Abstract
OBJECTIVES
This study sought to report the calcification pattern of the mitral valve annulus and its implications for procedural and safety outcomes in transcatheter aortic valve implantation.
METHODS
Between November 2018 and September 2019, a total of 305 patients had transcatheter aortic valve implants at our institution. The extent of calcification of the mitral valve annulus was analysed, and the impact on safety outcomes was evaluated.
RESULTS
The prevalence of mitral annular calcification (MAC) was 43%. Calcification of the mitral valve annulus was either less than or at least one-third of the posterior annulus (34% and 32%), the whole posterior annulus (28%) or the extension to the attachment of the anterior leaflets (7%). Severe circumferential MAC revealed moderate paravalvular leaks in 5/8 (63%) patients and was associated with right branch bundle block [odds ratio (OR) 2.01 (0.39–3.06); P = 0.098] and low cardiac output [OR 3.12 (1.39–7.04); P = 0.033]. Subannular calcification at the anterolateral trigonum represented a risk factor for left ventricular outflow tract injury [OR 3.54 (1.38–8.27); P = 0.001] in balloon-expandable valves, associated with relevant rhythm disorders [OR 2.26 (1.17–5.65); P = 0.014] and female gender (7/8, 88%). The 30-day all-cause mortality in circumferential MAC reaching into the anterior annulus (grade IV) compared to patients with less MAC (grade I–III) was 13% vs 2% with a mean valve size of 24.6 vs 25.7 mm.
CONCLUSIONS
Extensive MAC was associated with moderate paravalvular leaks, with implications for the prosthesis size and survival in transcatheter aortic valve implants. In severe MAC, we recommend implanting oversized self-expandable prostheses, the goal being to reduce the risk of right branch bundle block and paravalvular leaks.
Subj collection
122, 125
Keywords: Calcification pathology, Mitral valve annulus, Transcatheter aortic valve implantation
Mitral annular calcification (MAC) represents a chronic degenerative process in the fibrous base of the mitral valve (MV) that more commonly affects the posterior rather than the anterior annulus [1].
INTRODUCTION
Mitral annular calcification (MAC) represents a chronic degenerative process in the fibrous base of the mitral valve (MV) that more commonly affects the posterior rather than the anterior annulus [1]. In general, it contrasts with rheumatic valve disease, in which fibrosis affects the commissural tissue and leaflets with late extension to the annulus. The pathophysiology of MAC is still a topic of controversy. The most common theory is continuous degeneration of the annulus throughout life [2], with the disorientation and thickening of collagen fibres, an increase in fatty tissue and a decline in mucopolysaccharides. However, in the majority of cases, calcification originates from previous valvular disease.
In a transcatheter aortic valve implant (TAVI), more than mild mitral regurgitation is an established risk factor with regard to long-term survival [3]. The role of extensive MAC due to its direct anatomical relationship to the aortic valvular apparatus is not well studied, and the relevance with regard to TAVI is currently unclear.
Our primary objective was to study the calcification pattern of the mitral annulus and its implications for safety and valve type in TAVI.
METHODS
From November 2018 to September 2019, a total of 305 intermediate- (EuroSCORE I 10.0–24.9) and high-risk patients (EuroSCORE I ≥25.0) with high-grade aortic stenosis underwent transfemoral (n = 294, 96%) and, only in the case of a contraindication, transapical (n = 11, 4%) aortic valve implants at our institution.
After approval by the local ethics committee (reference number: 061/20-ek), informed consent to perform the procedure was obtained from each patient. Preoperatively, each patient was examined by transthoracic and transoesophageal echocardiography and multislice computed tomography (CT) with contrast medium. Preoperative CT angiography images were reconstructed using a 3-dimensional workstation (syngo.via, Siemens Healthcare GmbH, Erlangen, Germany). After centreline reconstruction, the circumferential and vertical segments involved were analysed and the various mitral annulus diameters were measured. On the basis of diagnostic measurements obtained from transoesophageal echocardiography and multislice CT, individually selected transcatheter-based heart valve prostheses were used.
Classification of mitral valve annulus calcification pattern
According to a proposed classification system by Alain Carpentier, the valve must be analysed systematically using segmental analysis [2]. The phenotypic type of the calcification pattern was analysed with regard to the extent of the circumferential and vertical MAC. Circumferentially, the calcification process involved either (i) less than one-third of the posterior annulus; (ii) at least one-third of the posterior annulus; (iii) the entire posterior annulus; or (iv) circumferential reaching into the anterior annulus [2]. Vertically, the calcification process was restricted to the annulus itself or extended (i) to the leaflet tissue; (ii) to the ventricular myocardium; or (iii) to the anterior papillary muscle [2]. With regard to the vertical extent of the MAC, the categories are not mutually exclusive, but overlapping between the single categories was rare. Of course, every patient with leaflet or myocardium involvement had calcification of the annulus.
Definition of risk factors and clinical parameters
Postsurgical complications were recorded according to the updated standardized end point definitions for TAVI [4]. The degree of a paravalvular leak was assessed by transoesophageal echocardiography in the midoesophageal long-axis view using the pressure half-time (mild: >400 ms; moderate: 200–400 ms; severe: <200 ms) and the width of the regurgitant jet within the left ventricular outflow tract (LVOT) (<30%; 30–50%; >50%) [5]. In addition, paravalvular leaks were identified after the release of the prosthesis by aortic root angiography according to the Sellers classification [6]. Pacemaker rate, mean transprosthetic gradients and the rare risk of subannular LVOT injury were documented. Relevant rhythm disorders included complete heart block or persistent high-degree atrioventricular block, increase of PR interval or QRS duration ≥20 ms in patients with pre-existing right branch bundle block (RBBB), left branch bundle block, first degree atrioventricular block or pre-existing intraventricular conduction delay with QRS duration ≥120 ms according to Rodes-Cabau et al. [7] Mortality was defined as periprocedural death within 72 h after the initial TAVI. All-cause mortality was defined as within and after 30 days during the follow-up period.
Set-up and technical aspects of transcatheter aortic valve implantation
All patients underwent the procedure by a multidisciplinary team consisting of a cardiac surgeon, interventional cardiologist and anaesthetist in a hybrid operating room (Artis zeego system, Siemens Inc., Forchheim, Germany) under general anaesthesia. The TAVI implantation technique has been described previously [8]. After successful deployment, angiographic assessment was performed to prove no paravalvular leak and to ascertain good valve function. Extubation was attempted in the operating room to assess the neurological status. All patients underwent standardized postoperative management in our intensive care unit.
Data collection and follow-up protocol
Data were retrospectively collected. Preoperative and perioperative data and postoperative outcomes were analysed. After TAVI, patients were seen in our outpatient clinic after 12 months. Consequently, follow-up was complete in all patients.
Statistical analysis
Statistical analyses were performed using SPSS statistical software (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA). For the statistical analyses, data were 100% complete. Continuous variables are expressed as mean ± standard deviation for Gaussian distribution; otherwise, median values were given with the 25th percentile to 75th percentile range. Categorical data are given as proportions. Logistic regression analysis for binary data using univariate analysis with regard to circumferential MAC reaching into the anterior annulus (grade IV) was performed using odds ratios (ORs) with a 95% confidence interval. A Kaplan–Meier analysis was performed for all-cause 30-day mortality of TAVI and stratified by MAC. A P-value of <0.05 was considered statistically significant.
RESULTS
Demographics and previous surgical approach
Patient demographics and clinical risk factors are shown in Table 1. The median age at TAVI of these patients was 80.0 years (range 76.3–83.0 years). From this cohort, 131 (43%) had MAC and 174 (57%) did not have MAC. Grading of MAC severity was performed using Carpentier’s surgical classification in pre-TAVI CT scans (Fig. 1). A total of 59% of the cases of MAC were present in women, whereas 41% were present in men.
Table 1:
Baseline patient characteristics
| Patient characteristics | n = 305 (100%) |
|---|---|
| Demographics | |
| Age (years) | 80 (76–83) |
| Male | 156 (51) |
| LVEF | 55 (44–60) |
| EuroSCORE | 22 (13–38) |
| EuroSCORE II | 6 (3–10) |
| NYHA functional class | |
| III | 208 (68) |
| IV | 23 (8) |
| CCS class | |
| III | 16 (5) |
| IV | 3 (1) |
| Chronic health conditions and risk factors | |
| Hypertension | 288 (94) |
| COPD | 29 (10) |
| Diabetes mellitus | 118 (39) |
| Dialysis on admission | 5 (2) |
| Hypercholesterolaemia | 216 (71) |
| Carotid artery disease | 30 (10) |
| Peripheral artery disease | 22 (7) |
| Previous interventional approach | |
| PTCA with stent | 95 (31) |
Data are presented as n (%) or median (interquartile range).
CCS: Canadian Cardiovascular Society; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PTCA: percutaneous transluminal coronary angioplasty.
Figure 1:
Grading of mitral annulus calcification severity according to Carpentier’s surgical classification in pretranscatheter aortic valve implantation computed tomography scans [2]. Circumferential classification: I = less than one-third of the posterior annulus; II = at least one-third of the posterior annulus; III = the entire posterior annulus; and IV = circumferential reaching into the anterior annulus.
Periprocedural and postprocedural clinical data
Periprocedural and postprocedural outcomes are presented in Table 2. The Sapien S3 prosthesis was used in 213 (70%) patients, whereas the Evolut-R prostheses were implanted in 22 (7%) and the Symetis AcurateNeo in 70 (23%). The mean valve size was 26.0 ± 0.3 mm. Predilatation was performed in 89% (n = 272) and postdilatation in 11% (n = 34). The median procedural time was 40.0 min (35.0–52.5 min) with a median dose area product of 2727.0 mGy cm2 (1819.8–4411.5 mGy cm2). The intensive care unit stay was 2.0 days (1.0–2.0). A total of 23% of the patients (n = 70) developed postinterventional rhythm disorders, and the reintubation rate was 2% (n = 7). Disabling stroke was present in 2% (n = 5). All-cause mortality within 30 days was low at 4% (n = 11).
Table 2:
Periprocedural and postprocedural clinical data for all patients and for patients with circumferentially extended mitral annulus calcification
| Overall data (n = 305) | I (n = 42) | II (n = 39) | III (n = 34) | IV (n = 8) | |
|---|---|---|---|---|---|
| Prosthesis type | |||||
| Sapien S3 | 213 (70) | 26 (62) | 28 (72) | 23 (70) | 6 (75) |
| Evolut R | 22 (7) | 6 (14) | 4 (10) | 2 (6) | 0 (0) |
| Symetis | 70 (23) | 10 (24) | 7 (18) | 8 (24) | 2 (25) |
| Valve size (mm) | 26.0 ± 0.3 | 26.1 ± 0.9 | 25.5 ± 0.8 | 25.5 ± 0.7 | 24.6 ± 2.1 |
| Predilatation | 272 (89) | 36 (86) | 34 (87) | 31 (94) | 7 (88) |
| Postdilatation | 34 (11) | 6 (14) | 1 (3) | 2 (6) | 0 (0) |
| Procedural time (min) | 40 (35–53) | 40.0 (35–55) | 39.0 (30–50) | 50.0 (35–65) | 42.5 (35–53) |
| Median fluoroscopy time (min) | 8.1 (7–11) | 10.2 (7–13) | 8.2 (6–10) | 8.4 (7–11) | 7.7 (7–9) |
| Dose area product (mGy cm2) | 2727 (1820–4412) | 3092 (1981–5734) | 2526 (1692–3574) | 2291 (1787–3701) | 2170 (1835–2710) |
| Contrast medium dose (ml) | 75 (60–102) | 80.0 (60–106) | 75.0 (60–103) | 75.0 (60–107) | 70.0 (62–97) |
| Rhythm disorders | 70 (23) | 10 (24) | 5 (13) | 9 (27) | 3 (38) |
| Reintubation | 7 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (13) |
| Non-disabling stroke | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Disabling stroke | 5 (2) | 1 (2) | 0 (0) | 1 (3) | 0 (0) |
| Low cardiac output | 4 (1) | 0 (0) | 0 (0) | 1 (3) | 1 (13) |
| Periprocedural mortality (≤72 h) | 5 (2) | 0 (0) | 0 (0) | 2 (6) | 1 (13) |
| All-cause mortality | |||||
| Within 30 days | 11 (4) | 0 (0) | 0 (0) | 0 (0) | 1 (13) |
| After 30 days | 7 (2) | 1 (2) | 2 (5) | 3 (9) | 0 (0) |
| ICU stay (days) | 2 (1–2) | 2.0 (1–2) | 2.0 (1–2) | 2.0 (1–2) | 1.5 (1–3) |
| Entire hospital stay (days) | 12 (9–17) | 12.0 (10–15) | 14.0 (10–17) | 13.0 (8–18) | 15.0 (11–20) |
| Observed follow-up period (days) | 159 (31–297) | 162 (86–315) | 94.0 (40–262) | 176 (71–294) | 183 (82–205) |
Data are presented as n (%) or median (interquartile range).
ICU: intensive care unit; S3: Edwards Sapien S3 prosthesis.
Phenotypic type of calcification patterns of the mitral valve annulus
Circumferential analysis showed 4 patterns of MV annulus calcification (Table 3): (i) less than one-third of the posterior annulus (n = 42, 34%); (ii) at least one-third of the posterior annulus (n = 39, 32%); (iii) the entire posterior annulus (n = 34, 28%); and (iv) circumferential reaching into the anterior annulus (n = 8, 7%).
Table 3:
Mitral annular calcification analysis
| Circumferential extension | n (%) | Vertical extension | n (%) |
|---|---|---|---|
| I—Less than one-third of the posterior annulus | 42 (34) | I—restricted to the annulus | 87 (69) |
| II—at least one-third of the posterior annulus | 39 (32) | II—extended to the leaflet tissue | 16 (13) |
| III—entire posterior annulus | 34 (28) | III—extended to the ventricular myocardium | 22 (17) |
| IV—circumferential extension into the anterior annulus | 8 (7) | IV—extended to the anterior papillary muscle | 2 (2) |
Data are presented as n (%).
ICU: intensive care unit.
Vertical analysis showed 4 patterns of MV annulus calcification: (i) the calcification process was restricted to the annulus itself (n = 87, 69%), (ii) extended to the leaflet tissue (n = 16, 13%), (iii) to the ventricular myocardium (n = 22, 17%) or (iv) to the anterior papillary muscle (n = 2, 2%).
Implications of safety outcomes
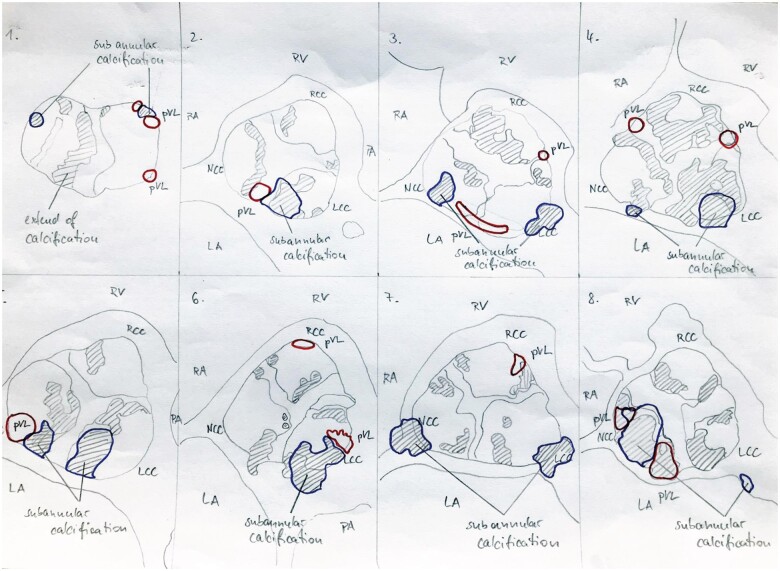
Circumferential MAC reaching into the anterior annulus revealed moderate paravalvular leaks in 5/8 patients [63%, OR 2.19 (1.21–5.46); P = 0.002; Fig. 2]. The intraoperative and postoperative data of the 8 patients with severe MAC are presented in Table 4. Circumferential MAC was associated with RBBB [OR 2.01 (0.39–3.06); P = 0.1] and low cardiac output [OR 3.12 (1.39–7.04); P = 0.033; Table 5]. In case of circumferential MAC, we found a trend towards a higher rate of new atrioventricular block III and atrial fibrillation [OR 1.03 (0.38–2.78); P = 0.23 and OR 1.49 (0.60–3.70); P = 0.2]. Risk factor analysis revealed that circumferential MAC was associated with a smaller mean prosthesis diameter [OR 0.07 (0.01–0.78); P = 0.03]. All-cause mortality within 30 days in patients with circumferential MAC reaching into the anterior annulus (grade IV) compared to patients with less MAC (grade I–III) was 13% (1/8) vs 2% (2/115) with a mean valve size of 24.6 vs 25.7 mm.
Figure 2:
Precise location of the extent of calcification with anatomical landmarks, subannular calcification and paravalvular leaks in all 8 patients with severe mitral annulus calcification (IV). Blue line = subannular calcification; red line = paravalvular leakage.
Table 4.
Separate intraoperative and postoperative data of the 8 patients with severe mitral annulus calcification (grade IV)
| Patient (n) | Valve type | Prosthesis size (mm) | Transfemoral access | PVL | RBBB | LVOT injury | Subannular calcification | Other complications | Cardiovascular deaths |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sym | 27 | 1 | 1 | 1 | 0 | 1 | Intrinsic tachycardia | 0 |
| 2 | S3 | 23 | 1 | 1 | 0 | 0 | 1 | Pericardial tamponade with LCO and anterolateral thoracotomy, acute kidney injury, mesenteric ischaemia | 1 |
| 3 | S3 | 26 | 1 | 2 | 0 | 0 | 1 | Right middle and lower lobe pneumonia, delirium | 0 |
| 4 | S3 | 26 | 1 | 2 | 1 | 0 | 1 | None | 0 |
| 5 | S3 | 20 | 1 | 2 | 0 | 0 | 0 | Urinary tract infection | 0 |
| 6 | Sym | 25 | 1 | 1 | 0 | 0 | 1 | None | 0 |
| 7 | S3 | 26 | 1 | 2 | 0 | 0 | 1 | Pseudoaneurysm of the right groin | 0 |
| 8 | S3 | 23 | 1 | 2 | 0 | 0 | 1 | New AV block III, pacemaker implant, new paroxysmal atrial fibrillation with tachycardia, pneumonia | 0 |
AV: atrioventricular; LCO: low cardiac output; LVOT: left ventricular outflow tract; PVL: paravalvular prosthetic leak; RBBB: right branch bundle block; S3: Sapien S3; Sym: Boston Scientific Acurate Symetis prosthesis.
Table 5:
ORs for severe mitral annulus calcification (grade IV)
| Data | OR (95% CI) | P-value |
|---|---|---|
| Valve diameter ≥23 mm | 0.07 (0.01–0.78) | 0.030 |
| Moderate paravalvular leak | 2.19 (1.21–5.46) | 0.002 |
| RBBB | 2.01 (0.39–3.06) | 0.10 |
| AV block III | 1.03 (0.38–2.78) | 0.23 |
| Postsurgical new AF | 1.49 (0.60–3.70) | 0.20 |
| Postsurgical lung oedema | 2.60 (0.48–6.31) | 0.08 |
| LCO | 3.12 (1.39–7.04) | 0.033 |
Data are presented as ORs (95% CI) and P-values.
AF: atrial fibrillation; AV: atrioventricular; CI: confidence interval; LCO: low cardiac output; OR: odds ratio; RBBB: right branch bundle block.
Subannular calcification at the anterolateral trigone represented a risk factor for LVOT injury [OR 3.54 (1.38–8.27); P = 0.001] in balloon-expandable valves and was associated with relevant rhythm disorders [OR 2.26 (1.17–5.65); P = 0.014] and female gender (7/8, 88%).
The 30-day mortality of a transcatheter aortic valve implant
With regard to 30-day mortality, a Kaplan–Meier analysis was performed and stratified by MAC (Fig. 3). In the first graph (Fig. 3A), 88% of the patients with circumferential MAC reaching into the anterior mitral annulus were alive after 30 days, whereas 96% of the patients without MAC survived (P = 0.14). In the second graph (Fig. 3B), no MAC was compared to any MAC with 93% survival after 30 days (P = 0.48). In the third graph (Fig. 3C), no MAC was compared with MAC including only the entire posterior to circumferential annulus with a mean survival of 92% (P = 0.012).
Figure 3:
Kaplan–Meier analysis for all-cause mortality within 30 days of a transcatheter aortic valve implant stratified by MAC. (A) No MAC versus less than one-third, one-third, entire posterior annulus and circumferential MAC. (B) No MAC versus any MAC. (C) No MAC versus MAC including the entire posterior annulus to circumferential MAC reaching into the anterior mitral annulus. MAC: mitral annulus calcification.
DISCUSSION
MAC represents a chronic degenerative process in the fibrous base of the MV and more commonly affects the posterior rather than the anterior annulus [1]. MAC may be initiated by factors such as shear stress passing the MV, inflammation and dysregulation of regulators of mineral metabolism [9]. Calcification was often seen at the ventricular portion of the annulus between the left ventricular myocardium and the leaflet base because the highest mechanical shear forces occur in that area and at the same time the leaflet base contacts the myocardium in each diastole. In patients in whom MAC is severe, it may invade the interannular fibrosa with resulting leaflet immobility [10]. Extensive MAC due to its direct anatomical neighbourhood in relation to the aortic valvular apparatus is still a topic of controversy, and the relevance with regard to TAVI is currently unclear.
Our results indicate that at least some degree of MAC was present in almost half of the patients with severe aortic stenosis evaluated for TAVI. However, severe MAC, defined as circumferential calcification reaching into the anterior annulus, was present in only 3% of the entire study cohort. MAC is more often seen in women [11]. In our study, MAC was present in 59% of women and in 41% of men. This result is consistent with the results in the current literature [11–13].
Of utmost importance was the fact that severe circumferential MAC was found to be strongly associated with moderate paravalvular leaks across the aortic prosthesis. Furthermore, severe MAC was associated with RBBB and low cardiac output.
The central role of the atrioventricular plane influences safety outcome and survival by different mechanisms. Implanting a prosthesis may be complicated by the inability to place an adequately sized prosthetic valve, which can lead to periprosthetic leakage, which might be one reason why severe MAC was associated with a smaller prosthesis diameter. However, Abramowitz et al. found similar rates of postprocedural paravalvular regurgitation and aortic valve gradients in patients with and without MAC (4% vs 2%); hence, their study may be underpowered. Ancona et al. [13] found a trend (without significance) towards a difference for aortic regurgitation > mild in patients with severe compared to those with non-severe MAC (5% vs 8%; P = 0.39). In contrast, in our study, circumferential calcific deposits reaching into the anterior annulus led not just to a more oval-shaped aortic annulus but also to a preferential spot for moderate paravalvular leaks in 5/8 patients (63%, Fig. 2 and Table 4).
We found that severe MAC was associated with RBBB; we also found a trend towards a higher rate of new atrioventricular block III and atrial fibrillation. These findings were not significant, and we found no association with the number of new pacemaker implants. In a recent study, the rate of new permanent pacemaker insertions for patients with severe MAC was 26% and 8% in patients with non-severe MAC (P = 0.01) [10]. Boerlage-Van Dijk et al. [14] found MAC and pre-existing RBBB as independent predictors for permanent pacemaker insertion after TAVI. There may be 2 explanations for the higher rate of RBBB in severe MAC. First, it may be due to the direct extension of calcific deposits to the region of the interventricular septum at the junction of the membranous and muscular areas. A second explanation might be the diffuse degenerative conduction disease that is frequently seen in MAC. Finally, the injury caused by the valvuloplasty balloon and the stent frame of the valve is more likely to result in a high-grade atrioventricular block. Thus, to reduce both severe paravalvular leaks and the risk of RBBB in patients with extensive MAC, we recommend implanting an oversized self-expandable prosthesis. Of course, oversizing can increase the risk of annular rupture or fistula formation. Possible substrates for annular rupture are severe subannular calcification in close proximity to the region of the muscular LVOT, twin icicles, balloon-expandable valves, multislice CT-based area oversizing ≥20% and higher frequency of postdilatation [15–17]. Possible substrates for the formation of fistulas are congenital or acquired sinus of Valsalva aneurysms, trauma and infections.
Previous studies have demonstrated a strong association between MAC and increased cardiovascular and all-cause mortality [10, 12, 18–20]. Nevertheless, in a recent study, this has been questioned: Okuno et al. [21] found that patients with severe MAC and coexisting MV disease had an increased risk of all-cause and cardiovascular death at 30 days and 1 year, whereas patients with isolated severe MAC without MV disease had survival comparable to that of patients without severe MAC and MV disease. However, these findings may be biased because severe MAC was present in this study in 18% (172/967) of the patients compared to our findings of 3%. In addition, it is unusual to find severe MAC without MV disease. In most patients, MAC had little influence on MV function because, unlike in rheumatic valve disease, there is the usual sparing of the leaflet commissures [22]. However, in patients with severe MAC, extensive annular calcification may involve leaflet commissures reaching deeply into the anterior annulus and interannular fibrosa.
Several authors suggest that MAC is a marker for atherosclerotic disease burden and end-organ damage [10]. MAC seems to be associated with cardiovascular [18, 23], metabolic [12, 24], inflammatory [25, 26] and haemostatic [27] risk factors responsible for the increased risk of mortality [10, 11]. Renal function and serum phosphate level are strongly associated with MAC, considering that mineral bone disease is a key feature of chronic renal insufficiency [12, 24].
Limitations
Limitations of the current study include its retrospective, single-centre design and its limited follow-up. Despite the fact that 43% of the patients with severe aortic stenosis have MAC, we cannot entirely rule out a certain bias due to any other confounding factors such as the type of implanted prosthesis used. In addition, no validated classification system for the assessment of MAC in CT currently exists. Therefore, we used the qualitative classification system suggested by Carpentier et al. [2] Severe MAC was found to be a significant predictor of all-cause mortality in the current study. Several mechanisms related to these results were determined previously.
CONCLUSION
In conclusion, near half of the patients with severe valvular stenosis were found to have MAC in TAVI. Extensive MAC was associated with moderate paravalvular leaks, with implications for the prosthesis size and for survival in TAVI. In severe MAC, we recommend implanting oversized self-expandable prostheses to reduce the risk of RBBB and paravalvular leaks. Considering the unsatisfactory results of TAVI in patients with severe MAC, surgical aortic valve replacement may remain a valid alternative for these patients. Hopefully, a single classification system will emerge that encompasses MV annulus morphology, annulus lesion and location and extent of associated calcification in a clinically meaningful manner. The study represents our initial clinical experience with this special patient subgroup and acknowledges the superiority of an interdisciplinary team approach [28]. The clinical value may well increase in the approaching era of transcatheter MV implants.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
After approval by the local ethics committee, we herein confirm written consent obtained from each patient in accordance with the Declaration of Helsinki to report and publish the individual patient data obtained. A statement on consent to participate from each patient in the current study was obtained.
Conflict of interest: none declared.
Glossary
- ABBREVIATIONS
- CT
Computed tomography
- LVOT
Left ventricular outflow tract
- MAC
Mitral annulus calcification
- MV
Mitral valve
- OR
Odds ratio
- RBBB
Right branch bundle block
- TAVI
Transcatheter aortic valve implant
Contributor Information
Martin Haensig, Department of Cardiothoracic Surgery, Central Clinic Hospital of Bad Berka, Bad Berka, Germany.
Thomas Kuntze, Department of Cardiothoracic Surgery, Central Clinic Hospital of Bad Berka, Bad Berka, Germany.
David Lopez Gonzalez, Department of Cardiothoracic Surgery, Central Clinic Hospital of Bad Berka, Bad Berka, Germany.
Harald Lapp, Department of Cardiology and Internal Medicine, Central Clinic Hospital of Bad Berka, Bad Berka, Germany.
Philipp Lauten, Department of Cardiology and Internal Medicine, Central Clinic Hospital of Bad Berka, Bad Berka, Germany.
Tamer Owais, Department of Cardiothoracic Surgery, Central Clinic Hospital of Bad Berka, Bad Berka, Germany; Department of Cardiothoracic Surgery, Cairo University, Cairo, Egypt.
Presented at the 34th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 8–10 October 2020.
Author contributions
Martin Haensig: Conceptualization; Formal analysis; Investigation; Methodology; Validation; Writing—original draft. Thomas Kuntze: Conceptualization; Supervision. David Lopez Gonzalez: Formal analysis; Methodology; Writing—original draft. Harald Lapp: Conceptualization; Methodology; Supervision. Philipp Lauten: Conceptualization; Formal analysis; Investigation; Methodology; Writing—original draft. Tamer Owais: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—original draft.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Cai Cheng, Gregory Pattakos and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Mejean S, Bouvier E, Bataille V, Seknadji P, Fourchy D, Tabet JY et al. Mitral annular calcium and mitral stenosis determined by multidetector computed tomography in patients referred for aortic stenosis. Am J Cardiol 2016;118:1251–7. [DOI] [PubMed] [Google Scholar]
- 2. Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcification of the mitral valve annulus: pathology and surgical management. J Thoracic Cardiovasc Surg 1996;111:718–29; discussion 29–30. [DOI] [PubMed] [Google Scholar]
- 3. Muratori M, Fusini L, Tamborini G, Ghulam Ali S, Gripari P, Fabbiocchi F et al. Mitral valve regurgitation in patients undergoing TAVI: impact of severity and etiology on clinical outcome. Int J Cardiol 2020;299:228–34. [DOI] [PubMed] [Google Scholar]
- 4. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403–18. [DOI] [PubMed] [Google Scholar]
- 5. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 2011;32:205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sellers RD, Levy MJ, Amplatz K, Lillehei CW. Left retrograde cardioangiography in acquired cardiac disease: technic, indications and interpretations in 700 cases. Am J Cardiol 1964;14:437–47. [DOI] [PubMed] [Google Scholar]
- 7. Rodes-Cabau J, Ellenbogen KA, Krahn AD, Latib A, Mack M, Mittal S et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol 2019;74:1086–106. [DOI] [PubMed] [Google Scholar]
- 8. Cribier A, Litzler PY, Eltchaninoff H, Godin M, Tron C, Bauer F et al. Technique of transcatheter aortic valve implantation with the Edwards-Sapien heart valve using the transfemoral approach. Herz 2009;34:347–56. [DOI] [PubMed] [Google Scholar]
- 9. Cavalcanti LRP, Sa M, Perazzo AM, Escorel Neto AC, Gomes RAF, Weymann A et al. Mitral annular calcification: association with atherosclerosis and clinical implications. Curr Atheroscler Rep 2020;22:9. [DOI] [PubMed] [Google Scholar]
- 10. Abramowitz Y, Kazuno Y, Chakravarty T, Kawamori H, Maeno Y, Anderson D et al. Concomitant mitral annular calcification and severe aortic stenosis: prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur Heart J 2017;38:1194–203. [DOI] [PubMed] [Google Scholar]
- 11. Massera D, Kizer JR, Dweck MR. Mechanisms of mitral annular calcification. Trends Cardiovasc Med 2020;30:289–95. [DOI] [PubMed] [Google Scholar]
- 12. Fox CS, Vasan RS, Parise H, Levy D, O'Donnell CJ, D'Agostino RB et al. ; Framingham Heart Study. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation 2003;107:1492–6. [DOI] [PubMed] [Google Scholar]
- 13. Ancona MB, Giannini F, Mangieri A, Regazzoli D, Jabbour RJ, Tanaka A et al. Impact of mitral annular calcium on outcomes after transcatheter aortic valve implantation. Am J Cardiol 2017;120:2233–40. [DOI] [PubMed] [Google Scholar]
- 14. Boerlage-Van Dijk K, Kooiman KM, Yong ZY, Wiegerinck EM, Damman P, Bouma BJ et al. Predictors and permanency of cardiac conduction disorders and necessity of pacing after transcatheter aortic valve implantation. Pacing Clin Electrophysiol 2014;37:1520–9. [DOI] [PubMed] [Google Scholar]
- 15. Girdauskas E, Owais T, Fey B, Kuntze F, Lauer B, Borger MA et al. Subannular perforation of left ventricular outflow tract associated with transcatheter valve implantation: pathophysiological background and clinical implications. Eur J Cardiothorac Surg 2017;51:91–6. [DOI] [PubMed] [Google Scholar]
- 16. Barbanti M, Yang TH, Rodes Cabau J, Tamburino C, Wood DA, Jilaihawi H et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013;128:244–53. [DOI] [PubMed] [Google Scholar]
- 17. Tochii M, Muramatsu T, Amano K, Ishikawa M, Hoshino N, Miyagi M et al. “Twin icicle” calcifications cause aortic annular rupture. Ann Thorac Surg 2018;106:e53–5. [DOI] [PubMed] [Google Scholar]
- 18. Barasch E, Gottdiener JS, Marino Larsen EK, Chaves PH, Newman AB. Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study). Am J Cardiol 2006;97:1281–6. [DOI] [PubMed] [Google Scholar]
- 19. Ramaraj R, Manrique C, Hashemzadeh M, Movahed MR. Mitral annulus calcification is independently associated with all-cause mortality. Exp Clin Cardiol 2013;18:e5–7. [PMC free article] [PubMed] [Google Scholar]
- 20. Volzke H, Haring R, Lorbeer R, Wallaschofski H, Reffelmann T, Empen K et al. Heart valve sclerosis predicts all-cause and cardiovascular mortality. Atherosclerosis 2010;209:606–10. [DOI] [PubMed] [Google Scholar]
- 21. Okuno T, Asami M, Khan F, Praz F, Heg D, Lanz J et al. Does isolated mitral annular calcification in the absence of mitral valve disease affect clinical outcomes after transcatheter aortic valve replacement? Eur Heart J Cardiovasc Imaging 2020;21:522–32. [DOI] [PubMed] [Google Scholar]
- 22. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol 2015;66:1934–41. [DOI] [PubMed] [Google Scholar]
- 23. Allison MA, Cheung P, Criqui MH, Langer RD, Wright CM. Mitral and aortic annular calcification are highly associated with systemic calcified atherosclerosis. Circulation 2006;113:861–6. [DOI] [PubMed] [Google Scholar]
- 24. Ix JH, Shlipak MG, Katz R, Budoff MJ, Shavelle DM, Probstfield JL et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2007;50:412–20. [DOI] [PubMed] [Google Scholar]
- 25. Elmariah S, Budoff MJ, Delaney JA, Hamirani Y, Eng J, Fuster V et al. Risk factors associated with the incidence and progression of mitral annulus calcification: the multi-ethnic study of atherosclerosis. Am Heart J 2013;166:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varol E, Aksoy F, Ozaydin M, Erdogan D, Dogan A. Relationship between mean platelet volume and mitral annular calcification. Blood Coagul Fibrinolysis 2013;24:189–93. [DOI] [PubMed] [Google Scholar]
- 28. Kiefer P, Seeburger J, Noack T, Schroter T, Linke A, Schuler G et al. The role of the heart team in complicated transcatheter aortic valve implantation: a 7-year single-centre experience. Eur J Cardiothorac Surg 2015;47:1090–6. [DOI] [PubMed] [Google Scholar]