Abstract
Muscle fibers are syncytial postmitotic cells that can acquire exogenous nuclei from resident muscle stem cells, called satellite cells. Myonuclei are added to muscle fibers by satellite cells during conditions such as load-induced hypertrophy. It is difficult to dissect the molecular contributions of resident versus satellite cell-derived myonuclei during adaptation due to the complexity of labeling distinct nuclear populations in multinuclear cells without label transference between nuclei. To sidestep this barrier, we used a genetic mouse model where myonuclear DNA can be specifically and stably labeled via nonconstitutive H2B-GFP at any point in the lifespan. Resident myonuclei (Mn) were GFP-tagged in vivo before 8 wk of progressive weighted wheel running (PoWeR) in adult mice (>4-mo-old). Resident + satellite cell-derived myonuclei (Mn+SC Mn) were labeled at the end of PoWeR in a separate cohort. Following myonuclear isolation, promoter DNA methylation profiles acquired with low-input reduced representation bisulfite sequencing (RRBS) were compared to deduce epigenetic contributions of satellite cell-derived myonuclei during adaptation. Resident myonuclear DNA has hypomethylated promoters in genes related to protein turnover, whereas the addition of satellite cell-derived myonuclei shifts myonuclear methylation profiles to favor transcription factor regulation and cell-cell signaling. By comparing myonucleus-specific methylation profiling to previously published single-nucleus transcriptional analysis in the absence (Mn) versus the presence of satellite cells (Mn+SC Mn) with PoWeR, we provide evidence that satellite cell-derived myonuclei may preferentially supply specific ribosomal proteins to growing myofibers and retain an epigenetic “memory” of prior stem cell identity. These data offer insights on distinct epigenetic myonuclear characteristics and contributions during adult muscle growth.
Keywords: DNA methylation, hypertrophy, RRBS, satellite cells
INTRODUCTION
The presence of Pax7+ muscle stem cells (referred to as satellite cells) alters muscle transcriptional profiles in response to loading (1, 2). Using a Pax7-driven inducible then constitutive reporter, fluorescent-activated sorting, and single cell/nucleus RNA-sequencing, preliminary evidence suggests that muscle fiber nuclei newly acquired from satellite cells (myonuclei coexpressing the reporter and myosin transcripts) may have a unique nascent transcriptional profile after fusing to adult muscle fibers during short-term, load-induced hypertrophy (3). Otherwise, little is known about how satellite cell-derived myonuclei that integrate into multinuclear muscle cells coalesce with preexisting myonuclei to regulate long-term myofiber homeostasis in vivo. The reason for this lack of knowledge is largely technical. For epigenetic profiling, labeling and long-term tracking of resident and externally sourced nuclei in an in vivo syncytium cannot be accomplished without inducible, nucleus-associated, lineage-restricted reporters where the fluorescence tag is nonconstitutive and nontransferable between cells and nuclei.
Using the muscle fiber-specific human skeletal actin promoter Tet-ON system to drive histone 2B green fluorescent protein (HSA-GFP) mouse (4), we can fluorescently tag myonuclei with high specificity before (i.e., resident myonuclei) and following a perturbation (i.e., resident + satellite cell-derived myonuclei). GFP integration into nucleosomes is stable and occurs only during the specified labeling period, and the vast majority of myonuclei acquired during muscle hypertrophy are presumed to be from satellite cells (5, 6). In the current investigation, we fluorescently labeled myonuclei at the beginning and at the end of progressive weighted wheel running (PoWeR, a potent skeletal muscle hypertrophic and metabolic stimulus for mice that results in myonuclear accretion; 7, 8) and purified them via fluorescent-activated myonuclear sorting (FAMnS) for DNA methylation analysis. With this strategy, resident myonuclei present at the onset of training that persisted throughout the training period were labeled with GFP in the first group (Mn), whereas the entire post-training myonuclear pool (resident myonuclei present at the onset of training plus satellite cell-derived myonuclei acquired throughout training) were GFP-labeled in the latter group (Mn+SC Mn); the contribution of satellite cell-derived myonuclei can therefore be deduced by comparing these two groups. We then collated global myonuclear DNA methylome profiles to previously published single nucleus RNA-sequencing (snRNA-seq) data from PoWeR mice in the absence (Mn) versus the presence of satellite cells (Mn+SC Mn) (1, 9). This study provides initial evidence for how myonuclei acquired from stem cells during muscle remodeling may contribute to long-term adaptations in postmitotic muscle fibers and influence global methylation profiles that are known to be altered by hypertrophic exercise training in humans (10–12).
METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Kentucky. Mice were housed in a temperature and humidity-controlled room, maintained on a 14:10-h light-dark cycle, and food and water were provided ad libitum. Animals were euthanized via a lethal dosage of sodium pentobarbital injected intraperitoneally followed by cervical dislocation.
Male HSA-rtTA+/−-H2B-GFP+/− (HSA-GFP) mice were generated as previously described by us (4, 7, 9, 13). HSA-GFP mice were aged until 4 mo, once developmental muscle growth had ceased and resident myonuclei were largely established (6). One cohort of adult mice were treated with low-dose doxycycline (0.5 mg/mL doxycycline in drinking water with 2% sucrose) during unweighted wheel acclimation before 8 wk of voluntary PoWeR exercise (biological triplicate), whereas a second group identically engaged in PoWeR but received doxycycline for 72 h during the last week of training (biological triplicate), as described previously in Wen et al. (7). Expression of histone-bound GFP only occurs in the presence of doxycycline, and the half-life of doxycycline in mice is a matter of hours. Wheel running performance between groups was similar and is reported elsewhere (7). After 8 wk of PoWeR, myonuclei from plantaris muscles were processed following a 48-h wheel lock and overnight fast, purified via FAMnS, then analyzed using myonucleus-specific low-input RRBS, as described by us (7, 13, 14) (Fig. 1A).
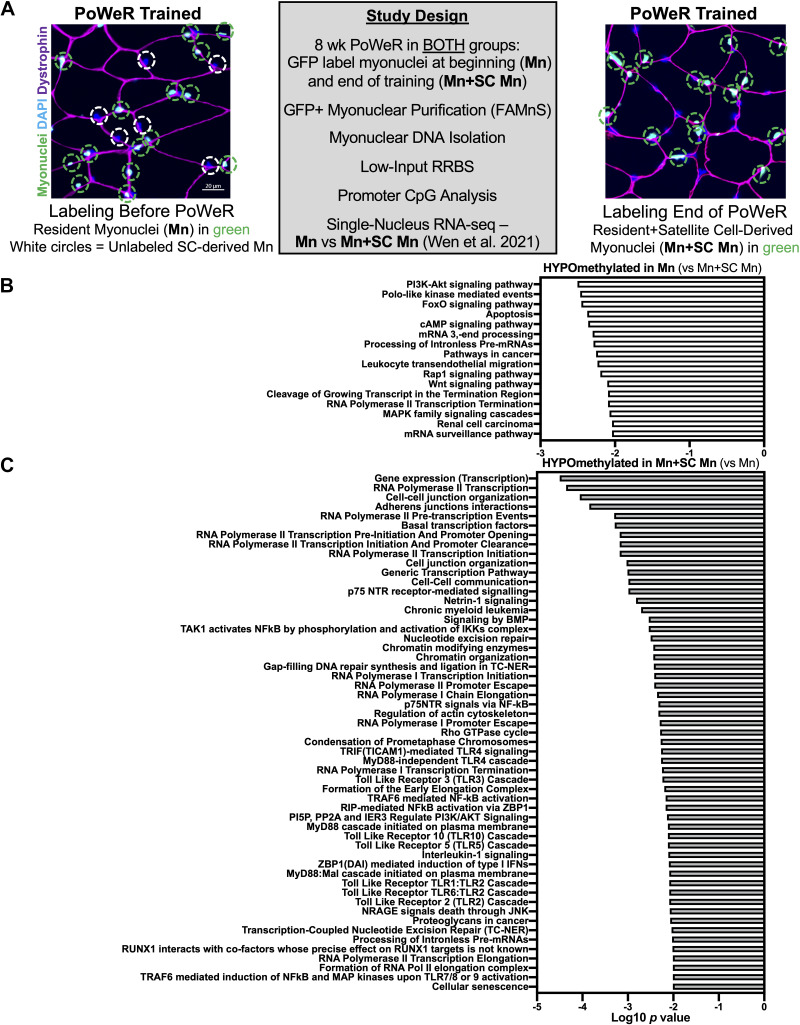
Figure 1.
Global methylation differences in resident myonuclei (Mn) versus resident + satellite cell-derived myonuclei (Mn+SC Mn) after 8 wk of progressive weighted wheel running (PoWeR). A: study design and fluorescent histochemistry representative images on 8-wk PoWeR plantaris muscle cross sections in HSA-GFP mice treated at the beginning versus the end of PoWeR; green circles denote GFP-labeled myonuclei (Mn), and white circles denote myonuclei acquired by satellite cells (SC) during PoWeR that were not labeled at the end of training. B: pathway over-representation analysis of hypomethylated promoters in Mn versus Mn+SC Mn following PoWeR. C: pathway over-representation analysis of hypomethylated promoters in Mn+SC Mn vs. Mn following PoWeR. n = 3 males per group. HSA-GFP, histone 2B green fluorescent protein; Mn, myonuclei; SC, satellite cells.
Fluorescent histochemistry for representative images from plantaris muscles (Fig. 1A) was performed as previously described (7). Frozen plantaris muscles were Dounce homogenized in a physiological buffer and GFP/propidium iodide-labeled myonuclei were sorted via FAMnS (7, 13, 14). The magnitude of myonuclear accretion previously reported by us with PoWeR using orthogonal techniques (1, 15) was greater via FAMnS when comparing Mn+SC Mn to Mn samples (127,322 ± 13,097 vs. 51,825 ± 16,011, respectively, means ± SD, from similar amounts of starting material). The difference could be 1) methodological (FAMnS vs. histochemistry and single fiber analyses), 2) biological secondary to some loss of GFP signal from histone turnover that may occur during exercise training (16), and/or 3) technical due to the FAMnS gating strategy (preference for the brightest GFP+ myonuclei). Myonuclear DNA was extracted using the Qiagen DNA MicroKit (Hilden, Germany) with carrier RNA, and low-input Msp1 RRBS was performed by Zymo Research using 6 ng of genomic DNA (Irvine, CA) (7, 13). RRBS data were processed using MethylSig (17), with a minimum cutoff of 10 times coverage per CpG site in each sample (7). Promoters were defined as within 1 kb upstream of the transcription start site (7). Ribosomal DNA (rDNA) methylation was mapped and quantified as previously described by us (14). Differentially methylated sites were determined using a β binomial distribution and a false discovery rate (FDR) of <0.05, as determined by the Benjamini–Hochberg method. Pathway analysis was conducted using ConcensusPathDB with KEGG and Reactome databases using default settings (18). Single nucleus RNA-sequencing data from 4-wk PoWeR plantar flexor muscles (soleus) in the presence and absence of satellite cells (depletion initiated at 4 mo of age in female Pax7-DTA mice) after a 24-h wheel lock and overnight fast was from our previous investigations (1, 9). Heatmaps were generated using GraphPad Prism.
RESULTS
At the pathway level in Mn, promoters of genes in PI3K-AKT, FoxO, Wnt, and cancer pathways were hypomethylated relative to Mn+SC Mn (Fig. 1B); these included at least one CpG site in Atg12, Fgf15, Flt1, Gadd45b, Hey1, Rac1, and Wnt3 (FDR < 0.05). Conversely, relative to Mn, Mn+SC Mn displayed hypomethylation in promoters of genes associated with gene expression (e.g., transcription factors), as well as genes related to cell-cell junctions and communication (Fig. 1C); these included at least one CpG in Btg2; Cdh1, 2, and 3; Cxcl3; Drosha; Gata3; Myc; Rybp; Ski; Smad4, 5, and 6; and Timp3 (FDR < 0.05). Satellite cell activation is associated with widespread alterations in transcription factor composition (19, 20) as well as cell–cell communication factors (2, 3, 21, 22). Myogenic progenitor cells can also have a long-term DNA methylation “memory” of prior environmental exposures (23), so the contribution of satellite cell–derived myonuclear methylation profiles to total myonuclear methylation profiles after PoWeR could be partly attributable to a residual satellite cell identity. A promoter region of Hoxa6 was hypomethylated in Mn+SC Mn relative to Mn (3 CpG sites, 32% versus 9% on average across sites, range 76%–0%, respectively, FDR < 0.05). The consequences of this are unclear, but emerging evidence suggests the Hoxa6 promoter is hypermethylated in cancer cells (24, 25), and Hox genes appear highly regulated at the methylation level in muscle (26), specifically with exercise and aging (27, 28).
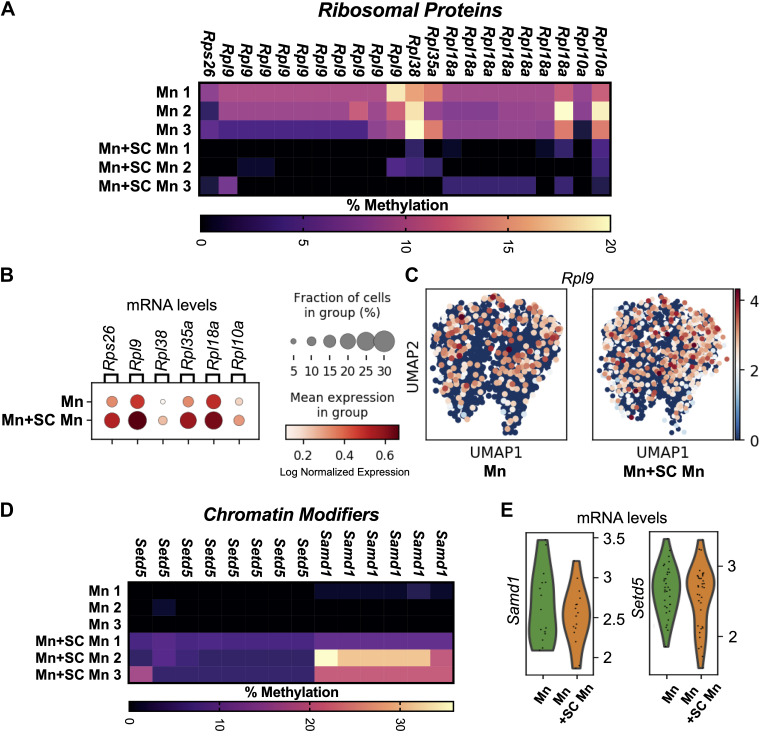
We recently reported that promoter regions across an array of ribosomal proteins were hypermethylated in Mn after PoWeR relative to sedentary age-matched controls (7). In Mn+SC Mn, the methylation signal was diluted and CpGs in promoters of ribosomal proteins were shifted to be relatively hypomethylated compared to resident myonuclei after training (FDR < 0.05); these included Rps26, Rpl10a, Rpl18a, Rpl35a, Rpl38, and 10 CpGs in Rpl9 (Fig. 2A). Ribosomal protein promoters are CG-rich (29, 30), and promoter CpG methylation regulates transcription of ribosomal proteins (31). Furthermore, a cluster of CpGs around the rDNA 28S transcription termination area was hypermethylated (sites 12946, 12993, 12998, 13070, and 13080, 20% vs. 11% average across sites, respectively, FDR < 0.05), whereas CpG site 43510 in the rDNA enhancer region was hypomethylated (49% vs. 27%, respectively, FDR < 0.05) in Mn+SC Mn versus Mn. Coordinated methylation of ribosomal proteins and rDNA may indicate distinct ribosome biogenesis regulation between myonuclear populations. Satellite cell fusion is complete after 4 wk of PoWeR (1). We recently reported plantar flexor muscle snRNA-seq data following 4 wk of PoWeR in the absence versus presence of satellite cells using the Pax7-DTA satellite cell depletion model (1, 9); this can be leveraged as a method to explore adaptations in Mn versus Mn+SC Mn, respectively. snRNA-seq revealed that Mn+SC Mn had significantly higher levels of Rps26 (Log2FC = 0.79, FDR = 0.027), Rpl35a (Log2FC = 0.96, FDR = 0.0005), and Rpl9 (Log2FC = 0.63, FDR = 0.035) relative to Mn, with Rpl9 having the highest expression (Fig. 1, B and C); higher mRNA corresponds with promoter region hypomethylation. Collectively, ribosomal proteins delivered by satellite cell fusion may support muscle fiber adaptation, which is attenuated in the absence of satellite cells beyond 4 wk (1, 21, 32, 33).
Figure 2.
Differential promoter DNA methylation and gene expression in resident myonuclei (Mn) vs. resident + satellite cell-derived myonuclei (Mn+SC Mn) following progressive weighted wheel running (PoWeR). A: DNA methylation of promoter CpGs in ribosomal protein genes in resident myonuclei (Mn) and resident + satellite cell-derived myonuclei (Mn+SC Mn) (FDR < 0.05); a gene listed multiple times = multiple distinct promoter CpGs. B: ribosomal protein gene expression from skeletal muscle snRNA-sequencing (myosin heavy chain-expressing nuclei) following 4 wk of PoWeR in the soleus in the absence of satellite cells (Mn) and the presence of satellite cells (Mn+SC Mn), data from Wen et al. (9). C: Uniform Manifold Approximation and Projection (UMAP) for dimension reduction plots showing expression of RPL9 with PoWeR (FDR < 0.05) in myonuclei, which had promoter-wide hypomethylation differences in Mn+SC Mn vs. Mn. D: myonuclear promoter DNA methylation of Samd1 and Setd5 (FDR < 0.05); a gene listed multiple times = multiple distinct promoter CpGs. E: Single myonucleus gene expression of epigenetic regulators Samd1 and Setd5 after PoWeR; data from Wen et al. (9). n = 3 males per group for methylation analyses, and N = 1 females per group for snRNA-seq. FDR, false discovery rate; Mn, myonuclei; SC, satellite cells; snRNA, single nucleus RNA.
Mn had >5 hypomethylated promoter CpGs in Samd1 and Setd5 relative to Mn+SC Mn (FDR < 0.05, Fig. 1D). Both were relatively higher at the transcript level in Mn by 4 wk of PoWeR (Log2FC = 0.45 and 0.39, respectively), but this was not statistically significant (Fig. 1E). Samd1 (34, 35), and Setd5 (36, 37) were recently identified as powerful chromatin modifiers. The SETD family member Setd3 was also hypomethylated with resistance training and retraining in human muscle tissue concomitant with increased gene expression (11). Again, this differential methylation may be suggestive of specialized roles for distinct myonuclear pools during long-term adult muscle fiber adaptation. Other chromatin modifying enzymes had at least one hypermethylated promoter region CpG in Mn relative to Mn+SC Mn (Arid2, Kdmb3, Kdm4b, Phf2, Prmt7, Rbb4, Rela, and Smarce1). In resident myonuclei, relative promoter hypermethylation across an array of chromatin-modifying genes and more targeted hypomethylation is consistent with the hypothesis that trained myonuclei may underpin the more “refined” transcriptional response to exercise observed in well-conditioned muscle (7, 38–40).
DISCUSSION
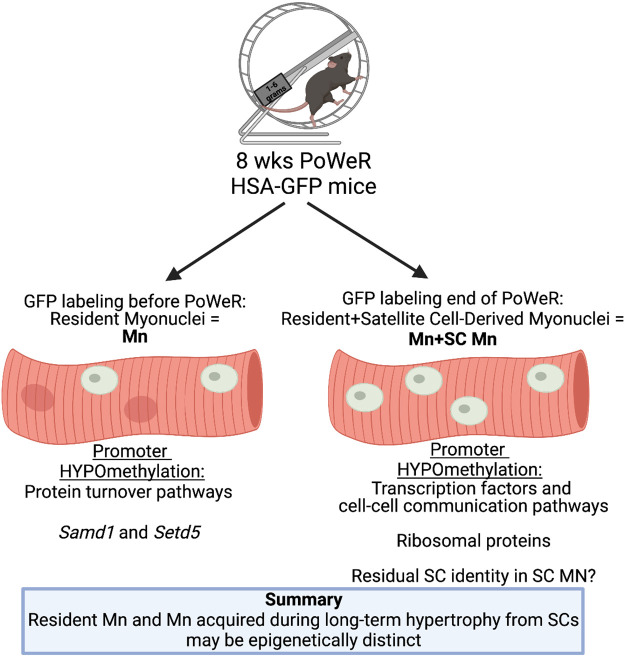
This investigation revealed: 1) epigenetic profiles of Mn versus Mn+SC Mn after PoWeR appear distinct, likely due to the contribution of satellite cell fusion in the latter; 2) resident myonuclei seem geared toward anabolic/catabolic processes, consistent with evidence suggesting that these nuclei are principal drivers of hypertrophic processes in adult muscle (7, 22), whereas satellite cell-derived myonuclei may be epigenetically programmed for transcription factor regulation and cell-cell communication; 3) promoter CpG methylation may relate to transcription of ribosomal proteins; 4) satellite cell-derived myonuclei could support growth processes by contributing ribosomal macromolecular components to myofibers; and 5) myonuclei acquired by satellite cells during exercise may have a methylation memory of their pre-myonuclear identity (Fig. 3).
Figure 3.
Summary of promoter region methylation results from the current investigation. Mn, myonuclei; PoWeR, progressive weighted wheel running; SC, satellite cells. Figure created with BioRender.com.
Ribosomal proteins are preferentially synthesized in growing cells (41), and the stoichiometry of ribosomal proteins correlates with growth rate (42). Manipulation of specific ribosomal proteins influences numerous aspects of anabolism (43), which implicates them as regulators of muscle cell size and function via ribosome specialization (44, 45) and/or ribosome biogenesis (46). For instance, Rpl9 knockout inhibits cell growth via inactivation of Nf-κB in cancer cells (47), whereas Rpl9 mutations influence ribosome biogenesis and cellular metabolism (48). Rpl35a is hypomethylated and over-expressed in human muscle tissue following resistance training and retraining (11), perhaps indicative of satellite cell activity and/or fusion. Nuclear domains in myotubes may depend on ribosome localization and accessibility (49). The influx of specific ribosomal proteins (and other factors) from a subset of myonuclei donated by satellite cells during exercise-induced adult muscle fiber growth may therefore influence the long-term maintenance of myonuclear domains (1, 3, 9, 22). In light of our findings, the possibility of distinct myonuclear contributions from satellite cell-derived myonuclei at different phases of adaptation deserves further consideration (50). Lastly, ribosomal proteins are markedly higher in myoblasts versus myotubes (51) and produced in relative excess in mammalian myogenic cells upon terminal differentiation (52–54); these in vitro data provide additional evidence of a residual stem cell identity in satellite cell-derived myonuclei and lend support to our in vivo observations.
We provide the first insights on molecular contributions of resident versus satellite cell-derived myonuclei to long-term adult muscle fiber adaptations, but our study is not without limitations. Some GFP signal in resident myonuclei may had been lost during training due to histone replacement (16), so epigenetic differences in Mn+SC Mn versus Mn could be partially attributable to a subset of resident myonuclei with high histone turnover and diminished label. Genomic coverage using low-input myonuclear RRBS is also somewhat limited, so advancements in DNA methylation detection technology will help elucidate the epigenetic contributions of distinct nuclear populations with greater resolution. Future investigations using satellite cell-specific Tet-On systems (55), nucleus-specific genetic reporters, snRNA-seq, and myonuclear epigenetic analyses such as whole-genome bisulfite sequencing and ATAC-seq will provide more detailed information on whether satellite cell-derived myonuclei acquired during adult muscle fiber hypertrophy retain a memory of satellite cell identity, and how they contribute to prolonged adaptation. Differential contributions of endogenous versus externally sourced myonuclei to muscle adaptation could also have consequences for the design and interpretation of long-term muscle epigenetic investigations aimed at evaluating a global “muscle memory” of prior training (7, 10, 11), where myonuclear number may fluctuate over time (8, 15, 56).
DATA AVAILABILITY
RRBS data are deposited in GEO GSE180433. Processed data are available upon reasonable request.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grant K99/R00 AG063994 (to K.A.M). Funding was also provided by NIH grants AR060701 and DK119619 (via John McCarthy and Charlotte Peterson of the University of Kentucky Center for Muscle Biology).
DISCLOSURES
Y.W. is sole proprietor of Myoanalytics LLC, and he has been providing muscle image analysis consultation for the University of Alabama in the last year. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
K.A.M. conceived and designed research; K.A.M., C.M.D., F.v.W., and Y.W. performed experiments; K.A.M. and Y.W. analyzed data; K.A.M. interpreted results of experiments; K.A.M. prepared figures; K.A.M. and Y.W. drafted manuscript; K.A.M., C.M.D., F.v.W., and Y.W. edited and revised manuscript; K.A.M., C.M.D., F.v.W., and Y.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Keith Booher of Zymo Research for technical assistance, Jennifer Strange of the University of Kentucky Flow Cytometry Core for expertise in FAMnS, Drs. Davis Englund and Bailey Peck for carrying out the previously published snRNA-seq experiments (GSE 163207 from Wen et al. 9), Taylor Valentino and Dr. Brooks Mobley for assistance with mouse colony management, and Drs. John McCarthy and Charlotte Peterson of the University of Kentucky Center for Muscle Biology for support and encouragement as well as grant funding.
REFERENCES
- 1.Englund DA, Figueiredo VC, Dungan CM, Murach KA, Peck BD, Petrosino JM, Brightwell CR, Dupont AM, Neal AC, Fry CS, Accornero F, McCarthy JJ, Peterson CA. Satellite cell depletion disrupts transcriptional coordination and muscle adaptation to exercise. Function (Oxf) 2: zqaa033, 2021. doi: 10.1093/function/zqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murach KA, Vechetti IJ Jr, Van Pelt DW, Crow SE, Dungan CM, Figueiredo VC, Kosmac K, Fu X, Richards CI, Fry CS, McCarthy JJ, Peterson CA. Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy. Function (Oxf) 1: zqaa009, 2020. doi: 10.1093/function/zqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murach KA, Peck BD, Policastro RA, Vechetti IJ, Van Pelt DW, Dungan CM, Denes LT, Fu X, Brightwell CR, Zentner GE, Dupont-Versteegden EE, Richards CI, Smith JJ, Fry CS, McCarthy JJ, Peterson CA. Early satellite cell communication creates a permissive environment for long-term muscle growth. iScience 24: 102372, 2021. doi: 10.1016/j.isci.2021.102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwata M, Englund DA, Wen Y, Dungan CM, Murach KA, Vechetti IJ Jr, Mobley CB, Peterson CA, McCarthy JJ. A novel tetracycline-responsive transgenic mouse strain for skeletal muscle-specific gene expression. Skelet Muscle 8: 33, 2018. doi: 10.1186/s13395-018-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murach KA, White SH, Wen Y, Ho A, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet Muscle 7: 14, 2017. doi: 10.1186/s13395-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen Y, Dungan CM, Mobley CB, Valentino T, von Walden F, Murach KA. Nucleus type-specific DNA methylomics reveals epigenetic “memory” of prior adaptation in skeletal muscle. Function (Oxf) 2: zqab038, 2021. doi: 10.1093/function/zqab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murach KA, Mobley CB, Zdunek CJ, Frick KK, Jones SR, McCarthy JJ, Peterson CA, Dungan CM. Muscle memory: myonuclear accretion, maintenance, morphology, and miRNA levels with training and detraining in adult mice. J Cachexia Sarcopenia Muscle 11: 1705–1722, 2020. doi: 10.1002/jcsm.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen Y, Englund DA, Peck BD, Murach KA, McCarthy JJ, Peterson CA. Myonuclear transcriptional dynamics in response to exercise following satellite cell depletion. iScience 24: 102838, 2021. doi: 10.1016/j.isci.2021.102838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner DC, Seaborne RA, Sharples AP. Comparative transcriptome and methylome analysis in human skeletal muscle anabolism, hypertrophy and epigenetic memory. Sci Rep 9: 4251, 2019. doi: 10.1038/s41598-019-40787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seaborne RA, Strauss J, Cocks M, Shepherd S, O'Brien TD, van Someren KA, Bell PG, Murgatroyd C, Morton JP, Stewart CE, Sharples AP. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci Rep 8: 1898, 2018. doi: 10.1038/s41598-018-20287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharples AP, Seaborne RA. Exercise and DNA methylation in skeletal muscle. In: Sports, Exercise, and Nutritional Genomics: Current Status and Future Directions, edited by Barh D, Ahmetov I.. London: Elsevier, 2019, p. 211–229. [Google Scholar]
- 13.von Walden F, Rea M, Mobley CB, Fondufe-Mittendorf Y, McCarthy JJ, Peterson CA, Murach KA. The myonuclear DNA methylome in response to an acute hypertrophic stimulus. Epigenetics 15: 1151–1162, 2020. doi: 10.1080/15592294.2020.1755581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueiredo VC, Wen Y, Alkner B, Fernandez-Gonzalo R, Norrbom J, Vechetti IJ Jr, Valentino T, Mobley CB, Zentner GE, Peterson CA, McCarthy JJ, Murach KA, von Walden F. Genetic and epigenetic regulation of skeletal muscle ribosome biogenesis with exercise. J Physiol 599: 3363–3384, 2021. doi: 10.1113/JP281244. [DOI] [PubMed] [Google Scholar]
- 15.Dungan CM, Murach KA, Frick KK, Jones SR, Crow SE, Englund DA, Vechetti IJ Jr, Figueiredo VC, Levitan BM, Satin J, McCarthy JJ, Peterson CA. Elevated myonuclear density during skeletal muscle hypertrophy in response to training is reversed during detraining. Am J Physiol Cell Physiol 316: C649–C654, 2019. doi: 10.1152/ajpcell.00050.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohsawa I, Kawano F. Chronic exercise training activates histone turnover in mouse skeletal muscle fibers. FASEB J 35: e21453, 2021. doi: 10.1096/fj.202002027RR. [DOI] [PubMed] [Google Scholar]
- 17.Park Y, Figueroa ME, Rozek LS, Sartor MA. MethylSig: a whole genome DNA methylation analysis pipeline. Bioinformatics 30: 2414–2422, 2014. doi: 10.1093/bioinformatics/btu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herwig R, Hardt C, Lienhard M, Kamburov A. Analyzing and interpreting genome data at the network level with ConsensusPathDB. Nat Protoc 11: 1889–1907, 2016. doi: 10.1038/nprot.2016.117. [DOI] [PubMed] [Google Scholar]
- 19.Machado L, Esteves de Lima J, Fabre O, Proux C, Legendre R, Szegedi A, Varet H, Ingerslev LR, Barrès R, Relaix F, Mourikis P. In situ fixation redefines quiescence and early activation of skeletal muscle stem cells. Cell Rep 21: 1982–1993, 2017. doi: 10.1016/j.celrep.2017.10.080. [DOI] [PubMed] [Google Scholar]
- 20.van Velthoven CTJ, de Morree A, Egner IM, Brett JO, Rando TA. Transcriptional profiling of quiescent muscle stem cells in vivo. Cell Rep 21: 1994–2004, 2017. doi: 10.1016/j.celrep.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20: 56–69, 2017. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murach KA, Fry CS, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Fusion and beyond: satellite cell contributions to loading‐induced skeletal muscle adaptation. FASEB J 35: e21893, 2021. doi: 10.1096/fj.202101096R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharples AP, Polydorou I, Hughes DC, Owens DJ, Hughes TM, Stewart CE. Skeletal muscle cells possess a “memory” of acute early life TNF-α exposure: role of epigenetic adaptation. Biogerontology 17: 603–617, 2016. doi: 10.1007/s10522-015-9604-x. [DOI] [PubMed] [Google Scholar]
- 24.Li D, Bai Y, Feng Z, Li W, Yang C, Guo Y, Lin C, Zhang Y, He Q, Hu G, Li X. Study of promoter methylation patterns of HOXA2, HOXA5, and HOXA6 and its clinicopathological characteristics in colorectal cancer. Front Oncol 9: 394, 2019. doi: 10.3389/fonc.2019.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishida Y, Natsume A, Kondo Y, Takeuchi I, An B, Okamoto Y, Shinjo K, Saito K, Ando H, Ohka F, Sekido Y, Wakabayashi T. Epigenetic subclassification of meningiomas based on genome-wide DNA methylation analyses. Carcinogenesis 33: 436–441, 2012. doi: 10.1093/carcin/bgr260. [DOI] [PubMed] [Google Scholar]
- 26.Tsumagari K, Baribault C, Terragni J, Chandra S, Renshaw C, Sun Z, Song L, Crawford GE, Pradhan S, Lacey M, Ehrlich M. DNA methylation and differentiation: HOX genes in muscle cells. Epigenetics Chromatin 6: 25, 2013. doi: 10.1186/1756-8935-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voisin S, Jacques M, Landen S, Harvey NR, Haupt LM, Griffiths LR, Gancheva S, Ouni M, Jähnert M, Ashton KJ, Coffey VG, Thompson J‐LM, Doering TM, Gabory A, Junien C, Caiazzo R, Verkindt H, Raverdy V, Pattou F, Froguel P, Craig JM, Blocquiaux S, Thomis M, Sharples AP, Schürmann A, Roden M, Horvath S, Eynon N. Meta‐analysis of genome‐wide DNA methylation and integrative omics of age in human skeletal muscle. J Cachexia Sarcopenia Muscle 12: 1064–1078, 2021. doi: 10.1002/jcsm.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner DC, Gorski PP, Maasar MF, Seaborne RA, Baumert P, Brown AD, Kitchen MO, Erskine RM, Dos-Remedios I, Voisin S, Eynon N, Sultanov RI, Borisov OV, Larin AK, Semenova EA, Popov DV, Generozov EV, Stewart CE, Drust B, Owens DJ, Ahmetov II, Sharples AP. DNA methylation across the genome in aged human skeletal muscle tissue and muscle-derived cells: the role of HOX genes and physical activity. Sci Rep 10: 15360, 2020. doi: 10.1038/s41598-020-72730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roepcke S, Zhi D, Vingron M, Arndt PF. Identification of highly specific localized sequence motifs in human ribosomal protein gene promoters. Gene 365: 48–56, 2006. doi: 10.1016/j.gene.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 30.Yoshihama M, Uechi T, Asakawa S, Kawasaki K, Kato S, Higa S, Maeda N, Minoshima S, Tanaka T, Shimizu N, Kenmochi N. The human ribosomal protein genes: sequencing and comparative analysis of 73 genes. Genome Res 12: 379–390, 2002. doi: 10.1101/gr.214202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahpour A, Scruggs BS, Smiraglia D, Ouchi T, Gelman IH. A methyl-sensitive element induces bidirectional transcription in tata-less CpG island-associated promoters. PloS One 13: e0205608, 2018. doi: 10.1371/journal.pone.0205608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Englund DA, Murach KA, Dungan CM, Figueiredo VC, Vechetti IJ Jr, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle hypertrophy in response to lifelong physical activity. Am J Physiol Cell Physiol 318: C1178–C1188, 2020. doi: 10.1152/ajpcell.00090.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stielow B, Zhou Y, Cao Y, Simon C, Pogoda H-M, Jiang J, Ren Y, Phanor SK, Rohner I, Nist A, Stiewe T, Hammerschmidt M, Shi Y, Bulyk ML, Wang Z, Liefke R. The SAM domain-containing protein 1 (SAMD1) acts as a repressive chromatin regulator at unmethylated CpG islands. Sci Adv 7: eabf2229, 2021. doi: 10.1126/sciadv.abf2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stielow B, Simon C, Liefke R. Making fundamental scientific discoveries by combining information from literature, databases, and computational tools – an example. Comput Struct Biotechnol J 19: 3027–3033, 2021. doi: 10.1016/j.csbj.2021.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sessa A, Fagnocchi L, Mastrototaro G, Massimino L, Zaghi M, Indrigo M, Cattaneo S, Martini D, Gabellini C, Pucci C, Fasciani A, Belli R, Taverna S, Andreazzoli M, Zippo A, Broccoli V. SETD5 regulates chromatin methylation state and preserves global transcriptional fidelity during brain development and neuronal wiring. Neuron 104: 271–289. e13, 2019. doi: 10.1016/j.neuron.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Hausmann S, Lyu R, Li T-M, Lofgren SM, Flores NM, Fuentes ME, Caporicci M, Yang Z, Meiners MJ, Cheek MA, Howard SA, Zhang L, Elias JE, Kim MP, Maitra A, Wang H, Bassik MC, Keogh M-C, Sage J, Gozani O, Mazur PK. SETD5-coordinated chromatin reprogramming regulates adaptive resistance to targeted pancreatic cancer therapy. Cancer Cell 37: 834–849.e13, 2020. doi: 10.1016/j.ccell.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE, Gordon PM. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (1985) 116: 693–702, 2014. doi: 10.1152/japplphysiol.01366.2013. [DOI] [PubMed] [Google Scholar]
- 39.Schmutz S, Däpp C, Wittwer M, Vogt M, Hoppeler H, Flück M. Endurance training modulates the muscular transcriptome response to acute exercise. Pflugers Arch 451: 678–687, 2006. doi: 10.1007/s00424-005-1497-0. [DOI] [PubMed] [Google Scholar]
- 40.Damas F, Ugrinowitsch C, Libardi CA, Jannig PR, Hector AJ, McGlory C, Lixandrão ME, Vechin FC, Montenegro H, Tricoli V, Roschel H, Phillips SM. Resistance training in young men induces muscle transcriptome-wide changes associated with muscle structure and metabolism refining the response to exercise-induced stress. Eur J Appl Physiol 118: 2607–2616, 2018. doi: 10.1007/s00421-018-3984-y. [DOI] [PubMed] [Google Scholar]
- 41.Tushinski RJ, Warner JR. Ribosomal proteins are synthesized preferentially in cells commencing growth. J Cell Physiol 112: 128–135, 1982. doi: 10.1002/jcp.1041120119. [DOI] [PubMed] [Google Scholar]
- 42.Slavov N, Semrau S, Airoldi E, Budnik B, van Oudenaarden A. Differential stoichiometry among core ribosomal proteins. Cell Rep 13: 865–873, 2015. doi: 10.1016/j.celrep.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Liao W-J, Liao J-M, Liao P, Lu H. Ribosomal proteins: functions beyond the ribosome. J Mol Cell Biol 7: 92–104, 2015. doi: 10.1093/jmcb/mjv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaillou T. Ribosome specialization and its potential role in the control of protein translation and skeletal muscle size. J Appl Physiol (1985) 127: 599–607, 2019. doi: 10.1152/japplphysiol.00946.2018. [DOI] [PubMed] [Google Scholar]
- 45.Jiao J, Kavdia K, Pagala V, Palmer L, Finkelstein D, Fan Y, Peng J, Demontis F. An age-downregulated ribosomal RpS28 protein variant regulates the muscle proteome. G3 (Bethesda) 11: jkab165, 2021. doi: 10.1093/g3journal/jkab165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen Y, Alimov AP, McCarthy JJ. Ribosome biogenesis is necessary for skeletal muscle hypertrophy. Exerc Sport Sci Rev 44: 110–115, 2016. doi: 10.1249/JES.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baik IH, Jo G-H, Seo D, Ko MJ, Cho CH, Lee MG, Lee Y-H. Knockdown of RPL9 expression inhibits colorectal carcinoma growth via the inactivation of Id-1/NF-κB signaling axis. Int J Oncol 49: 1953–1962, 2016. doi: 10.3892/ijo.2016.3688. [DOI] [PubMed] [Google Scholar]
- 48.Lezzerini M, Penzo M, O'Donohue M-F, Marques Dos Santos Vieira C, Saby M, Elfrink HL, Diets IJ, Hesse A-M, Couté Y, Gastou M, Nin-Velez A, Nikkels PGJ, Olson AN, Zonneveld-Huijssoon E, Jongmans MCJ, Zhang GJun, van Weeghel M, Houtkooper RH, Wlodarski MW, Kuiper RP, Bierings MB, van der Werff Ten Bosch J, Leblanc T, Montanaro L, Dinman JD, Da Costa L, Gleizes P-E, MacInnes AW. Ribosomal protein gene RPL9 variants can differentially impair ribosome function and cellular metabolism. Nucleic Acids Res 48: 770–787, 2020. doi: 10.1093/nar/gkz1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ralston E, McLaren RS, Horowitz JA. Nuclear domains in skeletal myotubes: the localization of transferrin receptor mRNA is independent of its half-life and restricted by binding to ribosomes. Exp Cell Res 236: 453–462, 1997. doi: 10.1006/excr.1997.3753. [DOI] [PubMed] [Google Scholar]
- 50.Goh Q, Song T, Petrany MJ, Cramer AA, Sun C, Sadayappan S, Lee S-J, Millay DP. Myonuclear accretion is a determinant of exercise-induced remodeling in skeletal muscle. eLife 8: e44876, 2019. doi: 10.7554/eLife.44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agrawal M, Bowman L. Transcriptional and translational regulation of ribosomal protein formation during mouse myoblast differentiation. J Biol Chem 262: 4868–4875, 1987. doi: 10.1016/S0021-9258(18)61276-1. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs FA, Bird RC, Sells BH. Differentiation of rat myoblasts: regulation of turnover of ribosomal proteins and their mRNAs. Eur J Biochem 150: 255–263, 1985. doi: 10.1111/j.1432-1033.1985.tb09015.x. [DOI] [PubMed] [Google Scholar]
- 53.Krauter KS, Soeiro R, Nadal-Ginard B. Unco-ordinate regulation of ribosomal RNA and ribosomal protein synthesis during L6E9 myoblast differentiation. J Mol Biol 142: 145–159, 1980. doi: 10.1016/0022-2836(80)90042-x. [DOI] [PubMed] [Google Scholar]
- 54.Zahradka P, Larson DE, Sells BH. Regulation of ribosome biogenesis in differentiated rat myotubes. Mol Cell Biochem 104: 189–194, 1991. [PubMed] [Google Scholar]
- 55.Brett JO, Arjona M, Ikeda M, Quarta M, de Morrée A, Egner IM, Perandini LA, Ishak HD, Goshayeshi A, Benjamin DI, Both P, Rodríguez-Mateo C, Betley MJ, Wyss-Coray T, Rando TA. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat Metab 2: 307–317, 2020. doi: 10.1038/s42255-020-0190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murach KA, Dungan CM, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. “Muscle memory” not mediated by myonuclear number? Secondary analysis of human detraining data. J Appl Physiol 127: 1814–1816, 2019. doi: 10.1152/japplphysiol.00506.2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RRBS data are deposited in GEO GSE180433. Processed data are available upon reasonable request.