Abstract
Single chain antibody fragment (scFv) is a promising agent for imaging and targeted therapy. The objective of the study is to evaluate a kit formulation for 99mTc labeling of scFv for tumor imaging. The scFv was engineered to contain a cysteine tag to accommodate the specific conjugation of HYNIC and subsequent 99mTc labeling. The labeling conditions were formulated to allow instantaneous one-pot quantitative labeling. The reproducibility of labeling was evaluated at various time points during kit storage at −20 °C. In vitro cell binding experiments and HPLC analysis were performed to assess binding affinity and radiolabel stability, respectively. In vivo tumor targeting study was performed in xenograft models with biodistribution studied at 1, 3, and 24 h post-injection. The optimized kit with 5 μg SnF2, pH 5.5, and 50 μg GH along with as low as 15 μg of HYNIC-cys-scFv provided high labeling yield (>95%), high specific activity (1.8 × 107 Ci/Mol), and robust reproducibility with shelf life up to 90 days when stored at −20 °C. The in vitro cell binding study showed the labeled scFv maintained the binding capability with an apparent KD of ~27 nM. The animal study using tumor-bearing mice showed high tumor uptake at 16.9%ID/g 24 h post-injection along with rapid blood clearance (0.18%ID/g) and kidney excretion (44%ID/g), resulting in very high contrast (tumor/muscle >200:1). A kit formulation for 99mTc labeling of scFvs targeting mesothelioma was developed based on specific HYNIC conjugation and GH (Glucoheptonate) as a coligand, producing not only high specific activity, but also improved tumor uptake. This convenient one-pot labeling method has the potential for translation into clinical use and is applicable to other scFvs as well.
Graphical Abstract

Antibody imaging can provide a quantitative means of molecular characterization of cell surface antigenic profile in vivo, which can in turn provide information useful for diagnosis, patient stratification, monitoring response to therapy, and assessing potential adverse effects, thus contributing to the ongoing development of personalized cancer treatment.1,2 Engineered antibody fragments, such as diabodies, minibodies, and single chain antibody fragments (scFv), have been successfully employed for radioactive imaging of cancer in preclinical models and are poised to bring same-day imaging into clinical development.3–7
Direct labeling of monoclonal antibody with 99mTc has been reported previously but has not been widely applied in routine practice due to a number of drawbacks in the radiolabeling technique that have significant impact on the kinetics and biodistribution.8–11 These issues include the rather harsh condition required for labeling that affects antibody stability, the low radiochemical purity of labeled preparation that requires subsequent purification, the instability of the radiolabel, and the lack of a convenient method for use in the nuclear medicine clinic.10–12 The kit format for one-pot labeling with high efficiency can effectively address this problem, as kits for 99mTc labeling have been used in the clinic.13,14
We hereby report a kit-based labeling method for labeling monoclonal antibody fragment with 99mTc. The scFv M40 that we isolated previously for targeting mesothelioma15,16 was employed for evaluating the 99mTc labeling kit. The resulted 99mTc-M40 was evaluated for tumor cell binding in vitro and tumor targeting in vivo in a mesothelioma xenograft model.
RESULTS
Synthesis and Radiolabeling.
The scFv was engineered to contain a C terminal free cysteine (cys-scFv). The site-specific conjugation (HYNIC-cys-scFv) was achieved via reaction of cys-scFv with 3-N-maleimido-6-hydraziniumpyridine hydrochloride (MHPH) at the molar ratio of 3:1 (Figure 1). The conjugation resulted in about one HYNIC per antibody as determined by the nitrobenzaldehyde assay. The subsequent 99mTc labeling (99mTc-HYNIC-cys-scFv) were optimized by varying labeling conditions including the amount of SnF2 (5, 10, and 20 μg), pH (5.5, 6.0, and 6.5), and the amount of the coligand GH (Glucoheptonate) (25, 50, and 100 μg). ITLC and HPLC assays were used to assess labeling efficiency and in vitro stability to allow instant one-pot quantitative labeling. Although the labeling efficiencies were >90% under all the labeling conditions mentioned, the labeling at the optimized one-pot formulation (5 μg SnF2, pH 5.5, and 50 μg GH) along with 10 mCi of 99mTcO4− and as low as 15 μg of HYNIC-cys-scFv provides not only high labeling yield (>95%) but also a remarkable specific activity (1.8 × 107 Ci/Mol) with reproducible results (red line in Figure 2 top panel).
Figure 1.

Scheme of conjugation and radiolabeling.
Figure 2.

In vitro assay of the 99mTc labeling. Top: Reproducibility of radiolabeling of the optimal kit formulation at different storage time in freezer. Bottom: In vitro tumor cell binding of 99mTc labeled scFv, showing that the labeled scFv maintained binding affinity (27 nM) (line with green square for nonspecific binding).
In Vitro Evaluation.
The labeled product was evaluated in vitro for cell binding capability, and stability in phosphate buffered saline (PBS) at room temperature and serum at 37 °C for up to 24 h. The in vitro cell study confirmed the labeled scFv maintained the binding capability with a KD of 27 nM (Figure 2). The 99mTc-labeled scFv (99mTc-HYNIC-cys-scFv) appearing at 14.8 min in HPLC profile was found to be stable in both PBS and serum with 100% intact at 1 and 6 h (Figure 3), and with 98% and 91% intact at 24 h incubation in PBS and serum respectively, while the 99mTc labeling of cys-scFv M40 without HYNIC and with GH (Glucoheptonate) as the ligand remained only 89% intact after 3 h incubation in PBS and 81.4% after 1 h incubation in mouse serum (Figure 3).
Figure 3.
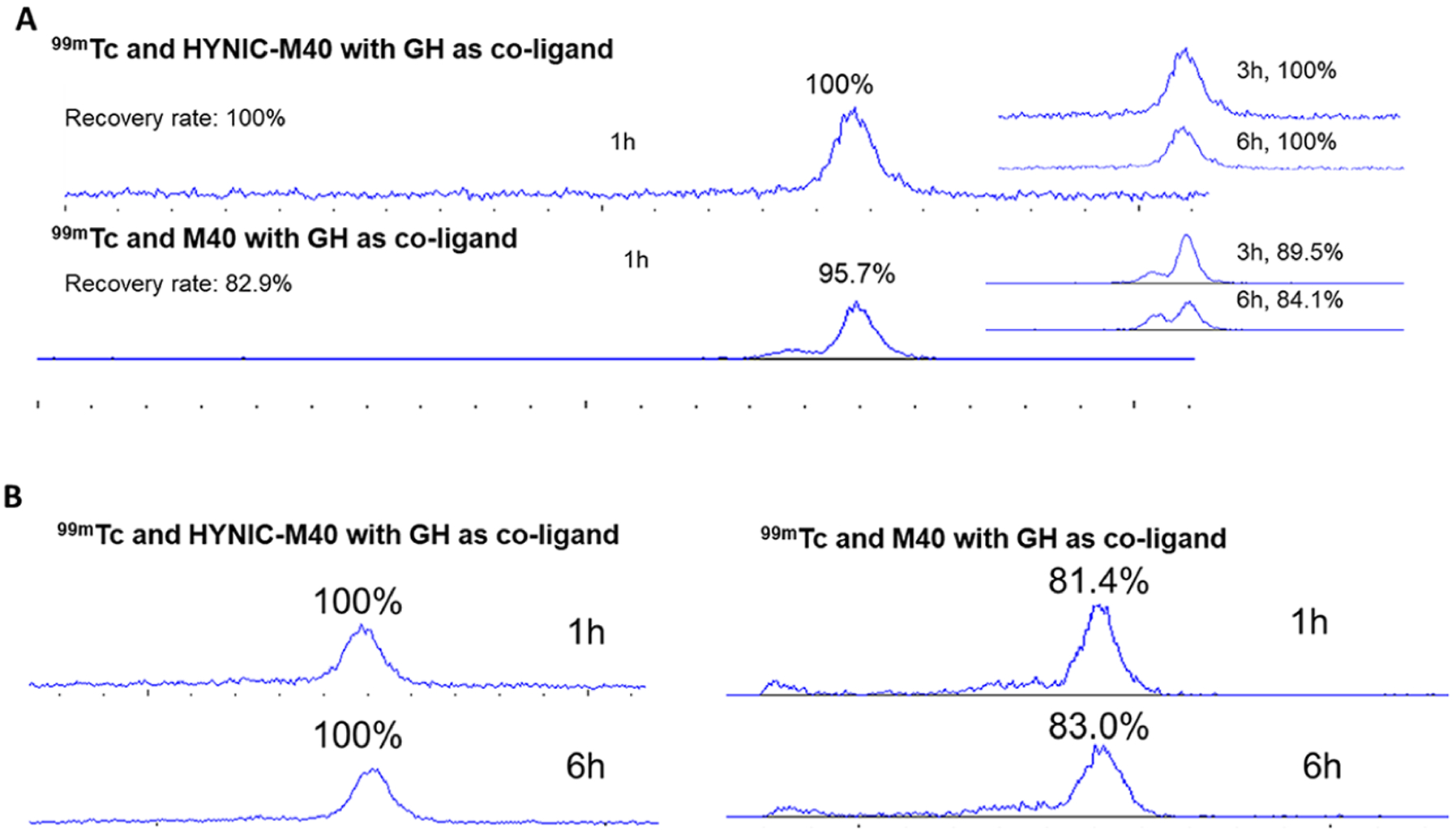
HPLC analysis (radioachromatogram profile) of stability of different 99mTc labeling of M40 antibody with (HYNIC-M40) or without HYNIC tag (M40) in PBS at room temperature (Panel A) and CD mouse serum (Panel B) at 37 °C. M40 monomer peak at 14.8 min are shown as a percentage (%) of total radioactivity.
In Vivo Study.
Animal studies in tumor bearing mice were performed to confirm tumor targeting in vivo. Biodistribution was obtained at 1, 3, and 24 h post-injection (Figure 4). The tumor uptake is the second highest at 5.46%ID/g and 7.82% ID/g for 1 and 3 h post-injection of 99mTc-M40, respectively, with the highest uptake in kidneys at 96.5%ID/g and 129%ID/g, respectively. The uptake in liver and most other organs/tissues is ≲1%ID/g. A high tumor uptake was observed (16.9% ID/g) at 24 h post-injection, along with rapid blood clearance (0.18%ID/g) and kidney excretion to a relatively low level (44%ID/g), providing a very high contrast between tumor and normal tissues (Figure 4).
Figure 4.

Animal study of 99mTc-labeled scFv in nude mice bearing human mesothelioma xenograft tumor. Top: Biodistribution of 99mTc-labeled scFv at 1, 3, and 24 h post-injection. Bottom: The high tumor/nontumor ratio is confirmed by the high contrast tumor images by microSPECT/CT at 24 h post-injection of 1.0 mCi of 99mTc-labeled scFv.
DISCUSSION
Antibody fragments such as scFv possessing desired specificity and binding affinity are promising agents for in vivo tumor imaging and targeted therapy development. By combining phage antibody library selection with direct selection on living tumor cells and tissues, we have identified human scFvs that bind to human tumor cells in situ,15,17–20 and, furthermore, those that are selectively internalized into tumor cells and thus capable of mediating intracellular delivery of small-molecule payloads21,22 or isotopes to tumor cells.16,20 This study is to develop an improved kit formulation for 99mTc labeling of scFv for tumor imaging, with high specific activity and excellent in vivo tumor targeting.
We have previously employed a tricarbonyl labeling approach for hexa-histidine tagged scFvs and demonstrated the specific targeting of this antibody, M40, as reported.16,20 In this study, we achieved site-specific conjugation of HYNIC chelator to an engineered scFv bearing a C terminal cysteine, and developed a one-pot labeling process in a kit format for scFv labeling. The HYNIC in conjugate not only serves as coordination group for 99mTc labeling, but also provide protection for the cysteine group during storage that avoids the reducing steps of cysteine tagged scFv each time immediately prior to 99mTc labeling. This instant labeling kit also provides significant improvement in specific activity (>50-fold) and enhanced tumor uptake in vivo (4-fold) compared to tricarbonyl labeling as previously reported.16 With these improvements, tumor as small as 52 mg was clearly imaged by SPECT 24 h after injection of 99mTc labeled M40 (Figure 5).
Figure 5.

SPECT images (A) and 3D rending images (B, C, D) of different sized tumors (at mass of 52 mg on right side in A and B and left side in C and D, and 650 mg for big tumor) received 0.5–1.0 mCi of 99mTc labeled M40 in nude mice 24 h before. Tumor as small as 52 mg can be detected.
For 99mTc radiolabeling using HYNIC, it has been well recognized that the coligand plays an important role for pharmacokinetics and biodistribution as reported by others.13,23–27 In this study, GH was investigated as coligand for HYNIC conjugated scFv. GH alone or together with other molecules has been reported for 99mTc labeling at mild conditions to form an excellent radiotracer for tumor imaging.28 Although the exact structure of final 99mTc-HYNIC-M40 with GH as coligand is not known, the in vitro binding assay (Figure 2) and in vitro stability tests in both phosphate buffer and serum (Figure 3) have demonstrated that the final labeling products exhibit excellent binding capability and labeling stability in both PBS and serum. To test if the final product is possible another compound, e.g., 99mTc(V)-O-GH complexing with M40 in absence of HYNIC, a control experiment of 99mTc labeling was performed with GH and antibody M40 without HYNIC tag under similar conditions. As shown in Figure 3, the resulting labeled product was not stable when incubated in buffer or serum, with a new peak close to 13.6 min in retention time, which possibly suggests the dimerization of cysteine scFvs in the 99mTc complexes, while there were no such new peaks when HYNIC was present along with GH to form the target complex of 99mTc-HYNIC-M40. Together, these results suggest that 99mTc labeled HYNIC-M40 with GH as coligand results in excellent labeling efficiency, biodistribution, and tumor targeting, while the exact structure remains uncertain, and more detailed characterization is needed. Among other coligands for 99mTc labeling of HYNIC functionalized molecules, tricine and ethylenediamine-N,N′-diacetic acid (EDDA) are the most extensively reported ones with success.23–27 More work to further optimize and improve the 99mTc labeling of site-specific conjugated HYNIC-M40 is ongoing to compare these different coligands, e.g., GH, tricine, or EDDA, or even combinations with two coligands: tricine/TPPTS (trisodium triphenylphosphine-3,3′,3″-trisulfonate), as reported by Zhao’s group.13
A kit formulation for 99mTc labeling based on site-specific HYNIC conjugation has been developed. This kit formulation provides improved specific activity of the radiolabeling and remarkable in vivo tumor targeting of the resulted radiotracer. It has the potential to provide a path to clinical testing and use. It may also be applicable to labeling other scFvs and peptides to facilitate their translation into the clinic.
CONCLUSIONS
A kit formulation for 99mTc labeling based on specific HYNIC conjugation and coligand was successfully developed for a novel tumor-targeting scFv. The kit formulation allows highly specific and robust antibody labeling, and has the potential for translation to clinic use.
MATERIALS AND METHODS
Antibody Production and Preparation of HYNIC Conjugates.
The scFv gene was subcloned from phage into the secretion vector that imparts a cysteine and a hexahistidine tag to the C terminus of the scFv using methods that we have described previously.17–19 Soluble scFv was harvested from the bacterial periplasm and purified by immobilized metal affinity chromatography and gel filtration.16,17,19,20 Following over-night dialysis in PBS, antibody purity was determined by gel electrophoresis, and concentration was determined using NanoDrop (Thermo Scientific, Wilmington, DE).
HYNIC conjugation: 0.1 mL of solution of purified M40 scFv (3 mg/mL) in phosphate buffer (pH 8.0) was mixed with 5 μL solution of DMF containing 3-N-maleimido-6-hydraziniumpyridine hydrochloride from Solulink (San Diego, CA) at molar ratio of 3:1 (HYNIC/M40), and the resulting solution was incubated at room temperature for 20 min. The M40-HYNIC conjugate was purified using Zeba desalt spin column with phosphate buffer (pH 7.0). The ratio of M40 to HYNIC of final product is determined by nitrobenzaldehyde assay, where p-nitrobenzaldehyde reacts with the hydrazine group on the HYNIC to change the hydrazino group to a corresponding hydrazone group resulting in an increase in absorbance at 386 nm for the determination.29 Final concentration of conjugated M40-HYNIC was about 2.5 mg/mL.
Antibody Radiolabeling.
The 99mTc labeling (99mTc-HYNIC-cys-scFv) was first optimized by varying labeling conditions including the amount of SnF2 (5, 10, and 20 μg), pH (5.5, 6.0, and 6.5), and various coligands GH (25, 50, and 100 μg) to allow instant one-pot quantitative labeling with labeling yield above 90% and excellent in vitro stability. The kits containing sodium glucoheptonate (GH), SnF2, saline, and M40-HYNIC conjugate in 10 mL vial glass was formulated to a final volume of 5 mL and stored in the freezer (−20 °C). For 99mTc labeling, the kit was brought to room temperature. 50 μL of the above solution was sequentially mixed with 10 μL of 0.01 N HCl and 100–200 μL of 99mTc Pertechnate (5–10 mCi), incubated at room temperature for 5–10 min, followed by labeling efficiency test with ITLC (Instant Thin-Layer Chromatography) and size exclusion-HPLC with 0.2 M phosphate buffer (pH 7.0) as eluant (Superose 12 10/30GL from GE healthcare, Marlborough, MA) at the flow rate of 1.0 mL/min. The final 99mTc labeled M40-HYNIC (99mTc-M40) was purified on PD10 column and eluted by phosphate buffer (pH 7.0). Both size exclusion-HPLC and ITLC analysis were used to characterize the final purified product 99mTc-M40. The chromatographic strips spotted 2.0 μL of the radiolabeled complex were developed in 0.9% NaCl as the mobile phase for determination of the percentage of free 99mTcO4 at the front (Rf = 1) with labeled scFv and others near original spot (Rf = 0). The percentage of insoluble and “colloidal” species were also identified (Rf = 0) with 99mTcO4 and 99mTc labeled scFv at front (Rf = 1) by ITLC, using albumin-soaked ITLC-SG strips using a mobile phase of acetonitrile/NH4OH/H2O (1:1:3; v/v). Strips were scanned using a TLC-scanner (Bioscan System).
To determine the radiolabeling reproducibility, the kit was kept in freezer (−20 °C) for up to 3 months. Three kits were used for 99mTc labeling at the storage time point of 7-, 21-, 35-, 60-, and 90-days. The labeling efficiency was determined by ITLC and size-exclusion HPLC as described above.
In Vitro Stability.
The stability of final purified 99mTc labeled antibodies was evaluated in vitro under both conditions of PBS incubation and serum incubation by size-exclusion HPLC. Briefly, the final 99mTc labeled antibodies were incubated with PBS at room temperature and serum at 37 °C. At designated time of incubation, samples were analyzed by size-exclusion HPLC. The radioactivity eluted through HPLC was also checked against the total activity injected for a recovery in percentage to determine the loss due to “colloid” or insoluble retained on the column.
In Vitro Cell Culture Assay.
The tumor cell binding experiment was performed as described previously.19,20 Briefly, 1 million M28 cells were seeded per well and incubated in RMPI-1640 medium containing 10% fetal bovine serum at 37 °C for 3 h. Approximately 150 kBq 99mTc-labeled scFv in a final concentration of 1 nmol/L was added to the medium. A fixed amount of 99mTc-M40 was spiked with increasing amount of unlabeled ligand to attain the upper end of desired concentration range (1 to 100 nM). The cells with added 99mTc-M40 were incubated at 37 °C in 5% CO2 for various time periods. The radioactivity was measured on a γ-counter and expressed as the percentage of applied activity normalized to 1 million cells. As a control for nonspecific uptake, the experiment was repeated with the addition of a 10-fold excess of cold scFv.
Animal Study Approval.
Animal procedures were performed according to protocols approved by Institutional Animal Care and Use Committee at both University of California, San Francisco and University of Virginia.
Xenograft Model.
Six-week-old male nu/nu mice were purchased from Charles River Laboratories. One million M28 cells in 100 μL of PBS were administered subcutaneously to seed xenograft tumors in right flanks of the animal. Growing tumors were palpated, and the diameters were measured by calipers. The experiment started when tumor on the right flank reaches the size of 4–6 mm in diameter.
Biodistribution Study.
Tumor-bearing nude mice in groups of 4 animals were injected with 18.5–37 MBq (0.5–1.0 mCi) of 99mTc-labeled M40 (5–10 μg of M40). The mice were euthanized and dissected at 1, 3, and 24 h post-injection, and blood, tumor, and major organ specimens were collected and weighed. The radioactivity in these tissues was measured against known activity standards using a γ-counter (Wizard, PerkinElmer, Milwaukee WI). The percent injected dose per gram (%ID/g) was determined.
SPECT/CT Imaging.
Mice were imaged with a small animal SPECT/CT system (Gamma Medica) as previously reported.16,20 For anatomical correlation, CT was first performed after injection. SPECT imaging was initiated 1, 3, and 24 h after injection.
Statistical Analysis.
All quantitative data are reported as mean ± SD. Statistical analysis was performed using a two-tailed t test; data differences were considered statistically significant for P values of 0.05 or less.
ACKNOWLEDGMENTS
This work was supported in part by NIH R01CA135358 and the American Cancer Society (IRG-97-150-10) to Jiang He, R01CA118919 and R01CA129491 to Bin Liu, R01CA223767 to both Jiang He and Bin Liu, R01CA154561 to Youngho Seo, and CCSG P30CA44579 to University of Virginia Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.bioconjchem.0c00319
The authors declare no competing financial interest.
Contributor Information
Jiang He, Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, Virginia 22908, United States; Department of Radiology and Biomedical Imaging, University of California, San Francisco, California 94143, United States;.
Jinjin Feng, Department of Radiology and Biomedical Imaging, University of California, San Francisco, California 94143, United States.
Yang Su, Department of Anesthesia, University of California, San Francisco, California 94143, United States.
Youngho Seo, Department of Radiology and Biomedical Imaging, University of California, San Francisco, California 94143, United States; UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, California 94143, United States.
Bin Liu, Department of Anesthesia, University of California, San Francisco, California 94143, United States; UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, California 94143, United States.
REFERENCES
- (1).Wu AM (2014) Engineered antibodies for molecular imaging of cancer. Methods 65, 139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Moek KL, Giesen D, Kok IC, de Groot DJA, Jalving M, Fehrmann RSN, Lub-de Hooge MN, Brouwers AH, and de Vries EGE (2017) Theranostics Using Antibodies and Antibody-Related Therapeutics. J. Nucl. Med 58, 83S–90S. [DOI] [PubMed] [Google Scholar]
- (3).Adumeau P, Sharma SK, Brent C, and Zeglis BM (2016) Site-Specifically Labeled Immunoconjugates for Molecular Imaging–Part 1: Cysteine Residues and Glycans. Mol. Imaging Biol 18, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Fu R, Carroll L, Yahioglu G, Aboagye EO, and Miller PW (2018) Antibody Fragment and Affibody ImmunoPET Imaging Agents: Radiolabelling Strategies and Applications. ChemMedChem 13, 2466–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Xavier C, Blykers A, Laoui D, Bolli E, Vaneyken I, Bridoux J, Baudhuin H, Raes G, Everaert H, Movahedi K, et al. (2019) Clinical Translation of [68Ga]Ga-NOTA-anti-MMR-sdAb for PET/CT Imaging of Protumorigenic Macrophages. Mol. Imaging Biol 21, 898–906. [DOI] [PubMed] [Google Scholar]
- (6).Frigerio B, Morlino S, Luison E, Seregni E, Lorenzoni A, Satta A, Valdagni R, Bogni A, Chiesa C, Mira M, et al. (2019) Anti-PSMA 124I-scFvD2B as a new immuno-PET tool for prostate cancer: preclinical proof of principle. J. Exp. Clin. Cancer Res 38, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rios X, Compte M, Gómez-Vallejo V, Cossío U, Baz Z, Morcillo MÁ, Ramos-Cabrer P, Alvarez-Vallina L, and Llop J (2019) Immuno-PET Imaging and Pharmacokinetics of an Anti-CEA scFv-based Trimerbody and Its Monomeric Counterpart in Human Gastric Carcinoma-Bearing Mice. Mol. Pharmaceutics 16, 1025–1035. [DOI] [PubMed] [Google Scholar]
- (8).Bridger GJ, Abrams MJ, Padmanabhan S, Gaul F, Larsen S, Henson GW, Schwartz DA, Longley CB, Burton CA, and Ultee ME (1996) A comparison of cleavable and noncleavable hydrazinopyridine linkers for the 99mTc labeling of Fab’ monoclonal antibody fragments. Bioconjugate Chem. 7, 255–64. [DOI] [PubMed] [Google Scholar]
- (9).Kim I, Kobayashi H, Yoo TM, Kim MK, Le N, Han ES, Wang QC, Pastan I, Carrasquillo JA, and Paik CH (2002) Lowering of pI by acylation improves the renal uptake of 99mTc-labeled anti-Tac dsFv: effect of different acylating reagents. Nucl. Med. Biol 29, 795–801. [DOI] [PubMed] [Google Scholar]
- (10).Nawaz S, Mullen GED, Blower PJ, and Ballinger JR (2017) A 99mTc-labelled scFv antibody fragment that binds to prostate-specific membrane antigen. Nucl. Med. Commun 38, 666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Solomon VR, Gonzalez C, Alizadeh E, Bernhard W, Hartimath SV, Barreto K, Geyer CR, and Fonge H (2018) 99mTc(CO)3+ labeled domain I/II-specific anti-EGFR (scFv)2 antibody fragment for imaging EGFR expression. Eur. J. Med. Chem 157, 437–446. [DOI] [PubMed] [Google Scholar]
- (12).Bala G, Crauwels M, Blykers A, Remory I, Marschall ALJ, Dübel S, Dumas L, Broisat A, Martin C, Ballet S, et al. (2019) Radiometal-labeled anti-VCAM-1 nanobodies as molecular tracers for atherosclerosis - impact of radiochemistry on pharmacokinetics. Biol. Chem 400, 323–332. [DOI] [PubMed] [Google Scholar]
- (13).Zhao M, and Li Z (2012) A single-step kit formulation for the (99m)Tc-labeling of HYNIC-Duramycin. Nucl. Med. Biol 39, 1006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kumar P, Singh B, Ghai A, Hazari PP, Mittal BR, and Mishra AK (2015) Development of a single vial kit formulation of [99mTc]-labeled doxorubicin for tumor imaging and treatment response assessment-preclinical evaluation and preliminary human results. J. Labelled Compd. Radiopharm 58, 242–9. [DOI] [PubMed] [Google Scholar]
- (15).((15)) An F, Drummond DC, Wilson S, Kirpotin DB, Nishimura SL, Broaddus VC, and Liu B (2008) Targeted drug delivery to mesothelioma cells using functionally selected internalizing human single-chain antibodies. Mol. Cancer Ther 7, 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Iyer AK, Su Y, Feng J, Lan X, Zhu X, Liu Y, Gao D, Seo Y, Vanbrocklin HF, Broaddus VC, et al. (2011) The effect of internalizing human single chain antibody fragment on liposome targeting to epithelioid and sarcomatoid mesothelioma. Biomaterials 32, 2605–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Liu B, Conrad F, Cooperberg MR, Kirpotin DB, and Marks JD (2004) Mapping tumor epitope space by direct selection of single-chain Fv antibody libraries on prostate cancer cells. Cancer Res. 64, 704–10. [DOI] [PubMed] [Google Scholar]
- (18).Ruan W, Sassoon A, An F, Simko JP, and Liu B (2006) Identification of clinically significant tumor antigens by selecting phage antibody library on tumor cells in situ using laser capture microdissection. Mol. Cell. Proteomics 5, 2364–2373. [DOI] [PubMed] [Google Scholar]
- (19).Roth A, Drummond DC, Conrad F, Hayes ME, Kirpotin DB, Benz CC, Marks JD, and Liu B (2007) Anti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cells. Mol. Cancer Ther 6, 2737–46. [DOI] [PubMed] [Google Scholar]
- (20).He J, Wang Y, Feng J, Zhu X, Lan X, Iyer AK, Zhang N, Seo Y, VanBrocklin HF, and Liu B (2010) Targeting prostate cancer cells in vivo using a rapidly internalizing novel human single-chain antibody fragment. J. Nucl. Med 51, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sherbenou DW, Aftab BT, Su Y, Behrens CR, Wiita A, Logan AC, Acosta-Alvear D, Hann BC, Walter P, Shuman MA, et al. (2016) Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J. Clin. Invest 126, 4640–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Su Y, Liu Y, Behrens CR, Bidlingmaier S, Lee NK, Aggarwal R, Sherbenou DW, Burlingame AL, Hann BC, Simko JP, et al. (2018) Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight 2018 (3), No. e121497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Korde A, Mallia M, Shinto A, Sarma HD, Samuel G, and Banerjee S (2014) Improved kit formulation for preparation of (99m)Tc-HYNIC-TOC: results of preliminary clinical evaluation in imaging patients with neuroendocrine tumors. Cancer Biother.Radiopharm 29, 387–94. [DOI] [PubMed] [Google Scholar]
- (24).Ortiz-Arzate Z, Santos-Cuevas CL, Ocampo-García BE, Ferro-Flores G, García-Becerra R, Estrada G, Gómez-Argumosa E, and Izquierdo-Sánchez V (2014) Kit preparation and biokinetics in women of 99mTc-EDDA/HYNIC-E-[c(RGDfK)]2 for breast cancer imaging. Nucl. Med. Commun 35, 423–32. [DOI] [PubMed] [Google Scholar]
- (25).Medina-García V, Ocampo-García BE, Ferro-Flores G, Santos-Cuevas CL, Aranda-Lara L, García-Becerra R, Ordaz-Rosado D, and Melendez-Alafort L (2015) A freeze-dried kit formulation for the preparation of Lys(27)(99mTc-EDDA/HYNIC)-Exendin(9–39)/99mTc-EDDA/HYNIC-Tyr3-Octreotide to detect benign and malignant insulinomas. Nucl. Med. Biol 42, 911–6. [DOI] [PubMed] [Google Scholar]
- (26).Mukherjee A, Korde A, Shinto A, Sarma HD, Kamaleswaran K, and Dash A (2019) Studies on batch formulation of a freeze dried kit for the preparation of 99mTc-HYNIC-TATE for imaging neuroendocrine tumors. Appl. Radiat. Isot 145, 180–186. [DOI] [PubMed] [Google Scholar]
- (27).D’Alessandria C, di Gialleonardo V, Chianelli M, Mather SJ, de Vries EFJ, Scopinaro F, Dierck RA, and Signore A (2010) Synthesis and optimization of the labeling procedure of 99mTc-HYNIC-Interleukin-2 for In vivo imaging of activated T lymphocytes. Mol. Imaging Biol 12, 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Alam SS, Junaid S, and Ahmed SM (2016) Evaluation of Technetium-99m glucoheptonate single photon emission computed tomography for brain tumor imaging. Asian J. Neurosurg 11, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).King TP, Zhao SW, and Lam T (1986) Preparation of protein conjugates via intermolecular hydrozone linkage. Biochemistry 25, 5774–5779. [DOI] [PubMed] [Google Scholar]


