Abstract
People with autism spectrum disorder (ASD) exhibit a variety of medical morbidities at significantly higher rates than the general population. Using an established monkey model of naturally occurring low sociality, we investigated whether low-social monkeys show an increased burden of medical morbidities compared to their high-social counterparts. We systematically reviewed the medical records of N = 152 (n = 73 low-social; n = 79 high-social) rhesus macaques (Macaca mulatta) to assess the number of traumatic injury, gastrointestinal, and inflammatory events, as well as the presence of rare medical conditions. Subjects’ nonsocial scores, determined by the frequency they were observed in a nonsocial state (i.e., alone), and macaque Social Responsiveness Scale-Revised (mSRS-R) scores were also used to test whether individual differences in social functioning were related to medical morbidity burden. Medical morbidity type significantly differed by group, such that low-social monkeys incurred higher rates of traumatic injury compared to high-social monkeys. Nonsocial scores and mSRS-R scores also significantly and positively predicted traumatic injury rates, indicating that monkeys with the greatest social impairment were most impacted on this health measure. These findings from low-social monkeys are consistent with well-documented evidence that people with ASD incur a greater number of traumatic injuries and receive more peer bullying than their neurotypical peers, and add to growing evidence for the face validity of this primate model.
Keywords: animal model, autism spectrum disorder, medical morbidities, rhesus macaque, social behavior, Social Responsiveness Scale
Lay Summary:
People with autism exhibit multiple medical problems at higher rates than the general population. We conducted a comprehensive medical record review of monkeys that naturally exhibit differences in sociality and found that low-social monkeys are more susceptible to traumatic injuries than high-social monkeys. These results are consistent with reports that people with autism also incur greater traumatic injury and peer bullying and add to growing evidence for the validity of this monkey model.
INTRODUCTION
People with autism spectrum disorder (ASD) exhibit pervasive social interaction difficulties and repetitive behaviors that negatively impact daily functioning. These core behavioral symptoms are evident early in development and do not resolve over time (American Psychiatric Association, 2013). Scientific progress in the biological detection and pharmacological treatment of ASD has been impeded by the difficulty of studying brain signaling pathways directly in ASD patients and matched controls, and overreliance on model organisms that fundamentally lack the sophisticated cognitive and behavioral abilities critical for modeling ASD’s core symptoms. Indeed, over 90% of new central nervous system drugs fail in clinical trials (Kola & Landis, 2004), and over 50% of those failures are attributable to false positives from poorly selected animal models (Garner et al., 2017). Given these constraints, there is significant value in developing and studying refined animal models with more behavioral and biological homology to the human disorder (Capitanio & Emborg, 2008; Parker et al., 2018).
Rhesus monkeys are an ideal model organism by which to advance this objective. Like humans, they are highly social, have complex social cognitive abilities, and display natural, stable, and pronounced individual differences in social behavior (Phillips et al., 2014). The frequency that animals are observed in a nonsocial state can be used to classify monkeys as naturally low-social versus socially competent, high-social animals (Parker et al., 2018). We have found both in unobtrusive observational studies conducted in subjects’ outdoor half-acre field corrals and in experimental laboratory studies that naturally low-social male monkeys initiate fewer affiliative interactions, spend less time in physical contact and grooming with conspecifics, and show greater social information processing abnormalities (e.g., face recognition deficits and impaired species-typical gaze aversion in response to conspecific aggression) compared to their socially competent peers (Capitanio, 1999, 2002; Parker et al., 2018; Sclafani et al., 2016).
We recently refined the macaque Social Responsiveness Scale (mSRS) (Feczko et al., 2016) to enable more reliable measurement of quantitative variation in autistic-like traits in rhesus monkeys. This effort resulted in the mSRS-Revised (mSRS-R) (Talbot et al., 2020). The mSRS was derived from the SRS, an instrument originally developed for use in humans (Constantino et al., 2003). The SRS and mSRS-R both provide a quantitative measure of species typical and atypical social functioning (including assessment of autistic or autistic-like traits) in natural social settings (Constantino et al., 2003; Constantino et al., 2007; Talbot et al., 2020); higher scores on these instruments indicate greater social impairment. SRS scores have been shown to be continuously distributed across the general human population (Constantino, 2011). At the extreme of the population distribution, higher SRS scores overlap significantly with an ASD diagnosis (Constantino et al., 2003; Pine et al., 2006). These findings have enabled the SRS to be used in humans as a research tool to measure the presence of autistic traits in members of the general population (Chan et al., 2017; Constantino & Gruber, 2012), and as a clinical screening tool for ASD (Constantino et al., 2003; Constantino et al., 2007).
Consistent with the human SRS (Constantino, 2011), we found that autistic-like traits in rhesus monkeys are continuously distributed across the general monkey population (Talbot et al., 2020). As would be expected if the mSRS-R is measuring intrinsic social traits, we found that neither age nor rank correlated with monkeys’ mSRS-R scores. Importantly, in a sample of N = 233 male monkeys, those that scored greater than 1.5 standard deviations above the mean on nonsocial behavior frequency (i.e., low-social monkeys) exhibited significantly more autistic-like traits than those that scored less than 1.5 standard deviations below the mean on this measure (i.e., high-social monkeys). We also found that mSRS-R scores predicted individuals’ social classification (low-social vs. high-social) with 96% accuracy. These findings indicate that male rhesus monkeys exhibit pronounced individual differences in autistic-like traits and that naturally low-social male monkeys exhibit an increased burden of them (Talbot et al., 2020).
Having assessed aspects of the face validity of this monkey model, we next sought to evaluate its construct validity, by pursuing a translational ASD biomarker discovery effort. We identified low cerebrospinal fluid (CSF) concentration of the neuropeptide arginine vasopressin (AVP) as a robust marker of monkey low sociality (Parker et al., 2018). We subsequently tested whether this finding translated to human patients and found that CSF AVP concentration accurately differentiated ASD cases and controls, and that ASD children with the lowest CSF AVP concentrations had the greatest clinical symptom severity (Oztan et al., 2018; Parker et al., 2018). We have also tested the predictive value of AVP, by mining over 900 banked CSF samples collected during standard of care from 0 to 3-month-old human neonates. We found that individuals diagnosed with ASD later in childhood have lower neonatal CSF AVP concentrations compared to those who do not later receive an ASD diagnosis (Oztan et al., 2020). Finally, on the strength of these translational CSF biomarker findings, we launched a “first in class” pilot AVP treatment trial. Using a double-blind, randomized, placebo-controlled trial design, we found that AVP treatment markedly enhances social abilities in children with ASD (Parker et al., 2019). This accumulating behavioral and biological evidence thus suggests that naturally occurring low sociality in male rhesus monkeys provides a useful face and construct valid approach by which to further model and interrogate ASD.
In addition to ASD’s core behavioral symptoms, patients with ASD exhibit a variety of well-documented medical morbidities at significantly higher rates than the general population (Bauman, 2010; Cawthorpe, 2017; Hand et al., 2020; Kohane et al., 2012; Muskens et al., 2017). These include gastrointestinal disorders (Buie et al., 2010; Cawthorpe, 2017; Chandler et al., 2013; Gorrindo et al., 2012; Hand et al., 2020; Mazurek et al., 2013; Neuhaus et al., 2018; Restrepo et al., 2020; Wang et al., 2011; Wasilewska & Klukowski, 2015), inflammatory dyscrasias (Cawthorpe, 2017), as well as a variety of other conditions, such as cardiac disease (Hand et al., 2020), congenital anomalies (Cawthorpe, 2017; Timonen-Soivio et al., 2015), and seizures/epilepsy (Hand et al., 2020; Isaksen et al., 2013; Lewine et al., 1999; Muñoz-Yunta et al., 2008; Parmeggiani et al., 2010; Viscidi et al., 2013). Furthermore, people with ASD have been shown to be at increased risk for unintentional injury (Cavalari & Romanczyk, 2012; Lee et al., 2008; McDermott et al., 2008), self-injurious behavior (SIB) (Akram et al., 2017; Hand et al., 2020; McDermott et al., 2008; Rattaz et al., 2015; Siegel et al., 2015; Slingsby et al., 2017; Summers et al., 2017), physical abuse (Duan et al., 2015; Mandell et al., 2005; Slingsby et al., 2017), violent crime (Christoffersen, 2019), and/or peer bullying (Hoover & Kaufman, 2018; Hwang et al., 2018; Sterzing et al., 2012). People with ASD also suffer greater injury-related mortality compared to the general population (Fluegge, 2017).
The prevalence and severity of co-occurring medical morbidities in people with ASD have also been linked to the severity of their autistic symptoms (Adams et al., 2011; Cavalari & Romanczyk, 2012; Gorrindo et al., 2012; Lee et al., 2008; McClintock et al., 2003; Wang et al., 2011). For example, GI symptoms strongly correlate with ASD symptom severity as assessed by the Autism Treatment Evaluation Checklist (Adams et al., 2011). Further, individuals with ASD and co-occurring GI disorders have significantly greater social impairment as determined by SRS scores compared to both ASD-only and GI disorder-only groups (Gorrindo et al., 2012). Similar findings have also been reported for the relationship between ASD symptom severity and traumatic injuries. Children with more severe ASD symptomatology (as determined by higher composite scores on the Pervasive Developmental Disorder Behavior Inventory) (I. L. Cohen et al., 2003) are more likely to engage in risk-taking behaviors and sustain more frequent and severe injuries than children with ASD who are less impaired (Cavalari & Romanczyk, 2012).
The goal of the present study was to systematically and comprehensively review the medical records of male rhesus monkeys previously identified as low-social or high-social to determine the presence and severity of their co-occurring medical morbidities. We hypothesized that low-social monkeys would show an increased burden of medical morbidities compared to high-social monkeys. We also hypothesized that nonsocial behavior frequency and mSRS-R scores would individually and positively predict medical morbidity burden.
METHODS
Subjects and study site
Subjects were N = 152 male rhesus macaques (Macaca mulatta) that had been born and reared at the California National Primate Research Center (CNPRC) in Davis, CA. Subjects lived outdoors in one of 24 half-acre (0.2 ha) field corrals, measuring 30.5 m wide × 61 m deep × 9 m high. Each corral contained up to 145 animals of all ages and both sexes. Subjects were tattooed as infants and dye-marked periodically to facilitate easy identification for husbandry- and research-related procedures.
Monkeys had ad libitum access to Lixit-dispensed water. Primate laboratory chow was provided twice daily, and fruit and vegetable supplements were provided weekly. Various toys, swinging perches, and other forms of enrichment in each corral, along with outdoor and social housing, provided a stimulating environment. The present study comprised only a records review and thus did not require IACUC approval. All procedures for the broader studies (see next paragraph) in which the animals were classified were ethically reviewed and approved by the Institutional Animal Care and Use Committees at the University of California, Davis and Stanford University. These procedures complied with the Guide for the Care and Use of Laboratory Animals (Council, N. R, 2011) and National Institutes of Health policies on the care and use of animals. The CNPRC and University of California, Davis, are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International).
Behavioral observations, social classification, and medical record review period
Unobtrusive behavioral observations had been previously conducted on monkeys in four separate study cohorts while they were in their home field corrals as previously described (Parker et al., 2018; Talbot et al., 2020). For Cohorts 1, 3, and 4, observers conducted 10-min focal samples on subjects during two observation periods per day, 4 days per week, for 2 weeks. The behavior of individual monkeys was recorded at 30-s (Cohort 1) or 15-s (Cohorts 3 and 4) intervals using instantaneous sampling. For Cohort 2, we adopted a scan sampling approach, enabling us to score multiple animals in the same group at the same time. Each observer conducted scan samples for a given corral during two observation periods per day. In each observation period, scan sampling was conducted at 20-min intervals, at a rate of 18 scans per day, for a total of 5 days. During each scan, the subjects in each corral were identified, and observers then recorded the behavior. The same five social behaviors were recorded for all cohorts (i.e., the ethogram was the same regardless of sampling technique): nonsocial (subject is not within an arm’s reach of any other animal and is not engaged in play), proximity (subject is within arm’s reach of another animal), contact (subject is touching another animal in a nonaggressive manner), groom (subject is engaged in a dyadic interaction with one animal inspecting the fur of another animal using its hands and/or mouth), and play (subject is involved in chasing, wrestling, slapping, shoving, grabbing, or biting accompanied by a play face [wide eyes and open mouth without bared teeth] and/or a loose, exaggerated posture and gait; the behavior must have been deemed nonaggressive to be scored). Both sampling methods estimate the durations of behavior (Altmann, 1974). After completion of data collection, the total frequency of nonsocial behavior was then summarized across all of the behavior samples collected for each subject. Monkeys were then rank ordered on total nonsocial behavior within each study cohort. Monkeys with the greatest frequency of nonsocial behavior were classified as low-social, and monkeys with the lowest frequency of nonsocial behavior (and therefore the highest frequency of all pro-social behaviors) were classified as high-social (as detailed below).
Subjects previously classified as low-social or high-social and included in the present study were as follows: Cohort 1, N = 30 animals (n = 15 low-social and n = 15 high-social); Cohort 2, N = 48 animals (n = 23 low-social; n = 25 high-social); Cohort 3, N = 34 animals (n = 15 low-social and n = 19 high-social); and Cohort 4, N = 40 animals (n = 20 low-social and n = 20 high-social). The present study thus included a total of n = 73 low-social and n = 79 high-social monkeys, which ranged in age from 1.85 to 10.04 years (M = 5.70, SD = 1.51). Ages were recorded on each subject’s individual project end date. This date also signified the end of their medical record review period. Project end date codes included death (either natural or via humane euthanasia), shipment to another facility, assignment to a subsequent project involving experimental procedures, or still living in the CNPRC colony when medical records were exported on June 26, 2019.
Rank, nonsocial equivalence score, and mSRS-R ratings
An individual’s rank may impact social behavior (Vessey, 1984) and health (Vandeleest et al., 2016) in non-human primates. We therefore included evaluation of rank in the present study. Rank was assessed in each corral by CNPRC behavioral management personnel on an approximately monthly basis by recording aggressive and submissive interactions following food provisioning. Because each corral contained a different number of males, using the raw rank was ineffective as a direct measure that could be compared across all subjects. For example, being the fifth-ranked male in a corral containing six males is both quantitatively and qualitatively different than being the fifth-ranked male in a corral containing 20 males. Thus, for analyses, rank was calculated as the proportion of males in the group that the focal individual outranked, such that the highest-ranked individual had a value of 1 and the lowest-ranked individual had a value of 0 (Linden et al., 2019). Rank was assessed during the period each animal was under behavioral observation, such that it was directly associated with subjects’ social classification (low-social vs. high-social).
As three of the study cohorts had been observed and classified using instantaneous sampling methods (i.e., Cohorts 1, 3, and 4), whereas one study cohort had been observed and classified using scan sampling methods (i.e., Cohort 2) (Altmann, 1974), we created a nonsocial equivalence score by Z-scoring nonsocial behavior frequency within each study cohort to enable comparison of animals across the four different cohorts (N = 152).
Finally, mSRS-R scores were available from N = 91 animals. These subjects had been rated by observers within 24 h of the completion of each subject’s behavioral observation period (see above). Observers rated each subject on a 36-item original mSRS (Feczko et al., 2016), which we had modified from a four-point to a seven-point Likert scale (1 = total absence of the trait, 7 = extreme manifestation of the trait) for each item (Talbot et al., 2020). Prior to final summary, questions written in the infrequent direction were reverse scored such that higher scores always indicated greater impairment. Since only 17 of the original 36 mSRS items exhibited consistent inter-rater and test-retest reliability, here we extracted and tabulated ratings for the 17 reliable items, which form the basis of the mSRS-R (Talbot et al., 2020). Example items include: “Is socially awkward. Does not respond appropriately to social cues, e.g., play initiations,” “Behaves in ways that seem strange or bizarre for others of comparable age/rank/sex categories,” “Stares or gazes off into space more so than others of the same age/sex class,” “Is too silly or makes inappropriate noises, e.g., odd vocalizations.” Ratings had been conducted using identical methods across study cohorts, so the mSRS-R data did not require normalization. Nonsocial equivalence scores and mSRS-R scores were used to test whether the frequency observed alone in nonsocial behavior and quantity of autistic-like traits would positively predict medical morbidity burden.
Medical record review
We initially constructed a medical event scoring spreadsheet from the exported electronic medical records of all study subjects from CNPRC’s Web Vitals database. This database utilizes the Systematized Nomenclature of Medicine – Clinical Terms (SNOMED CT), a standardized, multilingual vocabulary of clinical terminology (Rouse, 2010), for electronic recording of medical events by anatomical topography, morphology, function, etiology, procedure, treatment, and discharge diagnosis. All diagnoses were confirmed by CNPRC veterinarians. Within this spreadsheet, we first categorized medical events as gastrointestinal (GI), inflammation, trauma, or “other,” per the descriptions in the respective sections that follow. All data obtained from Web Vitals were initially included and reduced to one line per diagnosis before event scoring was conducted. Therefore, a single event may have incorporated multiple diagnoses (e.g., multiple traumas that occurred on the same date). This then served as a referencing index for morbidity codes and events when subsequently reviewing the subjects’ physical medical jackets, which was necessary to confirm the accuracy of the electronic medical records. Thus, the medical jacket was considered the primary source for documentation of the subjects’ medical histories, such that any events that were documented electronically but could not be verified in the medical jackets were excluded from event scoring and subsequent analysis. Additionally, any events meeting scoring criteria that were not documented electronically but were included in the medical jackets, were included in event scoring and subsequent analysis. Once cross-referencing with the physical medical jacket was completed, the medical events were tallied to calculate each subject’s morbidity score in each category. Summative morbidity scores were then converted to rates in the final analyses, as described below. Experimenters were blinded to social classification (i.e., low-social vs. high-social), nonsocial equivalence score, and mSRS-R score during the medical record collection, review, and scoring periods. Data collection was conducted using standard methodology for retrospective cohort studies (Woodward, 1999). A study flow diagram is presented in Figure 1.
FIGURE 1.
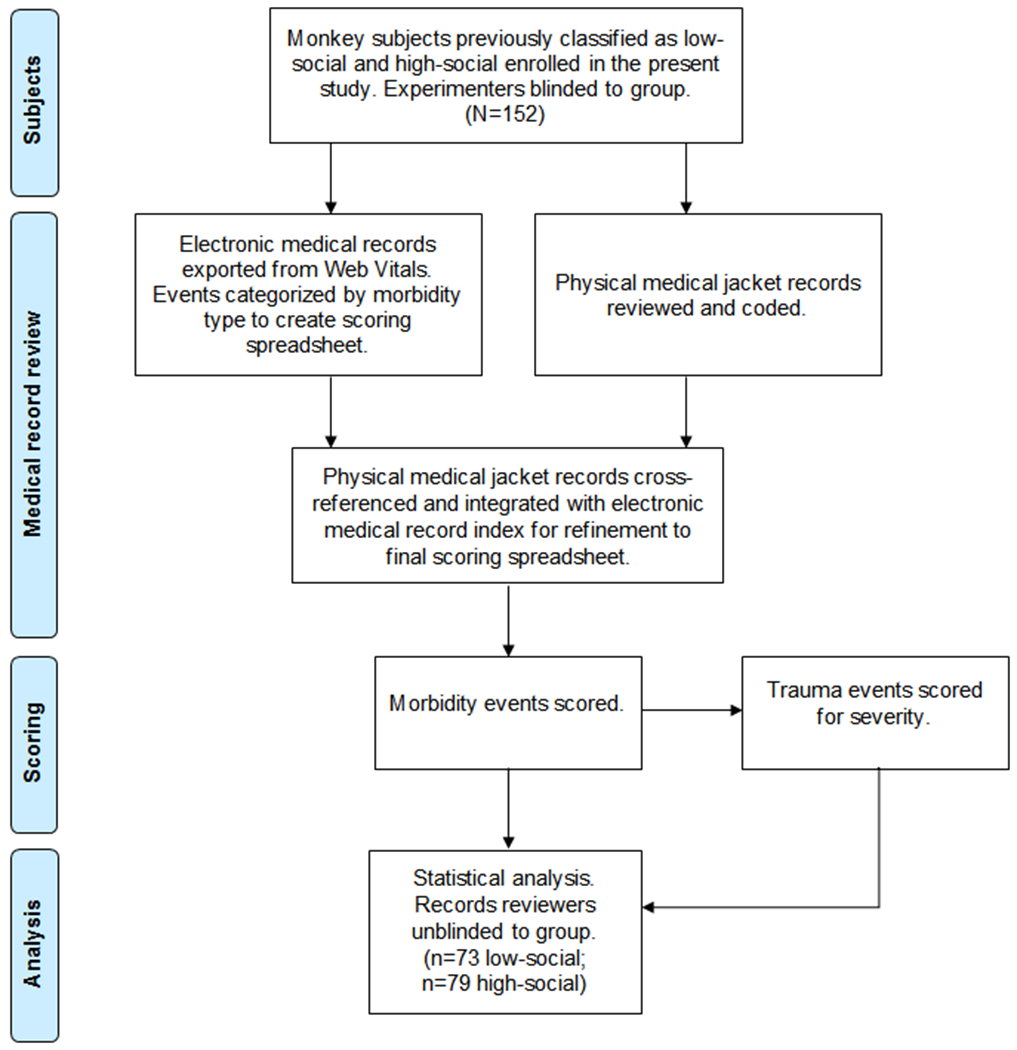
Flow diagram of study procedures. The flow diagram details the progress from social classification through statistical analysis for this medical record review study. Quantitative social behavior observations were previously conducted for four monkey cohorts and a subset of the animals were classified as low-social or high-social monkeys (see description in text). Electronic and physical medical records were then reviewed and scored, and data analyzed as detailed in the figure (N = 152)
GI event coding
A GI event was defined as one or more GI-related diagnoses that occurred on the same date requiring hospitalization and/or treatment at the subject’s location with confirmed resolution as documented in the medical jacket. Resolution was confirmed once normalized signs had been recorded on two consecutive days without resumption of signs thereafter (i.e., liquid or semi-solid recorded in stool column with accompanying note of “decline in stool, variable stool, liquid stool, or semi-solid stool” before the subject was discharged for the event), with the resolution date coded as the first date the signs normalized.
Trauma event coding
A trauma event was defined as one or more injury-related diagnoses that occurred on the same date requiring hospitalization and/or treatment at the subject’s location with confirmed resolution as documented in the medical jacket. Wound dehiscence or infection of an injury that was previously confirmed as an event was not coded as a new event. Injuries that were attributed to any cause other than conspecific aggression and clearly described in the subjects’ medical jackets as such were not included in trauma event scoring. For example, any injuries that were sustained as a result of SIB were treated as “other” events (see below). All descriptors of traumatic injuries encompassing each event were also recorded in the event scoring spreadsheet and used to assign severity scores to each in order to calculate an overall trauma severity score for each subject. This is described in more detail in the trauma severity scoring section that follows.
Inflammation event coding
An inflammation event was defined as one or more inflammation-related diagnoses that occurred on the same date requiring hospitalization and/or treatment at the subject’s location with confirmed resolution as documented in the medical jacket. To be considered an inflammation event, the diagnoses had to be mutually exclusive from events in the other morbidity categories and involve an inflammatory process. For example, purulent discharge from an infected traumatic wound was not considered an inflammation diagnosis as the trauma was the likely etiology of the inflammation and infection. Conversely, events including conditions such as dermatitis, where no inciting injury could be identified, were considered inflammation events. Furthermore, diarrhea or any inflammation diagnosis in the GI tract, (e.g., colitis, enteritis) was always scored as a GI event (as described above) and never as an inflammation event.
Other event coding
An “other” event was defined as all occurrences of medical diagnoses which did not fulfill the criteria for inclusion in any of the morbidity categories in the sections above. For example, this included congenital anomalies (e.g., inguinal hernia), acquired cardiac disease (e.g., cardiac murmurs), and disorders of undetermined etiology or pathogenesis (e.g., kyphosis, strabismus). Furthermore, given the overrepresentation of SIB in individuals with ASD, we felt it was pertinent to assess this traumatic injury etiology individually; however, due to their rarity, SIB events were included in this category. SIB events were defined as one or more injury-related diagnoses with any explicit description of self-directed injurious behavior that occurred on the same date requiring hospitalization and/or treatment at the subject’s location with confirmed resolution as documented in the medical jacket. Because “other” events were so uncommon (despite having distinct disease morphology) they were collectively categorized and scored. Finally, medical events were excluded from further study if they were only identified by special diagnostic modalities (e.g., necropsy, ultrasound) and not preceded by diagnosis via physical examination or gross observation.
Trauma severity scoring
To better understand the impact of trauma events, and potentially differences between these events based on social classification and quantitative behavioral measures, we examined the fine-grained medical records that followed each trauma event. A single trauma event may involve one or more injuries, and those injuries can differ in severity. Thus, in addition to calculating the total number of injuries observed and the mean number of injuries observed per trauma event, we assessed the severity of these injuries and calculated the mean severity of the injuries per trauma event. We first developed a novel rating scale modeled after the human Abbreviated Injury Scale (AIS), the first consensus-derived, widely implemented injury severity scale used in practice (Gennarelli & Wodzin, 2006). Utilizing its ordinal classifications, we characterized the injury types that were assigned to each category. This was necessary due to a lack of access to a list of AIS codes and the limitations of the physical records (i.e., AIS scores usually account for organ damage, e.g., which, generally, could not be addressed here). From this, each traumatic injury was assigned a score as defined in Table 1. In addition, any injuries noted as “communicating” or “coalescing” were scored as a single injury. “Communicating” and “coalescing” described injuries that appeared to be superficially distinct but were in fact connected in subcutaneous tissues. Any injuries that received treatment but became infected, necrotic, and/or fully dehisced before resolution and/or where “delayed healing” were explicitly noted were reassigned a score one higher than the original score. Any injuries where qualifiers (e.g., minor, moderate, severe, major, deep) were noted in the medical jacket were assigned a different trauma severity score if those descriptors indicated that they exceeded or failed to meet the basic criteria provided in Table 1. These individual injury scores were then summed to determine each subject’s overall trauma severity score, which was subsequently divided by the number of trauma events to calculate the mean event trauma severity score, which was the outcome measure for the analysis.
Table 1.
Trauma severity scale
| Severity category | Severity score | Definition |
|---|---|---|
| Minor | 1 | • Superficial abrasions, lacerations, bites, punctures, nail avulsions, and scratches that were not full thickness and did not require sutures or skin glue • Mild, focal bruising and/or mild soft tissue swelling/edema that were not directly associated with another trauma • Minor “lameness” or “non-weight bearing” with unknown etiology |
| Moderate | 2 | • Full thickness abrasions, lacerations, bites, punctures, nail avulsions, scratches, deglovings, and flaps that required sutures or skin glue but did not involve muscles, tendons, and/or fascia, require amputation, dehisce, or become infected or necrotic • Hematomas that were not directly associated with another trauma nor the result of venipuncture or surgery • Moderate swelling • Maceration that did not require sutures and/or glue • Superficial generalized trauma and bruising spanning a large body surface area where no specific body part was identified and/or ‘multiple’ was indicated • Moderate and/or severe bruising and/or edema/swelling • Digit and/or tail tip dislocations that did not require surgical amputation • Minor bone fractures • Moderate “lameness” or “non-weight bearing” with unknown etiology • Superficial and/or minor cornea trauma |
| Serious | 3 | • Muscle, tendon, and/or fascia involvement without complete transection • Surgical amputation of a body part due to necrosis and/or wound dehiscence, moderate-severe inflammation/edema, fracture of a digit or tail tip, and/or bone exposure • Initial infection/purulent discharge and/or necrosis later requiring sutures, skin glue, or surgical amputation • Cartilage exposure • Maceration requiring sutures and/or glue • Bone fracture • Crushing • Moderate or greater eye trauma |
| Severe | 4 | • Muscle or tendon completely bisected, transected, or avulsed • Severe crushing • Traumatic amputations |
| Critical | 5 | • Intensive critical care necessary for survival • Head crushing |
| Maximum | 6 | • Caused death or required humane euthanasia |
Statistical analyses
All analyses were performed in JMP 14 Pro for Windows, with additional post hoc analyses performed in SAS 9.3, as required. We adopted both family-level gatekeeping tests, and a general “winnowing” strategy to avoid multiple testing (and false discovery) (Blankenberger et al., 2018; Chu et al., 2004; Parker et al., 2018). We first tested the hypothesis that medical morbidity events would differ between low-social and high-social monkeys. To do so, we adopted a repeated measures approach, where each monkey acted as its own control, and thus differences in age, corral, rank, and any other variables were corrected for in the model. Specifically, we used a repeated measures mixed model, where monkey nested within group (i.e., low-social vs. high-social) was the subject, group was the between-subject factor, morbidity type was the within-subject factor, and the group-by-morbidity-type interaction explicitly tested whether the count of each morbidity event type (i.e., GI, inflammation, trauma, other) differed by group. In order to control for the duration that each monkey was retrospectively observed, observation duration was included in the model. Both morbidity event counts and observation duration were log-transformed. This log-log regression not only controls for duration but calculates a rate within the model, thereby allowing the rate of morbidity events to change as a function of duration. This is a standard approach to these kinds of data (Woodward, 1999). The assumptions of mixed models (homogeneity of variance, normality of error, and linearity) were confirmed post hoc. Significant group-by-morbidity interactions were examined using Bonferroni-corrected planned contrasts. N = 152 monkeys were included in this analysis.
We next tested whether quantitative nonsocial equivalence scores (N = 152 monkeys) and mSRS-R scores (N = 91 monkeys) individually predicted medical morbidity event frequency in the medical events that differed by group. For these analyses, we adopted a log–log general linear model approach. Here, the log of event count is predicted in a model that includes the log of the age at medical record review (i.e., the duration in which the events could occur), as well as the predictor variable (either the nonsocial equivalence score or the mSRS-R score for the separate analyses). This is equivalent to dividing by the age at medical record review but has the distinct advantage of allowing curved effects of observation duration. This is because observation duration is raised to a power modeled by the regression, a technique widely used in physiology and allometry (Grafen & Hails, 2002). In contrast, a simple rate, where event counts are divided by the observation duration raised to the fixed power of 1, assumes a constant chance of trauma per month, regardless of age, which is clearly untrue. These analyses were further controlled for (blocked by): the corral the animal was housed in, study cohort, age at behavioral observation, and rank. We first examined these models for collinearity, as collinearity in these kinds of data can lead to false negatives, and simplified them following best practice (Grafen & Hails, 2002; Thabane et al., 2013; Woodward, 1999). Thus, age at observation was collinear with age at medical record review and was removed because age at medical record review was inherent to the log–log approach, and because age at observation contributed less to the R2 of the model. Rank was collinear to the relationship between our predictor variables and the outcome variable, reducing the apparent significance of the predictor. As including rank did not improve the R2 of the model, nor did it change the outcomes, it was removed following best practice for linear models (Grafen & Hails, 2002; Thabane et al., 2013).
Finally, with the additional information readily available from the fine-grained trauma records, we tested the hypotheses that total traumatic injury frequency and severity would also differ between low-social and high-social monkeys (N = 152). We initially performed these analyses including rank, and the interaction between rank and group. As before, including rank did not improve the model, introduced collinearities, and did not change the overall pattern of results. Therefore, rank was removed from the final analyses following best practice for linear models (Grafen & Hails, 2002; Thabane et al., 2013). Only if group differences were significant did we test whether nonsocial equivalence score (N = 152) or mSRS-R score (n = 91) predicted any of these outcomes. These analyses were performed as before (i.e., with the log of age at medical record review, study cohort, and corral included as control variables). Total injuries and injuries per event were logged; severity per event was untransformed. The predictor variable was either group, nonsocial equivalence score, or mSRS-R score as described above.
For all analyses, the assumptions of GLM (homogeneity of variance, normality of error, and linearity) were confirmed post hoc, and suitable transformations applied as described. We also checked the raw data to make sure that when groups did not differ in their mean response, that there was also not a difference in variance or distribution. These model refinement steps follow best practice for “Sensitivity Analysis” in human subjects research (Thabane et al., 2013).
The advanced methods employed here greatly increase power and reduce sample size (often by 5-fold to 10-fold) over analyses such as T-Tests or simple regression, while simultaneously also reducing false positives due to spurious environmental interactions (Fisher, 1935; Garner, 2014; Gaskill & Garner, 2020; Grafen & Hails, 2002; Richter et al., 2009; Richter et al., 2010). However, no formal a priori power calculations similar to those used for simpler analyses exist for the analyses employed here. As we and others have advocated (Festing, 2014, 2018; Gaskill & Garner, 2020), the best approach to a priori power calculations for advanced analyses is Mead’s resource equation (Mead, 1988). Mead’s resource equation provides a sample size above which additional subjects will have little impact on power; we therefore powered the experiment accordingly.
Effect sizes are given as partial eta squared (ηp2) and also as equivalent partial correlation coefficients (rp) where appropriate. ηp2 is the universal measure of effect size for all linear models from which other measures can be derived (e.g., ηp2 = (rp)2 for continuous effects). Note, however, that ηp2 can be misleading as it gives relatively small values for effects that we would consider meaningful, and progressively so for larger sample sizes. For instance, rp = 30% is considered a medium effect size by Cohen, where ηp2 = 9%; similarly, a large effect size, according to Cohen is rp = 50%, where ηp2 = 25% (J. Cohen, 1988; Gaskill & Garner, 2020).
RESULTS
Characteristics of the low-social and high-social monkey groups are provided in Table 2. Low-social and high-social monkeys did not differ by rank, age at social classification, or age at medical record review. As expected, low-social and high-social monkeys did differ significantly on their nonsocial equivalence and mSRS-R scores, such that low-social monkeys were more frequently observed alone in nonsocial behavior and were rated as having a greater burden of autistic-like traits compared to high-social monkeys.
Table 2.
Characteristics of low-social and high-social monkeys
| Social classification | N | Rank | Age at social classification | Age at medical record review | Non-social equivalence score | mSRS-R score |
|---|---|---|---|---|---|---|
| High-social | 79 | 0.502 ± 0.032 | 3.14 ± 0.135 | 5.57 ± 0.170 | 0.456 ± 0.011 | 43.9 ± 2.02 |
| Low-social | 73 | 0.482 ± 0.034 | 3.36 ± 0.140 | 5.85 ± 0.177 | 0.729 ± 0.011 | 59.0 ± 2.13 |
| P = 0.6686 | P = 0.2737 | P = 0.2701 | P < 0.0001 | P < 0.0001 |
Subjects’ sociality influenced the rate of morbidity events differentially for the morbidity types (F3,450 = 3.4884; N = 152; ηp2 = 2.273%; p = 0.0158). Planned contrasts showed that only trauma events differed by group, with low-social monkeys incurring higher rates of trauma than high-social monkeys (F1,597.4 = 9.895; ηp2 = 1.629%; p = 0.0017; Figure 2). Similarly, nonsocial equivalence scores and mSRS-R scores significantly and positively predicted trauma rates (nonsocial: F1,127 = 8.2031; N = 152; rp = 0.2463; ηp2 = 6.067%; p = 0.0049; mSRS-R: F1,71 = 5.1654; N = 91; rp 0.2604; ηp2 = 6.782%; p = 0.0261). These findings were unchanged if rank was included in the analysis.
FIGURE 2.
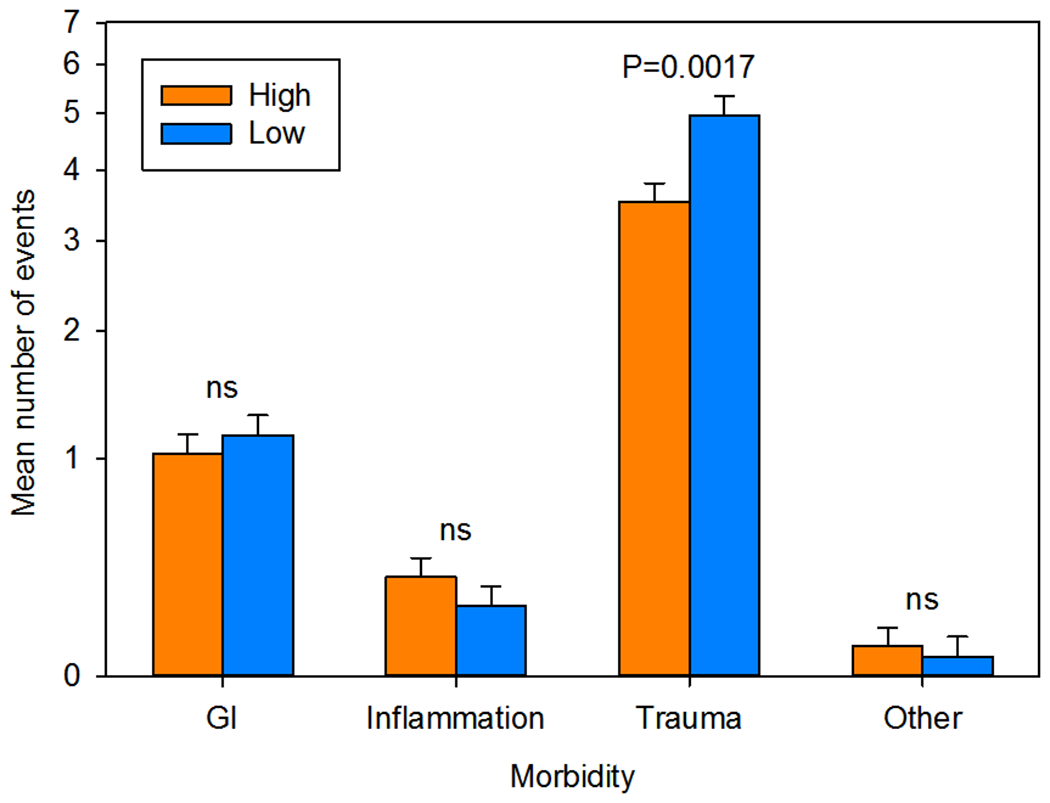
Medical morbidities by group. Only trauma events differed significantly between low-social (n = 73) and high-social (n = 79) monkeys. Data are plotted as the LSM ± SE number of morbidity events. The LSM was calculated at the mean log age at medical record review (66 months). Data were analyzed and are plotted on a log scale
Because single trauma events may have consisted of multiple traumatic injuries, we also analyzed group differences for this measure and found that group significantly predicted total injuries received, with low-social monkeys incurring more injuries than high-social monkeys (F1,127 = 7.1343; N = 152; ηp2 = 5.319%; P = .0086; Figure 3). Follow-up analyses showed that the nonsocial equivalence score significantly and positively predicted total injuries (F1,127 = 8.8669; N = 152; rp = 0.2555; ηp2 = 6.525%; p = 0.0035; Figure 4(a)), and that mSRS-R score likewise significantly and positively predicted total injuries (F1,71 = 7.3990; N = 91; rp = 0.3072; ηp2 = 9.438%; p = 0.0082; Figure 4(b)), such that the more socially impaired the monkey, the greater the burden of their traumatic injuries. Group did not predict the number of injuries per event (F1,127 = 1.634; N = 152; ηp2 = 1.270%; p = 0.2035), or the mean severity per injury (F1,127 = 0.2122; N = 152; ηp2 = 0.167%; p = 0.6459). As a result, these outcomes were not investigated further. These findings were unchanged if rank or its interaction with group was included in the analysis.
FIGURE 3.
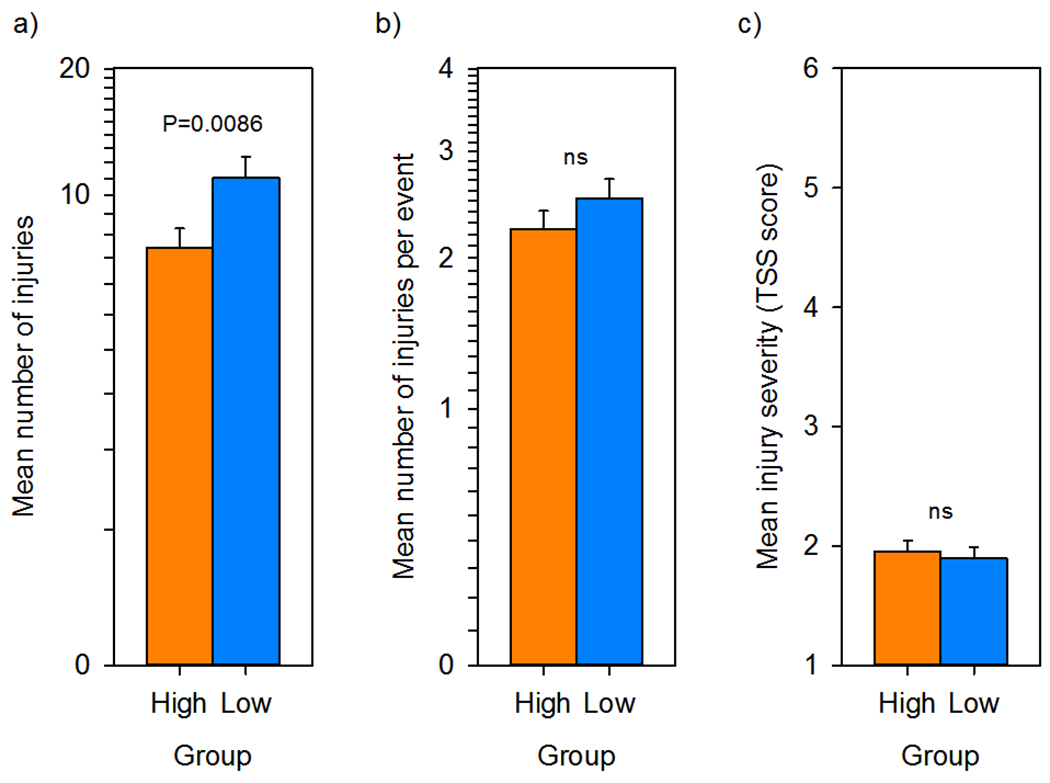
Fine-grained trauma-related medical record review by group. (a) The mean number of total injuries in the medical record was significantly greater in low-social (n = 73) compared to high-social (n = 79) monkeys. (b) The mean number of injuries per trauma event did not differ by group. (c) The mean trauma severity scale score per injury did not differ by group. All data are plotted as LSM ± SE. The LSM was calculated at the mean log age at medical record review (66 months). (a) and (b) Are plotted and analyzed on a log scale; (c) is untransformed. LSM, least square mean
FIGURE 4.
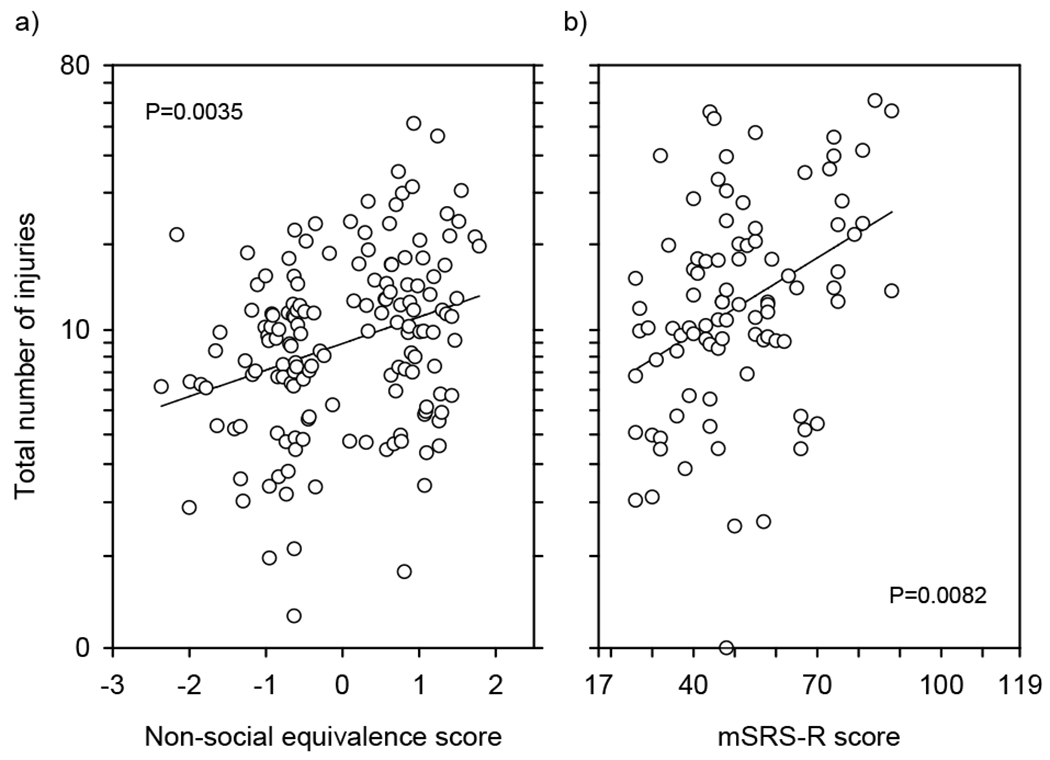
Relationships between sociality measures and total number of traumatic injuries received. (a) Nonsocial equivalence score significantly and positively predicts total number of traumatic injuries received (N = 152). (b) mSRS-R score significantly and positively predicts total number of traumatic injuries received (N = 91). Data are plotted corrected (partialled) for other terms in the model. Thus, we figured the least squares line for each data point and then plotted the data point as the residual from this expected value. This is directly equivalent to the calculations for plotting an LSM ± SE. These values were then scaled to the same range as the original data. Given the log–log model, this means that each data point is corrected from the observed age to the mean log age of the animals in the data set. This is 66 months of age for (a) and 67 months of age for (b). The data are plotted on a log scale to reflect the linearity and homogeneity of variance of the underlying analysis
DISCUSSION
The present study extended our established monkey model of naturally occurring low sociality to investigate whether low-social monkeys exhibit medical morbidities at significantly higher rates than their more socially competent counterparts. We also assessed whether individual differences in nonsocial behavior frequency and autistic-like trait burden predicted medical morbidity severity. Here, we performed a comprehensive medical record review of subjects previously classified as low-social or high-social to determine the presence and severity of their medical morbidities. We found that low-social monkeys experienced significantly higher rates of traumatic events and traumatic injuries than high-social monkeys. We also found that individual differences in monkeys’ nonsocial equivalence scores and mSRS-R scores positively predicted trauma rates and total number of injuries received, such that monkeys with the greatest social impairment were most impacted on these health measures. Our findings are in keeping with clinical reports documenting that people with ASD likewise exhibit a greater number of traumatic injuries relative to those in the general population (Cavalari & Romanczyk, 2012; Jain et al., 2014; Lee et al., 2008; McDermott et al., 2008) and that those with greater ASD symptomatology sustain more frequent and more severe injuries than those who are less impaired (Cavalari & Romanczyk, 2012).
Traumatic injuries have a variety of etiologies, including those that are self-directed or unintentional, as well as those that are the result of violent crime, physical abuse, or peer bullying. Almost all studies that have investigated associations between ASD and traumatic injuries have reported on the significantly increased prevalence of traumatic injuries overall in the ASD population (Cavalari & Romanczyk, 2012; Hand et al., 2020; Jain et al., 2014; Lee et al., 2008; McDermott et al., 2008; Vohra et al., 2016), with SIB consistently being the most overrepresented etiology (Hand et al., 2020; McDermott et al., 2008; Vohra et al., 2016), even in one study where the overall trauma results were equivocal (Kalb et al., 2016). There have been some conflicting reports on the increased likelihood of unintentional injury (Diguiseppi et al., 2018; Jernbro et al., 2020), with one report even citing the diagnosis of ASD as a protective factor (Jain et al., 2014). However, there has been more unanimity in terms of the increased prevalence of violent crime (Christoffersen, 2019), physical abuse (Duan et al., 2015; Mandell et al., 2005; Slingsby et al., 2017), and peer bullying (Hoover & Kaufman, 2018; Hwang et al., 2018; Sterzing et al., 2012) in ASD, thus painting a complex picture of how these traumatic injuries occur in this population.
The medical records in this study generally limited our interpretation of traumatic injuries in monkeys to SIB, unintentional injury, and conspecific aggression. SIB is a unique form of injury that has been studied singularly and extensively in relation to ASD (Akram et al., 2017; Rattaz et al., 2015; Siegel et al., 2015; Slingsby et al., 2017). Therefore, we felt it was pertinent to code SIB as a separate morbidity category initially to determine its specific relationship to our subjects’ sociality. Notably, SIB is less commonly reported in outdoor-reared and outdoor-housed rhesus macaques (Gottlieb et al., 2013; Rommeck et al., 2009), and indeed was scored in only 7 of the 1182 medical events recorded here (and was thus combined and analyzed with other rare events as noted above). Unintentional injury was difficult to fully assess because our subjects were not observed at all times they were on study. Despite this, we were generally able to determine if traumatic injuries were perpetrated by corral mates based on detailed medical record descriptions. In this regard, the clinical picture of these traumatic injuries in low-social monkeys appears to mimic the complex clinical picture of traumatic injuries in people with ASD that are not attributed to self-directed behavior. Namely, these injuries arise most consistently from intentional, external sources. In the case of low-social monkeys, we have previously found that low-social monkeys exhibit deficits in social information processing and show diminished capacity to respond appropriately to species-typical behavioral cues (Capitanio, 2002; Sclafani et al., 2016). In particular, low-social monkeys show increased latencies to display species-typical gaze aversion to conspecific aggression, which may place them at greater risk of experiencing trauma from conspecifics compared to socially competent monkeys.
Interestingly, this study did not find group differences in any other medical morbidities evaluated here. This may be due to inclusion of subjects in the low-social classification that were not phenotypically at the behavioral extreme of impaired social functioning. Perhaps only monkeys at the very extreme of low sociality would show significant nontrauma-related morbidities, whereas even minor social cognitive deficits may confer a greater risk of incurring traumatic injury. This is because injuries can be both a primary manifestation and a secondary consequence of social impairments and the expected inappropriate behavioral responses which accompany them. This line of reasoning also implies that traumas simply may be more directly reflective of social deficits compared to the other morbidities, which, conversely, are more susceptible to the influence of other nonsocial factors. Stress, for example, has been shown to contribute significantly to the prevalence of both nontrauma-related morbidities (Gottlieb et al., 2018) and SIB (Gottlieb et al., 2013; Novak, 2003) in monkeys. This and other factors may have confounded the results for those morbidities where they were more likely to impart greater influence. Otherwise, a lack of evidence in the other morbidity categories could generally signify that co-occurring medical morbidities documented in people with ASD do not map specifically onto the sociality measures of this model.
A number of best practices, both in data gathering, and in data analysis, mitigated the risk of false positive and false negative findings associated with this sort of epidemiological study. In particular, in data gathering, clear inclusion/exclusion criteria and blinding prevent unintentional bias in both epidemiological and laboratory studies (Landis et al., 2012; McCann et al., 2016; Sena et al., 2010). In this study, we chose to include all animals that had been previously observed in our research program, and for which we had clearly assigned a high-social or low-social designation. The assessor of the medical records for these animals was blind to their social classification. While these animals were not formally randomized, they were essentially randomly selected on the basis of their social behavior. We also avoided the chance of false positives due to idiosyncrasies, such as observer effects (Chesler et al., 2002) or phenotype-by-environment interactions (Fisher, 1935; Richter et al., 2009; Richter et al., 2010), by including four study cohorts of animals observed in four different calendar years. We adopted advanced analytical methods that controlled for extraneous variables and maximized power. We also stuck to clear a priori hypotheses to avoid fishing or multiple testing and committed to reporting both positive and negative results. Finally, we adopted both family-level gatekeeping tests and a general “winnowing” strategy to avoid unnecessary multiple testing as described in the statistical analysis section.
The present study nevertheless had several limitations that merit discussion. First, as mentioned above, study participants in the low-social classification may not have consistently represented the behavioral extreme of social functioning, which may have significantly impacted the prevalence of nontrauma-related morbidities in this group. A follow-up study is thus warranted in monkey subjects that more consistently represent the behavioral extreme of low sociality. Second, while it seems much more likely that low sociality leads to being attacked and injured by other animals, it is also possible that a history of being attacked leads to social withdrawal and impaired social functioning. We have previously reported that 3–4-month-old infant monkeys, later classified in adulthood as low-social, show impairment in species-typical gaze aversion to conspecific aggression compared to age-matched infants which develop typically (Sclafani et al., 2016). Further research is nevertheless required to confirm the causal direction of this observed relationship. Third, despite the magnitude of detail provided by both the electronic and physical medical jacket records, it was nearly impossible to fully account for the etiology of traumatic injuries in all instances without having had constant observation of the subjects. This, in turn, may have led to overrepresentation of specific traumatic injury types and underrepresentation of others (e.g., SIB), potentially skewing the data. To avoid false discovery and potential rater bias in this regard, we inherently applied a rigid and conservative approach to data collection and subsequent scoring, which may have excluded some events that potentially met criteria for inclusion in the study. Fourth, due to ASD’s male-biased prevalence (Maenner et al., 2020), significantly more scientific information was available from male ASD research participants for the purposes of modeling in monkeys behavioral symptoms and assessing co-occurring medical morbidities. The present study sample consequently included only male monkey subjects. However, ASD does impact females. Growing evidence also indicates that girls with ASD need to display higher levels of autistic traits to garner medical attention and tend to be diagnosed at later ages than boys on the spectrum (Loomes et al., 2017), suggesting that ASD may be under-detected and may manifest differently in girls. As our scientific understanding of ASD in female humans grows, it will be imperative to model these findings in female monkeys. Lastly, psychological stress (e.g., rearing environment and corral disruption) and other factors (e.g., personality) could not be measured and assessed in the context of the current study design. These have been shown to contribute significantly to the prevalence of specific morbidities (e.g., GI disorders, stereotypies, and SIB) in monkeys (Gottlieb et al., 2013; Gottlieb et al., 2018; Kinnally et al., 2019; Lutz et al., 2003) and are also likely to be correlated with social cognitive deficits (e.g., decreased engagement in affiliative interactions and contact/grooming time and increased latency to gaze aversion) (Capitanio, 1999, 2002). Therefore, it is impossible to know what sort of role these factors may have played in the study’s health measures. By incorporating additional measures in future studies to account and/or control for these factors, we could perhaps better assess their influence on both health status and social classification or include only those morbidity events where these factors did not impart significant influence.
In conclusion, there is significant value in developing and studying refined animal models with more behavioral and biological homology to ASD. Not only has this monkey model enabled us to study social aspects of this disorder (Sclafani et al., 2016; Talbot et al., 2020) but also to identify and target specific biological pathways associated with these behaviors to achieve streamlined translation and clinical impact (Oztan et al., 2018; Oztan et al., 2020; Parker et al., 2018; Parker et al., 2019). As medical morbidities continue to be a consistently overlooked and poorly understood facet of ASD, having an animal model that recapitulates at least some of ASD’s co-occurring health problems will enable rigorous experimental research that may help improve our knowledge and clinical management of this disorder.
ACKNOWLEDGMENTS
The authors thank Erna R. Tarara, Shannon K. Seil, Kyle J. Bone, Jesus E. Madrid, Joshua A. Herrington, Alicia M. Bulleri, and Kylee M. Beck for helping with behavioral observations. The authors thank the CNPRC staff for maintaining the health and well-being of the primates. This research was supported by the Simons Foundation (SFARI #274472 to Karen J. Parker; SFARI #342873 to Karen J. Parker), the National Institutes of Health (R21HD079095 to Karen J. Parker; R01 HD087048 to Karen J. Parker, P51OD011107 CNPRC base operating grant; T35OD010989 fellowship to Adam K. Myers), and the Department of Psychiatry and Behavioral Sciences at Stanford University.
Funding information
Department of Psychiatry, Stanford University; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R01HD087048, R21HD079095; NIH Office of the Director, Grant/Award Numbers: P51OD011107, T35OD010989; Simons Foundation Autism Research Initiative, Grant/Award Numbers: SFARI #274472, SFARI #342873
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- Adams JB, Johansen LJ, Powell LD, Quig D, & Rubin RA (2011). Gastrointestinal flora and gastrointestinal status in children with autism – Comparisons to typical children and correlation with autism severity. BMC Gastroenterology, 11(1), 22. 10.1186/1471-230x-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram B, Batool M, Rafi Z, & Akram A (2017). Prevalence and predictors of non-suicidal self-injury among children with autism spectrum disorder. Pakistan Journal of Medical Sciences, 33(5), 1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann J (1974). Observational study of behavior: Sampling methods. Behaviour, 49(3), 227–267. 10.1163/156853974x00534 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association. [Google Scholar]
- Bauman ML (2010). Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurotherapeutics, 7(3), 320–327. 10.1016/j.nurt.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberger WB, Weber EM, Chu DK, Geronimo JT, Theil J, Gaskill BN, Pritchett-Corning K, Albertelli MA, Garner JP, & Ahloy-Dallaire J (2018). Breaking up is hard to do: Does splitting cages of mice reduce aggression? Applied Animal Behaviour Science, 206, 94–101. 10.10161/j.applanim.2018.06.003 [DOI] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinyo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, … Winter H. (2010). Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics, 125(Suppl 1), S1–S18. 10.1542/peds.2009-1878C [DOI] [PubMed] [Google Scholar]
- Capitanio JP (1999). Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology, 47(4), 299–320. [DOI] [PubMed] [Google Scholar]
- Capitanio JP (2002). Sociability and responses to video playbacks in adult male rhesus monkeys (Macaca mulatta). Primates, 43(3), 169–177. 10.1007/bf02629645 [DOI] [PubMed] [Google Scholar]
- Capitanio JP, & Emborg ME (2008). Contributions of non-human primates to neuroscience research. The Lancet, 371(9618), 1126–1135. 10.1016/s0140-6736(08)60489-4 [DOI] [PubMed] [Google Scholar]
- Cavalari RN, & Romanczyk RG (2012). Caregiver perspectives on unintentional injury risk in children with an autism spectrum disorder. Journal of Pediatric Nursing, 27(6), 632–641. 10.1016/j.pedn.2011.07.013 [DOI] [PubMed] [Google Scholar]
- Cawthorpe D (2017). Comprehensive description of comorbidity for autism spectrum disorder in a general population. The Permanente Journal, 21, 16–88. 10.7812/TPP/16-088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W, Smith LE, Hong J, Greenberg JS, & Mailick MR (2017). Validating the social responsiveness scale for adults with autism. Autism Research, 10(10), 1663–1671. 10.1002/aur.1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Carcani-Rathwell I, Charman T, Pickles A, Loucas T, Meldrum D, Simonoff E, Sullivan P, & Baird G (2013). Parent-reported gastro-intestinal symptoms in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(12), 2737–2747. 10.1007/s10803-013-1768-0 [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, & Mogil JS (2002). Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neuroscience and Biobehavioral Reviews, 26(8), 907–923. 10.1016/s0149-7634(02)00103-3 [DOI] [PubMed] [Google Scholar]
- Christoffersen MN (2019). Violent crime against children with disabilities: A nationwide prospective birth cohort-study. Child Abuse & Neglect, 98, 104150. 10.1016/j.chiabu.2019.104150 [DOI] [PubMed] [Google Scholar]
- Chu L-R, Garner JP, & Mench JA (2004). A behavioral comparison of New Zealand white rabbits (Oryctolagus cuniculus) housed individually or in pairs in conventional laboratory cages. Applied Animal Behaviour Science, 85(1–2), 121–139. 10.1016/j.applanim.2003.09.011 [DOI] [Google Scholar]
- Cohen IL, Schmidt-Lackner S, Romanczyk R, & Sudhalter V (2003). The PDD behavior inventory: A rating scale for assessing response to intervention in children with pervasive developmental disorder. Journal of Autism and Developmental Disorders, 33(1), 31–45. 10.1023/a:1022226403878 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). L. Erlbaum Associates. [Google Scholar]
- Constantino JN (2011). The quantitative nature of autistic social impairment. Pediatric Research, 69(5 Pt 2), 55R–62R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, & Reich W (2003). Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders, 33(4), 427–433. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social Responsiveness Scale, Second Edition (SRS-2). Western Psychological Services. [Google Scholar]
- Constantino JN, Lavesser PD, Zhang YI, Abbacchi AM, Gray T, & Todd RD (2007). Rapid quantitative assessment of autistic social impairment by classroom teachers. Journal of the American Academy of Child & Adolescent Psychiatry, 46(12), 1668–1676. 10.1097/chi.0b013e318157cb23 [DOI] [PubMed] [Google Scholar]
- Council NR (2011). Guide for the care and use of laboratory animals (8th ed.). The National Academies Press. [PubMed] [Google Scholar]
- Diguiseppi C, Levy SE, Sabourin KR, Soke GN, Rosenberg S, Lee L-C, Moody E, & Schieve LA (2018). Injuries in children with autism spectrum disorder: Study to explore early development (SEED). Journal of Autism and Developmental Disorders, 48(2), 461–472. 10.1007/s10803-017-3337-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G, Chen J, Zhang W, Yu B, Jin Y, Wang Y, & Yao M (2015). Physical maltreatment of children with autism in Henan province in China: A cross-sectional study. Child Abuse & Neglect, 48, 140–147. 10.1016/j.chiabu.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Feczko EJ, Bliss-Moreau E, Walum H, Pruett JR Jr., & Parr LA (2016). The macaque social responsiveness scale (mSRS): A rapid screening tool for assessing variability in the social responsiveness of rhesus monkeys (Macaca mulatta). PLoS One, 11(1), e0145956. 10.1371/journal.pone.0145956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festing MF (2014). Randomized block experimental designs can increase the power and reproducibility of laboratory animal experiments. ILAR Journal, 55(3), 472–476. 10.1093/ilar/ilu045 [DOI] [PubMed] [Google Scholar]
- Festing MF (2018). On determining sample size in experiments involving laboratory animals. Laboratory Animals, 52(4), 341–350. 10.1177/0023677217738268 [DOI] [PubMed] [Google Scholar]
- Fisher RA (1935). The design of experiments. Oliver & Boyd. [Google Scholar]
- Fluegge K (2017). Injury mortality in autism. American Journal of Public Health, 107(7), e3. 10.2105/AJPH.2017.303829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP (2014). The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR Journal, 55(3), 438–456. 10.1093/ilar/ilu047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JP, Gaskill BN, Weber EM, Ahloy-Dallaire J, & Pritchett-Corning KR (2017). Introducing therioepistemology: The study of how knowledge is gained from animal research. Lab Animal (NY), 46(4), 103–113. 10.1038/laban.1224 [DOI] [PubMed] [Google Scholar]
- Gaskill BN, & Garner JP (2020). Power to the people: Power, negative results and sample size. Journal of the American Association for Laboratory Animal Science, 59(1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli TA, & Wodzin E (2006). AIS 2005: A contemporary injury scale. Injury, 37(12), 1083–1091. 10.1016/j.injury.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, & Levitt P (2012). Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Research, 5(2), 101–108. 10.1002/aur.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, & McCowan B (2013). Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): Animal’s history, current environment, and personality. American Journal of Primatology, 75(10), 995–1008. 10.1002/ajp.22161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, del Rosso L, Sheikhi F, Gottlieb A, McCowan B, & Capitanio JP (2018). Personality, environmental stressors, and diarrhea in rhesus macaques: An interactionist perspective. American Journal of Primatology, 80(12), e22908. 10.1002/ajp.22908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafen A, & Hails R (2002). Modern statistics for the life sciences. Oxford University Press. [Google Scholar]
- Hand BN, Angell AM, Harris L, & Carpenter LA (2020). Prevalence of physical and mental health conditions in Medicare-enrolled, autistic older adults. Autism, 24(3), 755–764. 10.1177/1362361319890793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DW, & Kaufman J (2018). Adverse childhood experiences in children with autism spectrum disorder. Current Opinion in Psychiatry, 31(2), 128–132. 10.1097/YCO.0000000000000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Kim YS, Koh YJ, & Leventhal BL (2018). Autism spectrum disorder and school bullying: Who is the victim? Who is the perpetrator? Journal of Autism and Developmental Disorders, 48(1), 225–238. 10.1007/s10803-017-3285-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksen J, Bryn V, Diseth TH, Heiberg A, Schjølberg S, & Skjeldal OH (2013). Children with autism spectrum disorders – The importance of medical investigations. European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society, 17(1), 68–76. 10.1016/j.ejpn.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Jain A, Spencer D, Yang W, Kelly JP, Newschaffer CJ, Johnson J, Marshall J, Azocar F, Tabb LP, & Dennen T (2014). Injuries among children with autism spectrum disorder. Academic Pediatrics, 14(4), 390–397. 10.1016/j.acap.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Jernbro C, Bonander C, & Beckman L (2020). The association between disability and unintentional injuries among adolescents in a general education setting: Evidence from a Swedish population-based school survey. Disability and Health Journal, 13(1), 100841. 10.1016/j.dhjo.2019.100841 [DOI] [PubMed] [Google Scholar]
- Kalb LG, Vasa RA, Ballard ED, Woods S, Goldstein M, & Wilcox HC (2016). Epidemiology of injury-related emergency department visits in the US among youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(8), 2756–2763. 10.1007/s10803-016-2820-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Martinez SJ, Chun K, Capitanio JP, & Ceniceros LC (2019). Early social stress promotes inflammation and disease risk in rhesus monkeys. Scientific Reports, 9(1), 7609. 10.1038/s41598-019-43750-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, Bickel J, Wattanasin N, Spence S, Murphy S, & Churchill S (2012). The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One, 7(4), e33224. 10.1371/journal.pone.0033224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola I, & Landis J (2004). Can the pharmaceutical industry reduce attrition rates? Nature Reviews. Drug Discovery, 3(8), 711–715. 10.1038/nrd1470 [DOI] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, … Silberberg SD (2012). A call for transparent reporting to optimize the predictive value of preclinical research. Nature, 490 (7419), 187–191. 10.1038/nature11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LC, Harrington RA, Chang JJ, & Connors SL (2008). Increased risk of injury in children with developmental disabilities. Research in Developmental Disabilities, 29(3), 247–255. 10.1016/j.ridd.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Lewine JD, Andrews R, Chez M, Patil AA, Devinsky O, Smith M, Kanner A, Davis JT, Funke M, Jones G, Chong B, Provencal S, Weisend M, Lee RR, & Orrison WW (1999). Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics, 104(3), 405–418. 10.1542/peds.104.3.405 [DOI] [PubMed] [Google Scholar]
- Linden JB, McCowan B, Capitanio JP, & Isbell LA (2019). Male-inflicted wounds have opposite effects on hair cortisol for captive male and female rhesus macaques (Macaca mulatta) following new group formation. Primates, 60(1), 51–62. 10.1007/s10329-018-0703-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56(6), 466–474. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, & Novak M (2003). Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. American Journal of Primatology, 60(1), 1–15. 10.1002/ajp.10075 [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee LC, Harrington RA, Huston M, … Dietz PM. (2020). Prevalence of autism spectrum disorder among children aged 8 years — Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveillance Summaries, 69(4), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Walrath CM, Manteuffel B, Sgro G, & Pinto-Martin JA (2005). The prevalence and correlates of abuse among children with autism served in comprehensive community-based mental health settings. Child Abuse & Neglect, 29(12), 1359–1372. 10.1016/j.chiabu.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, Murray DS, Freedman B, & Lowery LA (2013). Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. Journal of Abnormal Child Psychology, 41(1), 165–176. 10.1007/s10802-012-9668-x [DOI] [PubMed] [Google Scholar]
- McCann SK, Cramond F, Macleod MR, & Sena ES (2016). Systematic review and meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke: an update. Translational Stroke Research, 7(5), 395–406. 10.1007/s12975-016-0489-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock K, Hall S, & Oliver C (2003). Risk markers associated with challenging behaviours in people with intellectual disabilities: A meta-analytic study. Journal of Intellectual Disability Research, 47(6), 405–416. 10.1046/j.1365-2788.2003.00517.x [DOI] [PubMed] [Google Scholar]
- McDermott S, Zhou L, & Mann J (2008). Injury treatment among children with autism or pervasive developmental disorder. Journal of Autism and Developmental Disorders, 38(4), 626–633. 10.1007/s10803-007-0426-9 [DOI] [PubMed] [Google Scholar]
- Mead R (1988). The design of experiments: Statistical principles for practical applications. Cambridge University Press. [Google Scholar]
- Muñoz-Yunta JA, Ortiz T, Palau-Baduell M, Martí-Muñoz L, Salvadó-Salvadó B, Valls-Santasusana A, … Dürsteler C. (2008). Magnetoencephalographic pattern of epileptiform activity in children with early-onset autism spectrum disorders, 119(3), 626–634. 10.1016/j.clinph.2007.11.007 [DOI] [PubMed] [Google Scholar]
- Muskens JB, Velders FP, & Staal WG (2017). Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: A systematic review. European Child & Adolescent Psychiatry, 26(9), 1093–1103. 10.1007/s00787-017-1020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E, Bernier RA, Tham SW, & Webb SJ (2018). Gastrointestinal and psychiatric symptoms among children and adolescents with autism Spectrum disorder. Frontiers in Psychiatry, 9, 515. 10.3389/fpsyt.2018.00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak MA (2003). Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment, 59(1), 3–19. 10.1002/ajp.10063 [DOI] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Constantino JN, & Parker KJ (2020). Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proceedings of the National Academy of Sciences, 117(19), 10609–10613. 10.1073/pnas.1919050117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztan O, Garner JP, Partap S, Sherr EH, Hardan AY, Farmer C, Thurm A, Swedo SE, & Parker KJ (2018). Cerebrospinal fluid vasopressin and symptom severity in children with autism. Annals of Neurology, 84(4), 611–615. 10.1002/ana.25314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Oztan O, Tarara ER, Li J, Sclafani V, del Rosso LA, Chun K, Berquist SW, Chez MG, Partap S, Hardan AY, Sherr EH, & Capitanio JP (2018). Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Science Translational Medicine, 10(439), eaam9100. 10.1126/scitranslmed.aam9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, Summers JE, Hinman KE, Motonaga KS, Phillips JM, Carson DS, Fung LK, Garner JP, & Hardan AY (2019). A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Science Translational Medicine, 11(491), eaau7356. 10.1126/scitranslmed.aau7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani A, Barcia G, Posar A, Raimondi E, Santucci M, & Scaduto MC (2010). Epilepsy and EEG paroxysmal abnormalities in autism spectrum disorders. Brain and Development, 32(9), 783–789. 10.1016/j.braindev.2010.07.003 [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ’t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, & Voytko ML. (2014). Why primate models matter. American Journal of Primatology, 76(9), 801–827. 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine E, Luby J, Abbacchi A, & Constantino JN (2006). Quantitative assessment of autistic symptomatology in preschoolers. Autism, 10(4), 344–352. 10.1177/1362361306064434 [DOI] [PubMed] [Google Scholar]
- Rattaz C, Michelon C, & Baghdadli A (2015). Symptom severity as a risk factor for self-injurious behaviours in adolescents with autism spectrum disorders. Journal of Intellectual Disability Research, 59(8), 730–741. 10.1111/jir.12177 [DOI] [PubMed] [Google Scholar]
- Restrepo B, Angkustsiri K, Taylor SL, Rogers SJ, Cabral J, Heath B, Hechtman A, Solomon M, Ashwood P, Amaral DG, & Nordahl CW (2020). Developmental–behavioral profiles in children with autism spectrum disorder and co-occurring gastrointestinal symptoms. Autism Research, 13, 1778–1789. 10.1002/aur.2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter SH, Garner JP, Auer C, Kunert J, & Würbel H (2010). Systematic variation improves reproducibility of animal experiments. Nature Methods, 7(3), 167–168. 10.1038/nmeth0310-167 [DOI] [PubMed] [Google Scholar]
- Richter SH, Garner JP, & Würbel H (2009). Environmental standardization: Cure or cause of poor reproducibility in animal experiments? Nature Methods, 6(4), 257–261. 10.1038/nmeth.1312 [DOI] [PubMed] [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, & McCowan B (2009). Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. Journal of Applied Animal Welfare Science, 12(1), 61–72. 10.1080/10888700802536798 [DOI] [PubMed] [Google Scholar]
- Rouse M (2010). SNOMED CT (Systematized Nomenclature of Medicine — Clinical Terms). SearchHealthIT https://searchhealthit.techtarget.com/definition/SNOMED-CT. [Google Scholar]
- Sclafani V, del Rosso LA, Seil SK, Calonder LA, Madrid JE, Bone KJ, Sherr EH, Garner JP, Capitanio JP, & Parker KJ (2016). Early predictors of impaired social functioning in male rhesus macaques (Macaca mulatta). PLoS One, 11(10), e0165401. 10.1371/journal.pone.0165401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PMW, Howells DW, & Macleod MR (2010). Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biology, 8(3), e1000344. 10.1371/journal.pbio.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Smith KA, Mazefsky C, Gabriels RL, Erickson C, Kaplan D, … Developmental Disorders Inpatient Research, C. (2015). The autism inpatient collection: Methods and preliminary sample description. Molecular Autism, 6, 61. 10.1186/s13229-015-0054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingsby B, Yatchmink Y, & Goldberg A (2017). Typical skin injuries in children with autism Spectrum disorder. Clinical Pediatrics (Phila), 56(10), 942–946. 10.1177/0009922817705187 [DOI] [PubMed] [Google Scholar]
- Sterzing PR, Shattuck PT, Narendorf SC, Wagner M, & Cooper BP (2012). Bullying involvement and autism Spectrum disorders. Archives of Pediatrics & Adolescent Medicine, 166(11), 1058–1064. 10.1001/archpediatrics.2012.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J, Shahrami A, Cali S, D’Mello C, Kako M, Palikucin-Reljin A, Savage M, Shaw O, & Lunsky Y (2017). Self-injury in autism spectrum disorder and intellectual disability: Exploring the role of reactivity to pain and sensory input. Brain Sciences, 7 (12), 140. 10.3390/brainsci7110140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot CF, Garner JP, Maness AC, McCowan B, Capitanio JP, & Parker KJ (2020). A psychometrically robust screening tool to rapidly identify socially impaired monkeys in the general population. Autism Research, 13(9), 1465–1475. 10.1002/aur.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, Thabane M, Giangregorio L, Dennis B, Kosa D, Debono VB, Dillenburg R, Fruci V, Bawor M, Lee J, Wells G, & Goldsmith CH (2013). A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Medical Research Methodology, 13(1), 92. 10.1186/1471-2288-13-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen-Soivio L, Vanhala R, Malm H, Leivonen S, Jokiranta E, Hinkka-Yli-Salomäki S, Gissler M, Brown AS, & Sourander A (2015). The association between congenital anomalies and autism spectrum disorders in a Finnish national birth cohort. Developmental Medicine & Child Neurology, 57(1), 75–80. 10.1111/dmcn.12581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleest JJ, Beisner BA, Hannibal DL, Nathman AC, Capitanio JP, Hsieh F, Atwill ER, & McCowan B (2016). Decoupling social status and status certainty effects on health in macaques: A network approach. PeerJ, 4, e2394. 10.7717/peerj.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey SH (1984). Dominance among rhesus monkeys. Political Psychology, 5(4), 623–628. 10.2307/3791232 [DOI] [Google Scholar]
- Viscidi EW, Triche EW, Pescosolido MF, McLean RL, Joseph RM, Spence SJ, & Morrow EM (2013). Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PLoS One, 8(7), e67797. 10.1371/journal.pone.0067797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra R, Madhavan S, & Sambamoorthi U (2016). Emergency department use among adults with autism spectrum disorders (ASD). Journal of Autism and Developmental Disorders, 46(4), 1441–1454. 10.1007/s10803-015-2692-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Tancredi DJ, & Thomas DW (2011). The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. Journal of Developmental & Behavioral Pediatrics, 32(5), 351–360. 10.1097/dbp.0b013e31821bd06a [DOI] [PubMed] [Google Scholar]
- Wasilewska J, & Klukowski M (2015). Gastrointestinal symptoms and autism spectrum disorder: Links and risks - a possible new overlap syndrome. Pediatric Health, Medicine and Therapeutics, 6, 153–166. 10.2147/PHMT.S85717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M (1999). Epidemiology: Study design and data analysis. Chapman & Hall/CRC Press. [Google Scholar]


