind the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Evidence: Not stated Comment: Although it would have been possible to blind outcome assessment, we were unable to ascertain if this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Evidence: "One patient relapsed after randomisation and did not receive high dose chemotherapy (15‐day group). The analysis presented here thus concerns 56 patients in each group." Comment: We do not believe that the loss of 1 patient would have affected results |
| Selective reporting (reporting bias) | Unclear risk |
Evidence: All planned outcomes reported Comment: No published protocol. We did not request a copy of the protocol from the trialist |
| Other bias | High risk |
Evidence: Different dressings according to skin damage Comment: Different dressing protocols may have introduced a bias |
Engervall 1995.
| Methods |
Study design: Single centre RCT Sample size calculation: Not stated ITT analysis: Not stated Ethics and informed consent: Local ethics committee Registration number and name of registry: Not stated |
|
| Participants |
Population: "Patients with haematological malignancies and severe aplastic anaemia, in need of a permanent central venous catheter." Setting: In‐patient unit, Karolinska Hospital, Stockholm, Sweden Number: The abstract states "thirty‐two consecutive patients with haematological disorders . . . were randomly allocated to have their CVC bandages changed once (n=20) or twice (n=19) a week. However, the 'Methods' section of the paper states "Thirty‐one consecutive patients with haematological malignancies and one patient with severe aplastic anaemia, in need of a permanent CVC, were allocated randomly to have their CVC bandages changed, 16 in the once a week group and 16 in the twice a week group." In the results section, tables reported a total of 39 patients. It seems that 32 patients, who had a total of 39 catheters were randomised Age: Once‐weekly group: median 46 years, range 18‐85 years. Twice‐weekly group: median 50 years, range 22‐84 years Gender (male:female): Once‐weekly group: 8:8. Twice‐weekly group: 10:6 Skin complexion: Not stated Known allergies to dressings: Not stated Known history of current BSI: Not stated Inclusion criteria: Not stated Exclusion criteria: Not stated |
|
| Interventions |
Aim: To determine whether a reduction of dressings from twice to once weekly could be performed safely in neutropenic patients Intervention: Once‐weekly dressing changes Control: Twice‐weekly dressing changes Dressing protocol in both groups: "CVC changes were performed by the nurse responsible for the patient at the ward. The catheter exit site was cleaned with 70% ethanol and a transparent polyurethane dressing Tegaderm (3M) was applied to the area. No other bandages were used, thus allowing the attending nurse to inspect the exit site daily. The presence of erythema or other signs of infection was noted and documented. In the presence of erythema, a gauze dressing moistened with 10% ethanol with aluminium acetotartrate 10% was used. When erythema or other signs of infection had disappeared the patient returned to the allocated changing interval." Patients in the once‐weekly group had more extra dressings due to erythema compared to the twice‐weekly group (3%; 0‐91% once‐weekly group; 0%; 0‐17% twice‐weekly group; P value 0.08 expressed as extra dressings days per CVAD days) Deviation from planned dressing day: Not stated Number of dressing changes during dwell time of CVAD: Not stated Duration of follow‐up: Daily skin assessments until 120 days post CVAD insertion Numbers lost to follow‐up: 12 patients died (Once‐weekly group 6; Twice‐weekly group 6). 2 patients dislocated CVCs. 2 CVC tip cultures not obtained. 23 CVCs (14 Once‐weekly group and 9 Twice‐weekly group) for analysis Reason for CVAD insertion: In need of a permanent CVC Method of CVAD insertion: Inserted under aseptic conditions in an operating theatre Anatomical location of CVAD: 39 catheters were inserted in 32 patients' internal jugular (Once‐weekly group 2; Twice‐weekly group 2); external jugular (Once‐weekly group 5; Twice‐weekly group 4); subclavian (Once‐weekly group 13; Twice‐weekly group 13) and tunnelled subcutaneously for a distance of approximately 15 cm to an exit site at the anterior of the thorax Profession of CVAD inserter: Not stated Type of CVAD: Silicone catheter Number of CVAD lumens: Single Dwell time of CVAD: Once‐weekly group: median 39.5 days (range 8‐114 days); Twice‐weekly group: median 46 days (range 13‐120+ days) Study dates: Not stated |
|
| Outcomes |
Primary outcomes CRBSI: Not included. Suspected CRBSI: Local catheter infections defined as > 15 CFU at catheter tip culture. Positive blood culture defined as growth of bacteria in at least 1 sample from a peripheral vein, and for coagulase‐negative staphylococci growth in at least 2 of the 3 cultures taken. The CVC was removed aseptically. During granulocytopenia (< 0.5 x 109L‐1) 3 separate cultures were obtained (2 from a peripheral vein and 1 from the central line) for aerobic and anaerobic cultures at start of fever (temperature > 38.0°C on 2 occasions with at least a 4‐h interval, or > 38.5°C on 1 occasion). Additional blood cultures were obtained before change of antibiotic therapy in patients with a persistent fever All‐cause mortality: Reported Secondary outcomes Catheter‐site infection: Skin cultures at exit site graded into 2 categories: < 10 CFU per plate or ≥ 10 CFU per plate. CVC tip cultures Skin damage: Days with erythema at the exit site, temperature > 38°C, antibiotic therapy and the need for extra dressings. Erythema surrounding the exit site was graded into 2 categories: mild erythema, not requiring extra change of dressing or extensive erythema or other signs of local infection requiring extra daily changes Pain: Not included Quality of life: Not included Cost: Not included Other outcomes reported in the trial Number of catheters removed due to complications: The catheters were followed for the first 120 days after insertion Overall catheter survival time Validity of measures: Not stated Inter‐rater reliability: CVC changes were performed by the nurse responsible for the patient at the ward Time points: Skin cultures samples for bacterial culture were obtained from the skin at the exit site and from the skin next to the transparent dressing at the time of changing the bandages |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Evidence: Randomisation envelopes mixed manually Comment: This information was sought from the author; it was not reported in the publication |
| Allocation concealment (selection bias) | Unclear risk |
Evidence: Randomisation envelopes mixed manually Comment: This information was sought from the author; it was not reported in the publication |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Evidence: Not stated Comment: Not possible to blind the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Evidence: Not stated Comment: Not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Evidence: 6 participants died in each group. Results analysed by catheter, not by participant for most outcomes Comment: Equal numbers died in each group. Consequently we judged this element to be at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk |
Evidence: All planned outcomes reported Comment: Protocol not reviewed |
| Other bias | High risk |
Evidence 1: The trial was stopped early, following an interim analysis, when it became clear that differences in the primary outcome would not be found in the time available for the study, this may or may not indicate a potential bias Comment 1: Based on unequal numbers between the number of participant recruited (32) and the numbers reported in the tables (39), it seems as though results were based on the number of catheters, not the number of participants. Consequently, there is, potentially, risk of a 'Unit of analysis' error Evidence 2: Different dressings according to skin damage Comment 2: Different dressing protocols may have introduced a bias |
Rasero 2000.
| Methods |
Study design: Multi‐centre RCT Sample size calculation: Not stated ITT analysis: Not stated Ethics and informed consent: Ethical Committee of Azienda Ospedaliera Careggi, Florence, Italy Registration number and name of registry: Not stated |
|
| Participants |
Population: "Patients undergoing bone marrow transplantation (either autologous, allogeneic from sibling or unrelated donors, or recipients of autologous peripheral blood stem cells)." Setting: 7 Italian BMT centres Number: "399 consecutive patients were enrolled: 230 patients with a tunnelled CVC: 10‐day group 118/230 and 5‐day group 112/230; 169 patients with a non‐tunnelled CVC: 5‐day group 85/169 and 2‐day group 84/169." Age: Not reported Gender (male:female): Not reported Skin complexion: Not reported Known allergies to dressings: Not reported Known history of current BSI: Not reported Inclusion criteria: "Consecutive patients undergoing BMT (either autologous, allogeneic from sibling or unrelated donors, or recipients of autologous peripheral blood stem cells)." Exclusion criteria: "Patients with active cutaneous lesions at the site of CVC insertion at the time of enrolment, patients with known allergy to polyurethane dressings and patients with generalized dermatologic diseases." |
|
| Interventions |
Aim: To compare 2 different time interval protocols for CVC dressing in order to assess the effects on local infections and toxicity Intervention: Tunnelled CVC 10‐day dressing changes. Non‐tunnelled CVC 5‐day dressing changes Control: Tunnelled CVC 5‐day dressing changes. Non‐tunnelled CVC 2‐day dressing changes Dressing protocol in both groups: "A detailed protocol for CVC dressing under controlled sterile conditions was prepared, and all nurses involved in CVC maintenance were asked to adhere strictly to it for the whole study period; it was the responsibility of each Center’s coordinator to ensure the correct performance of the protocol. Sterile, polyurethane transparent adherent dressings (Tegaderm, 3M) were used for the CVC dressing." Number of dressing changes during dwell time of CVAD: Not stated Duration of follow‐up: Every dressing change until CVAD removal Numbers lost to follow‐up: Tunnelled CVC: 70/230. Non‐tunnelled: 70/169 Reason for CVAD insertion: BMT Method of CVAD insertion: Not stated Anatomical location of CVAD: Not stated Profession of CVAD inserter: Not stated Type of CVAD: Not stated Number of CVAD lumens: Not stated Dwell time of CVAD: Not stated Study dates: March 1996‐October 1997 |
|
| Outcomes |
Primary outcomes CRBSI: Not included Suspected CRBSI: Not included All‐cause mortality: Not included Secondary outcomes Catheter‐site infection: Cultures for bacterial and fungal agents were set up according to established methodologies used in the microbiology department of each Center’s central hospital laboratory Skin damage: Severity of local skin toxicity directly attributable to the dressing procedure itself. Cutaneous lesions were graded according to the ECOG scale. A specific data form sheet was made available for recording ECOG grading in each patient for each dressing. The following parameters were carefully checked at all dressing changes and at the time of CVC removal: erythema, swelling, tenderness, induration, pain, pruritus, and purulence Pain: Not included Quality of life: Not included Cost: Calculations were made using an exchange rate of USD 1 = ITL 1700. The actual (net) cost of a nurse in an Italian public hospital was about USD 10/hour. Calculations were based on the assumption that the mean hospital stay for an allogeneic patient with a tunnelled CVC was about 40 days (corresponding to a total of 20 dressing changes according to the standard protocol and to 4 changes in the new protocol); the assumption for an autologous BMT recipient with a non‐tunnelled CVC was about 20 days (corresponding to a total of 10 dressing changes in the standard protocol and 4 with the new one). Median time per dressing was calculated from the scheduled time of PNR (10 min), the Clock Survey from Azienda Ospedaliera Careggi, Florence (20 min), and the time measured at the bed‐side in the BMT Unit in Florence (13 min) Other outcomes reported in the trial None Validity of measures: Not stated Inter‐rater reliability: A detailed protocol for CVC dressing under controlled sterile conditions was prepared, and all nurses involved in CVC maintenance were asked to adhere strictly to it for the whole study period; it was the responsibility of each centre's co‐ordinator to ensure the correct performance of the protocol Time points: Skin swabs were taken from the site of CVC insertion in all patients enrolled in the study at the time of admission to the BMT Unit (basal sample) and later on at 10‐day intervals during the BMT procedure for the whole period of the patients’ stay in hospital |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Evidence: Not stated in the trial report Comment: We were unable to judge the adequacy of sequence generation |
| Allocation concealment (selection bias) | Unclear risk |
Evidence: Not stated Comment: We were unable to judge the adequacy of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Evidence: Not stated Comment: Not possible to blind the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Evidence: Not stated Comment: It would have been possible to blind assessment of the study outcomes, but this was not stated in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Evidence: All withdrawn patients accounted for Comment: All data complete |
| Selective reporting (reporting bias) | Unclear risk |
Evidence: All planned outcomes reported Comment: Protocol not reviewed |
| Other bias | Unclear risk |
Evidence: Comment: As no baseline data were published, it was unclear if groups were matched for important risk factors |
Timsit 2009.
| Methods |
Study design: Multi‐centre, 2 x 2 factorial, RCT Sample size calculation: Yes ITT analysis: Yes Ethics and informed consent: Grenoble University Hospital Ethics Committee, France Registration number and name of registry: NCT00417235 www.clinicaltrials.gov |
|
| Participants |
Population: "Patients expected to require an arterial catheter, central‐vein catheter, or both inserted for 48 hours or longer in ICU." Setting: 7 ICUs (2 medical, 2 surgical, 3 medical‐surgical) in 3 university and 2 general hospitals in France. Number: 1653 patients randomised: 416 in the 3‐day standard dressing group; 412 in the 3‐day CHGIS group; 412 in the 7‐day standard dressing group; 413 in the 7‐day CHGIS group Age: Median 63 years (IQR 50‐74) Gender (male:female): 1052:584 Skin complexion: Not stated Known allergies to dressings: Patients with a history of allergy to chlorhexidine or to transparent dressings were excluded Known history of current BSI: Not stated Inclusion criteria: "Patients older than 18 years expected to require an arterial catheter, central‐vein catheter, or both inserted for 48 hours or longer in ICU. CVC inserted in the study ICU or immediately before by the anaesthetist in the emergency unit or in the operating room. CVC inserted under maximal barrier precautions." Exclusion criteria: "Patients with a history of allergy to chlorhexidine or to transparent dressings. Pulmonary arterial, haemodialysis and PICCs were not included. Antiseptic and antibiotic impregnated CVCs were not included. CVC inserted under emergency conditions. CVC not inserted under maximal barrier precautions." |
|
| Interventions |
Aim: To assess superiority of CHGIS dressings (Biopatch, Ethicon, New Jersey, USA) regarding the rate of major CRIs (clinical sepsis with or without bloodstream infection) and non‐inferiority (less than 3% colonisation‐rate increase) of 7‐day versus 3‐day dressing changes Intervention: 7‐day CHGIS group and 7‐day standard dressing group Control: 3‐day CHGIS group and 3‐day standard dressing group Dressing protocol in both groups: "The same semipermeable transparent dressing (Tegaderm; 3M Inc, St Paul, Minnisota) were used in all 4 treatment groups. The dressing was changed 24 hours after catheter insertion (day 1) and then as randomised. The alcohol‐based povidone‐iodine solution was used for skin antisepsis during dressing changes. In the CHGIS group, the CHGIS dressing was applied to the entire skin surface at and around the insertion site. The semitransparent dressing was then applied. A new CHGIS was used at each dressing change." Deviation from planned dressing day: "Leakage or soiling prompted immediate dressing change." Number of dressing changes during dwell time of CVAD: "Median 3 dressing changes per catheter (IQR 1‐5)." Duration of follow‐up: Until 48 h after ICU discharge Numbers lost to follow‐up: 7‐day CHGIS group: 4 withdrew consent; 52 catheters/19 participants excluded from per protocol analysis 7‐day standard group: 3 withdrew consent; 57 catheters/22 participants excluded from per protocol analysis 3‐day CHGIS group: 4 withdrew consent; 54 catheters/29 participants excluded from per protocol analysis 3‐day standard group: 6 withdrew consent; 83 catheters/41 participants excluded from per protocol analysis Reason for CVAD insertion: ICU admission Method of CVAD insertion: "All study centers followed French recommendations for catheter insertion and care, which are similar to recommendations from the CDC. Maximal sterile barrier precautions (large sterile drape; surgical hand antisepsis; and mask, cap, sterile gloves, and gown) were used at catheter insertion. The insertion site was scrubbed with 4% aqueous povidone iodine solution (Betadine Scrub; Viatris Pharmaceuticals, Merignac, France), rinsed with sterile water, and dried with sterile gauze; an alcohol‐based antiseptic solution (5% povidone‐iodine in 70% ethanol) (Betadine Alcohol‐based Solution, Viatris) was then applied for at least 1 minutes, and sterile drapes were placed around the site." Anatomical location of CVAD: Jugular 560/2051; subclavian 819/2051; femoral 672/2051 Profession of CVAD inserter: Intensivist Type of CVAD: Not stated Number of CVAD lumens: 0 lumens 37/2051; 2 lumens 209/2051; 3 lumens 1805/2051 Dwell time of CVAD: "Median 6 days (IQR 4‐10)" Study dates: 20 December 2006‐20 May 2008 |
|
| Outcomes |
Primary outcomes CRBSI: Major CRI (defined as catheter‐related sepsis with or without bloodstream infection Catheter‐related clinical sepsis without bloodstream infection defined as fever ≥ 38.5°C or ≤ 36.5°C; catheter tip culture yielding ≥ 10³ CFU/ml; pus at the insertion site or resolution of clinical sepsis after catheter removal; absence of any other infectious focus CRBSI was defined as a combination of ≥ 1 positive peripheral blood cultures sampled immediately before or within 48 h after catheter removal; a quantitative catheter‐tip culture testing positive for the same micro‐organism or a differential time to positivity of blood cultures ≥ 2 h; no other infectious focus explaining the positive blood culture result Suspected CRBSI: Not included All‐cause mortality: Reported Secondary outcomes Catheter‐site infection: Skin colonisation assessed by the semi‐quantitative insertion‐site skin cultures at catheter removal Skin damage: The condition of the skin was described on a standardised form by the nurse in charge of the patients at each dressing change and at catheter removal, using the International Contact Dermatitis Research Group system (0, normal skin; 1, mild erythema; 2, red and slightly thickened skin; 3, intense redness and swelling with coalesced large blisters or spreading reaction) Pain: Not included Quality of life: Not included Cost: Not included Other outcomes reported in the trial None Validity of measures: French (Timsit) and US (Mermel) guidelines Inter‐rater reliability: Not stated Time points: Not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Evidence: "The randomization schedule, stratified by ICU, was developed using a Web‐based random‐number generator to select permuted blocks of 8 patients each." Comment: Adequate method for sequence generation |
| Allocation concealment (selection bias) | Unclear risk |
Evidence: Not stated in the trial report Comment: We were unable to judge the adequacy of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Evidence: "The study was not blinded for the investigators or ICU staff. Double‐blinding was not feasible, because visually identical sponges without chlorhexidine were not available and the nurses had to be informed of the dressing change interval." Comment: It was not possible to undertake blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Evidence: "The study was blinded for the microbiologists processing the skin and catheter cultures and for the assessors. A blinded procedure was used for the catheter cultures. Independent assessors conducted blind review of all suspected catheter infections." Comment: Adequate method for blinding outcome assessor used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Evidence: "1653 patients were enrolled, but subsequently 17 withdrew consent to participate, leaving 1636 available for inclusion in the ITT analysis." Comment: Similar numbers were reported in both groups in the ITT analysis |
| Selective reporting (reporting bias) | Low risk |
Evidence: Planned outcomes in methods section and in the protocol (clinicaltrials.gov) were reported in the paper Comment: Although we were unable to extract primary outcome data for this review (because of the way it was reported) the planned outcomes were reported in the paper |
| Other bias | Unclear risk |
Evidence: "The number needed to treat with CHGIS dressings was 117 catheters (95%CI, 86‐1020). Treatment for 10 days usually requires 3 dressings, each of which costs US$6 (2007 $), and the cost of preventing a single episode of major C‐RI can be estimated at $2106 (95%CI $1518‐$18360). The cost of managing a single case of major C‐RI ranges from $8000 to more than $28000, suggesting the CHGIS dressings may be a cost saving." Comment: Uncertain of the NNTB. All data presented per catheter rather than per patient. Author contacted |
Vokurka 2009.
| Methods |
Study design: Multicentre, RCT Sample size calculation: Not stated ITT analysis: Not stated Ethics and informed consent: Ethical consent not stated. Informed consent obtained Registration number and name of registry: Not stated |
|
| Participants |
Population: "Adults with acute myeloid leukaemia treated with intensive chemotherapy containing cytosine‐arabinoside (Ara‐C) and anthracyclines." Setting: Hemato‐Oncology Department, University Hospital Number: Once‐weekly (every 7 days) group: 39 participants. Twice‐weekly (every 3‐4 days) group: 42 participants Age: Once‐weekly group mean age 41.4 years (± 14.9). Twice‐weekly group mean age 49.9 years (± 10.7) Gender (male:female): Once‐weekly group: 19:20. Twice‐weekly group: 16:26 Skin complexion: Not stated Known allergies to dressings: Patients were excluded Known history of current BSI: Not stated Inclusion criteria: "Adults with acute myeloid leukaemia treated with intensive chemotherapy containing cytosine‐arabinoside (ara‐c) and anthracyclines were included in the observation." Exclusion criteria: "Patients with damaged skin at baseline, those allergic to disinfectant, acrylate, or polyurethane, and patients with radiotherapy of the chest in their history were excluded." |
|
| Interventions |
Aim: To gain experience and to verify whether prolonging the dressing change interval would really be of any benefit and be safe Intervention: Dressings changed once weekly (every 7 days) Control: Dressings changed twice weekly (every 3‐4 days) Dressing protocol in both groups: "Transparent polyurethane semi‐permeable occlusive dressings (Bioclusive, Johnson and Johnson). The dressing could be changed sooner in case of an unstitched, loose, or soiled dressing, insertion‐site inflammation, local cutaneous damage, in‐site bleeding, or other significant (technical) reason." Deviation from planned dressing day: Once‐weekly group: 58% dressing changes as per protocol; Twice‐weekly group: 80% dressing changes as per protocol Number of dressing changes during dwell time of CVAD: "Once‐weekly group: mean number of occlusive dressing changes 4.5 (± 2.4). Twice‐weekly group: mean number of occlusive dressing changes 5.9 (± 2.5)." Duration of follow‐up: "Local cutaneous damage was assessed daily." Numbers lost to follow‐up: All patients accounted for Reason for CVAD insertion: Treatment with intensive chemotherapy Method of CVAD insertion: "Povidone‐iodine was used for skin disinfection at the time of CVC insertion and before any occlusive dressing application." Anatomical location of CVAD: Vena subclavia Profession of CVAD inserter: Not stated Type of CVAD: Non‐tunnelled polyurethane CVCs Number of CVAD lumens: Once‐weekly group: 28 catheters with 1 lumen; 6 catheters with 2 lumens; 8 catheters with 3 lumens. Twice‐weekly group: 19 catheters with 1 lumen; 6 catheters with 2 lumens; 14 catheters with 3 lumens Dwell time of CVAD: Not stated Study dates: August 2003‐August 2005 |
|
| Outcomes |
Primary outcomes CRBSI: Not included Suspected CRBSI: Not included All‐cause mortality: Not included Secondary outcomes Catheter‐site infection: Infection rate and insertion‐site inflammation. The CVC insertion‐site inflammation was defined as local circular redness accompanied, in case of larger reactions, with swelling and pain or palpitation in the area surrounding the point of percutaneous insertion. Reported across both groups. Skin damage: Local cutaneous damage was assessed daily using local institutional grading (0: healthy skin, 1: erythema, 2: erythema with itching or dry desquamation, 3: moist desquamation, exfoliation, 4: deep ulceration, necrosis) Pain: Any pain or discomfort presented during the dressing change was evaluated by patients using visual analogue scoring (VAS) ranging from 0 to 10 (0: no pain, 5: moderate pain, 10: severe pain) Quality of life: Not included Cost: Not included Other outcomes reported in the trial Highest temperature and blood cultures for microbiological testing Tolerance Validity of measures: Not stated Inter‐rater reliability: Not stated Time points: Daily assessment of skin. Skin swabs for microbiological testing were obtained from the area around the CVC insertion‐site on any dressing change before local disinfection. The highest temperature was recorded on a daily basis and blood cultures for microbiological testing were taken from the CVC on the first occurrence of fever (> 38°C) and thereafter as indicated by the medical staff |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Evidence: "The patients were randomized by GraphPad StatMate (GraphPad Software Inc)" Comment: Computer generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk |
Evidence: "As for our randomized trial allocation, we used a Randomization PC Software to allocate the trial patients into the individual cohorts. We did not used sealed envelopes." Comment: The evidence for this 'bias' element was obtained from the trialist through email contact |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Evidence: Not possible Comment: Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Evidence: There was no information about outcome assessor blinding in the report Comment: Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Evidence: All of the enrolled patients were accounted for in the results Comment: An equal number of patients (3) in each group were withdrawn due to intolerance of the dressing Consequently, we judged that this domain was at low risk for bias |
| Selective reporting (reporting bias) | Unclear risk |
Evidence: All planned outcomes reported Comment: Protocol not reviewed |
| Other bias | Low risk |
Evidence: None reported Comment: As there were no 'other' biases reported, we judged this domain to be at low risk |
Abbreviations
BMT: bone marrow transplant BSI: blood stream infection CDC: Centers for Disease Control and Prevention CFU: colony forming unit CHGIS: chlorhexidine gluconate‐impregnated sponge CRBSI: catheter‐related bloodstream infection CRI: catheter‐related infection CVAD: central venous access device CVC: central venous catheter ECOG: Eastern Cooperative Oncology Group h: hour(s) HDC: high dose chemotherapy ICU: intensive care unit IQR: inter‐quartile range ITT: intention‐to‐treat (analysis) min: minute(s) NNTB: number needed to treat for an additional beneficial outcome PICC: peripherally inserted central catheter PNR: patient nurse ratio RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bystricka 2004 | Study protocol |
| Davidson 1986 | Comparison of different dressing types not frequencies |
| Dickerson 1989 | Letter to the editor (comment on a study comparing two types of dressings) |
| Hagerstrom 1994 | Conference abstract related to dressing methods ‐ unrelated to timing |
| Ishizuka 2011 | The wards involved were randomly allocated, not the patients |
| Lucas 1996 | Comparison of different dressing types not frequencies |
| Powell 1985 | Co‐interventions (frequency of administration set replacement) were different between different arms of the study |
| Samsoondar 1985 | Co‐interventions (frequency of administration set replacement) were different between different arms of the study Quasi‐randomisation |
| Young 1988 | Co‐interventions (frequency of administration set replacement) were different between different arms of the study |
| Zitella 2003 | Systematic literature review of central venous catheter site care for blood and marrow transplant recipients |
Characteristics of studies awaiting assessment [ordered by study ID]
Fessard 1994.
| Methods | Unknown |
| Participants | Paediatrics |
| Interventions | Frequency of dressing changes |
| Outcomes | Unknown |
| Notes | Prospective randomised trial to study the best time interval between catheter dressing: Study performed by the nurses of paediatric transplantation unit. Title found in a reference list. Awaiting paper from publishers |
Differences between protocol and review
Methods; Criteria for considering studies; Types of studies
Studies with co‐intervention excluded. Co‐interventions (frequency of administration set replacement) were different between different arms of the study. Not stated in the protocol.
Methods; Data collection and analysis; Measures of treatment effect
Event rates for dichotomous outcomes are presented as risk ratio (RR) and 95% confidence interval (CI). Not stated in the protocol.
Methods; Data collection and analysis; Summary of Findings Table
Summary of Findings Tables added.
Contributions of authors
Nicole Gavin: conceived, designed and coordinated the review. Extracted data, checked the quality of the data extraction, and analysed and interpreted data. Undertook and checked quality assessment. Performed statistical analysis and checked the quality of the statistical analysis. Completed the first draft of the review. Is the guarantor of the review.
Joan Webster: extracted data, checked the quality of the data extraction, and analysed and interpreted data. Undertook and checked quality assessment. Performed part of data analysis and interpretation. Checked the quality of the statistical analysis. Performed part of writing and editing the review. Advised on the review and approved the final review before submission.
Raymond Chan: Performed part of writing and editing the review. Advised on the review and approved the final review before submission.
Claire Rickard: Performed part of writing and editing the review. Advised on the review and approved the final review before submission.
Contributions of editorial base
Nicky Cullum: edited the protocol and the review: advised on methodology, interpretation and content. Approved the final review prior to publication. Sally Bell‐Syer: co‐ordinated the editorial process. Advised on methodology, interpretation and content. Edited the protocol and review. Ruth Foxlee: designed the search strategy and Rocio Rodriguez‐Lopez ran the searches.
Sources of support
Internal sources
-
Cancer Care Services & Centre for Clinical Nursing (Research & Development Unit), Royal Brisbane and Women’s Hospital, Brisbane, Australia.
For funding the salary and facilities for NG to conduct this systematic review
-
Griffith University, Brisbane, Australia.
For providing the supervision to undertake this systematic review as part of Nicole Gavin's Masters of Advanced Practice (Health Care Research)
External sources
-
Australasian Cochrane Centre, Australia.
For providing two‐day workshop: Developing a Protocol for a Systematic Review and Introduction to Analysis
For providing five‐day workshop: Cochrane Review Completion and Update Program
-
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Australian National Health and Medical Research Council through the Centre for Research Excellence in Nursing Interventions for Hospitalised Patients, Australia.
Nicole Gavin is supported for a PhD scholarship
Declarations of interest
Nicole C Gavin: none known Joan Webster: none known Raymond J Chan: none known Claire M Rickard: Claire Rickard is a Board Member of the Australian Intensive Care Foundation. She has carried out consultancy research on IV flushing for Becton Dickinson Medical and has received grants from commercial companies supporting research projects including those on IV dressings. The granting bodies did not undertake study design, procedures, data analysis or preparation of results for publication. She has received payment from commercial companies for educational lectures based on her research, and educational grants to support her conference attendance.
New
References
References to studies included in this review
Benhamou 2002 {published data only}
- Benhamou E, Fessard E, Com‐Nougue C, Beaussier PS, Nitenberg G, Tancrede C, et al. Less frequent catheter dressing changes decrease local cutaneous toxicity of high‐dose chemotherapy in children, without increasing the rate of catheter‐related infections: results of a randomised trial. Bone Marrow Transplantation 2002;29:653‐8. [DOI] [PubMed] [Google Scholar]
- Fessard E. Frequency of change of dressings two comparative trials with central venous catheters in children, hospitalized for severe chemotherapy and bone marrow transplantation [Fréquence de réfection des pansements]. Soins 1994;585/586:33‐8. [PubMed] [Google Scholar]
Engervall 1995 {published data only}
- Engervall P, Ringertz S, Hagman E, Skogman K, Bjorkholm M. Change of central venous catheter dressings twice a week is superior to once a week in patients with haematological malignancies. Journal of Hospital Infection 1995;29:275‐86. [DOI] [PubMed] [Google Scholar]
- Engervall P, Ringertz S, Lothman P, Hagman E, Skogman C, Bjorkholm M. A prospective, randomized study comparing change of central venous catheter dressings once or twice a week in patients with hematological malignancies. Journal of Haematology 1994;87(S1):67. [Google Scholar]
Rasero 2000 {published data only}
- Rasero L, Degl'Innocenti M, Mocali M, Alberani F, Boschi S, Giraudi A, et al. Comparison of two different CVC medications change time intervals in bone marrow transplant patients: the results of a multicentre randomised controlled trial [Confronto di due differenti protocolli nell'intervallo del cambio di medicazione per cateteri venosi centrali in pazienti trapiantati di midollo osseo: risultati di uno studio melticentrico randomizzato]. Assistenza Infermieristica e Ricerca 2000;19:122‐9. [PubMed] [Google Scholar]
- Rasero L, Degl'Innocenti M, Mocali M, Alberani F, Boschi S, Giraudi A, et al. Comparison of two different time interval protocols for central venous catheter dressing in bone marrow transplant patients: results of a randomized, multicenter study. Haematologica 2000;85:275‐9. [PubMed] [Google Scholar]
Timsit 2009 {published data only}
- Timsit JF, Schwebel C, Bouadma L, Geffroy A, Garrouste‐Orgeas M, Pease S, et al. Chlorhexidine‐impregnated sponges and less frequent dressing changes for prevention of catheter‐related infections in critically ill adults. JAMA 2009;301(12):1231‐41. [DOI] [PubMed] [Google Scholar]
Vokurka 2009 {published data only}
- Vokurka S, Bystricka E, Visokaiova M, Scudlova J. Once‐versus twice‐weekly changing of central venous catheter occlusive dressing in intensive chemotherapy patients: results of a randomized multicenter study. Medical Science Monitor 2009;15(3):CR107‐10. [PubMed] [Google Scholar]
References to studies excluded from this review
Bystricka 2004 {published data only}
- Bystricka E, Chvojkova I, Suvova J, Mullerova N, Vokurka S, Koza V, et al. Risks and benefit of double‐prolonged interval of central venous catheter dressing changes in patients treated by intensive chemotherapy ‐ proposal of a new randomised nurses' trial. Bone Marrow Transplantation 2004;33(S1):S295. [Google Scholar]
Davidson 1986 {published data only}
- Davidson LE. Dressing subclavian catheters. Nursing Times 1986;82(7):40. [PubMed] [Google Scholar]
Dickerson 1989 {published data only}
- Dickerson N, Horton P, Smith S, Rose RC. Clinically significant central venous catheter infections in a community hospital: association with type of dressing. The Journal of Infectious Diseases 1989;160(4):720‐2. [DOI] [PubMed] [Google Scholar]
Hagerstrom 1994 {published data only}
- Hagerstrom M, Matthleson K, Thuessen C. A randomised dressing method for haemodialysis patients with permanent central venous catheters. 4th European Conference on Advances in Wound Management proceedings 1994:abstract 197. [Google Scholar]
Ishizuka 2011 {published data only}
- Ishizuka M, Nagata H, Takagi K, Kubota K. Dressing change reduces the central venous catheter‐related bloodstream infection. Hepato‐Gastroenterology 2011;58:1882‐6. [DOI] [PubMed] [Google Scholar]
Lucas 1996 {published data only}
- Lucas H, Attard‐Montalto S. Central line dressings: study of infection rates. Paediatric Nursing 1996;8(6):21‐3. [PubMed] [Google Scholar]
Powell 1985 {published data only}
- Powell CR, Traetow MJ, Fabri PJ, Kudsk KA, Ruberg RL. Op‐site dressing study: a prospective randomized study evaluating povidone iodine ointment and extension set changes with 7‐day op‐site dressings applied to total parenteral nutrition subclavian sites. Journal of Parenteral and Enteral Nutrition 1985;9(3):443‐6. [DOI] [PubMed] [Google Scholar]
Samsoondar 1985 {published data only}
- Samsoondar W, Freeman JB. Colonization of intravascular monitoring devices. Critical Care Medicine 1985;13(9):753‐5. [DOI] [PubMed] [Google Scholar]
Young 1988 {published data only}
- Young GP, Alexeyeff M, Rusell DM, Thomas RJS. Catheter sepsis during parenteral nutrition: the safety of long‐term op‐site dressings. Journal of Parenteral and Enteral Nutrition 1988;12(4):365‐70. [DOI] [PubMed] [Google Scholar]
Zitella 2003 {published data only}
- Zitella L. Central venous catheter site care for blood and marrow transplant recipients. Clinical Journal of Oncology Nursing 2003;7(3):289‐98. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fessard 1994 {published data only}
- Fessard E, Baussier PS, Benrondhane S. Prospective randomized trial to study the best time interval between catheter dressing: Study performed by the nurses of pediatric transplantation unit. European Bone Marrow Transplant Nursing Journal 1994;2:7‐9. [Google Scholar]
Additional references
Camp‐Sorrell 2004
- Camp‐Sorrell D. Access Device Guidelines: Recommendations for Nursing Practice and Education. 2nd Edition. Pittsburgh: Oncology Nursing Society, 2004. [Google Scholar]
Casey 2010
- Casey AL, Elliott TSJ. Prevention of central venous catheter‐related infection: update. British Journal of Nursing 2010;19(2):78‐87. [DOI] [PubMed] [Google Scholar]
CDC 2002
- Centers for Disease Control and Prevention. Guidelines for the prevention of intravascular catheter‐related infections. Morbidity and Mortality Weekly Report 2002;51(No. RR‐10):1‐29. [PubMed] [Google Scholar]
CNSA 2007
- Cancer Nurses Society of Australia. Central Venous Access Devices: Principles for Nursing Practice and Education. Summary and Recommendations. Cancer Nurses Society of Australia, 2007. [Google Scholar]
Cutting 2008
- Cutting KF. Impact of adhesive surgical tape and wound dressings on the skin, with reference to skin stripping. Journal of Wound Care 2008;17(4):157‐62. [DOI] [PubMed] [Google Scholar]
DeSpain 1992
- DeSpain JD. Dermatologic toxicity of chemotherapy. Seminars in Oncology 1992;19(5):501‐7. [PubMed] [Google Scholar]
Dykes 2001
- Dykes PJ, Heggie R, Hill SA. Effects of adhesive dressings on the stratum corneum of skin. Journal of Wound Care 2001;10(2):7‐10. [DOI] [PubMed] [Google Scholar]
Dykes 2007
- Dykes PJ. The effect of adhesive dressing edges on cutaneous irritancy and skin barrier function. Journal of Wound Care 2007;16(3):97‐100. [DOI] [PubMed] [Google Scholar]
ECOG 2007
- Eastern Cooperative Oncology Group. Common Toxicity Criteria. http://ecog.dfci.harvard.edu/general/ctc.pdf (accessed on 16 November 2010).
Elkabir 2001
- Elkabir JJ, Khadra A. Wound dehiscence. MRCS Core Modules: Essential Revision Notes. Knutsford: Pastest, 2001. [Google Scholar]
Elliott 1998
- Elliott TS, Tebbs SE. Prevention of central venous catheter related infection. Journal of Hospital Infection 1998;40(3):193‐201. [DOI] [PubMed] [Google Scholar]
Gabriel 2005
- Gabriel J, Bravery K, Doughtery L, Kayley J, Malster M, Scales K. Vascular access: indications and implications for patient care. Nursing Standard 2005;9(26):45‐52. [DOI] [PubMed] [Google Scholar]
Gillies 2003
- Gillies D, Carr D, Frost J, O’Riordan E, Gunning R, O’Brien I. Gauze and tape and transparent polyurethane dressings for central venous catheters. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003827] [DOI] [PubMed] [Google Scholar]
Glean 2001
- Glean E, Edwards S, Faithfull S, Meredith C, Richards C, Smith M, et al. Interventions for acute radiotherapy induced skin reactions in cancer patients: the development of a clinical guideline recommended for use by the College of Radiographers. Journal of Radiotherapy in Practice 2001;2:75‐84. [Google Scholar]
Hadaway 2003
- Hadaway LC. Infusing without infecting. Nursing 2003;33(10):58‐64. [DOI] [PubMed] [Google Scholar]
Hayden 2005
- Hayden K, Goodman M. Chemotherapy: principles of administration. In: Yarbro CH, Frogge MR, Goodman M editor(s). Cancer Nursing: Principles and Practice. 6th Edition. Boston: Jones and Bartlett, 2005. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Hollingworth 2009
- Hollingworth H. Challenges in protecting peri‐wound skin. Nursing Standard 2009;24(7):53‐62. [DOI] [PubMed] [Google Scholar]
Hopewell 1990
- Hopewell JW. The skin: its structure and response to ionizing radiation. International Journal of Radiation Biology 1990;57(4):751‐73. [DOI] [PubMed] [Google Scholar]
Hunt 1997
- Hunt TK, Williams H. Wound healing and wound infection: what surgeons and anesthesiologists can do. Surgical Clinical of North America 1997;77(3):587‐606. [DOI] [PubMed] [Google Scholar]
INS 2011
- Infusion Nursing Society. Infusion nursing standards of practice. Journal of Infusion Nursing 2011;34(S1):S1‐S110. [Google Scholar]
IVNNZ 2012
- Intravenous Nursing New Zealand Incorporated Society. Provisional infusion therapy standards of practice. www.ivnnz.co.nz (accessed 6 August 2015).
Karwoski 2004
- Karwoski AC, Plaut RH. Experiments on peeling adhesive tapes from human forearms. Skin Research and Technology 2004;10(4):271‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lotti 1998
- Lotti T, Rodofili C, Benci M, Menchin G. Wound healing problems associated with cancers. Journal of Wound Care 1998;7(2):81‐4. [DOI] [PubMed] [Google Scholar]
Loveday 2014
- Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, et al. epic3: National evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospital in England. Journal of Hospital Infection 2014;86(S1):S1‐S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maki 1997
- Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter‐related bloodstream infection by use of an antiseptic‐impregnated catheter: a randomized, controlled trial. Annals of Internal Medicine 1997;127(4):257‐66. [DOI] [PubMed] [Google Scholar]
Mermel 2000
- Mermel LA. Prevention of intravascular catheter‐related infections. Annals of Internal Medicine 2000;132(5):391‐402. [DOI] [PubMed] [Google Scholar]
Mermel 2009
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical Practice Guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009;49:1‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Grady 2011
- O'Grady NP, Alexander M, Burns LA, Patchen Dellinger E, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter‐related infections. http://www.cdc.gov/hicpac/bsi/bsi‐guidelines‐2011.html (accessed 6 August 2015).
O'Rourke 1989
- O'Rourke K, Detsky AS. Meta‐analysis in medical research: strong encouragement for higher quality individual research efforts. Journal of Clinical Epidemiology 1989;42(10):1021‐4. [DOI] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Rippon 2007
- Rippon M, White R, Davies P. Skin adhesives and their role in wound dressings. Wounds UK 2007;3(4):76‐86. [Google Scholar]
RNAO 2005
- Registered Nurses’ Association of Ontario. Care and Maintenance to Reduce Vascular Access Complications. Toronto: Registered Nurses’ Association of Ontario, 2005. [Google Scholar]
Rosenthal 2003
- Rosenthal K. Pinpointing intravascular device infections. Nursing Management 2003;34(6):35‐43. [DOI] [PubMed] [Google Scholar]
Safdar 2002
- Safdar N, Maki DG. Inflammation at the insertion site is not predictive of catheter‐related bloodstream infection with short‐term, noncuffed central venous catheters. Critical Care Medicine 2002;30:2632–5. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. In: Higgins JPT, Green S (editors). Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org.
SIGN 2011
- Scottish Intercollegiate Guidelines Network. Search filters. http://www.sign.ac.uk/methodology/filters.html (accessed 24 July 2014).
Timsit 2012
- Timsit JF, Bouadma L, Ruckly S, Schwebel C, Garrouste‐Orgeas M, Bronchard R, et al. Dressing disruption is a major risk factor for catheter‐related infections. Critical Care Medicine 2012;40:1707‐14. [DOI] [PubMed] [Google Scholar]
Tortura 2000
- Tortura GJ, Grabowski SR. Principles of Anatomy and Physiology. 9th Edition. New York: John Wiley & Sons Inc, 2000. [Google Scholar]
Walshe 2002
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. Journal of Clinical Oncology 2002;20:3276–81. [DOI] [PubMed] [Google Scholar]
Wille 1993
- Wille JC, Blusse van Oud Alblas A, Thewessen EAPM. A comparison of two transparent film‐type dressings on central venous therapy. Journal of Hospital Infection 1993;23(2):113‐21. [DOI] [PubMed] [Google Scholar]
Wilson 2006
- Wilson J. Preventing infection associated with intravascular therapy. Infection Control in Clinical Practice. 3rd Edition. London: Elsevier, 2006. [Google Scholar]


