Abstract
Introduction
Phosphodiesterase 5 inhibitors are the predominant treatment option for erectile dysfunction.
Aim
This study evaluates the efficacy and safety of sildenafil orally disintegrating strips for the treatment of erectile dysfunction.
Methods
One hundred twenty erectile dysfunction patients were enrolled in a prospective, randomized, controlled crossover study and allocated into 2 groups of 60 participants. Patients were either treated with sildenafil strips or tablets for 8 weeks after which they crossed over into the alternate treatment formulation for another 8 weeks following a 4-week wash-out period. Each participant was assessed 8 times throughout the study period and their formulation preference registered at the end of the study.
Main outcomes and measures
Changes in the abridged International Index of Erectile Function (IIEF-5) score and Erection Hardness Score (EHS) resulting from sildenafil orally disintegrating strip or tablet treatments were the primary end points, with differences in onset of action, duration of action, and incidence of adverse events between the 2 formulations included as secondary end points.
Results
Both sildenafil formulations were effective in treating patients with erectile dysfunction. There was significant improvement of erectile function in term of IIEF-5 score and EHS from both formulations. The number and type of adverse events were also comparable. Likewise, there were no statistically significant differences between the earliest onset of action times and longest duration of action times. However, the results showed a 7.1-minute earlier onset of action time for orally disintegrating strips that may be considered as clinically meaningful by some patients.
Conclusion
Sildenafil orally disintegrating strips are a safe and effective alternative to the conventional tablet formulation for the treatment of erectile dysfunction. Sangkum P, Sirisopana K, Matang W, et al. Efficacy of the Orally Disintegrating Strip Sildenafil for the Treatment of Erectile Dysfunction: A Prospective, Randomized Trial. Sex Med 2021;9:100453.
Key Words: Sildenafil, Orally Disintegrating Strip, Erectile Dysfunction, Crossover Study
INTRODUCTION
Erectile dysfunction (ED) is defined as the inability to attain and/or maintain penile erection sufficient for satisfactory sexual performance.1 In an effort to estimate the prevalence of ED in Thailand, a nationwide study was conducted by the Thai Erectile Dysfunction Epidemiologic Study Group in 1998.2 From a population of 1,250 men aged 40–70 years residing in urban or municipal areas, ED was diagnosed in 37.5% of participants for whom ED prevalence increased with advancing age.3 Similarly, recent reports establish that the prevalence of ED is higher in populations with risk factors such as diabetes, hypertension, cardiovascular disease, and smoking.4, 5, 6, 7 By virtue of the nature of the condition, ED has negative effects not only on a patient's quality of life but also on that of their partner.8, 9, 10
Currently, phosphodiesterase 5 (PDE5) inhibitors are the predominant treatment for ED. PDE5 inhibitors act by suppressing the conversion of cyclic guanosine monophosphate (cGMP) to guanosine monophosphate (GMP) during penile erection. The ensuing increase in cGMP results in heightened and prolonged relaxation of smooth muscle, vasodilation of blood vessels, and subsequent improvement of penile erection. Introduced in 1998, sildenafil was the first PDE5 inhibitor to be approved for the treatment of ED. During its first 6 years on the market, sildenafil was used to treat more than twenty million men. Initial treatment regimens begin with 50 mg sildenafil, followed by dosage adjustments taking into account treatment responses and side effects. Sildenafil has an action duration of 8 hours with a Tmax of 30-60 minutes. However, Tmax can last longer in patients with a prolonged gastric emptying time.11 In addition, the efficacy of sildenafil decreases when it is used by diabetic patients or by those who take sildenafil with a high-fat diet.12 Tablet formulations are the conventional sildenafil delivery method.
Orally disintegrating films are a novel orodispersible drug delivery system that can dissolve rapidly and disperse its payload when placed in the mouth without the need for water.13 The film is hydrated by saliva in the oral cavity, thereby releasing the active ingredients for local and systemic absorption. Compared to tablets, orodispersible films provide a more convenient delivery method with a reduced risk of choking and a potentially accelerated onset. In a small sample study, Radiciono et al14 confirmed that there was no statistically significant difference between the 100 mg sildenafil orally disintegrating strip (ODS) formulation and the 100 mg tablet formulation in terms of pharmacokinetics. These results suggest that the orodispersible film formulation may represent a viable alternative to the current products for the treatment of ED, with additional benefits coming in the form of patient convenience and acceptability, which could enhance treatment compliance owing to the film's ease of use.
In Thailand, while the conventional sildenafil tablet formulation has been in use since 2000, alternative formulations such as the sildenafil ODS are not yet widely available. Comparisons of the different drug delivery methods have mainly focused on bioequivalence studies, with a dearth of clinical study data regarding the ODS formulation. In answer to this shortage, we conducted a prospective, randomized, controlled crossover study to compare the efficacy and safety of sildenafil ODS with that of conventional sildenafil tablets in a Thai population. In addition to the primary objective of assessing the efficacy of sildenafil strips for the treatment of ED, secondary objectives include determining onset of action and duration of action for sildenafil orally disintegrating strips while identifying treatment side effects and evaluating patient satisfaction.
MATERIALS AND METHODS
Study Design
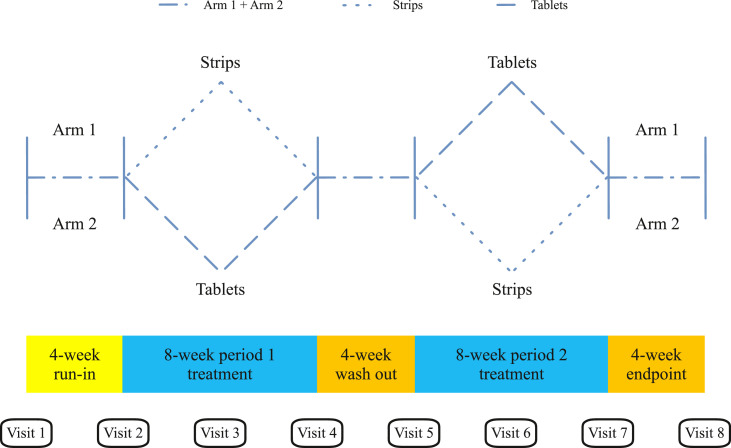
The sildenafil ODS trial was a prospective, randomized, controlled crossover study that evaluated the efficacy of sildenafil orally disintegrating strips (Hart-S sildenafil citrate, Pacific Biosciences Pte. Ltd., Singapore) and compared the ODS efficacy to that of sildenafil tablets (Sidegra sildenafil citrate, Government Pharmaceutical Organization, Thailand). Efficacy was assessed as a function of the abridged International Index of Erectile Function (IIEF-5) score and the Erection Hardness Score (EHS). The study consisted of 120 ED patients who were randomized and allocated into 2 groups of 60 participants (eg, Arm 1 and Arm 2). As shown in Figure 1, patients in Arm 1 were first treated with sildenafil strips for 8 weeks, with treatment pausing for a 4-week wash-out period after which treatment resumed with sildenafil tablets for 8 additional weeks. Alternatively, patients in Arm 2 were first treated with sildenafil tablets for 8 weeks, with treatment pausing for a 4-week wash-out period after which treatment resumed with sildenafil strips for 8 additional weeks. Throughout the study period, each participant was assessed every 4 weeks (eg, Visits 1 through 8) to build a dataset of all onset of action and duration of action time values. During each visit, patients completed the IIEF-5 and EHS questionnaires. Patient satisfaction and reports of adverse events were continually assessed and each patient's preference for either the sildenafil ODS or tablet formulation was registered at the end of the study.
Figure 1.
Schematic of the study protocol workflow. Following screening and enrollment, patient randomization was performed during the 4-week run-in period marking the clinical trial starting point. The study proceeded with an 8-week treatment period followed by a 4-week wash out period and then a second 8-week treatment period. Patients starting treatment with 1 sildenafil formulation crossed over to the other formulation during the wash out period between Visits 4 and 5. Eight visits at 4-week intervals were spaced throughout the trial period.
Study Population
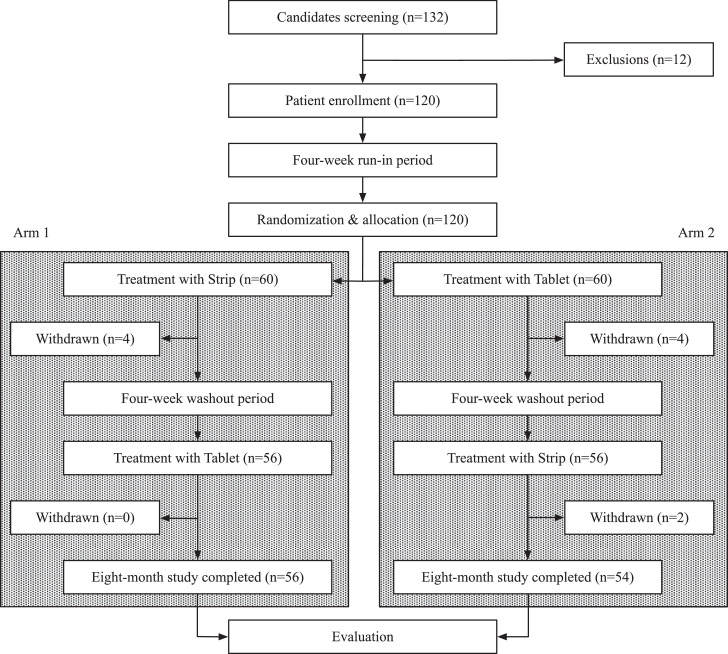
This study was conducted between November 2018 and December 2020. A total of 132 candidates were enrolled. Subsequently, eleven candidates withdrew after being informed about the details of the research protocol. One patient was later excluded because of their medical history, which involved a radical prostatectomy. One hundred twenty eligible patients started the sildenafil ODS study following the run-in period.
Figure 2 shows the flow diagram for study enrollees. Following the enrollment period, the remaining 120 ED patients were randomly allocated into 2 treatment arms, each consisting of 60 participants. Patients in Arm 1 were treated with sildenafil ODS for 8 weeks (period 1) followed by a 4-week pause in treatment (wash-out period). Subsequently, these patients crossed over and were treated with sildenafil tablets for another 8 weeks (period 2). Alternatively, patients in Arm 2 were treated with sildenafil tablets for 8 weeks (period 1) followed by a 4-week pause in treatment (wash-out period). Subsequently, these patients crossed over and were treated with sildenafil ODS for another 8 weeks (period 2). The study design afforded each patient the opportunity to use both treatment formulations, thereby informing their preference for one or the other.
Figure 2.
Patient disposition from enrollment to study conclusion. Out of 132 total candidates, 120 patients were enrolled in the study. From the 12 excluded candidates, one did not meet the inclusion criteria while the remaining eleven declined to participate after being informed of the protocol. During the first treatment period, 4 patients withdrew from each arm of the study. After the washout period and treatment crossover, 2 patients in Arm 2 withdrew during the second treatment period without completing their sildenafil ODS regimen.
The clinical trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Harmonized Tripartite Guidelines for Good Clinical Practice. The study protocol, information sheet, consent form, case report form, and all other relevant documentation were reviewed and approved by the institutional Human Research Ethics Committee. The study population was composed of male patients aged 18 years or older. Inclusion criteria screened for patients who had been diagnosed with ED for more than 3 months, who were unable to maintain an erection long enough to achieve successful sexual intercourse in 50% of attempts (or higher), and who have only 1 female sexual partner with whom sexual intercourse occurs at least 2 times per month. Study candidates who were contraindicated for or allergic to PDE5 inhibitors, had been diagnosed with ED as a result of spinal cord injury or radical prostatectomy, had a fasting blood sugar level over 270 mg/dL, or had untreated hypogonadism were excluded from the clinical trial. Written informed consent was obtained prior to the start of treatment for all patients. Participants who subsequently decided to withdraw from the clinical study were not required to provide a reason for withdrawal.
Treatment Formulations
Two drug delivery formulations were used in this study. Hart-S orally disintegrating strips each contain a dosage of 50 mg sildenafil citrate. Patients were prescribed 2 strips before sexual intercourse with a maximum dosage of 2 strips per week (eg, 100 mg per week). Each patient received a total of 16 strips for use during the 8-week treatment period involving sildenafil ODS. Sidegra tablets each contain a dosage of 100 mg sildenafil citrate. Patients were prescribed 1 tablet before sexual intercourse with a maximum dosage of 1 tablet per week (eg, 100 mg per week). Each patient received a total of 8 tablets for use during the 8-week treatment period involving conventional sildenafil tablets.
Study Assessments
The efficacy of both sildenafil formulations was assessed using a questionnaire to determine the IIEF-5 score and the EHS from patient assessments collected during both the treatment and non-treatment periods. The IIEF consists of 15 items and 5 domains and is a psychometrically valid and reliable method for determining efficacy of treatment in controlled clinical trials.15 The IIEF-5 is an abbreviated version that can be used as a diagnostic tool.16 ED severity can be classified on the IIEF-5 scale as follows: severe (5–7), moderate (8–11), mild to moderate (12–16), mild (17–21), and no ED (22–25). The EHS is a single-item, patient-reported outcome for scoring erection hardness.17 The 4 grade levels in the EHS are used to categorize the severity of ED. EHS grade 1 represents cases of severe ED. EHS grade 2 represents cases of moderate ED that do not allow for vaginal penetration. EHS grade 3 represents cases of mild ED. And EHS grade 4 signifies normal erectile function.
IIEF-5 and EHS results were compared as a measure of ODS and tablet efficacy. Safety profiles and adverse events were constantly evaluated after the start of treatment. Furthermore, values for the onset of action and duration of action for both sildenafil formulations were collected at visits 3, 4, 6, and 7 after 4 weeks of medication use. This data was collected through patient self-reporting with the aim of reflecting real-life situation outcomes. As indicated in Figure 1, patients were assessed every 4 weeks throughout the study regimen for a total of 8 mandated medical center visits. Patient preference between the 2 sildenafil formulations was assessed at the end of the study.
Statistical Analysis
Data analysis was performed using STATA version 14.1 (STATA Corp., TX, USA). Categorical variables were evaluated using the chi-squared test with the corresponding data being reported as numbers and percentages. Continuous variables were compared using a 2-sample t-test with the corresponding data being reported as median ± standard deviation. For univariate and multivariate analyses using the multilevel mixed-effects linear regression, a P value of less than .05 was considered statistically significant.
RESULTS
Patient demographics are presented in Table 1. The mean age of all participants was 64.48 ± 8.66 years. No significant difference in baseline characteristics was detected between the 2 treatment arm groups. Within the study population, the 3 most common associated diseases were hypertension (55.8%), dyslipidemia (51.7%), and benign prostatic hyperplasia (47.9%).
Table 1.
Study population demographics and baseline clinical characteristics
| Characteristic | ODS (n = 60) | Tablet (n = 60) | Overall (n = 120) | P value |
|---|---|---|---|---|
| Age (years); (mean ± SD) | 65.34 ± 8.99 | 63.61 ± 8.29 | 64.48 ± 8.66 | .276 |
| Body weight (kg); (mean ± SD) | 71.33 ± 11.07 | 71.56 ± 13.62 | 71.44 ± 12.36 | .917 |
| Height (cm); (mean ± SD) | 166.34 ± 5.36 | 166.97 ± 5.70 | 166.66 ± 5.52 | .534 |
| BMI (kg/m2); (mean ± SD) | 25.75 ± 3.75 | 25.61 ± 4.39 | 25.68 ± 4.07 | .850 |
| Underlying disease; n (%) | ||||
| Hypertension | 34 (56.67) | 33 (55) | 67 (55.83) | .854 |
| Diabetes mellitus | 14 (23.33) | 21 (35) | 35 (29.17) | .160 |
| Dyslipidemia | 29 (48.33) | 33 (55) | 62 (51.67) | .465 |
| Ischemic heart disease | 1 (1.67) | 1 (1.67) | 2 (1.67) | >.999 |
| Obstructive sleep apnea | 4 (6.67) | 2 (3.33) | 6 (5) | .402 |
| B.P.H. | 23 (38.98) | 34 (56.67) | 57 (47.90) | .054 |
| Obesity | 7 (11.67) | 11 (18.33) | 18 (15) | .306 |
| Severity of ED; n (%) | ||||
| Severe ED | 7 (11.67) | 10 (16.67) | 17 (14.17) | .247 |
| Moderate ED | 12 (20) | 14 (23.33) | 26 (21.67) | |
| Mild-moderate ED | 29 (48.33) | 18 (30) | 47 (39.17) | |
| Mild ED | 11 (18.33) | 14 (23.33) | 25 (20.83) | |
| No ED | 1 (1.67) | 4 (6.67) | 5 (4.17) |
BMI = body mass index; B.P.H. = benign prostatic hyperplasia; ED = erectile dysfunction; ODS = orally disintegrating strip; SD = standard deviation.
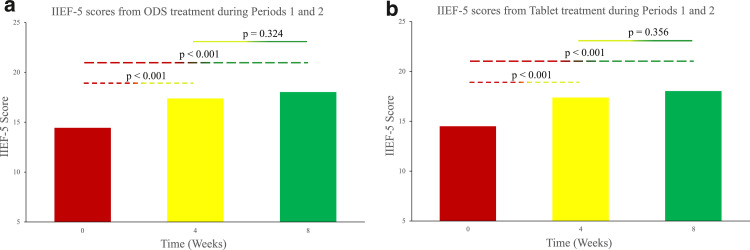
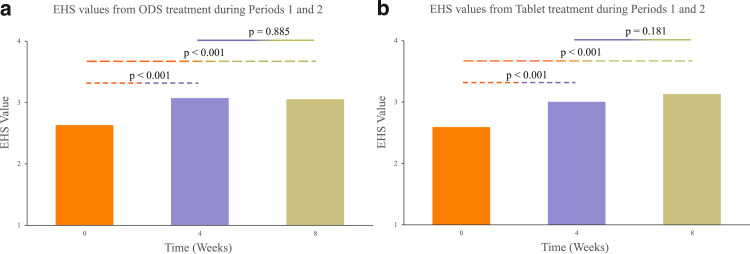
Both sildenafil formulations resulted in significantly improved erectile function after treatment. As a result of treatment with sildenafil ODS, IIEF-5 scores increased from 14.47 ± 4.83 to 17.38 ± 5.01 and 18.03 ± 4.83 at the 4-week and 8-week time points, respectively (P value < .05). Additionally, EHS values also increased from 2.63 ± 0.75 to 3.07 ± 0.74 and 3.05 ± 0.75 at the 4-week and 8-week time points, respectively (P value < .05). Concurrently, as a result of treatment with sildenafil tablets, IIEF-5 scores increased from 14.67 ± 5.27 to 17.46 ± 4.72 and 18.05 ± 5.00 at the 4-week and 8-week time points, respectively (P value < .05). Furthermore, EHS values also increased from 2.59 ± 0.75 to 3.00 ± 0.75 and 3.13 ± 0.74 at the 4-week and 8-week time points, respectively (P value < .05). Figure 3, Figure 4 show the changes in IIEF-5 scores and EHS values, respectively, as a function of time for each sildenafil formulation. The total ODS data set was collated by pooling questionnaire responses from Arm 1 patients during treatment period 1 and Arm 2 patients during treatment period 2. The total Tablet data set was collated by pooling questionnaire responses from Arm 1 patients during treatment period 2 and Arm 2 patients during treatment period 1.
Figure 3.
Improvements in IIEF-5 scores following treatment with the sildenafil ODS (A) and conventional tablet (B) formulations. The Week 0 IIEF-5 score represents the baseline value at the beginning of a treatment period. For patients in Arm 1, baseline IIEF-5 scores before ODS treatment were recorded during Visit 2. For patients in Arm 2, baseline IIEF-5 scores before ODS treatment were recorded during Visit 5. Likewise, baseline IIEF-5 scores before tablet treatment in Arms 1 and 2 were recorded during Visits 5 and 2, respectively. Statistically significant improvements in IIEF-5 scores appeared at both 4 and 8 weeks. There was no statistically significant difference between the scores at week 4 and week 8, for either the ODS or tablet formulation. Consequently, full treatment efficacy was achieved by week 4.
Figure 4.
Improvements in EHS values following treatment with the sildenafil ODS (A) and conventional tablet (B) formulations. The Week 0 EHS value represents the baseline value at the beginning of a treatment period. Baseline EHS values before ODS treatment in Arms 1 and 2 were recorded during Visits 2 and 5, respectively. Baseline EHS values before tablet treatment in Arms 1 and 2 were recorded during Visits 5 and 2, respectively. Statistically significant improvements in EHS values appeared at both 4 and 8 weeks. There was no statistically significant difference between the EHS values at week 4 and week 8, for either the ODS or tablet formulation. As a result, full treatment efficacy was achieved by week 4.
Changes in IIEF-5 scores and EHS values following sildenafil treatment were used to quantify treatment efficacy for the study population. A comparison of the changes in treatment efficacy between the sildenafil ODS and sildenafil tablet formulations yielded no statistically significant difference for the treatment of ED at identical dosage levels. Mean IIEF-5 scores for the sildenafil ODS and tablet formulations were 17.69 ± 4.93 and 17.75 ± 4.86, respectively (P value = .899). Mean EHS values for the sildenafil ODS and tablet formulations were 3.06 ± 0.75 and 3.07 ± 0.75, respectively (P value = .953). These results demonstrate that the efficacy of sildenafil strips was comparable to that of sildenafil tablets for the treatment of ED.
In addition to treatment efficacy, secondary response benchmarks such as onset of action times and duration of action times were evaluated. A comparison of the earliest onset of action times between the sildenafil ODS and tablet formulations showed no statistically significant difference in values (47.11 minutes and 54.21 minutes for sildenafil ODS and sildenafil tablets, respectively, P value = .580). Nonetheless, an onset of action time that was 7.1 minutes faster than the conventional response time may be considered clinically significant by some ED patients. Likewise, a comparison of the duration of action times between the sildenafil ODS and tablet formulations yielded no statistically significant difference in values (85.86 minutes and 90.84 minutes for sildenafil ODS and sildenafil tablets, respectively, P value = .745). Yet, a duration of action time that was 5 minutes longer than the experimental response time may be considered clinically significant by some ED patients.
Based on univariate and multivariate analyses, the results of this study demonstrate that neither sildenafil formulation, body weight, body mass index, nor time period with an ED diagnosis were associated with the treatment response, as shown in Table 2. There was no statistically significant difference in the extent of IIEF-5 scoring improvements when using either the ODS or tablet formulations. Similarly, increasing EHS values also showed no statistically significant difference when using either formulation. However, participant age, severity of ED, and attempt of medication use were significantly associated with IIEF-5 scoring changes after treatment.
Table 2.
Univariate and multivariate analyses of factors associated with IIEF-5 score improvements following sildenafil treatment
| Variable | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Coefficient (95% CI) | P value | Coefficient (95% CI) | P value | |
| Type of formulation | -0.04 (-0.47 to 0.40) | .865 | -0.04 (-0.47 to 0.39) | .862 |
| Medication use attempt | 0.59 (0.16–1.02) | .007 | 0.58 (0.16–1.01) | .009 |
| Severity of ED | 0.66 (0.52–0.80) | <.001 | 0.65 (0.52–0.78) | <.001 |
| Age | -0.09 (-0.19 to -0.002) | .044 | -0.07 (-0.14 to -0.004) | .038 |
| Body weight | 0.03 (-0.03 to 0.10) | .304 | ||
| BMI | 0.03 (-0.17 to 0.23) | .778 | ||
| Cigarette use | ||||
| Stopped > 3 months | 0.21 (-1.62 to 2.05) | .820 | ||
| Current smoker | -1.57 (-5.32 to 2.18) | .411 | ||
| Alcohol consumption | ||||
| Stopped > 3 months | 0.76 (-2.90 to 1.38) | .486 | ||
| Current drinker | 1.05 (-0.80 to 2.90) | .264 | ||
| ED duration (months) | 0.005 (-0.01 to 0.02) | .536 | ||
| Underlying disease | ||||
| Hypertension | -0.87 (-2.49 to 0.75) | .291 | ||
| Diabetes mellitus | -2.08 (-3.82 to -0.34) | .019 | ||
| Dyslipidemia | -0.57 (-2.18 to 1.04) | .490 | ||
| Obstructive sleep apnea | -0.48 (-4.21 to 3.24) | .799 | ||
| B.P.H. | -0.23 (-1.86 to 1.40) | .780 | ||
| Obesity (BMI > 30) | -1.71 (-3.93 to 0.52) | .133 | ||
BMI = body mass index; B.P.H. = benign prostatic hyperplasia; CI = confidence interval; ED = erectile dysfunction.
Notably, there were no serious or life-threatening adverse events during the course of the study. The adverse events affecting a small subset of the study population were minor and well documented from previous clinical studies (see Table 3). Furthermore, a comparison of the incidence of adverse events between the ODS and tablet formulations showed no significant difference. The only symptom that appeared for 1 formulation more often than the other was the flushing symptom, which was more common in the conventional tablet group (see Table 3).
Table 3.
Adverse events resulting from sildenafil ODS and tablet treatment
| Side effect | ODS, n (%) | Tablet, n (%) | P value |
|---|---|---|---|
| Headache | 26 (11.5) | 28 (12.3) | .785 |
| Flushing | 10 (4.4) | 21 (9.3) | .042 |
| Dyspepsia | 2 (0.9) | 1 (0.4) | .560 |
| Nasal congestion | 23 (10.2) | 28 (12.3) | .468 |
| Dizziness | 8 (3.5) | 4 (1.8) | .239 |
| Abnormal vision | 8 (3.5) | 15 (6.6) | .137 |
| Back pain | 0 (0) | 1 (0.4) | .318 |
| Myalgia | 3 (1.3) | 2 (0.9) | .649 |
| None | 152 (67.3) | 144 (63.4) | .393 |
| Other | 20 (8.9) | 20 (8.8) | .988 |
ODS = orally disintegrating strip.
At the end of the study, 47.3% of the treatment population preferred using the sildenafil ODS formulation whereas 52.7% preferred using the conventional sildenafil tablet formulation. Convenience was cited as a significant reason for preferring the tablet form over the ODS form.
DISCUSSION
Erectile dysfunction is a common condition experienced by elderly males. The estimated prevalence of ED in Asian men is projected to reach 200 million by the year 2025.18 Since the 2000 study, when the prevalence of ED in Thai men was benchmarked at 37.5%, this condition has come to be associated with other ailments such as hypertension, diabetes, and cardiovascular disease, all of which can affect an individual's overall health as well as the quality of life for couples.3 As the first-line therapy option for ED, PDE5 inhibitors are commonly accessible and broadly used. Following its regulatory approval in 1998,19 sildenafil became the first PDE5 inhibitor to be widely prescribed for the treatment of ED. Tablets continue to be the most prominent formulation for sildenafil. However, orally disintegrating films offer an alternative delivery method for ED treatment when patients desire a more natural or spontaneous response from their therapy options.20 Early orodissolvable film formulations highlighted the potential for high solubility, rapid onset of action, and improved bioavailability.21 Although bioequivalence studies involving sildenafil ODS have documented peak concentration (Cmax) terms and provided valuable pharmacokinetic profiles in healthy populations,14,22 there have been few studies demonstrating its efficacy.
The results of this study indicate that both formulations had comparable efficacy in the treatment of ED, as evidenced by statistically significant increases in IIEF-5 scores and EHS values. As a consequence of these improvements, treatment with sildenafil resulted in a shift in the severity of ED. The average baseline IIEF-5 score before treatment fell in the mild to moderate ED scale range (eg, IIEF-5 = 12–16). After treatment with either sildenafil formulation, the average IIEF-5 score shifted to the mild ED scale range (IIEF-5 = 17–21). Changes in EHS values also followed this trend as the initial moderate ED baseline values moved to mild ED post-treatment values. From a clinical perspective, patients with moderate ED are unable to have successful sexual intercourse because of insufficient penile erection, whereas patients with mild ED are able to have successful sexual activity despite a possible decrease in penile erection. Both the sildenafil ODS and tablet formulations significantly improved penile erection to the point where achieving successful sexual activity was likely.
Previously, a 2003 randomized, double-blind, placebo-controlled flexible dose study in Thailand23 documented comparable outcomes to those reported for the original sildenafil tablet (Viagra, Pfizer Inc., New York, NY, USA),19 using the initial IIEF scoring system. Similarly, the conventional tablet results from the present study were benchmarked to those reported by the ENDOTRIAL study,24 which examined the efficacy of sildenafil, tadalafil, and vardenafil in the treatment of ED using the abridged IIEF-5 scoring system. In terms of efficacy, improvements in the IIEF-5 scores were comparable between the 2 clinical trials. For the original sildenafil tablet in the ENDOTRIAL study, the IIEF-5 score increased by 3.52. Data from the present study showed an IIEF-5 score increase of 3.38, indicating that the efficacy of the generic tablet treatment was comparable to that of the original sildenafil treatment. With respect to EHS values, most patients were initially categorized at EHS grade 2 (moderate ED). Following treatment, EHS values significantly improved with patient EHS values shifting to grade 3 (mild ED), allowing for a higher likelihood of successful sexual activity.
Although there was no statistically significant difference in treatment efficacy, onset of action time, or duration of action time between the sildenafil ODS and tablet formulations, slight differences may still have clinical ramifications. In the present study, treatment with sildenafil ODS resulted in a 7.1-minute earlier onset of action time compared to the slower conventional tablet formulation. A faster onset of action time may be valuable to a number of patients. In addition to those patients with dysphagia or malabsorption, the ODS formulation may also benefit those who use the medication after a fat-rich meal or in the presence of alcohol as these conditions and scenarios directly affect sildenafil absorption.11
The adverse events experienced by patients in the present study were comparable to those reported in prior sildenafil trials. The most commonly encountered adverse events resulting from tablet use were headaches and flushing, accounting for 12.3% and 9.3% of the total incidence of adverse events, respectively. Morales et al reported similar incidence values for headaches and flushing at 16% and 10%, respectively.25 Although there was no statistically significant difference in adverse events between the sildenafil ODS and tablet formulations, flushing was more common in the conventional tablet group. As a result, patients who experience flushing after tablet use may encounter fewer adverse events upon switching to the ODS formulation.
The strength of this study derives from the implementation of a prospective, randomized, controlled crossover trial and the use of 2 established erectile function evaluation questionnaires to determine IIEF-5 scores and EHS values. Additionally, the IIEF-5 questionnaire was provided in the Thai language after having been validated for internal consistency, validity, and reliability.26 Also, the EHS questionnaire was crafted specifically for the development and study of sildenafil.17 By virtue of the crossover design, every patient had the opportunity to use each of the 2 sildenafil formulations for 8 weeks, providing enough time for patient preferences to develop.
The authors of the present study also recognize its limitations. First, the placebo group is absent. Because PDE5 inhibitors are now the standard treatment option for ED, a clinical trial that includes a placebo group was thought to be inappropriate for the patients. In addition, such a trial design may have attracted fewer candidates and would undoubtedly raise multiple ethical concerns. Second, the clinical trial was not conducted as a blind study. Because both sildenafil formulations were unique (eg, orally disintegrating strip vs tablet) it was impossible to disguise the identity of the formulation upon treatment administration. Therefore, an adequate washout period was included in the study design to mitigate the potential for bias. Third, values for the onset of action and duration of action were measured by the patients themselves using a watch. This data may not be completely accurate when compared to the stopwatch technique. Lastly, we could not compare the cost effectiveness between these 2 formulations because the ODS formulation is not currently marketed in Thailand. Even with these limitations, the authors believe that the crossover design has made the study results more consistent and more informative as a reference for the treatment of ED patients and for subsequent clinical studies.
CONCLUSIONS
The sildenafil ODS and conventional tablet formulations both demonstrated good clinical efficacy and safety for the treatment of ED. The present clinical trial data indicate that both formulations had comparable efficacy and safety profiles. Sildenafil ODS manifested a 7.1-minute faster onset of action time when compared to sildenafil tablets. Although not statistically significant, this difference in onset of action time may have clinical meaningfulness for some patients. Additionally, patients treated with sildenafil ODS exhibited fewer flushing symptoms. In cases where patients cannot tolerate treatment with sildenafil tablets because of flushing, the sildenafil ODS formulation may be a viable alternative.
STATEMENT OF AUTHORSHIP
Premsant Sangkum: Conceptualization, Methodology, Analysis and Interpretation of Data, Writing – Original Draft, Writing – Review & Editing, Resources; Kun Sirisopana: Acquisition of Data; Wijittra Matang: Acquisition of Data, Analysis and Interpretation of Data; Yada Phengsalae: Acquisition of Data, Analysis and Interpretation of Data; Panuwat Lertsithichai: Methodology, Analysis and Interpretation of Data; Chinnakhet Ketsuwan: Resources; Wachira Kochakarn: Funding Acquisition, Supervision; Wisoot Kongchareonsombat: Conceptualization, Writing – Review & Editing, Resources, Supervision.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: This clinical study was sponsored by the Government Pharmaceutical Organization of Thailand [R31061504] in terms of budget and materials, including the supply of both sildenafil formulations.
Detailed information on the sildenafil ODS trial can be accessed through the Thai Clinical Trial Registry using reference number 20181121002.
References
- 1.NIH Consensus Conference. Impotence NIH consensus development panel on impotence. Jama. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Thai Erectile Dysfunction Epidemiologic Study Group (TEDES) An epidemiological study of erectile dysfunction in Thailand (Part 1: Prevalence) J Med Assoc Thai. 2000;83:872–879. [PubMed] [Google Scholar]
- 3.Kongkanand A. Prevalence of erectile dysfunction in Thailand. Int J Androl. 2000;23(Suppl. 2):77–80. doi: 10.1046/j.1365-2605.2000.00022.x. Thai Erectile Dysfunction Epidemiological Study Group. [DOI] [PubMed] [Google Scholar]
- 4.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6:1232–1247. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 5.Javaroni V, Neves MF. Erectile dysfunction and hypertension: impact on cardiovascular risk and treatment. Int J Hypertens. 2012;2012 doi: 10.1155/2012/627278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim A, Ali M, Kiernan TJ, et al. Erectile dysfunction and ischaemic heart disease. Eur Cardiol. 2018;13:98–103. doi: 10.15420/ecr.2017.21.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovac JR, Labbate C, Ramasamy R, et al. Effects of cigarette smoking on erectile dysfunction. Andrologia. 2015;47:1087–1092. doi: 10.1111/and.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai T, Morgia G, Carrieri G, et al. An improvement in sexual function is related to better quality of life, regardless of urinary function improvement: results from the IDIProst® Gold Study. Arch Ital Urol Androl. 2013;85:184–189. doi: 10.4081/aiua.2013.4.184. [DOI] [PubMed] [Google Scholar]
- 9.Li HJ, Bai WJ, Dai YT, et al. An analysis of treatment preferences and sexual quality of life outcomes in female partners of Chinese men with erectile dysfunction. Asian J Androl. 2016;18:773–779. doi: 10.4103/1008-682X.159719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WG, Li P, Wang YS, et al. The effect of erectile dysfunction on quality of life in male kidney transplant recipients. Pak J Med Sci. 2014;30:361–365. [PMC free article] [PubMed] [Google Scholar]
- 11.Porst H, Burnett A, Brock G, et al. SOP conservative (medical and mechanical) treatment of erectile dysfunction. J Sex Med. 2013;10:130–171. doi: 10.1111/jsm.12023. [DOI] [PubMed] [Google Scholar]
- 12.Hatzimouratidis K, Giuliano F, Moncada I, et al. EAU Guidelines on erectile dysfunction, premature ejaculation, penile curvature and priapism. Eur Assoc Urol. 2016 https://www.uroweb.org/wp-content/uploads/EAU-Guidelines-Male-Sexual-Dysfunction-2016.pdf Available at: Accessed October 20, 2021. [Google Scholar]
- 13.Irfan M, Rabel S, Bukhtar Q, et al. Orally disintegrating films: a modern expansion in drug delivery system. Saudi Pharm J. 2016;24:537–546. doi: 10.1016/j.jsps.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radicioni M, Castiglioni C, Giori A, et al. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des Devel Ther. 2017;11:1183–1192. doi: 10.2147/DDDT.S124034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen RC, Cappelleri JC, Gendrano N., 3rd The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226–244. doi: 10.1038/sj.ijir.3900857. [DOI] [PubMed] [Google Scholar]
- 16.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 17.Mulhall JP, Goldstein I, Bushmakin AG, et al. Validation of the erection hardness score. J Sex Med. 2007;4:1626–1634. doi: 10.1111/j.1743-6109.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 18.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. Sildenafil Study Group. [DOI] [PubMed] [Google Scholar]
- 20.Jannini EA, Droupy S. Needs and expectations of patients with erectile dysfunction: an update on pharmacological innovations in phosphodiesterase type 5 inhibition with focus on sildenafil. Sex Med. 2019;7:1–10. doi: 10.1016/j.esxm.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosny KM, El-Say KM, Ahmed OA. Optimized sildenafil citrate fast orodissolvable film: a promising formula for overcoming the barriers hindering erectile dysfunction treatment. Drug Deliv. 2016;23:355–361. doi: 10.3109/10717544.2014.916763. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre LG, Olmedo IR, Nolasco AM, et al. Comparative bioavailability of sildenafil 50-mg film-coated tablets and 50-mg orally disintegrating films in healthy Mexican subjects: Results from a randomized, open-label, crossover study. Clin Pharmacol Drug Dev. 2019;8:404–410. doi: 10.1002/cpdd.599. [DOI] [PubMed] [Google Scholar]
- 23.Kongkanand A, Ratana-Olarn K, Ruangdilokrat S, et al. The efficacy and safety of oral sildenafil in Thai men with erectile dysfunction: a randomized, double-blind, placebo controlled, flexible-dose study. J Med Assoc Thai. 2003;86:195–205. [PubMed] [Google Scholar]
- 24.Jannini EA, Isidori AM, Gravina GL, et al. The ENDOTRIAL study: a spontaneous, open-label, randomized, multicenter, crossover study on the efficacy of sildenafil, tadalafil, and vardenafil in the treatment of erectile dysfunction. J Sex Med. 2009;6:2547–2560. doi: 10.1111/j.1743-6109.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 25.Morales A, Gingell C, Collins M, et al. Clinical safety of oral sildenafil citrate (VIAGRA) in the treatment of erectile dysfunction. Int J Impot Res. 1998;10:69–73. doi: 10.1038/sj.ijir.3900354. discussion 73-4. [DOI] [PubMed] [Google Scholar]
- 26.Sangkum P, Sukying C, Viseshsindh W, et al. Validation and reliability of a Thai version of the International Index of Erectile Dysfunction (IIEF) for Thai population. J Med Assoc Thai. 2017;100:S73–S79. [Google Scholar]