Abstract
Discovered in a large-scale screening of natural plant chemicals, Taxol/paclitaxel and the taxane family of compounds are surprisingly successful anti-cancer drugs, used in treatment of the majority of solid tumors, and especially suitable for metastatic and recurrent cancer. Paclitaxel is often used in combination with platinum agents and is administrated in a dose dense regimen to treat recurrent cancer.
The enthusiasm and clinical development were prompted by the discovery that Taxol binds beta-tubulins specifically found within microtubules and stabilizes the filaments, and consequently inhibits mitosis. However, questions on how paclitaxel suppresses cancer persist, as other specific mitotic inhibitors are impressive in pre-clinical studies but fail to achieve significant clinical activity. Thus, additional mechanisms, such as promoting mitotic catastrophe and impacting non-mitotic targets, have been proposed and studied. A good understanding of how paclitaxel, and additional new microtubule stabilizing agents, kill cancer cells will advance the clinical application of these common chemotherapeutic agents.
A recent study provides a potential non-mitotic mechanism of paclitaxel action, that paclitaxel-induced rigid microtubules act to break malleable cancer nuclei into multiple micronuclei. Previous studies have established that cancer cells have a less sturdy, more pliable nuclear envelope due to the loss or reduction of lamin A/C proteins. Such changes in nuclear structure provide a selectivity for paclitaxel to break the nuclear membrane and kill cancer cells over non-neoplastic cells that have a sturdier nuclear envelope.
The formation of multiple micronuclei appears to be an important aspect of paclitaxel in the killing of cancer cells, either by a mitotic or non-mitotic mechanism. Additionally, by binding to microtubule, paclitaxel is readily sequestered and concentrated within cells.
This unique pharmacokinetic property allows the impact of paclitaxel on cells to persist for several days, even though the circulating drug level is much reduced following drug administration/infusion. The retention of paclitaxel within cells likely is another factor contributing to the efficacy of the drugs.
Overall, the new understanding of Taxol/paclitaxel killing mechanism—rigid microtubule-induced multiple micronucleation—will likely provide new strategies to overcome drug resistance and for rational drug combination.
Keywords: Taxol, Paclitaxel, Taxanes, Microtubules, Mitosis, Nuclear envelope, Cell cycle, Drug resistance, Drug mechanism
The Taxane Family of Chemotherapeutic Drugs
The class of taxane drugs, including paclitaxel (tradename-Taxol) and docetaxel (tradename-Taxotere), is among the most effective anticancer agents commonly used in clinics today to treat several major cancers, including metastatic breast, ovarian, prostate, lung, pancreatic, and cervical cancers [1–7]. Currently, a cisplatin (or carboplatin)/paclitaxel regimen following debulking surgery is a standard frontline chemotherapy for ovarian cancer [1,8–12], and a dose intensive regimen of paclitaxel is also used in salvage treatment following recurrence [10–12].
Despite the impressive clinical success of paclitaxel as a frontline and salvage cancer therapy [12–14], a major challenge is the development of drug resistance in recurrent cancer [15–19]. Extensive investigations led to the proposal of a list of possible mechanisms for the important clinical question of paclitaxel resistance [7,20]. However, the common ability of cancer cells to acquire taxane resistance indicates that another major mechanism(s) has not yet been uncovered [15,19,21].
Since investigation of new microtubule-stabilizing agents, such as epothilones (ixabepilone), laulimalide, and discodermolide, is under development [22–24], our understanding of the mechanism of taxanes and other microtubule-stabilizing drugs is important and may have a significant clinical implication in the years to come [5,25,26].
Paclitaxel Binding to Beta-Tubulin within Microtubules and Their Stabilization
The discovery in the 1980s that paclitaxel binds and stabilizes microtubules [27–29] and inhibits mitosis [30,31] in culture cells propelled the development of the compound into a common anti-cancer drug [13]. Cell culture studies provided clear evidence that paclitaxel inhibited mitosis, and the mechanism that paclitaxel acts as a mitotic inhibitor quickly gained widespread acceptance and is now considered a dogma [7].
Generally, paclitaxel was thought to induce mitotic arrest and subsequently apoptosis in cancer cells [31–33] (Figure 1A). This idea seems reasonable and self-evident, as cancer cells exhibit uncontrolled growth and are usually more proliferative, and thus the targeting mitosis provides a specificity of paclitaxel for neoplastic compared to normal cells. Indeed, paclitaxel can cause significant off-target effects in normal, non-neoplastic cells that divide rapidly, such as hematopoietic cells [14] and cells of the hair follicle matrix [34], resulting in neutropenia and alopecia, respectively. Peripheral neuropathy is another dose limiting side effect of paclitaxel-induced microtubule stabilization [35].
Figure 1: Mechanisms of paclitaxel in instigating cancer cell death by mitotic and non-mitotic mechanisms.
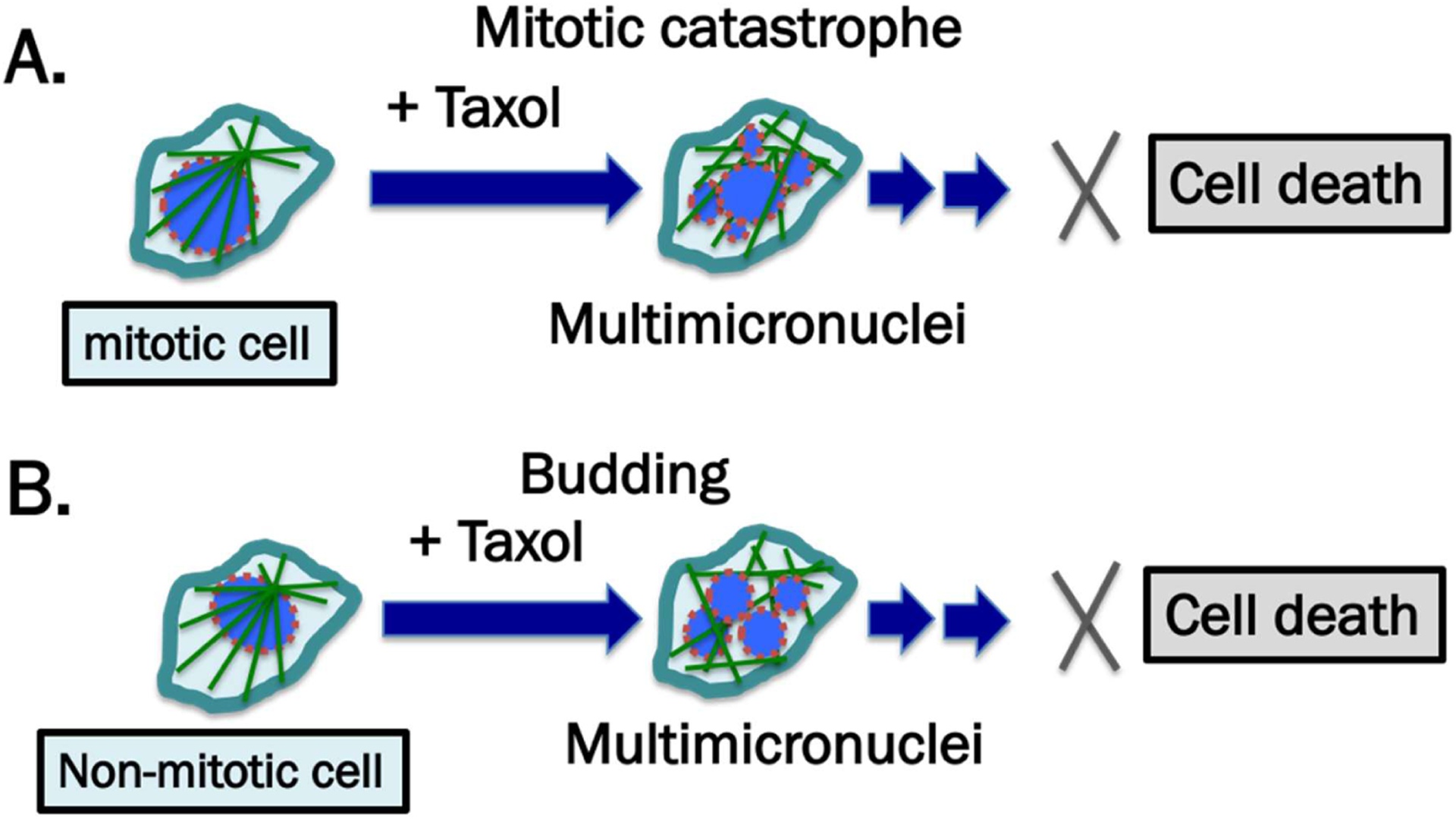
Mitotic or non-mitotic cancer cells generally have weakened nuclear lamina, depicted by the broken brown-colored outlines of nuclear envelope. (A) The generally accepted mechanism is that paclitaxel binds microtubules and interferes with their function in chromosome segregation during the mitotic phase of the cell cycle. The cells escape mitotic arrest and undergo mitotic catastrophe and aberrant chromosome segregation and the resulting multi-nucleated and lobulated cells subsequently undergo cell death. (B) In addition to mitotic cell death, a new proposal is that in non-mitotic cells, the rigid microtubule filaments induced by paclitaxel can promote massive formation of micronuclei and nuclear multiple micronucleation by nuclear budding in cells during interphase. The multi-nucleated and lobulated cells die, through as yet not-well-defined mechanisms.
Nevertheless, paclitaxel causes cancer cell death, rather than mere cytostatic [36–38], though how paclitaxel-induced cell growth arrest triggers death is not well understood [32,39,40]. However, binding and stabilization of microtubules is accepted as the key for the success of paclitaxel in cancer therapy [15,38]. Particularly, additional microtubule stabilizing molecules with chemical structures totally distinct from taxanes have been found to be effective anti-cancer agents [7,24,26,41]. Whether through mitotic or non-mitotic, apoptotic or non-apoptotic mechanisms, stabilization of microtubes has been found to be an amazingly optimal strategy in cancer therapy.
Anti-Mitotic Mechanism and Mitotic Catastrophe
Further careful studies of the effects of paclitaxel on cancer cells in culture revealed that the cells often escape mitotic arrest and undergo aberrant mitosis [40,42]. Thus, an unsuccessful mitosis in the presence of paclitaxel-induced microtubule malfunction, a phenomenon known as mitotic catastrophe, may be a major mechanism of cell killing [43]. Experiments using time-lapse video microscopy revealed that paclitaxel-treated cells become multi-nucleated, often a result of multi-polar division [40,44–46]. An aberrant mitosis that forms multiple micronuclei, or nuclear lobules, as a result of paclitaxel arresting microtubules, is believed to be the major mechanism of drug action [44,47] (Figure 1A). The formation of micronuclei following paclitaxel treatment was initially observed many years ago [48,49], though it was only followed more recently. Generally, the formation of micronuclei is thought to be the result of chromosome mis-segregation during mitosis [45–47] (Figure 1A), although new observation suggest that paclitaxel also prompts the formation of multiple micronuclei in non-mitotic cells [50] (Figure 1B), as discussed below.
Non-Mitotic Mechanisms and Prominent Formation of Multiple Micronuclei
One puzzle about the commonly accepted mechanism of paclitaxel action is the issue with mitosis as the target [51]. Unlike cells in tissue cultures, the neoplastic cells found in tumors in vivo are much less proliferative, with a doubling time significantly longer than cultured cells [42,52]. At any given time, only a small fraction of cancer cells are undergoing mitosis [37,38,42]. Thus, non-mitotic cells, in addition to cells undergoing mitosis, are likely targets of paclitaxel in cancer therapy [36]. Particularly in clinical settings, the susceptibility of cancer cells to killing by paclitaxel does not correlate with the proliferative index of the cancer [53]. This problem inspired the concept of “proliferative index paradox” [38], denoting that mitosis may not be a key target of paclitaxel or explain its efficacy as an anti-cancer agent [37,42,52,54].
Efforts to develop additional specific anti-mitotic agents inspired by the success of Taxol have not been successful [42,54,55], leading to skepticism about the rationale for targeting mitosis [37,52]. A few studies investigated and proposed non-mitotic mechanisms for paclitaxel in causing cancer cell cytotoxicity, including that paclitaxel influences bcl-2 phosphorylation [56]; paclitaxel targets microtubules involved in cellular transport [57]; and the drug impacts nuclear pores and transport [48]. Nevertheless, more investigations to identify a robust and general non-mitotic function of paclitaxel in targeting cancer cells seems warranted.
Indeed, recent studies showed that paclitaxel and other microtubule-stabilizing agents induce rigid microtubules that cause the breakage/fragmentation of the malleable nucleus of cancer cells, but not the sturdier nucleus present in normal cells [50] (Figure 1B). The paclitaxel-induced formation of multiple micronuclei is mitosis-independent, since paclitaxel-induced nuclear breakage still occurs when serum is removed to restrain growth, or in the presence of various mitotic inhibitors to suppress proliferation. Particularly, deletion of lmna gene (which encodes Lamin A/C proteins) sensitizes cells to nuclear breakage and death by paclitaxel [50]. Thus, a malleable nuclear envelope (caused by a reduction in Lamin A/C and perhaps other nuclear envelope structural proteins) underlies the specificity of microtubule stabilizing drugs such as paclitaxel in killing malignant cells.
The formation of multiple nuclear envelope fragments upon treatment of cancer cells with paclitaxel has been observed previously [48,49], though few studies have followed up the observation until recently. Generally, in the presence of paclitaxel to interfere with microtubule function, the formation of multiple micronuclei is thought to be a result of aberrant, multipolar mitosis [44–46].
In the absence of drugs, nuclear budding occurring in non-mitotic cells may be an important mechanism in producing micronuclei [58–61], as microtubules associating with the nuclear envelope physically pull and distort the structure [62–64]. Similarly, the proposal of a physical force exerted by paclitaxel-induced rigid microtubule filaments in breaking malleable cancer nuclei provides a non-mitotic mechanism to generate multiple micronuclei [50] (Figure 2). The LINC (Linker of nucleoskeleton and cytoskeleton) bridges linking the microtubules to nuclear envelope lamina likely provide the physical links to transmit the force in pulling the nuclear envelope protrusion [62,63]. The proposed mechanism provides a possible alternative explanation for the well-established dogma that paclitaxel targets mitosis in cancer therapy; rather, paclitaxel likely aims at the weakened nuclear envelope of malignant cells. Thus, paclitaxel can be predicted to be effective to treat cancer that shows a deformed nuclear envelope, such as in the case of the cervical cancer cells that can be detected by a PAP test [65,66]. The study provides a new realization that paclitaxel can induce the generation of micronuclei in cells at S phase by a non-mitotic mechanism [50].
Figure 2: Proposed mechanism for the paclitaxel-induced formation of multiple micronuclei in non-mitotic cells.

Cancer cells generally have weakened nuclear lamina, depicted by the broken brown outlines of nuclear envelope. A new proposal is that in nonmitotic cells, the rigid microtubule filaments induced by paclitaxel can promote massive formation of micronuclei through nuclear budding of cells during interphase. The paclitaxel-bound rigid microtubule bundles may physically pull and distort the nuclear envelope structure through the LINC (linking nucleus and cytoplasm) bridges, which connect microtubules and nuclear lamina. As a result, the malleable cancer nuclear envelope breaks into multiple micronuclei. The proposal of physical force exerted by paclitaxel-induced rigid microtubule filaments in breaking malleable cancer nuclei provides a non-mitotic mechanism to generate multiple micronuclei [50].
In addition, for paclitaxel to target proliferative, mitotic cells, the nuclear envelope malleability appears to be another characteristic of cancer versus benign cells targeted by paclitaxel. The loss or reduction of nuclear lamina proteins, especially Lamin A/C, in cancer cells has been previously noted [58–61]. Deletion of lmna gene encoding Lamin A/C is shown to lead to nuclear envelope malleability and paclitaxel-induced formation of micronuclei [50,67]. Thus, malleability of cancer nuclear envelope provides another specificity for paclitaxel.
Cell Death Mechanisms Triggered by Paclitaxel and the Involvement of Micronucleation
The generally accepted concept is that in cancer chemotherapy, paclitaxel induces apoptosis [32,68]. This appears to be an intuitively reasonable idea, and there are many reports on induction of apoptosis in cancer cells by paclitaxel [39,69,70]. However, more careful studies indicate, at least in some circumstances, caspase activation and the typical or canonical apoptotic pathway are not involved [49,71–73]. Until now, how paclitaxel may trigger apoptosis is uncertain [15,68]. The lack of in-depth understanding of the cell killing mechanism of such a successful and common chemotherapy drug such as paclitaxel is surprising
Although the cancer killing mechanism of paclitaxel is not well understood, likely the formation of micronuclei induced by paclitaxel is important, referred as “micronucleation” [47,50] (Figure 3). How the formation of micronuclei leads to cell death is not established yet. In the absence of paclitaxel, micronuclei often undergo catastrophic rupture [58–60,74], which may lead to aneuploidy and cell death. Another notion is that the micronuclei formed may trigger innate cellular DNA sensing and subsequent induced immune pathways, which then contributes to cancer killing activity [47].
Figure 3: Mechanisms of paclitaxel-induced breaking of nuclear envelope and multiple micronucleation in cancer killing efficacy.

Paclitaxel induces the breaking of nuclei of neoplastic cells and the formation of multiple micronuclei. The weaken nuclear envelope is depicted by the broken brown-colored outlines. The micronuclei are defective in membrane structure and have high propensity for rupture and release of chromatin material, resulting in compromised cellular structure and slow cell death.
A suggested model is that paclitaxel eliminates cancer cells by first inducing “micronucleation”, the breaking of malleable cancer nuclei into multiple micronuclei (Figure 3). The membrane and lamina envelope of these micronuclei are defective, and are easily compromised structurally, resulting in the release of DNA content (Figure 3). Similar ideas have been suggested, that paclitaxel induced a slow, passive cell death without triggering apoptosis [71].
Cellular Retention of Paclitaxel and Persistent Activity Within Cells
Paclitaxel has high binding affinity to beta-tubulin located in microtubule filaments [27], and the binding can approach 1-to-1 ratio [75,76]. In a cell culture study, a short-term exposure of cancer cells to paclitaxel produces a long-term, persistent inhibition of cell proliferation and induction of cell death [77]. In vivo, although paclitaxel is rapidly cleared from the circulation following infusion, the drug is retained in cells and activity persists for several days [77–79] (Figure 4). Presumably, the high concentration of paclitaxel within cells interferes with microtubule-dependent cellular functions several days after drug administration. The retention of paclitaxel within cancer cells likely is important for killing of cancer cells, but the persistent presence of paclitaxel in peripheral neurons and hair follicles also causes the well-known side effects of paclitaxel, such as peripheral neuropathy [35] and alopecia [80].
Figure 4: Retention of paclitaxel enables efficient killing of tumor cells.

During chemotherapy, paclitaxel (Taxol) is administrated to patients over 3–6 hours, and taxane concentration reaches a peak level in plasma by the end of drug infusion. Over the next 6 hours, paclitaxel level declines rapidly, and the drug is concentrated in cells (partly by binding to microtubules) several hundred times over the blood level (illustrated by red dots). Paclitaxel is present in high level inside cells for next 2–3 days by binding to the microtubules, and the drug triggers nuclear envelope breakage and the death of cancer cells over the next 2–3 days, but also causes damage of hair follicles and toxicity in peripheral neurons.
Microtubules are polymers of alpha- and beta-tubulin heterodimers [76,81], and play multiple roles in cellular functions [81,82]. Cellular microtubule networks are highly dynamic: the filaments are constantly extending and shortening, with a balance between the cellular pool of alpha- and beta-tubulin dimers and microtubule polymers, which are about half and half under normal conditions [75,82,83]. Paclitaxel promotes 90–100% of tubulin monomers to locate into polymerized forms [76,82–84]. Because of the importance of microtubules in multiple cellular functions, the homeostasis and the level of free tubulins is tightly regulated [85–87]. Tubulins control their own synthesis by autoregulation at the level of mRNA stability [86,87]. Thus, addition of paclitaxel to eliminate alpha- and beta-tubulin dimers (into polymers) stimulates production of new tubulins. Production of new tubulins will further sequester paclitaxel, until all available paclitaxel molecules are eliminated.
Tubulins are relatively stable, and the tubulin protein is removed by proteasome- (but not lysosome-) mediated degradation [88] and via degradation by cathepsin D [89]. Cells take up, sequester, and concentrate paclitaxel at several hundreds of times over the concentration found in the extracellular space [75]. Indeed, intracellular paclitaxel can be retained over several days after exposure, during which time the paclitaxel bound rigid microtubules persist [75,77,79]. The ability of cells to uptake and concentrate paclitaxel results in part from paclitaxel sequestration by binding to abundant microtubules and tubulins (estimated to be in the range of 10–20 μM inside cells) [75,82,83].
Thus, a special feature of the pharmacokinetics of paclitaxel is the long retention of the drug inside cells from sequestration by binding to the ample cellular microtubules, despite rapid clearance of the molecules in circulation [75,77,79]. We speculate that the prolong retention is likely a factor contributing to the success of paclitaxel efficacy over non-microtubular targeting mitotic inhibitors and other anti-neoplastic cytotoxic agents (Figure 4).
Prospects of the Microtubule Stabilizing Drugs with a Non-Mitotic Mechanism
Investigated in the 1970–1980s and entered into clinical use in the early 1990s [6,13,18], taxane/paclitaxel is still the most commonly used cancer drug today after treating millions of patients over the last 40+ years [13,23]. The development of taxanes for cancer therapy has been a celebrated success story [23,26], and new drugs with similar mechanism of actions as microtubule stabilization agents have a promising future in cancer treatment [24,41].
Paclitaxel is also used in combination with other agent(s), such as with doxorubicin (anthracycline) in metastatic breast cancer [2,3], and with Bevacizumab in lung cancer. The rationale combination of paclitaxel with other agents is an issue that oncologists must consider to increase the treatment response and efficacy [90,91]. Additional formulations such as Abraxane and liposomal taxane provide improvement on the delivery of paclitaxel and reduction of the hypersensitivity side effects [92,93]. Although the mechanism of paclitaxel drug action is still under study to gain a better understanding, microtubule stabilizing activity seems to be a key mechanism driving cancer killing activity [15,25]. A class of additional non-taxane microtubule-stabilizing agents, such as epothilones (ixabepilone), laulimalide, and discodermolide, isolated from microbiomes, sponges, and corals, respectively, is undergoing clinical development and testing in patient trials [22,24,41,94]. These new paclitaxel-like microtubule stabilizing agents may be useful for cancer that develops resistance to taxanes, and also for potential ability to be orally administrated, and have higher water solubility. Thus, continuing research and understanding of the microtubule stabilizing agents for their mechanism in efficient cancer cell killing will have a significant clinical implication in the years to come [13,23,26,91]. The newly uncovered non-mitotic mechanism of Taxol/paclitaxel in inducing breakage of cancer nuclear envelope [50]. (Figures 1–3) likely will prompt additional exploration and consideration in improving cancer chemotherapy using Taxol/paclitaxel and additional microtubule stabilizing agents.
Acknowledgement
We thank our lab members and alumni for the research work supporting this review paper. We acknowledge that many additional excellent works and publications are relevant to the discussed topic, but are not cited due to the limited scope of this review.
Funding
This work from our lab discussed and cited was supported by funds from grant NICHD R03HD071244 (E.R.S.), concept awards BC097189 and BC076832 from Department of Defense (USA). Grants R01 CA230916, R01 CA095071, R01 CA099471, and CA79716 to X-X Xu from NCI, NIH also contributed to the studies. Internal research funds from Sylvester Comprehensive Cancer Center and University of Miami also supported this work.
References
- 1.Bookman MA. Optimal primary therapy of ovarian cancer. Annals of Oncology. 2016. Apr 1; 27:i58–62. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich M, Diesing D, Villena-Heinsen C, Felberbaum R, Kolberg HC, Diedrich K. Taxanes in the first-line chemotherapy of metastatic breast cancer. European Journal of Gynaecological Oncology. 2004. Jan 1; 25(1):66–70. [PubMed] [Google Scholar]
- 3.Holmes FA. Paclitaxel combination therapy in the treatment of metastatic breast cancer: a review. In: Seminars in Oncology 1996. Oct 1; 23(5) Suppl 11:46–56. [PubMed] [Google Scholar]
- 4.Lemstrova R, Melichar B, Mohelnikova-Duchonova B. Therapeutic potential of taxanes in the treatment of metastatic pancreatic cancer. Cancer Chemotherapy and Pharmacology. 2016. Dec; 78(6):1101–11. [DOI] [PubMed] [Google Scholar]
- 5.Joshi M, Liu X, Belani CP. Taxanes, past, present, and future impact on non-small cell lung cancer. Anti-Cancer Drugs. 2014. Jun 1; 25(5):571–83. [DOI] [PubMed] [Google Scholar]
- 6.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). In: Seminars in Oncology 1993. Aug 1; 20(4) Suppl 3: 1–15. [PubMed] [Google Scholar]
- 7.Yang CP, Horwitz SB. Taxol®: the first microtubule stabilizing agent. International Journal of Molecular Sciences. 2017. Aug; 18(8):1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, Xu X, Hamilton TC. Focus on epithelial ovarian cancer. Cancer Cell. 2004. Jan 1; 5(1):19–24. [DOI] [PubMed] [Google Scholar]
- 9.Runowicz CD, Wiernik PH, Einzig AI, Goldberg GL, Horwitz SB. Taxol in ovarian cancer. Cancer. 1993. Feb 15;71(S4):1591–6. [DOI] [PubMed] [Google Scholar]
- 10.Baird RD, Tan DS, Kaye SB. Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nature reviews Clinical Oncology. 2010. Oct; 7(10):575–82. [DOI] [PubMed] [Google Scholar]
- 11.Baker VV. Salvage therapy for recurrent epithelial ovarian cancer. Hematology/Oncology Clinics. 2003. Aug 1; 17(4):977–88. [DOI] [PubMed] [Google Scholar]
- 12.Jain A, Dubashi B, Reddy KS, Jain P. Weekly paclitaxel in ovarian cancer-the latest success story. Current Oncology. 2011. Feb; 18(1):16–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: History and future challenges. Molecules. 2020. Jan; 25(24):5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995; 332 (15): 1004–14. [DOI] [PubMed] [Google Scholar]
- 15.Blagosklonny MV, Robey R, Sheikh MS, Fojo T. Paclitaxel-induced FasL-independent apoptosis and slow (non-apoptotic) cell death. Cancer Biology & Therapy. 2002. Mar 7; 1(2):113–7. [DOI] [PubMed] [Google Scholar]
- 16.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Annals of Oncology. 2007. Jul 1; 18:v3–8. [DOI] [PubMed] [Google Scholar]
- 17.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nature Reviews Cancer. 2010. Mar; 10(3):194–204. [DOI] [PubMed] [Google Scholar]
- 18.Mosca L, Ilari A, Fazi F, Assaraf YG, Colotti G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resistance Updates. 2021. Jan 1; 54:100742. [DOI] [PubMed] [Google Scholar]
- 19.Visconti R, Grieco D. Fighting tubulin-targeting anticancer drug toxicity and resistance. Endocrine-Related Cancer. 2017. Sep 1; 24(9):T107–17. [DOI] [PubMed] [Google Scholar]
- 20.Maloney SM, Hoover CA, Morejon-Lasso LV, Prosperi JR. Mechanisms of taxane resistance. Cancers. 2020. Nov; 12(11):3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003. Oct; 22(47):7280–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altaha R, Fojo T, Reed E, Abraham J. Epothilones: a novel class of non-taxane microtubule-stabilizing agents. Current Pharmaceutical Design. 2002. Sep 1; 8(19):1707–12. [DOI] [PubMed] [Google Scholar]
- 23.Ojima I, Lichtenthal B, Lee S, Wang C, Wang X. Taxane anticancer agents: a patent perspective. Expert Opinion on Therapeutic Patents. 2016. Jan 2; 26(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Mu X, Du G. Microtubule-stabilizing agents: new drug discovery and cancer therapy. Pharmacology & Therapeutics. 2016. Jun 1; 162:134–43. [DOI] [PubMed] [Google Scholar]
- 25.Florian S, Mitchison TJ. Anti-microtubule drugs. The Mitotic Spindle. 2016:403–21. [DOI] [PubMed] [Google Scholar]
- 26.Pabla N, Sparreboom A. CCR 20th anniversary commentary: BMS-247550—microtubule stabilization as successful targeted therapy. Clinical Cancer Research. 2015. Mar 15; 21(6):1237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manfredi JJ, Parness J, Horwitz SB. Taxol binds to cellular microtubules. The Journal of Cell Biology. 1982. Sep; 94(3):688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979. Feb; 277(5698):665–7. [DOI] [PubMed] [Google Scholar]
- 29.Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proceedings of the National Academy of Sciences. 1980. Mar 1; 77(3):1561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Current Medicinal Chemistry-Anti-Cancer Agents. 2002. Jan 1; 2(1):1–7. [DOI] [PubMed] [Google Scholar]
- 31.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews Cancer. 2004. Apr; 4(4):253–65. [DOI] [PubMed] [Google Scholar]
- 32.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003. Dec; 22(56):9075–86. [DOI] [PubMed] [Google Scholar]
- 33.Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2000. Jun 1; 88(11):2619–28. [DOI] [PubMed] [Google Scholar]
- 34.Purba TS, Ng’andu K, Brunken L, Smart E, Mitchell E, Hassan N, et al. CDK4/6 inhibition mitigates stem cell damage in a novel model for taxane-induced alopecia. EMBO Molecular Medicine. 2019. Oct; 11(10):e11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. European Journal of Cancer. 2006. Jan 1; 42(1):24–30. [DOI] [PubMed] [Google Scholar]
- 36.Field JJ, Kanakkanthara A, Miller JH. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorganic & Medicinal Chemistry. 2014. Sep 15; 22(18):5050–9. [DOI] [PubMed] [Google Scholar]
- 37.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nature Reviews Clinical Oncology. 2011. Apr; 8(4):244–50. [DOI] [PubMed] [Google Scholar]
- 38.Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Molecular Biology of the Cell. 2012. Jan 1; 23(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LG, Liu XM, Kreis W, Budman DR. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemotherapy and Pharmacology. 1999. Sep 1;44(5):355–61. [DOI] [PubMed] [Google Scholar]
- 40.Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? Journal of Cell Science. 2009. Aug 1; 122(15):2579–85. [DOI] [PubMed] [Google Scholar]
- 41.Hunt JT. Discovery of ixabepilone. Molecular Cancer Therapeutics. 2009. Feb 1; 8(2):275–81. [DOI] [PubMed] [Google Scholar]
- 42.Shi J, Mitchison TJ. Cell death response to anti-mitotic drug treatment in cell culture, mouse tumor model and the clinic. Endocrine-Related Cancer. 2017. Sep; 24(9):T83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morse DL, Gray H, Payne CM, Gillies RJ. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Molecular Cancer Therapeutics. 2005. Oct 1; 4(10):1495–504. [DOI] [PubMed] [Google Scholar]
- 44.Weaver BA. How Taxol/paclitaxel kills cancer cells. Molecular Biology of the Cell. 2014. Sep 15; 25(18):2677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zasadil LM, Andersen KA, Yeum D, Rocque GB, Wilke LG, Tevaarwerk AJ, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Science Translational Medicine. 2014. Mar 26;6(229):229ra43–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Zhou Y, Shi J. Post-slippage multinucleation renders cytotoxic variation in anti-mitotic drugs that target the microtubules or mitotic spindle. Cell Cycle. 2014. Jun 1; 13(11):1756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchison TJ, Pineda J, Shi J, Florian S. Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug? Open Biology. 2017. Nov 15; 7(11):170182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theodoropoulos PA, Polioudaki H, Kostaki O, Derdas SP, Georgoulias V, Dargemont C, et al. Taxol affects nuclear lamina and pore complex organization and inhibits import of karyophilic proteins into the cell nucleus. Cancer Research. 1999. Sep 15; 59(18):4625–33. [PubMed] [Google Scholar]
- 49.Merlin JL, Bour-Dill C, Marchal S, Bastien L, Gramain MP. Resistance to paclitaxel induces time-delayed multinucleation and DNA fragmentation into large fragments in MCF-7 human breast adenocarcinoma cells. Anti-Cancer Drugs. 2000. Apr 1; 11(4):295–302. [DOI] [PubMed] [Google Scholar]
- 50.Smith ER, Leal J, Amaya C, Li B, Xu XX. Nuclear Lamin A/C Expression Is a Key Determinant of Paclitaxel Sensitivity. Molecular and Cellular Biology. 2021. May 10: MCB-00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fürst R, Vollmar AM. A new perspective on old drugs: non-mitotic actions of tubulin-binding drugs play a major role in cancer treatment. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2013. Jul 15; 68(7):478–83. [PubMed] [Google Scholar]
- 52.Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clinical Cancer Research. 2012. Jan 1; 18(1):51–63. [DOI] [PubMed] [Google Scholar]
- 53.Schimming R, Mason KA, Hunter N, Weil M, Kishi K, Milas L. Lack of correlation between mitotic arrest or apoptosis and antitumor effect of docetaxel. Cancer Chemotherapy and Pharmacology. 1999. Feb 1; 43(2):165–72. [DOI] [PubMed] [Google Scholar]
- 54.Yan VC, Butterfield HE, Poral AH, Yan MJ, Yang KL, Pham CD, Muller FL. Why great mitotic inhibitors make poor cancer drugs. Trends in Cancer. 2020. Nov 1;6(11):924–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olziersky AM, Labidi-Galy SI. Clinical development of anti-mitotic drugs in cancer. Cell Division Machinery and Disease. 2017:125–52. [DOI] [PubMed] [Google Scholar]
- 56.Pucci B, Bellincampi L, Tafani M, Masciullo V, Melino G, Giordano A. Paclitaxel induces apoptosis in Saos-2 cells with CD95L upregulation and Bcl-2 phosphorylation. Experimental Cell Research. 1999. Oct 10; 252(1):134–43. [DOI] [PubMed] [Google Scholar]
- 57.Giannakakou P, Nakano M, Nicolaou KC, O’Brate A, Yu J, Blagosklonny MV, et al. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proceedings of the National Academy of Sciences. 2002. Aug 6; 99(16):10855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Capo-Chichi CD, Cai KQ, Smedberg J, Ganjei-Azar P, Godwin AK, Xu XX. Loss of A-type lamin expression compromises nuclear envelope integrity in breast cancer. Chinese Journal of Cancer. 2011. Jun; 30(6):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Capo-chichi CD, Cai KQ, Simpkins F, Ganjei-Azar P, Godwin AK, Xu XX. Nuclear envelope structural defects cause chromosomal numerical instability and aneuploidy in ovarian cancer. BMC Medicine. 2011. Dec; 9(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capo-Chichi CD, Yeasky TM, Smith ER, Xu XX. Nuclear envelope structural defect underlies the main cause of aneuploidy in ovarian carcinogenesis. BMC Cell Biology. 2016. Dec; 17(1):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith ER, George SH, Kobetz E, Xu XX. New biological research and understanding of P apanicolaou’s test. Diagnostic Cytopathology. 2018. Jun; 46(6):507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Current Opinion in Cell Biology. 2011. Feb 1; 23(1):47–54. [DOI] [PubMed] [Google Scholar]
- 63.Tapley EC, Starr DA. Connecting the nucleus to the cytoskeleton by SUN–KASH bridges across the nuclear envelope. Current Opinion in Cell Biology. 2013. Feb 1; 25(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tariq Z, Zhang H, Chia-Liu A, Shen Y, Gete Y, Xiong ZM, et al. Lamin A and microtubules collaborate to maintain nuclear morphology. Nucleus. 2017. Jul 4; 8(4):433–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papanicolaou GN. A new procedure for staining vaginal smears. Science. 1942. Apr 24;95(2469):438–9. [DOI] [PubMed] [Google Scholar]
- 66.Smith ER, Capo-Chichi CD, Xu XX. Defective nuclear lamina in aneuploidy and carcinogenesis. Frontiers in Oncology. 2018. Nov 20; 8:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith ER, Meng Y, Moore R, Jeffrey DT, Xu AG, Xu XX. Nuclear envelope structural proteins facilitate nuclear shape changes accompanying embryonic differentiation and fidelity of gene expression. BMC Cell Biology. 2017. Dec; 18(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McConkey DJ, Davis DW. Apoptosis and taxol therapy. Cancer Biology & Therapy. 2002. Mar 7;1(2):118–20. [DOI] [PubMed] [Google Scholar]
- 69.George Coukos MD, Rubin SC. Chemotherapy resistance in ovarian cancer: new molecular perspectives. Obstetrics & Gynecology. 1998. May 1; 91(5):783–92. [DOI] [PubMed] [Google Scholar]
- 70.Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxanes-induced apoptosis. Current Medicinal Chemistry-Anti-Cancer Agents. 2003. Jul 1; 3(4):291–306. [DOI] [PubMed] [Google Scholar]
- 71.Blagosklonny MV, Fojo T. Molecular effects of paclitaxel: myths and reality (a critical review). International Journal of Cancer. 1999. Oct 8; 83(2):151–6. [DOI] [PubMed] [Google Scholar]
- 72.Kitagawa K, Niikura Y. Caspase-independent mitotic death (CIMD). Cell Cycle. 2008. Apr 15; 7(8):1001–5. [DOI] [PubMed] [Google Scholar]
- 73.Panvichian R, Orth K, Day ML, Day KC, Pilat MJ, Pienta KJ. Paclitaxel-associated multimininucleation is permitted by the inhibition of caspase activation: a potential early step in drug resistance. Cancer Research. 1998. Oct 15; 58(20):4667–72. [PubMed] [Google Scholar]
- 74.Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013. Jul 3; 154(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proceedings of the National Academy of Sciences. 1993. Oct 15; 90(20):9552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmfeldt P, Sellin ME, Gullberg M. Predominant regulators of tubulin monomer–polymer partitioning and their implication for cell polarization. Cellular and Molecular Life Sciences. 2009. Oct; 66(20):3263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michalakis J, Georgatos SD, de Bree E, Polioudaki H, Romanos J, Georgoulias V, et al. Short-term exposure of cancer cells to micromolar doses of paclitaxel, with or without hyperthermia, induces long-term inhibition of cell proliferation and cell death in vitro. Annals of Surgical Oncology. 2007. Mar;14(3):1220–8. [DOI] [PubMed] [Google Scholar]
- 78.Koshiba H, Hosokawa K, Mori T, Kubo A, Watanabe A, Honjo H. Intravenous paclitaxel is specifically retained in human gynecologic carcinoma tissues in vivo. International Journal of Gynecologic Cancer. 2009. Apr 1; 19(4). [DOI] [PubMed] [Google Scholar]
- 79.Mori T, Kinoshita Y, Watanabe A, Yamaguchi T, Hosokawa K, Honjo H. Retention of paclitaxel in cancer cells for 1 week in vivo and in vitro. Cancer Chemotherapy and Pharmacology. 2006. Nov 1; 58(5):665–72. [DOI] [PubMed] [Google Scholar]
- 80.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. The Lancet Oncology. 2013. Feb 1; 14(2):e50–9. [DOI] [PubMed] [Google Scholar]
- 81.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annual Review of Cell and Developmental Biology. 1997. Nov; 13(1):83–117. [DOI] [PubMed] [Google Scholar]
- 82.Cassimeris L, Silva VC, Miller E, Ton Q, Molnar C, Fong J. Fueled by microtubules: does tubulin dimer/polymer partitioning regulate intracellular metabolism? Cytoskeleton. 2012. Mar; 69(3):133–43. [DOI] [PubMed] [Google Scholar]
- 83.Zhai Y, Borisy GG. Quantitative determination of the proportion of microtubule polymer present during the mitosis-interphase transition. Journal of Cell Science. 1994. Apr 1; 107(4):881–90. [DOI] [PubMed] [Google Scholar]
- 84.Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993. Mar 1; 32(11):2747–55. [DOI] [PubMed] [Google Scholar]
- 85.Caron JM, Jones AL, Kirschner MW. Autoregulation of tubulin synthesis in hepatocytes and fibroblasts. The Journal of Cell Biology. 1985. Nov; 101(5):1763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gasic I, Boswell SA, Mitchison TJ. Tubulin mRNA stability is sensitive to change in microtubule dynamics caused by multiple physiological and toxic cues. PLoS Biology. 2019. Apr 9; 17(4):e3000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin Z, Gasic I, Chandrasekaran V, Peters N, Shao S, Mitchison TJ, et al. TTC5 mediates autoregulation of tubulin via mRNA degradation. Science. 2020. Jan 3; 367(6473):100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huff LM, Sackett DL, Poruchynsky MS, Fojo T. Microtubule-disrupting chemotherapeutics result in enhanced proteasome-mediated degradation and disappearance of tubulin in neural cells. Cancer Research. 2010. Jul 15; 70(14):5870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson GV, Litersky JM, Whitaker JN. Proteolysis of microtubule-associated protein 2 and tubulin by cathepsin D. Journal of Neurochemistry. 1991. Nov; 57(5):1577–83. [DOI] [PubMed] [Google Scholar]
- 90.Herbst RS, Khuri FR. Mode of action of docetaxel–a basis for combination with novel anticancer agents. Cancer Treatment Reviews. 2003. Oct 1; 29(5):407–15. [DOI] [PubMed] [Google Scholar]
- 91.Barbuti AM, Chen ZS. Paclitaxel through the ages of anticancer therapy: exploring its role in chemoresistance and radiation therapy. Cancers. 2015. Dec; 7(4):2360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Design, Development and Therapy. 2015; 9:3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Advanced Drug Delivery Reviews. 2017. Dec 1; 122:20–30. [DOI] [PubMed] [Google Scholar]
- 94.Cao YN, Zheng LL, Wang D, Liang XX, Gao F, Zhou XL. Recent advances in microtubule-stabilizing agents. European Journal of Medicinal Chemistry. 2018. Jan 1; 143:806–28. [DOI] [PubMed] [Google Scholar]


