Abstract
OBJECTIVES
The incidence of pneumomediastinum (PNMD), its causes of development and its effect on prognosis in the coronavirus disease 2019 (COVID-19) are not clear.
METHODS
Between March 2020 and December 2020, 427 patients with real-time reverse transcriptase-polymerase chain reaction-confirmed COVID-19 admitted to the intensive care unit were analysed retrospectively. Using receiver operating characteristic analysis, the area under the curve (AUC) for initial invasive mechanical ventilation (MV) variables such as initial peak inspiratory pressure (PIP), PaO2/FiO2 (P/F ratio), tidal volume, compliance and positive end-expiratory pressure was evaluated regarding PNMD development.
RESULTS
The incidence of PNMD was 5.6% (n = 24). PNMD development rate was 2.7% in non-invasive MV and 6.2% in MV [odds ratio (OR) 2.352, 95% confidence interval (CI) 0.541–10.232; P = 0.400]. In the multivariate analysis, the independent risk factors affecting the development of PNMD were PIP (OR 1.238, 95% CI 1.091–1.378; P < 0.001) and P/F ratio (OR 0.982, 95% CI 0.971–0.994; P = 0.004). P/F ratio (AUC 0.815, 95% CI 0.771–0.854), PIP (AUC 0.780, 95% CI 0.734–0.822), compliance (AUC 0.735, 95% CI 0.677–0.774) and positive end-expiratory pressure (AUC 0.718, 95% CI 0.668–0.764) were the best predictors for PNMD development. Regarding the multivariate analysis, independent risk factors affecting mortality were detected as age (OR 1.015, 95% CI 0.999–1.031; P = 0.04), comorbidity (OR 1.940, 95% CI 1.100–3.419; P = 0.02), mode of breathing (OR 48.345, 95% CI 14.666–159.360; P < 0.001), PNMD (OR 5.234, 95% CI 1.379–19.857; P = 0.01), positive end-expiratory pressure (OR 1.305, 95% CI 1.062–1.603; P = 0.01) and tidal volume (OR 0.995, 95% CI 0.992–0.998; P = 0.004).
CONCLUSIONS
PNMD development was associated with the initial P/F ratio and PIP. Therefore, it was considered to be related to both the patient and barotrauma. PNMD is a poor prognostic factor for COVID-19.
Keywords: Severe acute respiratory syndrome coronavirus-2, Intensive care unit, Pneumomediastinum, Incidence, Prognosis
Severe acute respiratory syndrome coronavirus 2, the cause of the coronavirus disease 2019 (COVID-19), is associated with considerable morbidity and mortality [1].
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2, the cause of the coronavirus disease 2019 (COVID-19), is associated with considerable morbidity and mortality [1]. The number of patients requiring admission to the intensive care unit (ICU) has risen dramatically in the last 12 months with the COVID-19 pandemic, and the mortality risk is high among patients with severe disease in such settings [2]. The mortality rate is between 48% and 57% in patients with COVID-19 admitted to the ICU [3–5]. Baseline patient characteristics such as older age, male sex and comorbidities and risk factors such as high positive end-expiratory pressure (PEEP) or low PaO2/FiO2 (P/F) ratio have been widely investigated, and they are associated with a high case fatality rate in patients admitted to the ICU [3–7].
However, it is not well known whether the diagnosis of pneumomediastinum (PNMD) in patients with COVID-19 is associated with unfavourable outcomes and poor prognosis. Although the incidence of PNMD (either spontaneous or ventilation related) was reported as 12% in the severe acute respiratory syndrome pandemic, this remains unclear in patients with COVID-19. It has been hypothesized that several pathophysiological mechanisms, such as Macklin’s phenomenon, high PEEP values, increased risk of alveolar damage and infection-induced alveolar septal inflammation, cause the development of PNMD in patients with COVID-19 pneumonia [4–13]. However, it should be noted that these hypotheses have not yet been supported by strong evidence.
The present study primarily investigated the frequency of occurrence of PNMD in patients with COVID-19-related pneumonia who were admitted to ICU and the mechanisms causing PNMD in these patients. We also aimed to investigate whether PNMD in patients with COVID-19 is associated with prognosis.
METHODS
The present study was approved for the use of data from patients with COVID-19 treated in the ICU of the Bakırkoy Dr. Sadi Konuk Training and Research Hospital in Turkey by the ethics committee of the hospital (2021/05).
Patients
Between 15 March 2020 and 31 December 2020, a retrospective analysis was performed on 427 patients with real-time reverse transcriptase-polymerase chain reaction-confirmed COVID-19 who were admitted to the ICU.
Data on patients who received non-invasive mechanical ventilation (NIV) and invasive mechanical ventilation (MV) in the ICU due to COVID-19 pneumonia and those who were discharged or died were included, and patients who continued to receive treatment during the study period were excluded.
All patients were treated with the COVID-19 treatment guidelines published by the Ministry of Health Scientific Advisory Board [14].
Record of the invasive mechanical ventilation settings
In the present study, the initial values of MV variables such as PEEP, peak inspiratory pressure (PIP), tidal volume (TV), compliance and P/F ratio were recorded in patients who received invasive MV (called initial MV values). In patients with PNMD, MV variable values just before the development of the PNMD (referred to as PNMD-MV values) were also recorded (Fig. 1). Thus, in patients with PNMD, the initial MV values could be compared with the MV values just before PNMD development.
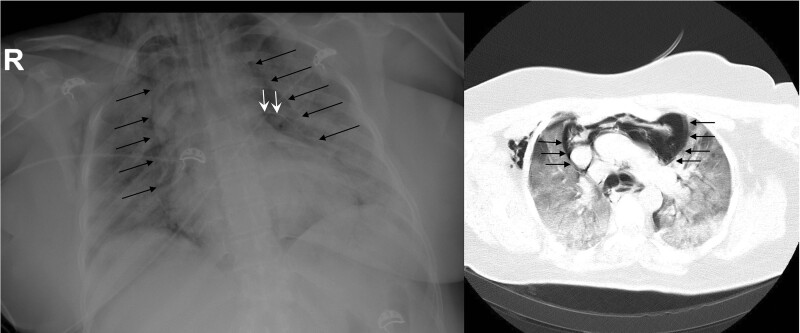
Figure 1:
Chest X-ray and chest tomography image of a female patient with pneumomediastinum in the intensive care unit (black arrow; mediastinal pleura, white arrow; pericardium).
Other prognostic factors and radiological examination
Patient characteristics and risk factors such as age, sex, comorbid disease and mortality in the ICU were also recorded.
High-resolution computed chest tomography (CT) was performed on all patients upon admission to the hospital. Chest X-ray was performed daily for each patient in the ICU. All chest X-rays were jointly reported by specialist radiologists, respiratory physicians and thoracic surgeons. During the follow-up, high-resolution CT of the thorax was performed in patients who had equivocal or atypical chest radiographs.
For each of the 427 patients, visual CT was evaluated on admission to the hospital, as described elsewhere [15]. The percentage of involvement in each lobe, as well as the overall lung ‘total severity score (TSS)’ was recorded. Each of the 5 lung lobes was assessed for the percentage of lobar involvement and classified as none (0%), minimal (1–25%), mild (26–50%), moderate (51–75%) or severe (76–100%), with corresponding scores of 0, 1, 2, 3 or 4. TSS was calculated by summing the 5 lobe scores ranging from 0 to 20 [15].
All patients were analysed to examine the incidence of PNMD and its effect on prognosis. To examine the relationship between invasive MV and PNMD, only patients undergoing MV were analysed.
Statistical analysis
The data were entered into the Statistical Package for the Social Sciences (IBM SPSS 14 Statistics for Windows, Version 23.0, Armonk, NY, USA). Descriptive statistics were used to summarize pertinent study information. Quantitative variables are presented as mean, maximum (max) and minimum (min) values and qualitative variables are presented as percentage values. The Student’s t-test was used for comparisons between the groups. The Pearson’s χ2 test was used for the analysis of qualitative variables; however, the Fisher’s exact test was used if the sample size was small. Non-parametric continuous variables, presented as median values, were compared using the Mann–Whitney U-test. Factors with a P-value of <0.05, as determined by univariate analysis, were considered potential factors in the multiple regression analysis. Therefore, some covariates were excluded from the models as they did not affect the development of PNMD or mortality in the univariate analysis. Since ventilation parameters were only in patients in the MV group, different multivariable logistic regression analyses were made when those parameters were statistically significant in the univariate analysis. The continuous variables were not categorized in the multivariable logistic regression analyses, and the stepwise regression analysis was used in the present study. Receiver operating characteristic (ROC) curves were drawn, and the areas under the ROC curves (AUCs) were calculated. The ‘optimal’ cut-off points calculated using ROC analysis for the development of PNMD in invasively ventilated patients were determined using the best sensitivity and specificity scores. Even if some MV parameters are not found to be significant in the multivariable logistic regression analyses, it was decided that they were added to the ROC analyses. Statistical significance was set at P-value <0.05.
RESULTS
The demographic and clinical characteristics of the patients are shown in Table 1. The patients had a median age of 59.9 (min = 19 years, max = 100 years, interquartile range = 18.7) and most of them were male (n = 288). At least 1 comorbidity was seen in 331 patients (87.5%). The prevalence of pre-existing lung disease was 14.1%. Radiologically, pneumonia was observed to be frequently bilateral (n = 382, 89.5%) and the mean TSS was 7.3 (min = 1, max = 18, interquartile range = 9.7). A total of 354 (82.9%) patients were supported with MV. PEEP, PIP, TV, P/F ratio and compliance values for patients with MV are shown in Table 1.
Table 1:
Demographics, clinical variables and MV settings data of the patients
| Variables | Outcomes |
|---|---|
| Age (years), mean ± SD | 59.9 ± 16.1 |
| Gender, n/% | |
| Female | 139/32.6 |
| Male | 288/67.4 |
| Comorbidity, n/% | 331/87.5 |
| Number of comorbidities, n/% | |
| Non | 96/22.5 |
| 1 | 115/26.9 |
| 2 | 93/21.8 |
| 3 or more | 123/28.8 |
| Pre-existing lung disease. n/% | 60/14.1 |
| Side of pneumonia, n/% | |
| Unilateral | 45/10.5 |
| Bilateral | 382/89.5 |
| TSS, mean ± SD | 7.3 ± 4.1 |
| Mode of breathing, n/% | |
| NIV | 73/17.1 |
| MV | 354/82.9 |
| PEEP (cmH2O),a mean ± SD | 8.8 ± 1.4 |
| PIP (cmH2O),a mean ± SD | 26.3 ± 4.8 |
| TV (ml/kg),a mean ± SD | 456.7 ± 84.2 |
| PaO2/FiO2 ratio (mmHg),a mean ± SD | 172.6 ± 68.5 |
| Compliance (ml/cmH2O),a mean ± SD | 32.9 ± 10.2 |
| PNMD development, n/% | |
| Yes | 24/5.6 |
| No | 403/94.4 |
| Pneumothorax, n/% | 11/2.6 |
| Status, n/% | |
| Discharge | 180/42.2 |
| Mortality | 247/57.8 |
Calculation was made in mechanically ventilated patients (n = 354).
MV: invasive mechanical ventilation; NIV: non-invasive mechanical ventilation; PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; PNMD: pneumomediastinum; SD: standard deviation: TSS: overall lung total severity score; TV: tidal volume.
Incidence of pneumomediastinum and factors affecting pneumomediastinum
During the follow-up period, 5.6% (n = 24) of patients had PNMD. The average time between ICU admission and the first documented PNMD was 4.2 days (min = 2 days, max = 25 days, interquartile range = 7). Of these patients with PNMD, 5 subsequently developed a pneumothorax (4 of them were treated using chest tube, 1 patient treating using nasal oxygen had a pneumothorax volume <5%). Isolated pneumothorax developed in 6 patients. In 2 patients having a tension PNMD, a mediastinal tube placement was performed under general anaesthetic to alleviate their haemodynamic instability with a right thoracoscopic surgery and a mediastinotomy.
On comparing the patients with and without PNMD, age (P = 0.009), PEEP (P < 0.001), PIP (P < 0.001), P/F ratio (P < 0.001) and compliance (P < 0.001) significantly influenced the development of PNMD. Sex, comorbidity, number of comorbidities, pre-existing lung disease history, bilateral pneumonia, TSS, supporting MV and TV were found not to affect PNMD development (Table 2). The PNMD development rate was 2.7% (n = 2) for NIV support and 6.2% (n = 22) for MV support; however, there was no statistical difference between the 2 modes of breathing [odds ratio (OR) 2.352, 95% confidence interval (CI) 0.541–10.232; P = 0.400]. PNMD developed on the second day in the NIV group, while it developed on the fifth day in the MV group (P = 0.116).
Table 2:
Comparisons between patients with PNMD and patients without PNMD
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Absence of PNMD (n = 403) | Presence of PNMD (n = 24) | P-value | OR | 95% CI | P-value | |
| Age (years), mean ± SD | 60.4 ± 15.9 | 51.2 ± 16.1 | 0.009 | 0.996 | 0.927–1.070 | 0.915 |
| Gender, n/% | 0.265 | |||||
| Female | 134/33.3 | 5/20.8 | ||||
| Male | 269/66.7 | 19/79.2 | ||||
| Comorbidity rate, n/% | 313/77.7 | 18/75.0 | 0.761 | |||
| Pre-existing lung disease, n/% | 56/13.9 | 4/16.7 | 0.761 | |||
| Side of pneumonia, | 1.000 | |||||
| Unilateral | 43/10.7 | 2/8.3 | ||||
| Bilateral | 360/89.3 | 22/91.7 | ||||
| TSS, mean ± SD | 7.2 ± 4.0 | 8.9 ± 5.4 | 0.198 | |||
| Mode of breathing, n/% | 0.400 | |||||
| NIV | 71/17.6 | 2/8.3 | ||||
| MV | 332/82.4 | 22/91.7 | ||||
| PEEP (cmH2O),a mean ± SD | 8.8 ± 1.4 | 9.8 ± 1.0 | <0.001 | 1.082 | 0.725–1.616 | 0.698 |
| PIP (cmH2O),a mean ± SD | 25.9 ± 4.4 | 32.3 ± 6.6 | <0.001 | 1.238 | 1.091–1.378 | <0.001 |
| TV (ml/kg),a mean ± SD | 457.4 ± 83.0 | 445.6 ± 102.3 | 0.580 | |||
| PaO2/FiO2 ratio (mmHg),a mean ± SD | 176.6 ± 67.6 | 111.7 ± 51.5 | <0.001 | 0.982 | 0.971–0.994 | 0.004 |
| Compliance (ml/cmH2O),a mean ± SD | 33.3 ± 10.2 | 26.5 ± 7.1 | <0.001 | 0.990 | 0.923–1.063 | 0.775 |
Calculation was made in mechanically ventilated patients (n = 354).
CI: confidence interval; MV: invasive mechanical ventilation; NIV: non-invasive mechanical ventilation; OR: odds ratio; PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; PNMD: pneumomediastinum; SD: standard deviation; TSS: overall lung total severity score; TV: tidal volume. Boldface indicates statistical significance.
In the multivariate analysis, the only independent risk factors affecting the development of PNMD were the PIP (OR 1.238, 95% CI 1.091–1.378; P < 0.001) and P/F (OR 0.982, 95% CI 0.971–0.994; P = 0.004).
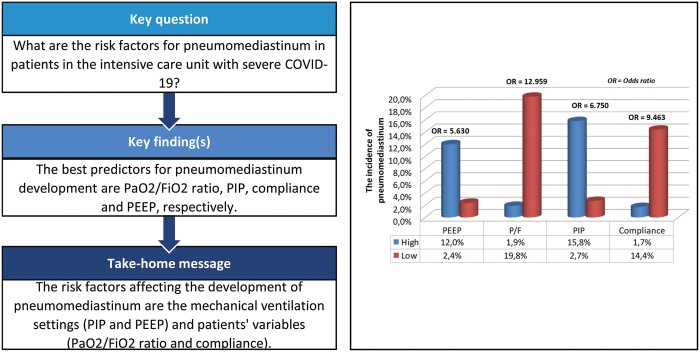
The best predictors for PNMD development were found to be P/F ratio (AUC 0.815, 95% CI 0.771–0.854) and PIP (AUC 0.780, 95% CI 0.734–0.822) (Table 3). The patients with the highest AUC values were divided into subgroups using optimal cut-off values in terms of P/F, PIP, compliance and PEEP. Patients with a high P/F ratio (n = 268) were found to develop statistically less PNMD than patients with a low P/F ratio (n = 86) (1.9% vs 19.8%, P < 0.001, OR 12.959, 95% CI 4.618–36.364). Patients who received high PIP (n = 95) developed statistically more PNMD than those who received low PIP (n = 259) (15.8% vs 2.7%, P < 0.001, OR 6.750, 95% CI 2.659–17.138), while patients with high compliance (n = 229) had less PNMD than those with low compliance (n = 125) (1.7% vs 14.4%, P < 0.001, OR 9.463, 95% CI 3.126–28.644). In addition, patients with high PEEP (n = 142) were found to have more PNMD than those who received low PEEP (n = 212) (12.0% vs 2.4%, P < 0.001, OR 5.630, 95% CI 2.027–15.638).
Table 3:
AUC for invasive mechanical ventilation variables were evaluated with regards to PNMD development in invasively ventilated patients
| a) Determination of AUC and threshold values | ||||||
|---|---|---|---|---|---|---|
| Variables | AUC | 95% CI | Cut-offa | Sensitivity | Specificity | P-value |
| PEEP | 0.718 | 0.668–0.764 | >9 | 77.2 | 62.3 | <0.001 |
| PIP | 0.780 | 0.734–0.822 | >29 | 68.1 | 73.4 | <0.001 |
| PaO2/FiO2 ratio | 0.815 | 0.771–0.854 | ≤120 | 77.2 | 79.2 | <0.001 |
| Compliance | 0.735 | 0.677–0.774 | ≤29 | 81.2 | 67.7 | <0.001 |
| TV | 0.535 | 0.482–0.588 | ≤300 | 13.6 | 96.3 | 0.681 |
| TSS | 0.578 | 0.529–0.625 | >13 | 29.1 | 91.5 | 0.271 |
| b) Comparison of PNMD incidence in subgroupsb | ||||||
| Variables | PNMD incidence, n/% | OR | 95% CI | |||
| PaO2/FiO2 | ||||||
| High PaO2/FiO2 (n = 268) | 5 (1.9) | 1 | ||||
| Low PaO2/FiO2 (n = 86) | 17 (19.8) | 12.959 | 4.618–36.364 | |||
| PIP | ||||||
| Low PIP (n = 259) | 7 (2.7) | 1 | ||||
| High PIP (n = 95) | 15 (15.8) | 6.750 | 2.659–17.138 | |||
| Compliance | ||||||
| High compliance (n = 229) | 4 (1.7) | 1 | ||||
| Low compliance (n = 125) | 18 (14.4) | 9.463 | 3.126–28.644 | |||
| PEEP | ||||||
| Low PEEP (n = 212) | 5 (2.4) | 1 | ||||
| High PEEP (n = 142) | 17 (12.0) | 5.630 | 2.027–15.638 | |||
Numerical values with the best sensitivity and specificities were accepted as cut-offs.
Using the determined cut-offs, patients were divided into subgroups according to their data above or below the threshold values.
AUC: area under the curve; CI: confidence interval; OR: odds ratio; PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; PNMD: pneumomediastinum; TSS: overall lung total severity score; TV: tidal volume. Boldface indicates statistical significance.
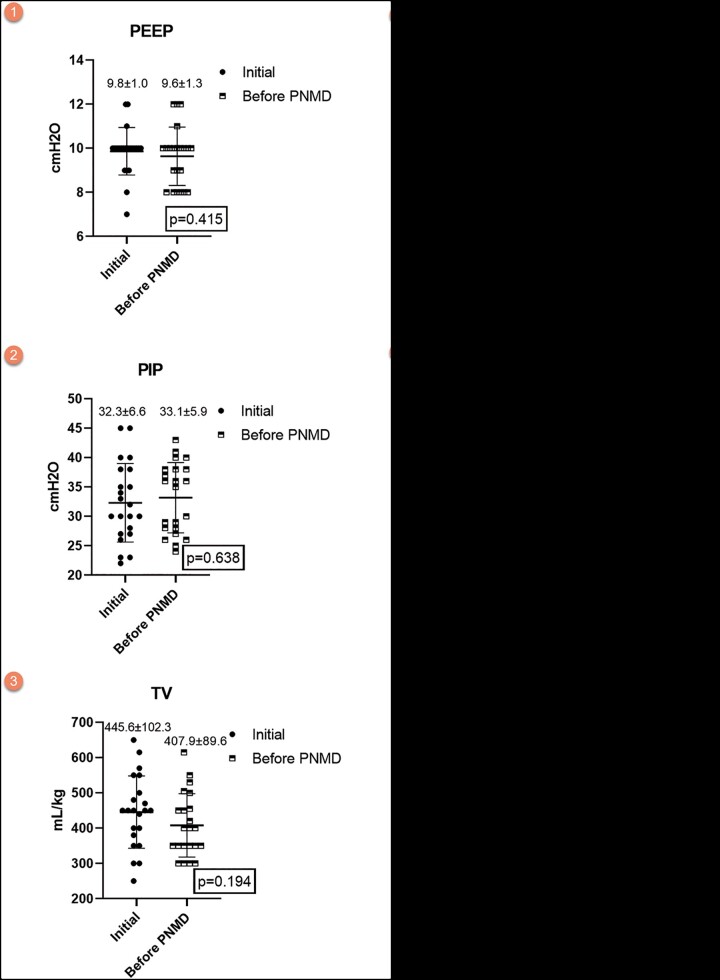
In patients with PNMD, when the initial MV values were compared with the MV values just before the development of PNMD, it was observed that PEEP was almost similar, whereas TV, P/F and compliance decreased in addition to the increase in PIP (Fig. 2). However, these changes were not statistically significant.
Figure 2:
Comparisons of the initial mechanical ventilation settings in patients with PNMD and the mechanical ventilation settings just before PNMD development. PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; PNMD: pneumomediastinum; TV: tidal volume.
Relationship between mortality and pneumomediastinum and the factors affecting mortality
Mortality was observed in 57.8% (n = 247) of the patients. Mortality was observed in 83.3% (n = 20) of the patients with PNMD (n = 24), this rate was 56.3% (n = 227) in those without PNMD (n = 403) (P = 0.009). Age (P < 0.001), comorbidity (P < 0.001), mode of breathing (P < 0.001), initial PEEP (P < 0.001), PIP (P = 0.003), TV (P = 0.002), P/F ratio (P < 0.001) and compliance (P = 0.003) were found to affect mortality. In 5 patients having a pneumothorax + PNMD, the mortality rate was 80% (n = 4), whereas it was 84.2% (n = 16) in 19 patients having an isolated PNMD (P = 1.000).
Considering the multivariate analysis, independent risk factors affecting mortality were age (OR 1.015, 95% CI 0.999–1.031; P = 0.04), comorbidity (OR 1.940, 95% CI 1.100–3.419; P = 0.02), mode of breathing (OR 48.345, 95% CI 14.666–159.360; P < 0.001), PNMD (OR 5.234, 95% CI 1.379–19.857; P = 0.01), initial PEEP (OR 1.305, 95% CI 1.062–1.603; P = 0.01) and TV (OR 0.995, 95% CI 0.992–0.998; P = 0.004) (Table 4).
Table 4:
Factors affecting mortality
| Variables | Univariate analysis |
Multivariate analysisa |
Multivariate analysisb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Discharge (n = 180) | Mortality (n = 247) | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age (years), mean ± SD | 56.2 ± 17.0 | 62.7 ± 14.8 | <0.001 | 1.015 | 0.999–1.031 | 0.04 | 1.015 | 0.999–1.032 | 0.04 |
| Gender, n/% | 0.901 | ||||||||
| Female | 58/32.2 | 81/32.8 | |||||||
| Male | 122/67.8 | 166/67.2 | |||||||
| Comorbidity rate, n/% | 124/68.9 | 207/83.8 | <0.001 | 1.940 | 1.100–3.419 | 0.02 | 2.179 | 1.183–4.014 | 0.01 |
| Pre-existing lung disease, n/% | 20/11.1 | 40/16.2 | 0.136 | ||||||
| Side of pneumonia, n/% | 0.757 | ||||||||
| Unilateral | 18/10.0 | 27/10.9 | |||||||
| Bilateral | 162/90.0 | 220/89.1 | |||||||
| TSS, mean ± SD | 7.1 ± 3.8 | 7.4 ± 4.3 | 0.841 | ||||||
| Mode of breathing, n/% | <0.001 | 48.345 | 14.666–159.360 | <0.001 | NA | NA | NA | ||
| NIV | 70/38.9 | 3/1.2 | |||||||
| MV | 110/61.1 | 244/98.8 | |||||||
| Pneumothorax, n/% | 0.767 | ||||||||
| Yes | 4/2.2 | 7/2.8 | |||||||
| No | 176/97.8 | 240/97.2 | |||||||
| PNMD development, n/% | 0.009 | 5.234 | 1.379–19.857 | 0.01 | 4.861 | 1.328–21.439 | 0.04 | ||
| Yes | 4/2.2 | 20/8.1 | |||||||
| No | 176/97.8 | 227/91.9 | |||||||
| PEEP (cmH2O),c mean ± SD | 8.5 ± 1.4 | 9.0 ± 1.4 | <0.001 | 1.305 | 1.062–1.603 | 0.01 | |||
| PIP (cmH2O),c mean ± SD | 25.3 ± 5.0 | 26.7 ± 4.7 | 0.003 | 0.989 | 0.930–1.053 | 0.743 | |||
| TV (ml/kg),c mean ± SD | 478.6 ± 85.4 | 446.8 ± 82.0 | 0.002 | 0.995 | 0.992–0.998 | 0.004 | |||
| PaO2/FiO2 ratio (mmHg),c mean ± SD | 190.7 ± 60.9 | 164.4 ± 70.2 | <0.001 | 0.996 | 0.993–1.001 | 0.125 | |||
| Compliance (ml/cmH2O),c mean ± SD | 35.0 ± 9.1 | 31.9 ± 10.5 | 0.003 | 0.998 | 0.970–1.028 | 0.940 | |||
Multivariate analysis was applied in all patients. Since ventilation parameters were only for patients in the MV group, those were not included in this analysis.
Multivariate analysis was made on patients in MV group (n = 354).
Calculation was made in mechanically ventilated patients (n = 354).
CI: confidence interval; MV: invasive mechanical ventilation; NA: not applıcable; NIV: non-invasive mechanical ventilation; OR: odds ratio; PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; PNMD: pneumomediastinum; SD: standard deviation; TSS: overall lung total severity score; TV: tidal volume. Boldface indicates statistical significance.
DISCUSSION
Spontaneous PNMD is a benign and rare condition, with an incidence of <1:44 000 [16]. It is a self-limiting condition that occurs when extraluminal gas enters the mediastinum [17, 18]. The most common cause of secondary PNMD is invasive MV. In patients admitted to the ICU for any reason, the incidence of PNMD varies between 7.4% and 36% and greatly depends on the underlying indication for MV [19–21]. As recent studies, the rate of PNMD has dropped below 10% [22]. In a prospective study investigating the effect of severe acute respiratory syndrome pneumonia, among 75 patients, 9 (12%) developed PNMD [9].
Three recent studies reported the rates of PNMD in patients with COVID-19 admitted to the ICU to be in the range of 9.4–13.6% [4–7]. Although the incidence in the current study (5.6%) was comparable with the incidences in these studies, it was found to be lower than the incidence in these previous studies. There may be several reasons for this. First, the current study included the MV and NIV groups. In a retrospective observational study investigating 154 patients with COVID-19 treated with NIV, PNMD occurred in 1.3% of the patients [8]. Second, these studies included hospitalized patients with COVID-19 during the early phase of the pandemic (February–April and March–April). The current study, however, comprised the first 10 months of the pandemic. Over time, the incidence may have decreased due to the standardization of the follow-up of patients with COVID-19 and the widespread use of lung protective ventilation (low TV, ∼6 ml/kg and a plateau airway pressure restricted to ∼28–30 cmH2O). In the initial months of the pandemic, it was widely suggested that the respiratory failure in patients with COVID-19 was due to viral pneumonia that progressed to acute respiratory distress syndrome (ARDS). Thus, several severely ill patients were mechanically ventilated at high pressures [4]. However, changes were made in the MV support in line with the new recommendations such as low TV ventilation, PEEP not exceeding 10 cmH2O and maximized up to 12 cmH2O, keeping SaO2 target values between 88% and 92% [23]. In addition, since the present study is a large patient series that specifically examines the relationship between COVID-19 and PNMD in the literature, the actual incidence may have decreased with the increase in the number of patients. This could have been because of decrease in the rate of progressive parenchymal inflammation in patients with the help of new treatment methods [24].
PNMD in patients with COVID-19 is poorly understood and is an uncommon clinical finding [10, 18, 25]. In the present study, PNMD in COVID-19 was associated with ventilatory variables. In the univariate analysis, it was determined that high initial PEEP, high initial PIP, low P/F ratio and low compliance increased the PNMD incidence, while in the multivariate analysis, only high PIP and low initial P/F ratio were found to affect PNMD development. Nevertheless, in patients with PNMD, although not statistically significant, it was observed that the PIP value was higher, and TV, P/F and compliance were lower on the day of just before the development of the PNMD than on the first ventilation day. The changes in ventilation variables over time do not affect the PNMD development. Similar to our study, there was no significant difference in the initial and maximum ventilation variables in a case series of 5 patients with PNMD [11].
The best predictors for PNMD development in COVID-19 were P/F ratio, PIP, compliance and PEEP. In the comparisons made using threshold values, it was found that PNMD increased 12.9 times in patients with low P/F, 6.7 times in those with high PIP, 9.4 times in those with low compliance and 5.6 times in those with high PEEP. Thus, the association between barotrauma and the presence of air outside the tracheobronchial tree in mechanically ventilated patients with COVID-19 should be considered. The present study is the first to compare PNMD development in terms of ventilation variables by determining threshold values using ROC analysis. In an NIV study using the threshold value for PEEP, PNMD occurred only in the high PEEP group in patients with severe COVID-19 (4.7%). However, the threshold value determined in this study was not identified by ROC analysis.
ARDS is a major risk factor for PNMD in patients with MV [19]. Historically, many studies involving patients with ARDS have revealed a relationship between both PIP and PEEP and PNMD [5, 21]. Although PIP and PEEP are frequently cited risk factors for pulmonary barotrauma, some studies have claimed that trans-alveolar pressure and alveolar distention, rather than airway pressures themselves, are the major factors that lead to barotrauma and ventilator-induced lung injury [5, 26]. However, since the effects of barotrauma on PNMD development were revealed with the results obtained in the present study, it cannot be inferred that the underlying COVID-19 parenchymal damage alone causes PNMD. Although TSS was found to be high in the PNMD group, the difference was not statistically significant supports this view. The possible effect of barotrauma is superimposed on the direct effect of lung damage related to COVID-19 pneumonia [8]. The current findings may support the emerging theories of lung damage in COVID-19. It must be considered that the combination of the barotrauma from high ventilator pressure and alveolar damage predisposes the patient cohort to PNMD [11]. The development of PNMD in the NIV group indicated the presence of alveolar damage. The development of PNMD in the NIV group indicated the presence of alveolar damage. Neither median minute ventilation nor the large swings in transpulmonary pressure resulting from spontaneous respiratory effort can be limited in NIV, by nature [27]. This may compound the reduced functional lung volume seen in COVID-19 pneumonia and ARDS, resulting in patient self-induced lung injury. In the early phases of ARDS, before the patient has fatigued or has been sedated, the high transpulmonary pressures associated with spontaneous vigorous inspiratory effort may contribute to the damage, it was termed ‘patient self-induced lung injury' [28].
Some studies indicated that pre-existing lung disease seem to have a role in the occurrence of PNMD, whereas others did not indicate [6,27,29]. In the present study, pre-existing lung disease history was found not to affect PNMD development. There could be several reasons for this. First, the rates of pre-existing lung disease vary from study to study. Second, the underlying lung disease subtype in studies may differ. Third and most importantly, it was thought that the primary main cause of PNMD in COVID-19 is the degree of lung destruction, not the underlying lung disease.
Independent risk factors affecting mortality were age, comorbidity, MV, PEEP, PNMD and TV. PNMD was found to increase the mortality risk 5.2 times. Similar to the present study, published studies on the relationship between PNMD and COVID-19 have found that the diagnosis of PNMD in patients with COVID-19 admitted to the ICU is associated with unfavourable outcomes and worse prognosis [6, 8, 10, 11]. Some studies have reported that PNMD has no statistically significant effect on mortality in patients with COVID-19 [4, 5]. The authors of those studies associated this with younger patients who developed PNMD [4].
The current study was presented to highlight the increased risk of this potentially life-threatening complication among the COVID-19 patient cohort and offer guidance for its management to physicians. Considering the association of PNMD with the MV settings (PIP and PEEP) and patients’ variables (P/F ratio and compliance), it was suggested to use the lung protective mechanism (lower PEEP and lower PIP) as possible to prevent PNMD. Moreover, it should be noted that PNMD should not be ignored in patients with low P/F ratio and compliance. To minimize the risk of barotrauma such as PNMD, patients should be ventilated with the least damaging settings possible to achieve adequate oxygenation. Considering the specific features of COVID-19 ARDS that may differ from non-COVID-19 ARDS, the message to take home, in the present study, that in patients requiring escalating PEEP and PIP, efforts should be focused on identifying potentially reversible causes and strategies to reduce the MV settings should be sought [4–6, 23].
Limitations
First, our study includes the incidence of PNMD in patients admitted to the ICU caused by COVID-19. Compared to patients with severe COVID-19 who require ICU admission, this incidence is likely to decrease in patients who do not require ICU admission. Second, PNMD may develop secondary to intubation [12]. Iatrogenic PNMD, although rare, is usually evident within 24 h after intubation [10]. In the current study, PNMD developed 2 days after intubation at the earliest. In addition, as CT was not performed frequently in all patients, minimal PNMDs developed in patients may have been overlooked.
CONCLUSION
PNMD is not uncommon in patients admitted to the ICU for COVID-19. High levels of MV variables such as PIP and PEEP and patient variables such as P/F and compliance were found to affect PNMD. This suggests that PNMD due to alveolar damage develops with the excess of parenchymal inflammation developing secondary to COVID-19 and the increase in MV variables used for the treatment of respiratory failure that develops as a result. Age, comorbidity, MV, PEEP, TV and PNMD increased the risk of mortality in severe COVID-19.
Funding
The authors received no specific funding for this work
Conflict of interest: The authors declare that there has no conflict of interest.
Author contributions
Servet Ö3zdemir:
Conceptualization; Data curation; Formal analysis; Investigation; Resources; Writing—original draft; Writing—review & editing. Deniz Özel Bilgi: Data curation; Formal analysis; Methodology; Software. Gülsüm Oya Hergünsel: Conceptualization; Supervision. Necati Çitak: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Validation; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Jose Belda-Sanchis, Yuji Shiraishi and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Glossary
- ABBREVIATIONS
- ARDS
Acute respiratory distress syndrome
- AUC
Area under the curve
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- CT
Chest tomography
- ICU
Intensive care unit
- MV
Mechanical ventilation
- NIV
Non-invasive mechanical ventilation
- OR
Odds ratio
- P/F ratio
PaO2/FiO2
- PEEP
Positive end-expiratory pressure
- PIP
Peak inspiratory pressure
- PNMD
Pneumomediastinum
- ROC
Receiver operating characteristic
- TSS
Total severity score
- TV
Tidal volume
Contributor Information
Servet Özdemir, Thoracic Surgery Department, Bakırköy Dr. Sadi Konuk Research and Education Hospital, İstanbul, Turkey.
Deniz Özel Bilgi, Anesthesiology and Reanimation Department, Bakırköy Dr. Sadi Konuk Research and Education Hospital, İstanbul, Turkey.
Gülsüm Oya Hergünsel, Anesthesiology and Reanimation Department, Bakırköy Dr. Sadi Konuk Research and Education Hospital, İstanbul, Turkey.
Necati Çitak, Thoracic Surgery Department, Bakırköy Dr. Sadi Konuk Research and Education Hospital, İstanbul, Turkey.
REFERENCES
- 1. Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med 2021;9:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Özdemir S, Bilgi DÖ, Köse S, Oya G. Pneumothorax in patients with coronavirus disease 2019 pneumonia with invasive mechanical ventilation. Interact CardioVasc Thorac Surg 2021;32:351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G et al. ; COVID-19 Lombardy ICU Network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020;180:1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGuinness G, Zhan C, Rosenberg N, Azour L, Wickstrom M, Mason DM et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology 2020;297:E252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lemmers DHL, Abu Hilal M, Bnà C, Prezioso C, Cavallo E, Nencini N et al. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res 2020;6:00385–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belletti A, Palumbo D, Zangrillo A, Fominskiy EV, Franchini S, Dell'Acqua A et al. ; COVID-BioB Study Group. Predictors of pneumothorax/pneumomediastinum in mechanically ventilated COVID-19 patients. J Cardiothorac Vasc Anesth 2021. Feb 6:S1053-0770(21)00103-8. doi: 10.1053/j.jvca.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards JA, Breitman I, Bienstock J, Badami A, Kovatch I, Dresner L et al. Pulmonary barotrauma in mechanically ventilated coronavirus disease 2019 patients: a case series. Ann Med Surg (Lond) 2020;61:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonio G, Federica S, Brambilla AM, Chiara C, Stella I, Francesco B et al. Occurrence of pneumothorax and pneumomediastinum in COVID-19 patients during non-invasive ventilation with continuous positive airway pressure. medRxiv 2020; doi:10.1101/2020.08.31.20185348. [Google Scholar]
- 9. Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Azzawi M, Douedi S, Alshami A, Al-Saoudi G, Mikhail J. Spontaneous subcutaneous emphysema and pneumomediastinum in COVID-19 patients: an indicator of poor prognosis? Am J Case Rep 2020;21:e925557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wali A, Rizzo V, Bille A, Routledge T, Chambers AJ. Pneumomediastinum following intubation in COVID-19 patients: a case series. Anaesthesia 2020;75:1076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Volpi S, Ali JM, Suleman A, Ahmed RN. Pneumomediastinum in COVID-19 patients: a case series of a rare complication. Eur J Cardiothorac Surg 2020;58:646–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 2020;21:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Ministry of Health Scientific Advisory Board. COVID-19 Treatment Guide 2020/2021. https://covid19.saglik.gov.tr/Eklenti/39061/0/covid-19rehberieriskinhastatedavisipdf.pdf Accessed date: 21.06.2021 [Article in Turkish].
- 15. Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol 2020;30:4407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macia I, Moya J, Ramos R, Morera R, Escobar I, Saumench J et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg 2007;31:1110–14. [DOI] [PubMed] [Google Scholar]
- 17. Byun CS, Choi JH, Hwang JJ, Kim DH, Cho HM, Seok JP. Vacuum-assisted closure therapy as an alternative treatment of subcutaneous emphysema. Korean J Thorac Cardiovasc Surg 2013;46:383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou C, Gao C, Xie Y, Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis 2020;20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simon M, Braune S, Laqmani A, Metschke M, Berliner C, Kalsow M et al. Value of computed tomography of the chest in subjects with ARDS: a retrospective observational study. Respir Care 2016;61:316–23. [DOI] [PubMed] [Google Scholar]
- 20. Caceres M, Braud RL, Maekawa R, Weiman DS, Garrett HE Jr. Secondary pneumomediastinum: a retrospective comparative analysis. Lung 2009;187:341–6. [DOI] [PubMed] [Google Scholar]
- 21. Gammon RB, Shin MS, Buchalter SE. Pulmonary barotrauma in mechanical ventilation. Patterns and risk factors. Chest 1992;102:568–72. [DOI] [PubMed] [Google Scholar]
- 22. Diaz R, Heller D. Barotrauma and mechanical ventilation. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2021. https://www.ncbi.nlm.nih.gov/books/NBK545226/ (8 August 2020, date last accessed). [PubMed]
- 23. Dondorp AM, Hayat M, Aryal D, Beane A, Schultz MJ. Respiratory support in COVID-19 patients, with a focus on resource-limited settings. Am J Trop Med Hyg 2020;102:1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolani S, Houari N, Haloua M, Alaoui Lamrani Y, Boubbou M, Serraj M et al. Spontaneous pneumomediastinum occurring in the SARS-COV-2 infection. IDCases 2020;21:e00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions: an interpretation of the clinical literature in the light of laboratory experiment. Medicine 1944;23:281–358. [Google Scholar]
- 27. Jones E, Gould A, Pillay TD, Khorasanee R, Sykes R, Bazo-Alvarez JC et al. Subcutaneous emphysema, pneumomediastinum, and pneumothorax in critically ill patients with coronavirus disease 2019: a retrospective cohort study. Crit Care Explor 2020;2:e0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 2020;323:2329–30. [DOI] [PubMed] [Google Scholar]
- 29. Tacconi F, Rogliani P, Leonardis F, Sarmati L, Fabbi E, De Carolis G et al. Incidence of pneumomediastinum in COVID-19: a single-center comparison between 1st and 2nd wave. Respir Investig 2021;59:661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]