Abstract
Age is one of the most important risk factors for the development of breast cancer. Nearly a third of all breast cancer cases occur in older women (aged ≥70 years), with most cases being oestrogen receptor-positive (ER+). Such tumours are often indolent and unlikely to be the ultimate cause of death for older women, particularly when considering other comorbidities. This Review focuses on unique clinical considerations for screening, detection, and treatment regimens for older women who develop ER+ breast cancers—specifically, we focus on recent trends for de-implementation of screening, staging, surgery, and adjuvant therapies along the continuum of care. Additionally, we also review emerging basic and translational research that will further uncover the unique underlying biology of these tumours, which develop in the context of systemic age-related inflammation and changing hormone profiles. With prevailing trends of clinical de-implementation, new insights into mechanistic biology might provide an opportunity for precision medicine approaches to treat patients with well tolerated, low-toxicity agents to extend patients’ lives with a higher quality of life, prevent tumour recurrences, and reduce cancer-related burdens.
Introduction
Breast cancer, as with most cancers arising in adults, is an age-related disease. Age is one of the most important risk factors, with nearly a third of all breast cancer cases diagnosed in patients older than 70 years and a peak incidence occurring between the ages of 60 and 80 years in White people, and between 40 and 50 years in Black, Asian, and Hispanic people.1–3 The vast majority of these cancers are oestrogen receptor-positive (ER+), and the proportion of ER+ tumours relative to other subtypes increases with age.4–6 Consistent with the favourable receptor status, these tumours grow slowly and are often less aggressive than tumours in younger patients, suggesting that tumorigenesis in these patients might largely be due to chronic exposure to tumour-promoting stimuli.5,7
Clinical management of older patients with early-stage ER+ breast cancer is challenging.8 Older patients typically develop tumours in the context of age-related inflammation,9 which might require different treatment considerations. Older patients typically have a greater degree of multimorbidity and polypharmacy burden than their younger counterparts (figure 1).10 As the International Society of Geriatric Oncology (SIOG) suggests, these patients are a heterogenous population, which warrants clinical use of validated comprehensive geriatric assessments. These assessments might reveal additional functional impairments, cognitive problems, nutritional issues, psychological distress, insufficient social support or engagement, and the presence of geriatric syndromes (figure 2).11–13 Further complicating optimal treatment strategies is the fact that older patients are often excluded in randomised controlled trials and have fewer treatment guidelines that are tailored to them than do younger patients with cancer.12–14 For example, one report showed that women who enrolled in clinical trials for breast cancer were nearly 8 years younger than the median age for individuals with the disease.12 Another report showed that only 24% of patients enrolled in oncology drug-approving trials were older than 70 years.13 Moreover, standard clinical trial endpoints, such as survival outcomes, are often less meaningful for older patients with cancer, for whom quality of life, treatment tolerability, and effects of treatment on functional measures might be more relevant. These challenges leave older patients particularly vulnerable to the risks of both overtreatment and undertreatment.11,15 Overtreatment can lead to diminished quality of life, financial burdens, and a cascade of low-value care; however, undertreating these patients might lead to an increased risk of tumour recurrence. These considerations make comprehensive geriatric assessments for these patients even more imperative; such multidomain assessments can help clinicians identify medical, psychosocial, and functional issues that are not readily detectable with routine evaluation.16 Despite being time-consuming, these validated tools can be a strong asset when devising a treatment plan, especially one in which omission of certain interventions is considered. When formulating care plans, clinicians should have discussions with patients and caregivers about goals for care and end of life.17
Figure 1: Challenges with treating older patients with ER+ breast cancer.
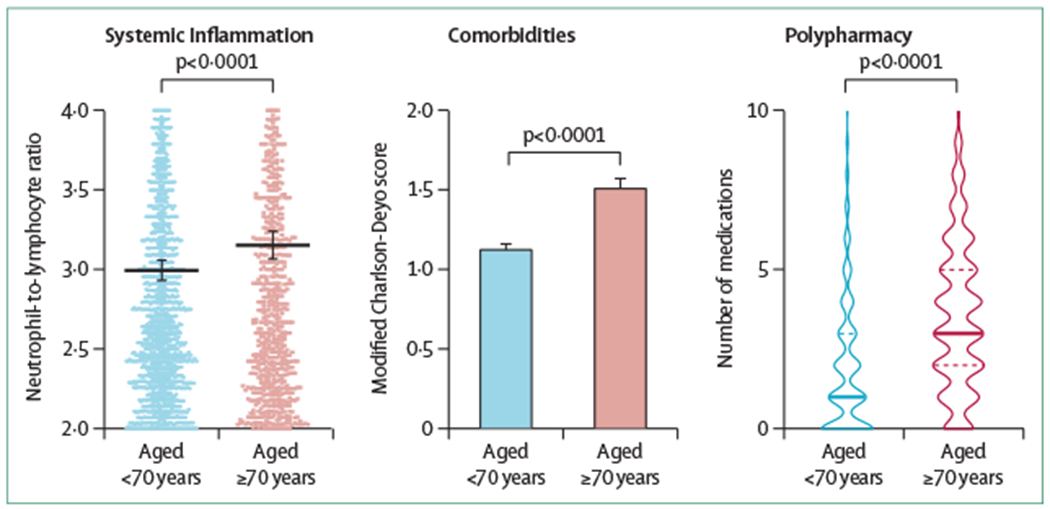
(A) One measure of age-related systemic inflammation is the neutrophil-to-lymphocyte ratio, which is measured routinely during blood analysis at check-ups and which increases with age. This age-related systemic inflammation is also seen in patients with breast cancer, for whom the neutrophil-to-lymphocyte ratio also increases with age. p<0·0001 when comparing patients younger than 70 years diagnosed with ER+ breast cancer (n=2701) and patients aged 70 years or older diagnosed with ER+ breast cancer (n=1182). (B) Older patients with ER+ breast cancer have more comorbidities than their younger counterparts as measured by the mean modified Charlson-Deyo score. p<0·0001 when comparing patients younger than 70 years diagnosed with ER+ breast cancer (n=3426) and patients aged 70 years or older diagnosed with ER+ breast cancer (n=1652). (C) Older patients with cancer are often prescribed multiple medications that might complicate or interact with treatments for their primary cancer. p<0.0001 when comparing patients younger than 70 years diagnosed with ER+ breast cancer (n=3426) and patients aged 70 years or older diagnosed with ER+ breast cancer (n=1652). Data shown in this figure are derived from the University of Pittsburgh Medical Center electronic medical record and cancer registry. For additional details, see the methods in Carleton et al.10 ER=oestrogen receptor.
Figure 2: The need for comprehensive geriatric assessment.
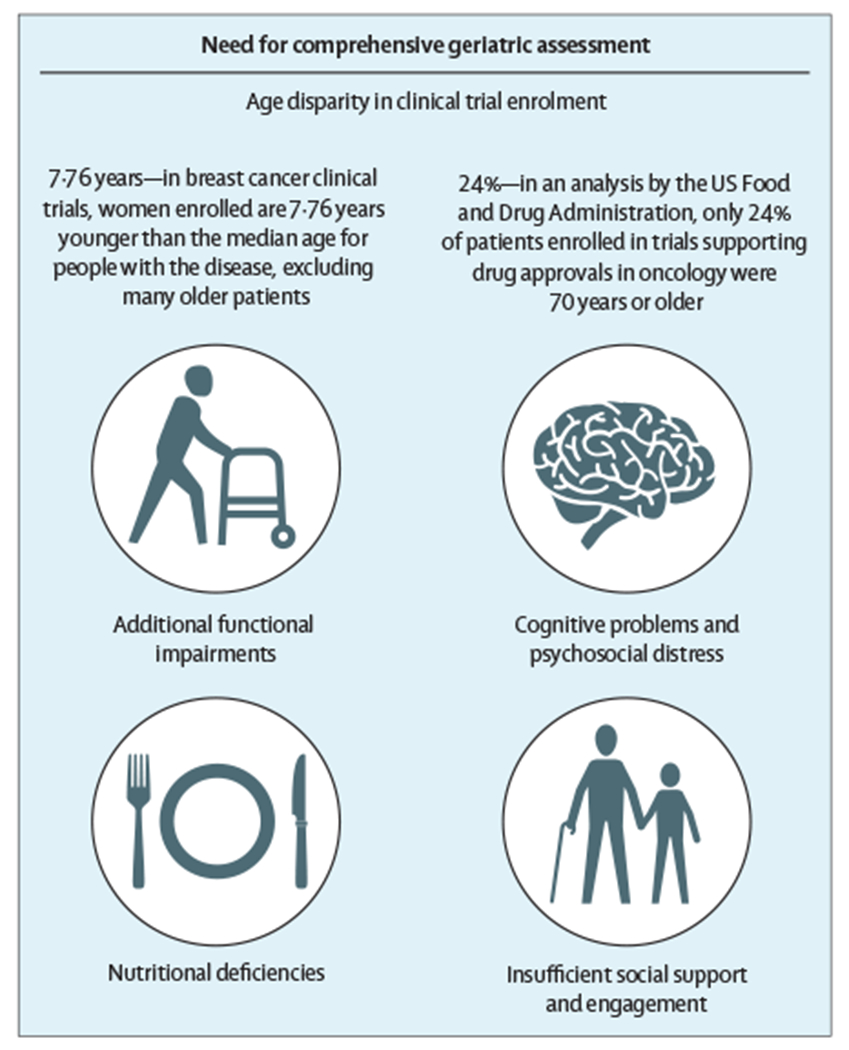
Although comorbidities and polypharmacy can be challenges, older women are also typically excluded from clinical trials. Thus, all women older than 70 years of age diagnosed with breast cancer should receive a comprehensive geriatric assessment, which might reveal additional insights that could influence therapeutic decision making. These can include additional functional impairments, cognitive or psychosocial distress, nutritional deficiencies, and a lack of social support or engagement.
To improve treatment for older patients with ER+ breast cancer, there has been a strong push to better understand the appropriate care regimen for the unique underlying biology of the tumours. De-escalation of some interventions has come to the forefront of discussions regarding cancer care for many older patients. In fact, there is currently a strong call for de-implementation of low-value care interventions,18–20 which are those healthcare services that are considered not to have a clinically meaningful benefit but do have a potential risk of adverse events.21 Omitting low-value care might reduce unnecessary interventions and treatment-related toxic effects, and might prevent diminished quality of life.22 An example relevant for older patients with ER+ breast cancer includes the Society of Surgical Oncology’s adoption of the American Board of Internal Medicine’s Choosing Wisely guidelines, which recommend against the use of sentinel lymph node biopsy (SLNB) for patients older than 70 years with ER+, clinically node-negative breast cancer.23 The comprehensive updated policies from the European Society of Breast Cancer Specialists (EUSOMA) and SIOG provide the latest clinical recommendations about the management of breast cancer in older patients. These recommendations provide a strong foundation for the treatment of older women with breast cancer, but they do not acknowledge some of the unique aspects of ER+ breast cancer or options to omit care because of favourable disease prognosis.24
In this Review, we expand on the aforementioned recommendations and outline the evidence and gaps in knowledge of current clinical de-implementation strategies across the spectrum, from screening to treatment in older women (≥70 years) with ER+ breast cancer, including axillary staging, breast-conserving surgical procedures, and adjuvant therapies (figure 3; panel). Furthermore, we review emerging areas of molecular medicine that provide insight into novel treatments for older patients, including those targeting cancer-promoting inflammation.
Figure 3: Considerations for treating older patients along a continuum of care.
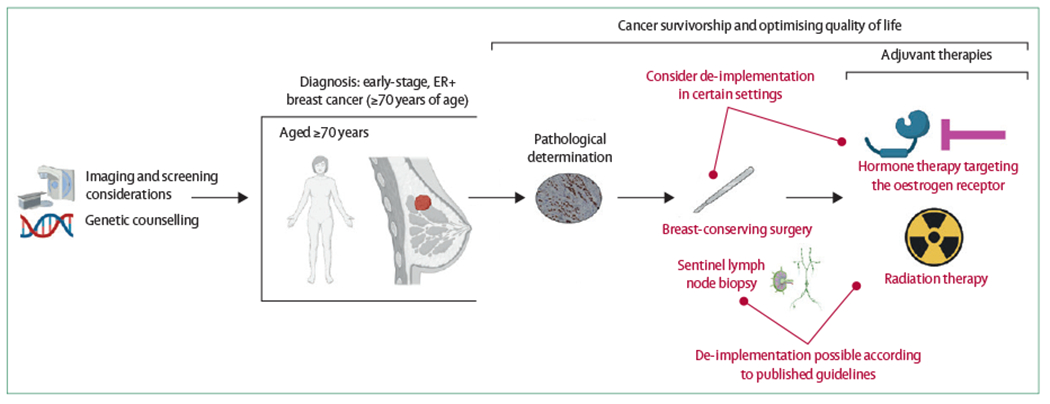
Options for de-implementation might occur at multiple times along the spectrum of care. Guidelines recommending against routine use of sentinel lymph node biopsy and radiation therapy are well established. Emerging evidence to omit upfront surgery and adjuvant endocrine therapy could also be options depending on a clinician’s assessment of the patient and the patient’s preferences. ER=oestrogen receptor.
Clinical trends in de-implementation in low-risk breast cancer in older women
Screening
The US Preventative Services Task Force guidelines do not recommend screening beyond the age of 74 years because of the scarcity of evidence from randomised controlled trials on the benefits of screening mammography in this older population. With the exception of the Swedish Two-County trial that invited women to screen every 33 months up to the age of 74 years, randomised controlled trials to date have not included women older than 70 years.28 However, there are compelling data on screening in older women from the American College of Radiology’s National Mammography Database that show reductions in false positives and increases in both cancer detection rates and the positive predictive value of biopsies done at increased ages, even beyond the age of 90 years.29 Mammographically detected cancers in women aged 75 years or older were much more likely to be diagnosed at a lower stage in several studies: only 12% of those assessed were node-positive at diagnosis compared with 38% of those clinically detected in the series of Malmgren and colleagues,30 and 11% of older women with screen-detected cancers had axillary metastases, as reported by Destounis and colleagues.31
There is an approximately 10-year delay in observing any benefit in breast cancer mortality from screening mammography.32 Instead of using 74 years of age as a screening termination target, national guidelines suggest stopping screening when the patient is no longer in good health (Society of Breast Imaging, National Comprehensive Cancer Network, and American Society of Breast Surgeons) or has a life expectancy of less than 10 years (American Cancer Society).33 Even the healthiest quartile of women older than 85 years has less than 10 years of life expectancy.34 An analysis of outcomes in women older than 65 years found that, even after correcting for prognostic index and potential confounders, breast cancer screening was associated with reduced all-cause mortality.35 This screening benefit was attenuated but persisted in women with reduced cognition. The authors of this analysis suggest that current algorithms might misclassify individuals as having a low life expectancy, resulting in cessation of screening that is inappropriately premature. Similar to these national guidelines, EUSOMA–SIOG recommend stopping screening after 75 years of age.24
Taken together, a detailed geriatric and life expectancy assessment in conjunction with patient preferences should guide decision making for continued screening. Clinicians should make every effort to rely on validated measures to estimate remaining life expectancy (eg, ePrognosis) and the potential benefits of continued screening. Screening after the age of 74 years can be effectively stopped, as per multiple society guidelines including the recent EUSOMA–SIOG recommendations.
Genetic screening
About 5–10% of breast cancers are related to a genetic predisposition. The possibility of a genetic predisposition in older patients should be considered, particularly when the personal and family history is suggestive of a cancer predisposition. In women diagnosed at age 65 years or older, the prevalence of risk genes has been estimated to be about 4–6%.36–38 Although the prevalence of pathogenic variants in BRCA1 and BRCA2 decreases in those older than 40 years, the prevalence of pathogenic variants in ATM, CHEK2, and PALB2 remains constant among patients aged 40–85 years.39 Older patients have a higher proportion of pathogenic variants in moderate-risk predisposition genes for hereditary breast cancer than do younger patients.37 Thus, genetic testing in older patients with breast cancer should be considered when appropriately based on personal and family history.40
Although the identification of a pathogenic variant in a breast cancer predisposition gene has implications for family members, its impact on subsequent cancer risk and medical management for older patients with breast cancer is less clear. Among individuals with a pathogenic variant in BRCA1 and BRCA2, women aged 71–80 years with a previous breast cancer diagnosis have an elevated breast cancer incidence of about 10 per 1000 person-years; however, there are no data specifically on the risk of developing a second primary breast cancer for those diagnosed with the first breast cancer at age 70 years or older.41,42 Compounding the sparse data on cancer risk in older patients is the scarcity of evidence on the benefits of enhanced surveillance and risk-reducing strategies. Although intensive surveillance has the potential to lead to early detection,35 the effect on survival outcomes in older people is largely unknown. Furthermore, although there is an increased risk of developing a second breast cancer in patients with a pathogenic variant in BRCA1 or BRCA2, contralateral risk-reducing mastectomy is unlikely to confer a survival benefit.43,44 There is currently no consensus nor are there standard guidelines on the management of older patients with a genetic breast cancer predisposition, and studies in this population are scarce. In a small retrospective cohort study of women older than 75 years with a pathogenic variant in BRCA1 or BRCA2, most patients elected to have no screening or screening with various schedules of mammography with or without MRI, whereas few patients elected to have risk-reducing mastectomy.45 Genetic testing for BRCA1 and BRCA2 might have implications for therapy, especially given the availability of poly ADP-ribose polymerase (PARP) inhibitors. However, with few older women included in the initial clinical trials, specific treatment tolerability with PARP inhibitors is currently less defined in older patients than in younger patients.46 Consistent with EUSOMA–SIOG recommendations, older women carrying pathological variants can be offered PARP inhibitors if their disease is progressive.
Axillary staging and breast surgery
The rationale for axillary surgery in breast cancer has evolved from the therapeutic benefit of locoregional clearance, to diagnostic staging to guide decision making on adjuvant therapy. SLNB has largely replaced axillary lymph node dissection as the standard of care for women with clinical N0 disease.47,48 Recent evidence has led to refining the diagnostic utility of SLNB, especially in older women.49–51 The Society of Surgical Oncology advocates for omission of SLNB in women aged 70 years or older with early-stage ER+, HER2− breast cancer.23 A large cohort study of more than 3000 older women, aged 70 years and older, at a single health-care system revealed that performing SLNB in this subgroup provided no outcome advantages with respect to locoregional recurrence or breast cancer-specific survival.10 Some recommendations, such as those from EUSOMA–SIOG, state that SLNB should only be omitted for frail patients with low-volume, luminal A-like tumours.24 However, emerging evidence posits that SLNB omission can be extended to many more patients: even omitting SLNB in the setting of higher-grade or higher-stage disease did not affect disease-free or locoregional recurrence-free survival. Even in the absence of a randomised trial, omission of SLNB from routine care is possible as: (1) there is limited diagnostic utility from the procedure; (2) with a clinically node-negative axilla, only some of these patients will actually have a pathologically positive node; and (3) in older women with early-stage disease, omitting SLNB will have a small absolute increase in recurrences without a detriment to their disease-free survival.10
Breast-conserving surgical procedures remain the standard of care for older patients with operable breast cancer, but there is a paucity of data regarding de-escalation of surgical procedures. These procedures are generally well tolerated and have a very low risk for perioperative mortality.52,53 Despite these outcomes, older women are known to be at increased risk of morbidity and mortality, such as postoperative neurocognitive dysfunction, because of the risks of both operation and anaesthesia, especially if they have preoperative functional or cognitive impairment.54–56 Additionally, depending on the extent of the operation and whether a patient needs to use the dominant arm for an assistive device, their dependency might be increased for days or weeks. In some cases, the decision to operate is challenged by the patient’s or family’s understanding, indecisiveness, or uncertainty about the patient’s ability to tolerate surgery. As it currently stands, most guidelines and recommendations, including those by EUSOMA–SIOG, posit that surgery should only be avoided for frail patients deemed non-surgical candidates because of multiple comorbidities, opting instead for treatment with primary endocrine therapy.57–59 A Cochrane review of seven randomised trials comparing surgical excision either with or without tamoxifen versus primary tamoxifen therapy alone in patients aged 70 years and older found that primary tamoxifen therapy was associated with inferior local disease control compared with excision alone, but it was non-inferior for overall survival.25 Similarly, the Bridging the Age Gap working group showed that primary endocrine therapy did not confer inferior breast cancer-specific survival in older women when compared with breast-conserving surgery.60
With this evidence, patients with low-risk ER+ disease are increasingly opting to forego surgery altogether in favour of primary endocrine therapy, as they correctly do not perceive their breast cancer to be a life-threatening ailment.61 Given the risk of postoperative cognitive and functional limitations and the low risk of breast cancer progression, surgical omission with primary endocrine therapy and regular follow-up could be a reasonable alternative treatment strategy for patients with ER+ disease. Prospective studies in this area are warranted, especially as the duration of tumour control with endocrine therapy alone might be limited to less than 5 years.62 If patients are anticipated to survive for longer than 5 years, then a surgical approach is favoured and should be discussed with the patient and caregivers. Clinicians can also use decision support interventions to further inform older women and their caregivers of the benefits and risks of surgical and endocrine treatment options.63
As our knowledge of optimal treatments for individual subtypes of breast cancer continues to improve, it is probable that de-escalation of breast cancer surgical procedures will be a possibility in selected patients, either by offering surgical procedures with lower risk of morbidity or no procedure at all. Development of surgical decision aids for both clinicians and their older patients might allow for more streamlined and individualised care in this patient population, which takes into consideration both clinical benefit and patient values.
Pathological determination of risk of recurrence
Although many luminal tumours in older women are considered low risk, identifying tumours that have an increased risk of recurrence is still a priority. Multigene commercial assays (OncotypeDX, MammaPrint, and others) are frequently used for such identification, but these assays are expensive and might not be required if pathological and immunohistological data are carefully evaluated. Data indicate that older women are both less likely to be tested with the 21-gene recurrence score assay (Oncotype DX; Genomic Health, Redwood City, CA, USA) and are less likely to have a high-risk recurrence score than are younger patients with ER+ cancers.64–66 Furthermore, older patients, when tested, are treated less frequently with adjuvant chemotherapy than are younger patients: only 3·6% of women aged 70 years or older with a recurrence score between 11 and 25 and 38·1% of women aged 70 years or older with a recurrence score of more than 25 receive adjuvant chemotherapy (compared with 8·6% and 74·0%, respectively, of patients aged 50–70 years).67 Older patients might also not receive as much benefit from adjuvant chemotherapy, even in the high-risk recurrence score group.68
An alternative, less expensive, and readily available approach to the recurrence score assay is to use the Magee Equations (Department of Pathology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA), a multivariable model derived from routine pathological and semiquantitative immunohistological data.69,70 The Magee Equations have been validated to estimate recurrence score with a fair degree of accuracy and have also been shown to have a strong chemopredictive value.70–72 A decision algorithm (Magee Decision Algorithm) has been described using Magee Equations data and mitosis scores to safely forgo molecular testing in 70% of cases sent for recurrence score testing.73,74 Evaluating the same dataset, we looked at the performance of the Magee Decision Algorithm in older patients (≥70 years).73 The dataset contained 350 older patients, of which 244 (70%) were classified, using the Magee Decision Algorithm, as: low-risk recurrence and not requiring oncotype testing. Of these 244 patients, 232 (95%) were accurately predicted recurrence score.73 These results in older patients are similar to the overall results previously published, suggesting that the Magee Decision Algorithm can be used in older patients for clinical decision making. The use of the Magee Decision Algorithm in routine practice is cost-effective and provides tremendous healthcare value. For every 100 clinical requests for OncotypeDX tests, routine use of the Magee Decision Algorithm for triaging translates to cost savings of about US$300 000 without any negative impact on patient care.73
Radiation therapy
The Cancer and Leukemia Group B (CALGB) 934350 and Postoperative Radiotherapy in Minimum-Risk Elderly II (PRIME II)51 studies support omission of adjuvant radiation therapy in older women with early-stage ER+ breast cancer. Addition of adjuvant radiation therapy significantly reduced the risk of locoregional recurrence (from 10–12% to 1–2%) but no differences were observed in distant metastases or overall survival. On the basis of these results, the National Comprehensive Cancer Network adopted anti-oestrogen therapy alone as an option for women aged 70 years or older with ER+, T1 disease.49 However, high rates of radiation therapy use are still observed, although there is high variability according to geographical region.10,75 Results from the Bridging the Age Gap group show that radiation therapy use in the adjuvant setting is largely determined by patient age and the clinician’s observed risk of recurrence and, interestingly, less driven by geriatric assessment.75 Overall, radiation therapy was well tolerated by older women, with only a transient decrease in quality of life. Even with tolerability, adjuvant radiation therapy can safely be de-escalated in older women in this setting; higher-grade or higher-stage tumours in the setting of cN0 disease do not increase the risk of recurrence.10
When these trials were designed in 2000–10, the duration of radiation therapy was 3–6 weeks, which caused both direct and indirect financial and physical burden to women undergoing treatment. Currently, many randomised trials have shown that shorter five-fraction treatments are equivalent in efficacy. The 10-year results from the UK FAST trial26 and 5-year results from the UK FAST-Forward trial27 have confirmed that five-fraction treatments are equivalent in efficacy to 3-week courses of radiation therapy. A prospective randomised study comparing five-fraction accelerated partial breast irradiation to 30-fraction whole-breast radiation therapy showed no difference in ipsilateral breast tumour recurrence but significantly greater reductions in acute and late toxic effects for accelerated partial breast irradiation.76 These radiation therapy regimens might be especially appealing moving forwards as adjuvant therapies for older patients for additional reassurance of local control.
Another important factor in determining adjuvant treatment strategies is compliance with therapy, which is about 50% for aromatase inhibitors on account of side-effects and the impact on quality of life.77–79 Population-based studies also show that, in this patient population, there is no difference in secondary breast cancer events when comparing aromatase inhibitors to radiation therapy alone, corroborating the fact that risk of distant metastases and breast cancer mortality is low in this population.80,81 Studies investigating therapeutic interventions should now also focus on patient-related quality of life, which was not examined in either the CALGB 9343 trial or the PRIME II trial. There is one ongoing study (EUROPA) that is designed to compare endocrine therapy alone with accelerated partial breast irradiation for women aged 70 years or older with early-stage breast cancer. The endpoints of this study are patient-reported outcomes, time to locoregional recurrence, distant metastases, and survival.82 Additionally, ongoing clinical trials should further aid decision making in this area, including PRIMETIME (ISRCTN41579286), PRECISION (NCT02653755), LUMINA (NCT01791829), and NATURAL (NCT03646955). Until more data are available, decisions about any adjuvant treatment in this population should consider all factors, including efficacy, effect on quality of life, comorbidities, direct and indirect costs, and patient preference.
Adjuvant endocrine therapy
Standard-of-care guidelines advocate for adjuvant endocrine therapy for a duration of at least 5 years,83 even though there is a low risk of recurrence after definitive management of early-stage ER+ breast cancer. Despite the high utilisation of endocrine therapy in older patients, multiple studies suggest non-adherence increases with increasing age, possibly owing to the susceptibility of older patients to systemic toxicity.84,85 In accordance with EUSOMA–SIOG, a crucial condition preceding the use of endocrine therapy should be a discussion about adherence to treatment and close monitoring of side-effects that might preclude long-term use.24 Interestingly, locoregional recurrence is reported to increase with endocrine therapy discontinuation,85 but discontinuation was not associated with a concomitant effect on overall survival or progression-free survival.84,85 In the CALGB 9343 trial of breast-conserving surgical procedures with subsequent radiation, older patients who adhered to endocrine therapy had lower cancer recurrence and higher overall survival, but equivalent cancer-specific mortality, than did older patients who did not adhere to endocrine therapy.86
Given the potential effects on quality of life, omission of endocrine therapy was studied in the older population, with findings that adjuvant endocrine therapy was associated with improved overall survival; however, there was no significant interaction with age.87 A further study reported improved overall survival with the use of endocrine therapy, although stage I patients accounted for only 42·3% of the cases.88 However, multiple studies suggest that outcomes do not improve with endocrine therapy if it is combined with adjuvant radiation.89 In a study of older patients with pT1N0 tumours after breast-conserving surgery, 5-year local recurrence rates were 1·5% for patients receiving radiation alone and 4·2% for patients receiving endocrine therapy alone, compared with 0·8% for patients receiving both.90 In a similar patient population using data from the Surveillance, Epidemiology, and End Results and Medicare linked database, patients who received endocrine therapy alone had a higher risk of a second breast cancer event than those receiving combined endocrine therapy and radiation therapy (hazard ratio 2·20, p=0·008), whereas patients receiving radiation therapy alone were not at a higher risk of a second breast cancer event.81 Additionally, data from a population-based study in a Danish cohort showed that, although the effect of adjuvant endocrine therapy is quite pronounced for younger patients, older women with small tumours (<10 mm) might not actually benefit from endocrine therapy.91 For these women, even with omission of endocrine therapy, their survival is similar to age-matched women without cancer in the general population.
Collectively, these studies suggest that omission of endocrine therapy is an acceptable approach in older patients with low-risk early-stage ER+ breast cancer, with the caveat that adjuvant radiation should be completed as per indication status after breast-conserving procedures.
When should clinicians escalate therapy?
Although most ER+ tumours diagnosed in older women are considered low risk according to both clinical examinations (ie, breast examination or ultrasound) and gene expression profiling, some situations might warrant additional therapy. Conflicting evidence about the role of adjuvant chemotherapy for older patients precludes clear conclusions of efficacy. For ER+, node-positive disease, a study using the US National Cancer Database found that, after adjustment for confounding factors, receipt of chemotherapy was associated with improved survival (hazard ratio 0·67).92 Notably, there were no geriatric assessments of patients, making real-world application of these data difficult. However, in a study from the Bridging the Age Gap group, chemotherapy was not associated with survival benefits in patients with ER+ disease.93 Luminal B-like tumours might also warrant escalation of therapy with either adjuvant chemotherapy or targeted agents (CDK4 or CDK6 inhibitors) in combination with endocrine therapy, but clinicians should exert caution with the use of multidrug chemotherapy regimens in older women.24 Due to toxic effects, dose modifications might be warranted earlier in treatment courses in older women.
After active treatment, there might be long-term sequelae, with which patients must then contend, including physical, functional, and psychosocial effects that can lead to patients requiring help with basic or instrumental daily activities. Although the impact of chemotherapy on quality of life could be transient,94 these implications might be less acceptable for older patients with fewer years of life remaining than for younger patients. It is the task of the medical oncologist and care team to minimise and prevent cancer morbidity and to ensure that cancer therapies help more than they harm. This evaluation should ideally occur at each clinic visit, as health status can change drastically for the better or the worse over short periods.
Notably, the Cancer and Aging Research Group-Breast Cancer score was validated to predict grade 3–5 chemotherapy toxicity in older adults with early-stage breast cancer.95 Among the eight predictors of toxicity, limited mobility and insufficient social support were both identified as important parameters. These findings further highlight that, in the setting of escalating therapy to include chemotherapy, clinicians should consider a comprehensive geriatric assessment before starting treatment.
Translational science: insights to guide new therapies
With the push for changes to the clinical approach for management of ER+ breast cancer in older patients, emerging evidence from preclinical studies might also inform new treatment strategies. Age-related alterations to hormones and hormone receptor biology, along with the persistence of a chronic inflammatory state, can all contribute to the development of cancer.
One of the hallmarks of ageing is an increase in systemic low-grade chronic inflammation, with one of the contributors being an increase in cells with a senescence-associated secretory phenotype (SASP).96–98 Persistent inflammation leads to a release of damage-associated molecular patterns, which are associated with increased secretion of multiple inflammatory cytokines.9 Although local inflammation can promote both pro-tumour and anti-tumour immune functioning, in the context of ageing, the inflammatory secretome is a major contributor to tumour development, leading to both a functional decline in immune surveillance (immunosuppressive microenvironment) and altered signalling within epithelial cells (figure 4).99,100
Figure 4: A systems biology view of the aged breast microenvironment.
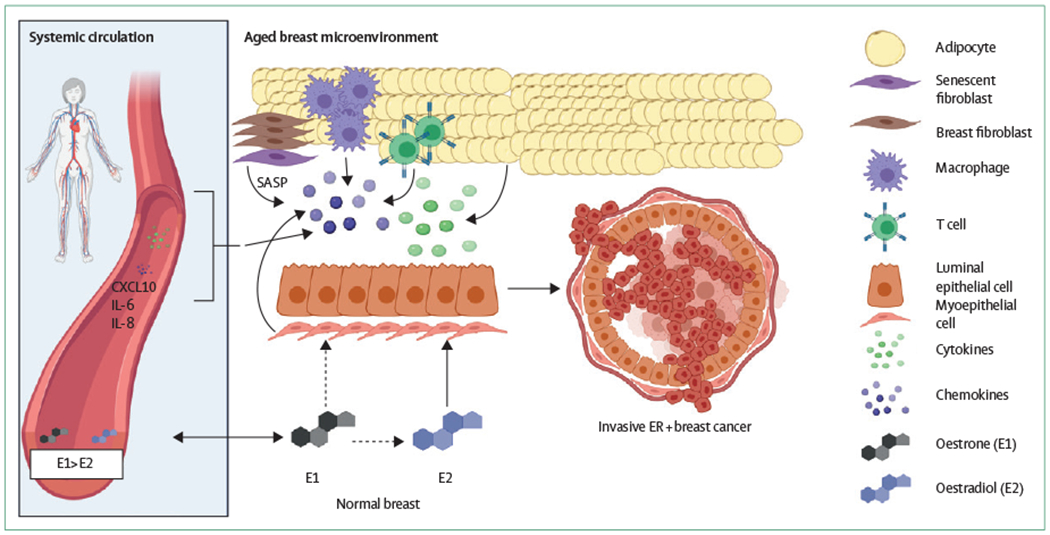
In the aged breast microenvironment, multiple cell types might contribute to the development of ER+ breast cancer. Fibroblasts might secrete pro-inflammatory, senescence-associated molecules; adipocytes might also take on a senescent phenotype; the adaptive immune system has a decreased responsiveness, and the myoepithelial cells might also secrete pro-inflammatory molecules. In turn, changes to the local oestrogen disposition might compound the age-associated inflammatory environment, leading to breast tumorigenesis. It is unclear whether or not systemic circulatory levels of inflammation and oestrogens are reflective of local changes. Dashed arrows show speculative links. CXCL10=C-X-C motif chemokine 10. ER=oestrogen receptor. IL=interleukin. SASP=senescence-associated secretory phenotype.
Ageing, hormones, and hormone receptor biology
There is a well established link between age, transcriptomic changes to ESR1 and altered hormone concentrations. When comparing pre-menopausal and post-menopausal patients with ER+ breast cancer, ESR1 was identified as the most differentially expressed and methylated gene.101,102 Aged breast cancers have hypomethylated ESR1, leading to increased expression of the gene and ER protein.
Age also changes the disposition of both circulating oestrogens and intra-tumoural oestrogens. In pre-menopausal women, the main oestrogen source for breast tissue is circulating oestradiol, which is secreted by the ovaries. However, in post-menopausal women, ovarian oestradiol is drastically reduced and the main form of unconjugated oestrogen in the plasma is oestrone, which is typically produced by aromatisation of androstenedione in a variety of peripheral tissues. Thus, an outstanding question remains as to how oestradiol still drives ER+ tumours in older women, in whom concentrations of circulating oestrone are greater than oestradiol. The prevailing model emanates from seminal work by Lønning and Dowsett indicating that, in post-menopausal women, oestrone undergoes equilibration with breast tissue and is further converted to oestradiol in the breast microenvironment by an isoform of the 17β-hydroxysteroid dehydrogenase enzyme.103,104 This work suggests that local breast production of oestradiol does largely not contribute to tumour development, and the potential for oestrone itself to drive tumorigenesis is probably low given that oestrone is a much weaker ligand for the ER.105,106 However, further work has found that oestrone is pro-inflammatory in the context of pre-existing inflammation, whereas oestradiol opposed these inflammatory properties.107 Notably, this study did not consider possible oestrone-to-oestradiol conversion and had sparse data on potentially physiologically relevant cytokines. Further validation is warranted, and studying how different ratios of oestrone to oestradiol might synergise with inflammation to drive tumour formation remains pertinent.
Ageing, inflammation, and inflammaging
Systemic chronic inflammation is a hallmark feature of ageing and is often called inflammaging.96,108 Evolutionarily, inflammation is a beneficial process used in the acute setting to transiently activate immune compartments to respond to a harmful threat; however, with age, the immune system becomes less able to deal with acute threats and it becomes chronically activated. Chronic inflammation supports the development of newly emergent tumours, causing them to behave as wounds that do not heal—continuous production of cytokines, chemokines, and growth factors within the tissue microenvironment fosters both tumour survival and host immune dysfunction and immunosuppression.9,109
In the breast, age-related remodelling of both the epithelium and stromal cell compartments occurs. A single-cell sequencing analysis of young and aged mammary tissue from mice revealed that multiple cell types contribute to local breast inflammation through the upregulation of cytokine gene expression, including myoepithelial cells, macrophages, and endothelial cells.110 Additional evidence suggests that much of the contribution of the pro-inflammatory microenvironment is related to the SASP, which is largely driven by aged fibroblasts.111 However, it appears that breast myoepithelial cells might be a key driver of inflammation and microenvironment remodelling, influencing not only the immune contexture but also neighbouring luminal epithelial cells.110,112
Establishing which cytokines (with a focus on chemokines) from systemic chronic inflammation functionally contribute to breast cancer development remains important. Finding reliable biomarkers that consistently mark age-related chronic inflammation has proved to be challenging.96 Plasma samples from patients with luminal breast cancer show age-related increases in interleukin (IL)-6, IL-8, and C-X-C motif chemokine 10 (CXCL10), but whether this reflects normal tissue ageing or is a result of tumour pathogenesis is unknown.113
The increase in systemic chronic inflammation is accompanied by concurrent changes in the proportions of immune cells and the function of the immune system. Decreased responsiveness of the adaptive immune system is an important feature of ageing.114 Notably, ageing is associated with the development of a subpopulation of GZMK+ CD8 T cells, which express markers of exhaustion, are pro-inflammatory, and secrete cytokines with a SASP. Additionally, the spectrum of T cells shifts to a predominantly memory T-cell pool, which leads to decreases in the T-cell receptor repertoire.115 In turn, these cells contribute to age-associated dysfunction of the immune system. In the breast, functional shifts in immune cell proportions occur, with increased bone marrow-derived macrophages, which propagate pro-tumour inflammation.110 Myeloid-derived suppressor cells are age-associated and might also be important: their long-term presence in tissues can impair waste clearance, disturb tissue proteostasis, and suppress the host immune response.116 However, their precise role in breast cancer pathogenesis, especially in older women, remains unclear.
Conclusions and future studies
Screening for breast cancer should continue as long as life expectancy exceeds 10 years; accuracy of screening mammography increases with age. Treatment strategies are evolving for older patients with early-stage breast cancer, with interventions and therapies that optimise patient preferences and quality of life. As evidence emerges showing that de-implementation strategies might be feasible for these older patients, ongoing discussions with the patient should remain a crucial aspect of care delivery. Patients might have wide-ranging reasons for utilising or omitting certain therapies, but communication strategies that focus on emphasising favourable prognoses and the low risk of both recurrences and cancer-related adverse events might help to guide patients’ decision-making.117 Clinicians treating older patients with early-stage ER+ breast cancer must consider the competing interests of quantity and quality of life. Depending on the patient, treatment might be foregone or adjusted to meet their specific goals of care. Therefore, clinicians should strive to provide better and more personalised care for older patients with early-stage ER+ early breast cancer than is currently provided, while considering the interaction of the influence of ageing on the patient (by taking into account comprehensive geriatric assessments, treatment toxicity prediction tools, quality of life, and life expectancy) and the distinct tumour biology.
Basic science research has the potential to add well tolerated, low-toxicity, pharmacological agents to treatment regimens and de-implement more invasive or toxic interventions. Evidence points to an age-related chronic inflammatory environment in the breast that can be pro-tumorigenic. Further studies exploring the crosstalk between oestrogen-driven signalling and inflammation should be a primary focus to better understand how and why ER+ breast cancers develop in older patients. A deeper understanding of cancer-promoting inflammation might also yield new therapeutic strategies, including the use of anti-inflammatories, to supplement ER-targeting therapies and allow for less invasive treatment and a better quality of life than are possible with current strategies. Many of the existing anti-inflammatories, such as metformin, aspirin, and statins, are well tolerated agents with low risks of adverse effects.109 Prospective studies are warranted to test whether these agents would be suitable chemopreventive agents, as aspirin has in some settings been shown to be a chemopreventive agent for colorectal cancer, or whether they might serve as complementary treatments.
Panel: Considerations and recommendations for screening and treatment for oestrogen receptor-positive (ER+) breast cancer in older patients.
Screening (imaging and genetics)
- Screening imaging
- Can continue for as long as life expectancy is more than 10 years; however, even the healthiest women are unlikely to have a life expectancy of more than 10 years beyond the age of 85 years
- Need to strongly consider comorbidities and competing causes of mortality, although current algorithms might misclassify women as having a low life expectancy, with resultant cessation of screening that is inappropriately premature
- Can stop screening with MRI in women older than 75 years at high risk
- Genetic testing
- Testing is always possible to help family members
- There is very little role for screening with MRI or risk-reducing mastectomies in those older than 75 years, even in the face of a pathogenic mutation
Surgery
Per Choosing Wisely guidelines and emerging evidence,10 it is recommended to not routinely perform sentinel lymph node biopsy in those aged 70 years or older with early-stage, clinically node-negative, ER+ tumours
- Breast-conserving surgery remains the standard of care but can be replaced with primary endocrine therapy for older patients who are frail and unfit for surgery or who prefer not to undergo surgery
- Omission of breast-conserving surgery might result in inferior local disease control but has been shown to be non-inferior to primary tamoxifen alone with respect to overall survival25
- Patient preference should be a strong consideration, as primary endocrine therapy with close observation can be a feasible strategy
- Functional impairments, cognitive or psychosocial distress, nutritional deficiencies, and insufficient social support or engagement might also prompt omission of surgery
Radiation oncology
Omission of radiation therapy can be strongly considered for patients with clinically node-negative, ER+ disease in accordance with the National Comprehensive Cancer Network guidelines if the patients are given adjuvant endocrine therapy
Accelerated partial breast irradiation might be a feasible short-course alternative to full cycles of radiation therapy, given the results of the FAST and FAST-Forward trials;26,27 this option might be especially appealing for older patients with ER+ disease
Medical oncology
Adjuvant endocrine therapy remains the standard of care for patients with early-stage ER+ disease
For patients for whom compliance to endocrine therapy might be an issue or for patients who desire not to take adjuvant endocrine therapy due to side-effects, radiation therapy in the adjuvant setting might be a feasible alternative for disease control
Genomic testing
The Magee Decision Algorithm can be used as a triaging method to decide whether or not to send for OncotypeDx recurrence score
Results from a study indicate that more than 70% of older patients with ER+ disease do not need OncotypeDx testing; thus, there is no need to order genomic testing on patients with a Magee score of less than 18 or patients with a score of 18-25 with mitosis score of 1
Consider sending assay if patient has node-positive disease, has a Magee score of more than 25, has a Magee score of 18-25 with a mitosis score of more than 1, or is particularly fit, in whom systemic chemotherapy would be considered
Survivorship, quality-of-life considerations, and comprehensive geriatric assessment
In general, for older women, breast cancer outcomes are better if a geriatric assessment is completed before treatment initiation than if it is completed after
Geriatric assessments that might be useful include the Comprehensive Geriatric Assessment, ePrognosis, PREDICT, and Cancer and Aging Research Group-Breast Cancer (CARG-BC) models; these assessments, along with patient preferences, should be incorporated as early as possible (ideally, into initial surgical consultation) to facilitate the identification of patients requiring further referral to a geriatric oncology clinic
Search strategy and selection criteria.
We used both MeSH terms (controlled language) and free-text terms and did searches on PubMed and Google Scholar for articles in English. The search query included “estrogen receptor positive breast cancer” AND “elderly” OR “older women”. More specific search combinations for treatments were then used, including the aforementioned terms and additional items; for example, “radiotherapy” OR “radiation therapy” was used to find articles related to considerations for radiation therapy. For searches related to basic science research, we searched using “elderly breast cancer” OR “aging and breast cancer” AND “inflammation” OR “inflammaging” OR “hormones” OR “hormone biology.” All searches were from inception of the database to May 31, 2021.
Acknowledgments
This work was supported by Susan G Komen Scholar awards (SAC110021 to AVL and SAC160073 to SO), the National Cancer Institute (5T32CA082084-20 and 1F30CA264963-01 to NC and R01CA187593 to WAB), the National Cancer Institute Cancer Center Support Grant (to GJvL), the UPMC Hillman Developmental Pilot Program (to AVL and SO), the Breast Cancer Research Foundation (to AVL, SO, and WAB [19-015]), the Shear Family Foundation (to AVL and SO), and the Magee Women’s Research Institute and Foundation (to GJvL). This Review was funded in part by the National Institutes of Health (NIH) under the award numbers listed above to NC, WAB, GJvL, AVL, and SO. No authors are employees of the NIH. The funders of the work had no role in the manuscript design, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Declaration of interests
PNB serves as chair of the Elsevier Breast Pathway; has received honoraria for speaking engagements from the Osler board review course on breast cancer, the Sino-American Network for Therapeutic Radiology and Oncology, the American College of Radiation Oncolology (ACRO), Rush University System for Health, Rush Oak Park Hospital, Franciscan Health, the Chicago Radiological Society, and the American Brachytherapy Society; is a board member for ACRO; and is on the guideline writing committee for Breast Reconstruction After Mastectomy, all outside of the submitted work. AMB has received consulting fees from Agendia, Biotheranostics, Myriad, Lilly, Pfizer, Novartis, Roche, Eisai, Daiichi, Seattle Genetics, and Puma, and has received payment for expert testimony from Pfizer, all outside of the submitted work. EJD is a member of the data safety monitoring board for Xoft, outside of the submitted work. SB is a consultant for Varian and Elsevier, and is on the data safety monitoring board for Xoft, outside of the submitted work. MTL has multiple patents; has served as the Chief Scientific Officer for Iovance; is currently the Chief Clinical Officer for Nurix; has stock or stock options with Nurix and iRepertoire; is chair of the SAB Alliance for Cancer Cell and Gene Therapy, President of the Translational Research Cancer Consortium, and Co-Director of the Society for Immunotherapy of Cancer Clinical Immuno-Oncology Network, all outside of the submitted work. SO is an External Advisory Board member for National Surgical Adjuvant Breast and Bowel Project (NSABP) and has received equipment, materials, or drugs from AstraZeneca, Illumina, H3 Biomedicine, and Blueprint Medicine, outside of the submitted work. AVL serves as chair of the Pittsburgh Foundation in Precision Medicine at the University of Pittsburgh Medical Center (UPMC); is an employee of UPMC Enterprises, Scientific Advisory Board member for Ocean Genomics, External Advisory Board member of NSABP; and has received research equipment, materials, or drugs from Illumina, GE, Boehringer Ingelheim, Blueprint Medicine, H3 Biomedicine, and AstraZeneca, all outside of the submitted work. WAB reports research funding from Koios Medical, outside of the submitted work. All other authors declare no competing interests.
Footnotes
For the ePrognosis measure see eprognosis.ucsf.edu
For the Magee Decision Algorithm see https://path.upmc.edu/onlineTools/mageeequations.html
References
- 1.DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019; 69: 438–51. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009; 27: 2758–65. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton SM, Oseni TO, Bababekov YJ, Hung YC, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg 2018; 153: 59–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson WF, Jatoi I, Devesa SS. Distinct breast cancer incidence and prognostic patterns in the NCI’s SEER program: suggesting a possible link between etiology and outcome. Breast Cancer Res Treat 2005; 90: 127–37. [DOI] [PubMed] [Google Scholar]
- 5.Benz CC. Impact of aging on the biology of breast cancer. Crit Rev Oncol Hematol 2008; 66: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014; 106: dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quong J, Eppenberger-Castori S, Moore D 3rd, et al. Age-dependent changes in breast cancer hormone receptors and oxidant stress markers. Breast Cancer Res Treat 2002; 76: 221–36. [DOI] [PubMed] [Google Scholar]
- 8.Punglia RS, Hughes KS, Muss HB. Management of older women with early-stage breast cancer. Am Soc Clin Oncol Educ Book 2015; 2015: 48–55. [DOI] [PubMed] [Google Scholar]
- 9.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140: 883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carleton N, Zou J, Fang Y, et al. Outcomes after sentinel lymph node biopsy and radiotherapy in older women with early-stage, estrogen receptor-positive breast cancer. JAMA Netw Open 2021;4: e216322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. J Oncol Pract 2018; 14: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999; 341: 2061–67. [DOI] [PubMed] [Google Scholar]
- 13.Ludmir EB, Mainwaring W, Lin TA, et al. Factors associated with age disparities among cancer clinical trial participants. JAMA Oncol 2019; 5: 1769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertagnolli MM, Singh H. Treatment of older adults with cancer—addressing gaps in evidence. N Engl J Med 2021; 385: 1062–65. [DOI] [PubMed] [Google Scholar]
- 15.DuMontier C, Loh KP, Bain PA, et al. Defining undertreatment and overtreatment in older adults with cancer: a scoping literature review. J Clin Oncol 2020; 38: 2558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014; 32: 2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rostoft S, O’Donovan A, Soubeyran P, Alibhai SMH, Hamaker ME. Geriatric assessment and management in cancer. J Clin Oncol 2021; 39: 2058–67. [DOI] [PubMed] [Google Scholar]
- 18.Baskin AS, Wang T, Berlin NL, Skolarus TA, Dossett LA. Scope and characteristics of choosing wisely in cancer care recommendations by professional societies. JAMA Oncol 2020; 6: 1463–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Baskin AS, Dossett LA. Deimplementation of the Choosing Wisely recommendations for low-value breast cancer surgery:a systematic review. JAMA Surg 2020; 155: 759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Sabel MS, Dossett LA. A framework for de-implementation in surgery. Ann Surg 2021; 273: e105–07. [DOI] [PubMed] [Google Scholar]
- 21.Oakes AH, Radomski TR. Reducing low-value care and improving health care value. JAMA 2021; 325: 1715–16. [DOI] [PubMed] [Google Scholar]
- 22.Derks MGM, van de Velde CJH, Giardiello D, et al. Impact of comorbidities and age on cause-specific mortality in postmenopausal patients with breast cancer. Oncologist 2019;24: e467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Society of Surgical Oncology. Don’t routinely use sentinel node biopsy in clinically node negative women ≥70 years of age with early stage hormone receptor positive, HER2 negative invasive breast cancer. July 12, 2016. https://www.choosingwisely.org/clinician-lists/sso-sentinel-node-biopsy-in-node-negative-women-70-and-over/ (accessed Dec 8, 2021). [Google Scholar]
- 24.Biganzoli L, Battisti NML, Wildiers H, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG) . Lancet Oncol 2021; 22: e327–40. [DOI] [PubMed] [Google Scholar]
- 25.Hind D, Wyld L, Reed MW. Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: Cochrane review. Br J Cancer 2007; 96: 1025–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunt AM, Haviland JS, Sydenham M, et al. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol 2020; 38: 3261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020; 395: 1613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011; 260: 658–63. [DOI] [PubMed] [Google Scholar]
- 29.Lee CS, Sengupta D, Bhargavan-Chatfield M, Sickles EA, Burnside ES, Zuley ML. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the national mammography database. JAMA Oncol 2017; 3: 1134–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malmgren JA, Parikh J, Atwood MK, Kaplan HG. Improved prognosis of women aged 75 and older with mammography-detected breast cancer. Radiology 2014; 273: 686–94. [DOI] [PubMed] [Google Scholar]
- 31.Destounis S, Arieno A, Santacroce A. Screening mammography: there is value in screening women aged 75 years and older. J Breast Imaging 2019; 1: 182–85. [DOI] [PubMed] [Google Scholar]
- 32.Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 2003; 361: 1405–10. [DOI] [PubMed] [Google Scholar]
- 33.Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015; 314: 1599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA 2001; 285: 2750–56. [DOI] [PubMed] [Google Scholar]
- 35.Schoenborn NL, Sheehan OC, Roth DL, et al. Association between receipt of cancer screening and all-cause mortality in older adults. JAMA Netw Open 2021; 4: e2112062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buys SS, Sandbach JF, Gammon A, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 2017; 123: 1721–30. [DOI] [PubMed] [Google Scholar]
- 37.Chavarri-Guerra Y, Hendricks CB, Brown S, et al. The burden of breast cancer predisposition variants across the age spectrum among 10 000 patients. J Am Geriatr Soc 2019; 67: 884–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurian AW, Bernhisel R, Larson K, et al. Prevalence of pathogenic variants in cancer susceptibility genes among women with postmenopausal breast cancer. JAMA 2020; 323: 995–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med 2021; 384: 440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2021; 19: 77–102. [DOI] [PubMed] [Google Scholar]
- 41.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003; 72: 1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317: 2402–16. [DOI] [PubMed] [Google Scholar]
- 43.Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev 2018; 4: CD002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, You R, Wang X, et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: a meta-analysis and systematic review. Clin Cancer Res 2016; 22: 3971–81. [DOI] [PubMed] [Google Scholar]
- 45.Salyer C, Kobelka C, Barrie A, Weintraub MR, Powell CB. Clinical characteristics and outcomes in elderly women with BRCA1 and BRCA2 mutations. Gynecol Oncol 2019; 154: 374–78. [DOI] [PubMed] [Google Scholar]
- 46.Liposits G, Loh KP, Soto-Perez-de-Celis E, et al. PARP inhibitors in older patients with ovarian and breast cancer: Young International Society of Geriatric Oncology review paper. J Geriatr Oncol 2019; 10: 337–45. [DOI] [PubMed] [Google Scholar]
- 47.Husted Madsen A, Haugaard K, Soerensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: a study from the Danish Breast Cancer Cooperative Group. Breast 2008; 17: 138–47. [DOI] [PubMed] [Google Scholar]
- 48.Marrazzo A, Taormina P, Gebbiab V, et al. Is sentinel lymph node biopsy more accurate than axillary dissection for staging nodal involvement in breast cancer patients? Chir Ital 2007; 59: 693–99. [PubMed] [Google Scholar]
- 49.Gradishar WJ, Moran MS, Abraham J, et al. NCCN guidelines® insights: breast cancer, version 4.2021. J Natl Compr Canc Netw 2021; 19: 484–93. [DOI] [PubMed] [Google Scholar]
- 50.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013; 31: 2382–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015; 16: 266–73. [DOI] [PubMed] [Google Scholar]
- 52.El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg 2007; 245: 665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westley T, Syrowatka A, Henault D, et al. Patterns and predictors of emergency department visits among older patients after breast cancer surgery: a population-based cohort study. J Geriatr Oncol 2018; 9: 204–13. [DOI] [PubMed] [Google Scholar]
- 54.de Glas NA, Bastiaannet E, Engels CC, et al. Validity of the online PREDICT tool in older patients with breast cancer: a population-based study. Br J Cancer 2016; 114: 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang V, Zhao S, Boscardin J, et al. Functional status and survival after breast cancer surgery in nursing home residents. JAMA Surg 2018; 153: 1090–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vacas S, Cole DJ, Cannesson M. Cognitive decline associated with anesthesia and surgery in older patients. JAMA 2021; 326: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilton J, Arnaout A, Clemons M. Primary endocrine therapy as an approach for patients with localized breast cancer deemed not to be surgical candidates. Curr Opin Support Palliat Care 2014; 8: 53–58. [DOI] [PubMed] [Google Scholar]
- 58.Osborn G, Jones M, Champ C, Gower-Thomas K, Vaughan-Williams E. Is primary endocrine therapy effective in treating the elderly, unfit patient with breast cancer? Ann R Coll Surg Engl 2011; 93: 286–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wink CJ, Woensdregt K, Nieuwenhuijzen GA, et al. Hormone treatment without surgery for patients aged 75 years or older with operable breast cancer. Ann Surg Oncol 2012; 19: 1185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyld L, Reed MWR, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer 2021; 142: 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angarita FA, Hoppe EJ, Ko G, Lee J, Vesprini D, Hong NJL. Why do older women avoid breast cancer surgery? A qualitative analysis of decision-making factors. J Surg Res 2021; 268: 623–33. [DOI] [PubMed] [Google Scholar]
- 62.Balakrishnan A, Ravichandran D. Early operable breast cancer in elderly women treated with an aromatase inhibitor letrozole as sole therapy. Br J Cancer 2011; 105: 1825–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wyld L, Reed MWR, Collins K, et al. Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices. Br J Surg 2021; 108: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gulbahce HE, White S, Herget KA, et al. 21-gene recurrence score testing utilization among older women from different races: a population-based study. J Geriatr Oncol 2021; 12: 206–11. [DOI] [PubMed] [Google Scholar]
- 65.Swain SM, Nunes R, Yoshizawa C, Rothney M, Sing AP. Quantitative gene expression by recurrence score in ER-positive breast cancer, by age. Adv Ther 2015; 32: 1222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shak S, Miller D, Howlader N, et al. Outcome disparities by age and 21-gene recurrence score®(RS) result in hormone receptor positive (HR+) breast cancer (BC). Ann Oncol 2016; 27: vi43. [Google Scholar]
- 67.Stemmer SM, Steiner M, Rizel S, et al. Ten-year clinical outcomes in N0 ER+ breast cancer patients with recurrence score-guided therapy. NPJ Breast Cancer 2019; 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kizy S, Altman AM, Marmor S, et al. 21-gene recurrence score testing in the older population with estrogen receptor-positive breast cancer. J Geriatr Oncol 2019; 10: 322–29. [DOI] [PubMed] [Google Scholar]
- 69.Flanagan MB, Dabbs DJ, Brufsky AM, Beriwal S, Bhargava R. Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol 2008; 21: 1255–61. [DOI] [PubMed] [Google Scholar]
- 70.Klein ME, Dabbs DJ, Shuai Y, et al. Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol 2013; 26: 658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhargava R, Esposito NN, O’Connor SM, et al. Magee Equations™ and response to neoadjuvant chemotherapy in ER+/HER2-negative breast cancer: a multi-institutional study. Mod Pathol 2021; 34: 77–84. [DOI] [PubMed] [Google Scholar]
- 72.Farrugia DJ, Landmann A, Zhu L, et al. Magee Equation 3 predicts pathologic response to neoadjuvant systemic chemotherapy in estrogen receptor positive, HER2 negative/equivocal breast tumors. Mod Pathol 2017; 30: 1078–85. [DOI] [PubMed] [Google Scholar]
- 73.Bhargava R, Clark BZ, Carter GJ, Brufsky AM, Dabbs DJ. The healthcare value of the Magee Decision Algorithm™: use of Magee Equations™ and mitosis score to safely forgo molecular testing in breast cancer. Mod Pathol 2020; 33: 1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhargava R, Clark BZ, Dabbs DJ. Breast cancers with Magee Equation score of less than 18, or 18-25 and mitosis score of 1, do not require Oncotype DX testing: a value study. Am J Clin Pathol 2019; 151: 316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Battisti NML, Hatton MQ, Reed MWR, et al. Observational cohort study in older women with early breast cancer: use of radiation therapy and impact on health-related quality of life and mortality. Radiother Oncol 2021; 161: 166–76. [DOI] [PubMed] [Google Scholar]
- 76.Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol 2020; 38: 4175–83. [DOI] [PubMed] [Google Scholar]
- 77.Hadji P, Ziller V, Kyvernitakis J, et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat 2013; 138: 185–91. [DOI] [PubMed] [Google Scholar]
- 78.Moon Z, Moss-Morris R, Hunter MS, Norton S, Hughes LD. Nonadherence to tamoxifen in breast cancer survivors: a 12 month longitudinal analysis. Health Psychol 2019; 38: 888–99. [DOI] [PubMed] [Google Scholar]
- 79.Wallace AS, Keene KS, Williams CP, et al. Radiation therapy utilization in Medicare beneficiaries with early-stage breast cancer. Cancer 2018; 124: 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buszek SM, Lin HY, Bedrosian I, et al. Lumpectomy plus hormone or radiation therapy alone for women aged 70 years or older with hormone receptor-positive early stage breast cancer in the modern era: an analysis of the National Cancer Database. Int J Radiat Oncol Biol Phys 2019; 105: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerber NK, Shao H, Chadha M, Deb P, Gold HT. Radiation without endocrine therapy in older women with stage I estrogen-receptor (ER) positive breast cancer is not associated with a higher risk of second breast cancer events. Int J Radiat Oncol Biol Phys 2021; published online May 8. 10.1016/j.ijrobp.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 82.Meattini I, Poortmans PMP, Marrazzo L, et al. Exclusive endocrine therapy or partial breast irradiation for women aged ≥70 years with luminal A-like early stage breast cancer (NCT04134598 - EUROPA): proof of concept of a randomized controlled trial comparing health related quality of life by patient reported outcome measures. J Geriatr Oncol 2021; 12: 182–89. [DOI] [PubMed] [Google Scholar]
- 83.Thomssen C, Balic M, Harbeck N, Gnant M. St Gallen/Vienna 2021: a brief summary of the consensus discussion on customizing therapies for women with early breast cancer. Breast Care (Basel) 2021; 16: 135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hancke K, Denkinger MD, König J, et al. Standard treatment of female patients with breast cancer decreases substantially for women aged 70 years and older: a German clinical cohort study. Ann Oncol 2010; 21: 748–53. [DOI] [PubMed] [Google Scholar]
- 85.La Rocca E, Meneghini E, Lozza L, et al. Older age and comorbidity in breast cancer: is RT alone the new therapeutic frontier? J Cancer Res Clin Oncol 2020; 146: 1791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Showalter SL, Meneveau MO, Keim-Malpass J, Camacho TF, Squeo G, Anderson RT. Effects of adjuvant endocrine therapy adherence and radiation on recurrence and survival among older women with early-stage breast cancer. Ann Surg Oncol 2021; 28: 7395–403. [DOI] [PubMed] [Google Scholar]
- 87.Ma SJ, Oladeru OT, Singh AK. Association of endocrine therapy with overall survival in women with small, hormone receptor-positive, ERBB2-negative breast cancer. JAMA Netw Open 2020; 3: e2013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeon YW, You SH, Lee JE, et al. Optimal treatment of breast cancer in women older than 75 years: a Korea Breast Cancer Registry analysis. Breast Cancer Res Treat 2019; 178: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reeder-Hayes KE, Wheeler SB, Meyer AM, et al. Adoption and effectiveness of de-escalated radiation and endocrine therapy strategies for older women with low-risk breast cancer. J Geriatr Oncol 2021; 12: 731–40. [DOI] [PubMed] [Google Scholar]
- 90.Dahn H, Wilke D, Walsh G, Pignol JP. Radiation and/or endocrine therapy? Recurrence and survival outcomes in women over 70 with early breast cancer after breast-conserving surgery. Breast Cancer Res Treat 2020; 182: 411–20. [DOI] [PubMed] [Google Scholar]
- 91.Christiansen P, Bjerre K, Ejlertsen B, et al. Mortality rates among early-stage hormone receptor-positive breast cancer patients: a population-based cohort study in Denmark. J Natl Cancer Inst 2011; 103: 1363–72. [DOI] [PubMed] [Google Scholar]
- 92.Tamirisa N, Lin H, Shen Y, et al. Association of chemotherapy with survival in elderly patients with multiple comorbidities and estrogen receptor-positive, node-positive breast cancer. JAMA Oncol 2020; 6: 1548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ring A, Battisti NML, Reed MWR, et al. Bridging The Age Gap: observational cohort study of effects of chemotherapy and trastuzumab on recurrence, survival and quality of life in older women with early breast cancer. Br J Cancer 2021; 125: 209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Battisti NML, Reed MWR, Herbert E, et al. Bridging the Age Gap in breast cancer: impact of chemotherapy on quality of life in older women with early breast cancer. Eur J Cancer 2021; 144: 269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magnuson A, Sedrak MS, Gross CP, et al. Development and validation of a risk tool for predicting severe toxicity in older adults receiving chemotherapy for early-stage breast cancer. J Clin Oncol 2021; 39: 608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019; 25: 1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Piber D, Olmstead R, Cho JH, et al. Inflammaging: age and systemic, cellular, and nuclear inflammatory biology in older adults. J Gerontol A Biol Sci Med Sci 2019; 74: 1716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vernot JP. Senescence-associated pro-inflammatory cytokines and tumor cell plasticity. Front Mol Biosci 2020; 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009; 139: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruhland MK, Coussens LM, Stewart SA. Senescence and cancer: an evolving inflammatory paradox. Biochim Biophys Acta 2016; 1865: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daraei A, Izadi P, Khorasani G, et al. Epigenetic changes of the ESR1 gene in breast tissue of healthy women: a missing link with breast cancer risk factors? Genet Test Mol Biomarkers 2017; 21: 464–70. [DOI] [PubMed] [Google Scholar]
- 102.Liao S, Hartmaier RJ, McGuire KP, et al. The molecular landscape of premenopausal breast cancer. Breast Cancer Res 2015; 17: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haynes BP, Straume AH, Geisler J, et al. Intratumoral estrogen disposition in breast cancer. Clin Cancer Res 2010; 16: 1790–801. [DOI] [PubMed] [Google Scholar]
- 104.Lønning PE, Helle H, Duong NK, Ekse D, Aas T, Geisler J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol 2009; 117: 31–41. [DOI] [PubMed] [Google Scholar]
- 105.Lønning PE, Haynes BP, Straume AH, et al. Exploring breast cancer estrogen disposition: the basis for endocrine manipulation. Clin Cancer Res 2011; 17: 4948–58. [DOI] [PubMed] [Google Scholar]
- 106.Lønning PE, Haynes BP, Straume AH, et al. Recent data on intratumor estrogens in breast cancer. Steroids 2011; 76: 786–91. [DOI] [PubMed] [Google Scholar]
- 107.Qureshi R, Picon-Ruiz M, Aurrekoetxea-Rodriguez I, et al. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metab 2020; 31: 1154–1172. [DOI] [PubMed] [Google Scholar]
- 108.Leonardi GC, Accardi G, Monastero R, Nicoletti F, Libra M. Ageing: from inflammation to cancer. Immun Ageing 2018; 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation—have anti-inflammatory therapies come of age? Nat Rev Clin Oncol 2021; 18: 261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li CM, Shapiro H, Tsiobikas C, et al. Aging-associated alterations in mammary epithelia and stroma revealed by single-cell RNA sequencing. Cell Rep 2020; 33: 108566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010; 5: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miyano M, Sayaman RW, Stoiber MH, et al. Age-related gene expression in luminal epithelial cells is driven by a microenvironment made from myoepithelial cells. Aging (Albany NY) 2017; 9: 2026–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berben L, Floris G, Kenis C, et al. Age-related remodelling of the blood immunological portrait and the local tumor immune response in patients with luminal breast cancer. Clin Transl Immunology 2020; 9: e1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mogilenko DA, Shpynov O, Andhey PS, et al. Comprehensive profiling of an aging immune system reveals clonal GZMK+ CD8+ T cells as conserved hallmark of inflammaging. Immunity 2021; 54: 99–115. [DOI] [PubMed] [Google Scholar]
- 115.Carrasco E, Gómez de Las Heras MM, Gabandé-Rodríguez E, Desdín-Micó G, Aranda JF, Mittelbrunn M. The role of T cells in age-related diseases. Nat Rev Immunol 2021; published online June 7. 10.1038/s41577-021-00557-4. [DOI] [PubMed] [Google Scholar]
- 116.Salminen A, Kaarniranta K, Kauppinen A. The role of myeloid-derived suppressor cells (MDSC) in the inflammaging process. Ageing Res Rev 2018; 48: 1–10. [DOI] [PubMed] [Google Scholar]
- 117.Wang T, Mott N, Miller J, et al. Patient perspectives on treatment options for older women with hormone receptor-positive breast cancer: a qualitative study. JAMA Netw Open 2020; 3: e2017129. [DOI] [PMC free article] [PubMed] [Google Scholar]


