Summary
Background
The contribution of alcohol to the large burden of oesophageal squamous cell carcinoma (ESCC) in east Africa remains uncertain and difficult to assess owing to complex consumption patterns of traditional and commercial drinks. We aimed to assess whether alcohol drinking, overall and at specific intake levels, contributes to ESCC risk in east Africa.
Methods
We did a hospital-based case-control study in Kenya, Tanzania, and Malawi, which included comprehensive assessment of a variety of locally consumed alcohol that we used to classify drinkers as exclusively low alcohol-by-volume (ABV; <30% ABV) drinkers or drinkers of some high-ABV drinks, as well as the number of drinks consumed, average weekly ethanol intake, and the contribution of each drink type to overall ethanol consumption. Cases were patients aged 18 years and older with incident primary ESCC, confirmed histologically for the majority of cases, and a clinical diagnosis for the remainder. Controls were frequency-matched on age and sex in a 1:1 ratio with cases. The controls were recruited from the same hospitals as cases and included outpatients, inpatients, and hospital visitors who did not have cancer or any other digestive disease. Consenting participants took part in face-to-face interviews in which they were asked whether they had ever consumed alcohol (the primary exposure variable); those who had were asked follow-up questions about their consumption habits for different alcoholic drinks.
Findings
1279 cases and 1346 controls were recruited between Aug 5, 2013, and May 24, 2020, including 430 cases and 440 controls from Kenya, 310 cases and 313 controls from Tanzania, and 539 cases and 593 controls from Malawi. 65 (4·8%) of 1344 cases were excluded. Consistent positive associations with ESCC risk were found for ever having consumed alcohol in Kenyan men and Tanzanian men, and for daily number of drinks and estimated ethanol intake in Kenya, Tanzania (both sexes) and Malawian women. Corresponding population-attributable fractions of ESCC for those reporting ever drinking alcohol (vs never drinking) were 65% (95% CI 52–78) in Kenyan men and 23% (<1–45) in Kenyan women, and 56% (95% CI 36–76) in Tanzanian men and 5% (0–42) in Tanzanian women. Increased risk and population-attributable fractions were almost entirely due to risks in high-ABV drinkers.
Interpretation
Alcohol appears to be a substantial contributor to ESCC risk in east Africa, particularly among men, and a large fraction of ESCC could be prevented by cessation or reduction of alcohol consumption. Future studies should consider independent ascertainment of alcohol intake to assess the potential of under-reporting in Malawi.
Funding
US National Cancer Institute, Wereld Kanker Onderzoek Fonds, and the IARC Environment and Lifestyle Epidemiology Branch.
Translations
For the Swahili and Chichewa translations of the abstract see Supplementary Materials section.
Introduction
Oesophageal cancer caused the sixth highest number of deaths from cancer worldwide in 2020 (544 076 deaths).1 Oesophageal squamous cell carcinoma (ESCC) was responsible for approximately 84% of 2018's estimated burden of oesophageal cancer cases.2 A high incidence of ESCC occurs in certain areas of the world such as in areas of eastern and southern Africa, whereas incidence is low in west Africa.3 In 2020, there were an estimated 3000 new oesophageal cancer cases in Kenya, 2600 in Tanzania, and 1800 in Malawi, with similar numbers of deaths due to oesophageal cancer in each country.1
Alcoholic beverages (ie, those containing ethanol) and its metabolite acetaldehyde are carcinogenic to humans (International Agency for Research on Cancer [IARC] Group 1), and they are causally related to ESCC.3 Alcohol consumption contributes to a substantial burden of ESCC in low incidence countries,4 but its contribution to ESCC in Africa is not firmly established. The contribution of alcohol consumption to ESCC might well differ between settings and by sex. A meta-analysis of alcohol consumption and ESCC in Africa.5 including data from six case-control studies6, 7, 8, 9, 10, 11 found a pooled odds ratio (OR) of 2·28 for ESCC associated with alcohol consumption (the definition of which varied by study). Case-control studies have found positive associations between alcohol consumption and ESCC in South Africa,6, 11, 12 in which synergistic effects with tobacco use were reported,6, 11 and in western Kenya,10, 13 including our findings in Kenya, of stronger alcohol-ESCC associations among smokers than those among non-smokers.13 ESCC risks increased linearly with daily number of drinks and ethanol intake. For the same estimated ethanol intake, ESCC risks were higher for drinking the strong, traditional, distilled spirit chang'aa (which is typically over 30% alcohol-by-volume [ABV]) than from other alcohols. Furthermore, population attributable fractions of ESCC cases for drinking two or more drinks daily compared to never drinking were 48% overall; 59% in men and 27% in women.13 In contrast, other studies have reported no association between alcohol and ESCC risk, including case-control studies in Tanzania in 2020,14 and older publications from studies in South Africa in 197415 and Zimbabwe in 1995,8 all of which found positive associations with tobacco smoking.
Research in context.
Evidence before this study
We identified a recent systematic review and meta-analysis of oesophageal squamous cell carcinoma (ESCC) risk factors in Africa from studies published until March 31, 2019, and updated it to June 20, 2021, by searching the Embase, PubMed, and Ovid databases using the search term “*esophag* AND (cancer OR carcinoma OR malignan*) AND (Africa OR [list of individual African countries])” and screening for studies assessing alcohol as a risk factor. The meta-analysis estimated a pooled odds ratio (OR) of 2·28 for alcohol consumption associated with ESCC risk. This OR was generated using eight estimates from six studies, four of which were in South Africa. Individual estimates showed a high degree of heterogeneity (ORs 0·90–5·24; I2 of 92·1%) and the exposure definition was not consistent between studies. Two additional studies published since this meta-analysis have also been inconsistent in their findings. Data from the ESCCAPE Kenya case-control study (included in this multi-country paper) found a positive association between ESCC and alcohol consumption including a strong interaction with tobacco (OR 2·6 in people who had never used tobacco, 9·0 in people who had ever used tobacco), whereas a case-control study done in Dar es Salaam, Tanzania, reported an OR of 0·91 for current versus never drinking.
Added value of this study
This three-country case-control study presents data from a harmonised comprehensive alcohol assessment that was sensitive to the multitude of traditional and commercial beverages consumed in each country's setting. These data were used to derive multiple alcohol exposure metrics. This approach has yielded both intracountry and intercountry positive associations for several alcohol exposure metrics, including ever drinking, daily number of drinks, estimated ethanol intake, and type of drinker (high vs low alcohol-by-volume [ABV] drinks), apart from men in Malawi for whom there was no positive association. These findings show that alcohol consumption contributes to the risk of ESCC in the east African high-incidence corridor, which was a factor previously downplayed because of inconsistent and uncompelling findings. The study also identified higher risks of ESCC among drinkers of high-alcohol distillations and spirits than among drinkers of low-ABV drinks, which is more prevalent among men than women. We speculate that under-reporting might explain the null findings for Malawian men.
Implications of all the available evidence
Although research into additional factors underpinning the perplexing geographical distribution of ESCC in east Africa is ongoing, this analysis provides immediately actionable information on a modifiable risk factor that, if effectively mediated, could result in a substantial reduction in disease burden, both of ESCC and other morbidities for which alcohol is responsible.
Exposure assessment of alcohol consumption in Africa requires careful setting-specific characterisation due to large variations in patterns of consumption and the variety of traditional and commercial alcoholic beverages consumed (legal and illegal). Alcohol consumption is generally higher in men than women, and there is a higher incidence of ESCC among men than among women in many settings.16 A cross-sectional study in 2015 in western Kenya, where ESCC incidence is high, showed that drinking prevalence was 55% in men and 9% in women and drinking prevalence was higher in tobacco users than non-users and in people in lower than higher socioeconomic groups.17 In the ESCC hotspot of northern Tanzania, as well as being common in adults,18 drinking is also common among people aged 15–24 years; in a 2012–13 cross-sectional community survey of people in this age bracket, there was a 20–45% prevalence of alcohol consumption in males and a 12–47% prevalence in females.19 In an attempt to curb alcohol misuse, spirit sachets were banned in 2005 in Kenya, in 2017 in Tanzania, and in 2012 in Malawi.20 In addition to commercially available alcoholic drinks, each setting has its own traditional beverages, including a widely consumed traditional beer fermented from maize, millet, or sorghum that is typically 2–4% ABV. These traditional alcoholic drinks include busaa in Kenya,21 mbege in Tanzania,22 and masese in Malawi (although a commercial version, chibuku, is also made).23 Much stronger (20–50% ABV) home distillations are also consumed, called chang'aa in Kenya,21 gongo in Tanzania,22 and kachasu in Malawi.24 Additionally, there are medium-strength (5–8% ABV) fermentations of locally sourced produce such as banana, palm, and coconut.25
We set out to assess whether alcohol consumption, overall and at specific intake levels, contributes to ESCC risk in east Africa. In this Article, we present results of a large comprehensive multicentre ESCC case-control study done in Africa. Building on previous published findings from Kenya,26 we now report these results together with those from Tanzania and Malawi. We examined the associations between alcohol consumption and risk of ESCC on the basis of detailed alcohol intake aggregated over a range of commercial and traditional drinks, including drinking frequency, type of drink (low ABV only or including high ABV spirits), drinking volume, and estimated ethanol consumption. Finally, we estimated population attributable fractions of ESCC incident cases associated with alcohol drinking.
Methods
Study design and participants
This hospital-based case-control study was done across three sites, in Kenya, Tanzania, and Malawi. The Esophageal Squamous Cell Carcinoma African PrEvention research (ESCCAPE) case-control studies are joint collaborations between IARC and Moi University in Kenya, The Kilimanjaro Clinical Research Institute in Tanzania, and the Malawi College of Medicine in Malawi. Recruitment in these ESCC case-control studies was done at the Moi Teaching and Referral Hospital in Eldoret, Kenya, the Kilimanjaro Clinical Medical Centre in Moshi, Tanzania (including patients from three periphery hospitals), and at the Queen Elizabeth Central Hospital in Blantyre, Malawi.
Cases were patients aged 18 years and older presenting with suspected incident first primary oesophageal cancer who, having undergone endoscopy with pinch biopsy, had received a histological diagnosis that did not rule out ESCC. In Tanzania, we also included cases diagnosed via barium swallow or clinical presentation of solid or liquid dysphagia with weight loss. We note that including cases on the basis of a clinical diagnosis only does not pose a major limitation in this setting due to the high proportion of ESCC in the total oesophageal cancer burden (>90%)2 and the concordance between clinical diagnosis and histological confirmation.14 Controls were recruited in a 1:1 ratio with cases, using age frequency (5-year age bands) and sex frequency matching to the distribution in cases. The controls were recruited from the same hospitals as cases and included outpatients, inpatients, and hospital visitors who did not have cancer or any other digestive disease. Signed or thumbprint informed consent was provided by all participants. The study received ethical approval from the IARC Ethics Committee (IEC 14/15) and institutional review boards for each site at which the study was done: the Institutional Research and Ethics Committee of Moi University (000921), Kenya; the National Institute for Medical Research (NIMR/HQ/R.8a/Vol.IX/1994) and the Tumaini University Kilimanjaro Christian Medical University College (830), Tanzania; and the National Health Sciences Research Committee (NHSRC 17/01/1712), Malawi. A detailed description of the study protocol is available online.
Procedures and outcomes
Consenting participants completed face-to-face interviews with trained research assistants in local dialects. Data were collected using mobile data collection apps (Open Data Kit in Kenya and Mobenzi Researcher in Tanzania and Malawi), which allowed mandatory data entry to be programmed for selected variables to prevent missing responses and for periodic quality checks to be made on the data being collected.
For the primary exposure variable, we asked every participant whether they had ever drank alcohol regularly (ie, at least one drink per week for 6 months). Participants who responded to having ever drunk alcohol were then asked questions on details of their consumption habits separately for different alcoholic drinks. For each drink, we asked participants how old they were at first and last consumption, to calculate drinking duration for each type of beverage. Intakes were assessed by asking how many drinks of a prespecified or participant-provided volume were consumed daily (in Kenya) or cumulatively over weekdays and during the weekend (in Tanzania and Malawi). The drinks in the questionnaire included four that were common to all countries: commercial beers, commercial spirits, traditional beers (busaa, mbege, and chibuku), and traditional spirits (chang'aa, gongo, and kachasu). In Tanzania, we also asked about the consumption of four additional drinks: dengelua, ulanzi, komoni, and banana wine. We ascertained the consumption of all other drinks in all countries as long as the drink could be specified. Drinkers were further categorised as high ABV and low ABV drinkers; low ABV drinkers comprised those who only consumed drinks containing less than 30% ABV (appendix 3 p 2) whereas people who consumed drinks with at least 30% ABV (ie, any of the commercial or traditional spirits) were considered high-ABV drinkers (who might have also, but not necessarily, consumed low-ABV alcohols).
For each drinki, based on the questionnaire data provided on frequency and volumes of consumption, weekly ethanol consumption was calculated: EtOHi=(Vi × EtOH% × 0·79), in which EtOHi is ethanol intake (g per week), Vi is the volume (mL per week), EtOH% is the ethanol concentration (presented as a decimal), and 0·79 is the specific gravity of ethanol. Ethanol concentrations for each type of drink were obtained from external literature and are summarised in appendix 3 (p 2). Average weekly ethanol intake (g per week) across all drinks was then calculated, considering that there are overlapping periods when different drinks were consumed:
in which Di is the drinking duration of an individual drink (years) and DT is the total duration a person drank any alcohol (years). For each drink, we also calculated the proportional contribution of the drink to the overall consumption of ethanol in the individual's lifetime as DiEtOHi ÷ (DTEtOHaverage). Information on the following variables and potential confounders was also obtained: age, sex, ethnicity, religion, highest education level, tobacco use, number of children, and family history of oesophageal cancer. Biospecimen samples (which could include saliva, blood, and urine) were also collected, but participants who completed the questionnaire but did not provide biospecimen samples were included in the study and the present analysis.
Statistical analysis
Measures of the exposure of interest (alcohol consumption) were: drinking status (never or ever), drinking status by strength (high [≥30%] ABV, low [<30%] ABV, or never), weekly number of drinks (0, <14, 14–27, 28–41, or ≥42), and estimated duration-weighted average weekly grams of ethanol (EtOHaverage; 0, <140 g, 150–349 g, 350–699 g, or ≥700 g). χ2 tests were used to investigate associations of possible confounders with ever alcohol drinking in controls. ORs and their 95% CIs of having ESCC associated with each exposure were estimated separately for each country using logistic regression models. In model 1 (OR1 [minimal adjustment]), ORs were estimated with adjustment for sex and age (10-year strata) to account for small imbalances in age and sex frequency matching. Model 2 (OR2 [full adjustment]) was further adjusted for smoked and smokeless tobacco use (ever or never), smoking frequency (a continuous variable: cigarettes [smoked tobacco] or uses [smokeless tobacco] per day), ethnicity, education, and religion. For all models, both sex-adjusted and sex-specific analyses were done to investigate effect modification by sex. To investigate specific additional risks associated with individual beverages, ORs were also generated per 10% increase in ethanol contributed from different drinks by adding these terms to models 1 and 2 one by one. Finally, for drinking status and drinking status by strength, assuming causality, we used the method from a 1993 study27 to estimate the population attributable fraction (PAF [%]), overall and by gender for each site, for the ESCC burden attributable to ever drinking (to estimate the proportion of patients with ESCC who would not have had ESCC if the patients who drank had been never drinkers) and if the drinking patterns of drinkers of high-ABV alcoholic beverages were altered to the patterns (types and intensity) of the low-ABV drinkers (to estimate the proportion of patients with ESCC who would not have had ESCC if the drinking patterns [ie, if the amount of ethanol consumed by drinkers of high-ABV drinks had matched that of drinkers of low-ABV drinks only]). In post-hoc analyses, we also explored the potential effect of misclassification of ever versus never alcohol status in Malawi (appendix 3 pp 9–10). Statistical analyses were done using R version 4.0.3.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The percentages of all participants who provided consent after being approached (before any later exclusions) were: 553 (97%) of 569 cases and 593 (94%) of 629 controls in Malawi; 322 (93%) of 345 cases and 313 (>99 %) of 314 controls in Tanzania; and 469 (95%) of 492 cases and 440 (92%) of 480 controls in Kenya. 430 cases (after excluding 39 [8%] people without histologically confirmed ESCC) and 440 controls were recruited from Kenya between Aug 5, 2013, and May 12, 2018. 310 cases, of which 210 (68%) were histology confirmed and 100 (32%) had a clinical diagnosis (after excluding 12 [4%] people with histology that ruled out ESCC) and 313 controls were recruited from Tanzania between Nov 10, 2015, and Dec 13, 2019. 539 cases (after excluding 14 [3%] people without histologically confirmed ESCC) and 593 controls were recruited from Malawi between June 1, 2017, and May 24, 2020. 60 (92%) of 65 of the people who were excluded due to non-ESCC histology across the three sites had adenocarcinoma. Men made up 282 (66%) of the 430 cases from Kenya, 237 (76%) of the 310 cases from Tanzania, and 311 (58%) of the 539 cases from Malawi (table 1). The mean age of all cases from Kenya was 59 years (SD 14), from Tanzania was 64 years (14), and from Malawi was 57 years (14).
Table 1.
Descriptive characteristics
|
Kenya |
Tanzania |
Malawi |
|||||
|---|---|---|---|---|---|---|---|
| Cases (n=430) | Controls (n=440) | Cases (n=310) | Controls (n=313) | Cases (n=539) | Controls (n=593) | ||
| Sex | |||||||
| Male | 282 (66%) | 272 (62%) | 237 (76%) | 237 (76%) | 311 (58%) | 335 (56%) | |
| Female | 148 (34%) | 168 (38%) | 73 (24%) | 76 (24%) | 228 (42%) | 258 (44%) | |
| Mean age at diagnosis (cases) or interview (controls), years (SD) | 59 (14) | 57 (15) | 64 (14) | 62 (14) | 57 (14) | 56 (15) | |
| Ethnicity | |||||||
| Kalenjin | 247 (57%) | 233 (53%) | .. | .. | .. | .. | |
| Luhya | 100 (23%) | 95 (22%) | .. | .. | .. | .. | |
| Luo | 37 (9%) | 32 (7%) | .. | .. | .. | .. | |
| Kikuyu | 17 (4%) | 29 (7%) | .. | .. | .. | .. | |
| Other | 29 (7%) | 51 (12%) | 60 (19%) | 36 (12%) | 41 (8%) | 69 (12%) | |
| Chagga | .. | .. | 199 (64%) | 230 (73%) | .. | .. | |
| Pare | .. | .. | 16 (5%) | 33 (11%) | .. | .. | |
| Maasai | .. | .. | 19 (6%) | 10 (3%) | .. | .. | |
| Meru | .. | .. | 16 (5%) | 7 (2%) | .. | .. | |
| Lomwe | .. | .. | .. | .. | 146 (27%) | 171 (29%) | |
| Ngoni | .. | .. | .. | .. | 95 (18%) | 99 (17%) | |
| Yao | .. | .. | .. | .. | 87 (16%) | 86 (15%) | |
| Chewa | .. | .. | .. | .. | 66 (12%) | 60 (10%) | |
| Sena | .. | .. | .. | .. | 58 (11%) | 53 (9%) | |
| Mang'anja | .. | .. | .. | .. | 46 (9%) | 55 (9%) | |
| Religion* | |||||||
| Christian | 399 (93%) | 422 (96%) | 187 (88%) | 201 (86%) | 445 (83%) | 526 (89%) | |
| Muslim | 2 (<1%) | 4 (1%) | 24 (11%) | 33 (14%) | 59 (11%) | 50 (8%) | |
| Other | 29 (7%) | 14 (3%) | 2 (<1%) | 1 (<1%) | 35 (6%) | 17 (3%) | |
| Education | |||||||
| None | 104 (24%) | 100 (23%) | 63 (20%) | 21 (7%) | 108 (20%) | 96 (16%) | |
| Some primary | 163 (38%) | 110 (25%) | 89 (29%) | 69 (22%) | 243 (45%) | 229 (39%) | |
| Completed primary | 85 (20%) | 91 (21%) | 118 (38%) | 162 (52%) | 67 (12%) | 82 (14%) | |
| Secondary or higher | 78 (18%) | 139 (32%) | 40 (13%) | 61 (19%) | 121 (22%) | 186 (31%) | |
| Median number of children (IQR) | 6 (4–8) | 6 (4–8) | 6 (4–8) | 5 (4–7) | 5 (3–7) | 5 (3–8) | |
| Family history of oesophageal cancer | |||||||
| No or unknown | 400 (93%) | 419 (95%) | 283 (91%) | 303 (97%) | 482 (89%) | 542 (91%) | |
| Yes | 30 (7%) | 21 (5%) | 27 (9%) | 10 (3%) | 57 (11%) | 51 (9%) | |
Data are n (%) unless otherwise stated. ..=not applicable.
Excluding 175 participants (97 cases and 78 controls) in Tanzania who were recruited during the first 15 months and when this question was not asked, and one case in Malawi who did not provide an answer.
To investigate potential confounders, alcohol drinking (ever or never) among controls was assessed in relation to sociodemographic variables (table 2). Drinking habits in the control participants who reported ever consuming alcohol are shown in the appendix 3 (p 5). Among controls, 148 (54%) of 272 men in Kenya, 114 (48%) of 237 men in Tanzania, and 217 (65%) of 335 men in Malawi reported ever drinking alcohol. Also, among controls, 43 (26%) of 168 women in Kenya, 28 (37%) of 76 women in Tanzania, and 35 (14%) of 258 women in Malawi reported ever drinking alcohol. Among controls in all three countries, the prevalence of ever having consumed alcohol did not differ significantly by age or education, but was higher among Christians than Muslims (except in Kenya where there were few Muslims) and higher among tobacco users than people who had never used tobacco.
Table 2.
Country-specific alcohol consumption in controls, overall and by sociodemographic factors
|
Kenya |
Tanzania |
Malawi |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ever consumed alcohol (n=191) | Never consumed alcohol (n=249) | p value | Ever consumed alcohol (n=142) | Never consumed alcohol (n=171) | p value | Ever consumed alcohol (n=252) | Never consumed alcohol (n=341) | p value | ||
| Sex | .. | .. | .. | .. | .. | .. | .. | .. | <0·0001 | |
| Male | 148/272 (54%) | 124/272 (46%) | <0·0001 | 114/237 (48%) | 123/237 (52%) | 0·086 | 217/335 (65%) | 118/335 (35%) | .. | |
| Female | 43/168 (26%) | 125/168 (74%) | .. | 28/76 (37%) | 48/76 (63%) | .. | 35/258 (14%) | 223/258 (86%) | .. | |
| Age, years | .. | .. | .. | .. | .. | .. | .. | .. | 0·60 | |
| 18–34 | 9/30 (30%) | 21/30 (70%) | 0·092 | 4/11 (36%) | 7/11 (64%) | 0·80 | 19/49 (39%) | 30/49 (61%) | .. | |
| 35–44 | 24/71 (34%) | 47/71 (66%) | .. | 13/27 (48%) | 14/27 (52%) | .. | 41/107 (38%) | 66/107 (62%) | .. | |
| 45–54 | 43/94 (46%) | 51/94 (54%) | .. | 26/56 (46%) | 30/56 (54%) | .. | 54/134 (40%) | 80/134 (60%) | .. | |
| 55–64 | 47/105 (45%) | 58/105 (55%) | .. | 41/80 (51%) | 39/80 (49%) | .. | 54/129 (42%) | 75/129 (58%) | .. | |
| 65–74 | 37/86 (43%) | 49/86 (57%) | .. | 33/81 (41%) | 48/81 (59%) | .. | 56/117 (48%) | 61/117 (52%) | .. | |
| 75+ | 31/54 (57%) | 23/54 (43%) | .. | 25/58 (43%) | 33/58 (57%) | .. | 28/57 (49%) | 29/57 (51%) | .. | |
| Ethnicity | .. | .. | .. | .. | .. | .. | .. | .. | 0·29 | |
| Kalenjin | 102/233 (44%) | 131/233 (56%) | 0·81 | .. | .. | <0·0001 | .. | .. | .. | |
| Luhya | 43/95 (45%) | 52/95 (55%) | .. | .. | .. | .. | .. | .. | .. | |
| Luo | 11/32 (34%) | 21/32 (66%) | .. | .. | .. | .. | .. | .. | .. | |
| Kikuyu | 14/29 (48%) | 15/29 (52%) | .. | .. | .. | .. | .. | .. | .. | |
| Other | 21/51 (41%) | 30/51 (59%) | .. | 13/53 (25%) | 40/53 (75%) | .. | 25/69 (36%) | 44/69 (64%) | .. | |
| Chagga | .. | .. | .. | 119/227 (52%) | 108/227 (48%) | .. | .. | .. | .. | |
| Pare | .. | .. | .. | 10/33 (30%) | 23/33 (70%) | .. | .. | .. | .. | |
| Lomwe | .. | .. | .. | .. | .. | .. | 80/171 (47%) | 91/171 (53%) | .. | |
| Ngoni | .. | .. | .. | .. | .. | .. | 43/99 (43%) | 56/99 (57%) | .. | |
| Yao | .. | .. | .. | .. | .. | .. | 28/86 (33%) | 58/86 (67%) | .. | |
| Chewa | .. | .. | .. | .. | .. | .. | 30/60 (50%) | 30/60 (50%) | .. | |
| Sena | .. | .. | .. | .. | .. | .. | 23/53 (43%) | 30/53 (57%) | .. | |
| Mang'anja | .. | .. | .. | .. | .. | .. | 23/55 (42%) | 32/55 (58%) | .. | |
| Religion | .. | .. | .. | .. | .. | .. | .. | .. | 0·036 | |
| Christian | 178/422 (42%) | 244/422 (58%) | 0·025 | 89/201 (44%) | 112/201 (56%) | <0·0001 | 230/526 (44%) | 296/526 (56%) | ||
| Muslim | 2/4 (50%) | 2/4 (50%) | .. | 1/33 (3%) | 32/33 (97%) | .. | 13/50 (26%) | 37/50 (74%) | ||
| Other | 11/14 (79%) | 3/14 (21%) | .. | 52/79 (66%) | 27/79 (34%) | .. | 9/17 (53%) | 8/17 (47%) | ||
| Education | .. | .. | .. | .. | .. | .. | .. | .. | 0·12 | |
| None | 46/100 (46%) | 54/100 (54%) | 0·28 | 11/21 (52%) | 10/21 (48%) | 0·38 | 31/96 (32%) | 65/96 (68%) | .. | |
| Some primary | 39/110 (35%) | 71/110 (65%) | .. | 29/69 (42%) | 40/69 (58%) | .. | 101/229 (44%) | 128/229 (56%) | .. | |
| Completed primary | 43/91 (47%) | 48/91 (53%) | .. | 69/162 (43%) | 93/162 (57%) | .. | 33/82 (40%) | 49/82 (60%) | .. | |
| Secondary or higher | 63/139 (45%) | 76/139 (55%) | .. | 33/61 (54%) | 28/61 (46%) | .. | 87/186 (47%) | 99/186 (53%) | .. | |
| Tobacco use (men) | .. | .. | .. | .. | .. | .. | .. | .. | <0·0001 | |
| Ever | 92/108 (84%) | 18/108 (16%) | <0·0001 | 51/58 (88%) | 7/58 (12%) | <0·0001 | 108/126 (86%) | 18/126 (14%) | .. | |
| Never | 56/162 (35%) | 106/162 (65%) | .. | 63/179 (35%) | 116/179 (65%) | 109/209 (52%) | 100/209 (48%) | .. | ||
| Tobacco use (women) | .. | .. | .. | .. | .. | .. | .. | .. | <0·0001 | |
| Ever | 21/27 (78%) | 6/27 (22%) | <0·0001 | 5/8 (62%) | 3/8 (38%) | 0·14 | 13/31 (42%) | 18/31 (58%) | .. | |
| Never | 22/141 (16%) | 119/141 (84%) | .. | 23/68 (34%) | 45/68 (66%) | .. | 22/227 (10%) | 205/227 (90%) | .. | |
Of the people in the control group who had ever drunk alcohol, traditional beers were the most consumed alcohol in both men and women (pp 5–6). Most men also consumed commercial beers, but a smaller proportion of women in Kenya and Malawi drank commercial beer. The proportion of drinkers of traditionally produced alcoholic beverages was generally higher than those of commercial ones, particularly among women. The proportion of male drinkers of strong distillations (chang'aa, gongo, or kachasu) was higher than the proportion of female drinkers of these across all three sites, although this difference was far less pronounced in Kenya than in Tanzania or Malawi. Median estimated ethanol intakes were higher in men than in women across all sites: in men, these intakes ranged from 256 g per week (IQR 164–499) in Tanzania to 324 g per week (126–792) in Malawi. In women, intakes ranged from 47 g per week (32–87) in Malawi to 163 g per week (81–428) in Kenya. Traditional beers were the largest contributor to ethanol intake among control drinkers in Malawi and commercial beers were the largest contributor in Tanzania. In Kenya, traditional beers and commercial beers contributed similarly to ethanol intake in men, but traditional beers contributed the most to intakes in women (with 0% of median intake in women contributed by commercial beers in Kenya).
When comparing those who had ever versus never consumed alcohol, minimally-adjusted ORs were generally positive, and they were larger in men than in women (except in Malawi; appendix 3 p 6). These estimates were attenuated following adjustment for tobacco and other sociodemographic factors (OR2; table 3). In Kenya, the ORs of developing ESCC associated with ever versus never drinking was 4·17 (95% CI 2·56–6·90) in men and 1·80 (0·94–3·44) in women, in Tanzania the ORs were 2·95 (95% CI 1·57–5·67) in men and 1·11 (0·47–2·65) in women, and in Malawi the ORs were 0·65 (95% CI 0·43–0·96) in men and 1·60 (0·94–2·74) in women (table 3). Notably, there was an apparent inverse association of ESCC with ever having consumed alcohol that was found exclusively among men in Malawi.
Table 3.
Association of alcohol consumption with oesophageal cancer risk in Kenya, Tanzania, and Malawi
|
Kenya |
Tanzania |
Malawi |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n=430) | Controls (n=440) | OR2 (95% CI) | Cases (n=310) | Controls (n=313) | OR2 (95% CI) | Cases (n=539) | Controls (n=593) | OR2 (95% CI) | ||
| Consumed alcohol | ||||||||||
| Both sexes | ||||||||||
| Never | 113 (26%) | 249 (57%) | 1 (ref) | 75 (24%) | 171 (55%) | 1 (ref) | 281 (52%) | 341 (58%) | 1 (ref) | |
| Ever | 317 (74%) | 191 (43%) | 2·88 (1·98–4·21) | 235 (76%) | 142 (45%) | 1·96 (1·20–3·20) | 258 (48%) | 252 (42%) | 0·87 (0·64–1·19) | |
| Men | ||||||||||
| Never | 40/282 (14%) | 124/272 (46%) | 1 (ref) | 35/237 (15%) | 123/237 (52%) | 1 (ref) | 105/311 (34%) | 118/335 (35%) | 1 (ref) | |
| Ever | 242/282 (86%) | 148/272 (54%) | 4·17 (2·56–6·90) | 202/237 (85%) | 114/237 (48%) | 2·95 (1·57–5·67) | 206/311 (66%) | 217/335 (65%) | 0·65 (0·43–0·96) | |
| Women | ||||||||||
| Never | 73/148 (49%) | 125/168 (74%) | 1 (ref) | 40/73 (55%) | 48/76 (63%) | 1 (ref) | 176/228 (77%) | 223/258 (86%) | 1 (ref) | |
| Ever | 75/148 (51%) | 43/168 (26%) | 1·80 (0·94–3·44) | 33/73 (45%) | 28/76 (37%) | 1·11 (0·47–2·65) | 52/228 (23%) | 35/258 (14%) | 1·60 (0·94–2·74) | |
| Never tobacco users | ||||||||||
| Never | 105/178 (59%) | 225/303 (74%) | 1 (ref) | 49/92 (53%) | 161/247 (65%) | 1 (ref) | 232/296 (78%) | 305/436 (70%) | 1 (ref) | |
| Ever | 73/178 (41%) | 78/303 (26%) | 2·42 (1·57–3·76) | 43/92 (47%) | 86/247 (35%) | 2·31 (1·28–4·24) | 64/296 (22%) | 131/436 (30%) | 0·75 (0·50–1·10) | |
| Ever tobacco users | ||||||||||
| Never | 8/252 (3%) | 24/137 (18%) | 1 (ref) | 26/218 (12%) | 10/66 (15%) | 1 (ref) | 49/243 (20%) | 36/157 (23%) | 1 (ref) | |
| Ever | 244/252 (97%) | 113/137 (82%) | 7·83 (3·36–20·20) | 192/218 (88%) | 56/66 (85%) | 1·49 (0·55–3·92) | 194/243 (80%) | 121/157 (77%) | 1·18 (0·68–2·06) | |
| Type of drinker by strength of beverage | ||||||||||
| Never drinker | 113 (26%) | 249 (57%) | 1 (ref) | 75 (24%) | 171 (55%) | 1 (ref) | 281 (52%) | 341 (58%) | 1 (ref) | |
| Low-ABV only | 40 (9%) | 52 (12%) | 1·44 (0·85–2·43) | 81 (26%) | 103 (33%) | 1·51 (0·90–2·53) | 71 (13%) | 88 (15%) | 0·83 (0·56–1·23) | |
| High-ABV | 277 (64%) | 139 (32%) | 3·73 (2·50–5·60) | 154 (50%) | 39 (12%) | 3·87 (2·04–7·38) | 187 (35%) | 164 (28%) | 0·90 (0·63–1·28) | |
| Average number of drinks, n per week | ||||||||||
| Never drinker | 113 (26%) | 249 (57%) | 1 (ref) | 75 (24%) | 171 (55%) | 1 (ref) | 281 (52%) | 341 (58%) | 1 (ref) | |
| <14 | 72 (17%) | 72 (16%) | 1·84 (1·16–2·90) | 32 (10%) | 40 (13%) | 1·55 (0·81–2·96) | 92 (17%) | 90 (15%) | 0·93 (0·64–1·36) | |
| 14–27 | 74 (17%) | 40 (9%) | 3·40 (2·03–5·75) | 29 (9%) | 44 (14%) | 1·04 (0·51–2·07) | 44 (8%) | 39 (7%) | 0·88 (0·52–1·51) | |
| 28–41 | 48 (11%) | 29 (7%) | 3·02 (1·67–5·54) | 22 (7%) | 20 (6%) | 1·59 (0·68–3·70) | 28 (5%) | 23 (4%) | 1·13 (0·60–2·15) | |
| ≥42 | 123 (29%) | 50 (11%) | 5·26 (3·18–8·82) | 152 (49%) | 38 (12%) | 4·82 (2·53–9·23) | 94 (17%) | 100 (17%) | 0·73 (0·48–1·10) | |
| Average ethanol intake, g per week | ||||||||||
| Never drinker | 113 (26%) | 249 (57%) | 1 (ref) | 75 (24%) | 171 (55%) | 1 (ref) | 281 (52%) | 341 (58%) | 1 (ref) | |
| <140 | 67 (16%) | 61 (14%) | 2·03 (1·27–3·26) | 39 (13%) | 35 (11%) | 1·95 (1·02–3·73) | 82 (15%) | 91 (15%) | 0·82 (0·56–1·21) | |
| 140–349 | 86 (20%) | 47 (11%) | 3·24 (1·98–5·35) | 46 (15%) | 61 (19%) | 1·03 (0·54–1·95) | 72 (13%) | 52 (9%) | 1·16 (0·74–1·84) | |
| 350–699 | 82 (19%) | 45 (10%) | 3·27 (1·97–5·49) | 54 (17%) | 30 (10%) | 2·09 (1·03–4·25) | 43 (8%) | 43 (7%) | 0·91 (0·54–1·53) | |
| ≥700 | 82 (19%) | 38 (9%) | 4·49 (2·59–7·87) | 69 (31%) | 16 (5%) | 6·25 (2·90–13·90) | 61 (11%) | 66 (11%) | 0·70 (0·43–1·11) | |
| Contribution from different drinks to total ethanol consumption, median % contribution | ||||||||||
| Traditional beer | 47% | 37% | 1·03 (0·97–1·09)* | 29% | 27% | 1·08 (0·96–1·21)* | 63% | 64% | 0·96 (0·91–1·02)* | |
| Commercial beer | 0% | 22% | 0·83 (0·77–0·90)* | 32% | 55% | 0·94 (0·85–1·02)* | 0 | 0 | 1·07 (0·99–1·16)* | |
| Chang'aa, gongo, or kachasu | 31% | 14% | 1·20 (1·12–1·30)* | 5% | 0 | 1·26 (1·06–1·53)* | 2% | 1% | 1·03 (0·96–1·10)* | |
| Commercial spirits | 0 | 0 | 0·77 (0·62–0·92)* | 0 | 0 | 0·92 (0·77–1·11)* | 0 | 0 | 0·90 (0·77–1·04)* | |
Data are n (%), unless otherwise indicated. Those who reported consuming at least one commercial or traditional distillation (eg, chang'aa, gongo, kachasu) of >30% ABV were categorised as high ABV drinkers. ORs were adjusted for age, sex, ethnicity, education and religion. All ORs were also adjusted for tobacco usage (ever or never) and tobacco frequency (number of tobacco products smoked or chewed per day) except in the cases of the tobacco use-specific models. ABV=alcohol by volume. OR=odds ratio.
Expressed per 10% absolute increase in ethanol from a given drink.
Except for in Malawi, the directions of the associations of alcohol with ESCC risk were consistent across countries for type of drinker (increased ESCC risks were specifically associated with high-ABV drinkers vs never drinkers), greater estimated weekly number of drinks (≥28 weekly drinks was associated with increased risk vs never drinking), and greater estimated average ethanol intake (≥350 g ethanol per week was associated with higher risk vs never drinking; table 3). In Malawi, sex-specific analyses (appendix 3 p 7) in women, revealed consistent positive associations of increased ESCC risk with the consumption of 140 g or more of ethanol per week. In contrast, associations in Malawi men were null, which attenuated the ORs in analyses on both sexes combined. Finally, after controlling for average ethanol intake, we examined whether there was an independent effect on ESCC risk of the type of drink and found specific additional risks linked to strong traditional distillations (chang'aa and gongo) in Kenya and Tanzania, whereas the type of drink did not affect risk in Malawi (table 3).
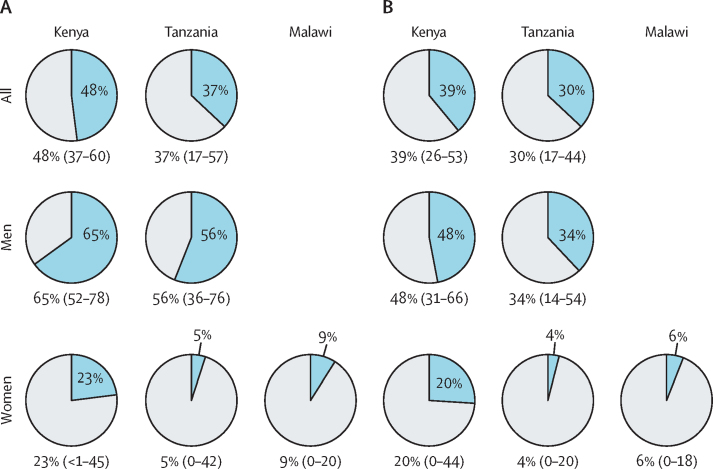
In Kenya, an interaction (p=0·018) was found between ever having consumed alcohol and ever having used tobacco for their effects on ESCC risk, with a higher odds ratio for ESCC in ever versus never consumers of alcohol among ever tobacco users than never tobacco users (table 3). Excluding men in Malawi (for whom positive associations between alcohol consumption and ESCC were not found), site and sex-specific PAFs—if ever drinkers were never drinkers (PAFever) and if high-ABV drinkers had the drinking patterns of only low-ABV drinkers (PAFhigh)—are shown in the figure. PAFever was 48% (95% CI 37–60) in Kenya and 37% (17–57) in Tanzania. PAFever was higher in Kenyan men (65% [95% CI 52–78]) than Kenyan women (23% [<1–45]), and was higher in Tanzanian men (56% [95% CI 36–76]) than Tanzanian women (5% [0–42]). PAFhigh was 39% (95% CI 26–53) in Kenya and 30% (17–44) in Tanzania. PAFhigh was higher in Kenyan men (48% [95% CI 31–66]) than Kenyan women (20% [0–44]), and was higher in Tanzanian men (34% [95% CI 14–54]) than Tanzanian women (4% [0–20]). In women in Malawi and Tanzania, PAFs were all low (<10%).
Figure.
PAFs (95% CI) of ESCC, overall and by sex
Data are shown for (A) ever versus never having consumed alcohol (ie, the estimated proportion of cases who would not have developed ESCC if everyone were a never drinker), and (B) those who consumed any high (>30%) ABV beverages versus those who consumed only low-ABV beverages (ie, the estimated proportion of cases who would not have developed ESCC if everyone had only consumed low-ABV drinks). Sex-specific PAFs in (A) were calculated from sex-specific model 2 odds ratios (model 2 was adjusted for age [10-year strata], smoked and smokeless tobacco use [ever or never], smoking frequency [a continuous variable: number of cigarettes or tobacco uses per day], ethnicity, education, and religion). The remaining PAFs were calculated using model 2 with both sexes combined. ESCC=oesophageal squamous cell carcinoma. PAF=population-attributable fraction.
A comparison of weekly ethanol intakes among controls from different drinks between high-ABV and low-ABV drinkers is shown in appendix 3 (p 8). In Kenya, for example, high-ABV drinkers would, on average, require a 57% reduction in weekly ethanol to reach the intake of low-ABV drinkers, roughly equating to cessation of all of their spirit intake, and a 32% reduction in traditional and commercial beer intake.
Finally, to explore the null associations in Malawian men, we investigated whether drinking status was systematically under-reported by conducting a recontacting exercise in this country alone. The findings of this investigation are described and reported in appendix 3 (pp 9–10), and they suggest that ever drinking might have been under-reported in this group (vs never drinking). However, we note that this exercise was done post-hoc and was limited in its design, and thus these findings are speculative.
Discussion
A reduction in harmful alcohol use is one of the main priorities of WHO global action plan for the prevention and control of non-communicable diseases.28 Despite being a known ESCC carcinogen,3 evidence of alcohol's role in east Africa's ESCC burden has been inconsistent. We did, to our knowledge, the largest multicentre ESCC case-control study in this region to date, with data from 1279 cases and 1346 controls. Undertaking detailed alcohol exposure assessment, we found consistent associations between ESCC risk and multiple alcohol exposure metrics in Kenya, Tanzania, and Malawi, including drinking status (ever drinking any alcohol and drinkers of strong alcohols), number of drinks per day, and ethanol intake. An association of ESCC and higher ethanol intakes was seen in Kenya and Tanzania (both sexes) and in Malawian women. Although low-ABV drinks contributed a large share of ethanol intake, namely traditional beers, specific additional risks associated with a 10% absolute increase in ethanol contribution from strong (>30% ABV) traditional distillations were observed in Kenya and Tanzania (table 3). This finding was reflected in large PAFs of ESCC cases attributable to different alcohol consumption habits, particularly in men, associated with altering the habits of drinkers whose drinking habits included high-ABV drinks to the habits of drinkers who only consumed low-ABV drinks in Kenya and Tanzania.
A standout finding of this analysis was the apparent null or negative association between alcohol and ESCC among men in Malawi. In men, positive associations were found between ESCC and ever versus never consuming alcohol in every country except for Malawian men in whom alcohol had no effect upon minimal adjustment and a protective effect after adjusting for tobacco. This clear outlier finding is contradictory to the known ESCC carcinogenic effect of alcohol, and alcohol consumption is expected to be higher in men. One possible explanation for this unexpected finding is that drinking status was systematically under-reported among men in Malawi, with a post-hoc recontacting exercise (appendix 3 pp 9–10) done in Malawi providing suggestive, though speculative, evidence to this effect.
Overall, our study had many strengths, including the systematic, sequential recruitment of cases over several years and histological confirmation of most cases. The minority of cases without histological confirmation poses no major limitation given the definitiveness of clinical diagnosis for late stage oesophageal cancer and a high (>90%) proportion of ESCC histology in these settings. Recruitment at sites in three high ESCC incidence countries allowed consistency to be investigated. A standout strength of the study was the detailed setting-appropriate alcohol exposure assessment, in which we obtained information on a wide variety of local alcohols whose contribution to overall alcohol intake is difficult to quantify and regulate in Africa because of under-reporting and variable ethanol concentrations, and by ensuring that consumption of traditional beverages was reported in the alcohol assessment. Although some minor differences in the wording of questions existed between Kenya and the other two sites, the derived metrics analysed were standardised between the three recruitment sites, further underlining the consistency in descriptive exposure characteristics and alcohol-ESCC associations between countries. Conversely, the self-reported nature of these metrics might have been subject to under-reporting, notably among men in Malawi but to some degree across the entire study population. Under-reporting has implications for alcohol exposure assessment in epidemiological studies in this and other settings, and might explain previous null associations between alcohol and ESCC risk in East Africa.14 Estimation of ethanol intake was also open to a large degree of error, relying on self-reported drinking volumes and frequencies, and sparse data on the ethanol content of a wide variety of local beverages of varying interdrink and intradrink type strength. Nevertheless, the categorisation of these values resulted in ORs with a broadly consistent positive trend between strata. Finally, not adjusting our analyses for potentially confounding additional putative ESCC risk factors beyond tobacco, such as dietary variables, might limit our findings, but we note the complexity of these other risk factors requires them to be analysed in their own right and do not expect them to have nullified any of the associations we observed.
A full understanding of the remarkable geographical variation in ESCC incidence rates worldwide, and in east Africa, is not known. A multifactorial picture of risk is beginning to emerge, with putative risk factors in this setting including tobacco, dietary micronutrient deficiencies, poor oral health and hygiene, very hot beverage consumption, and exposures to chemical carcinogens (such as polycyclic aromatic hydrocarbons or those present in unpiped drinking water).29 In conclusion, these alcohol-specific findings point to a substantial contribution to the ESCC burden in men across Kenya, Tanzania, and Malawi, with the exception of an uncertain role of alcohol in Malawian men. The large estimated PAFs of ESCC attributable to alcohol consumption, particularly among men, indicate that large absolute reductions in the cancer burden could be achieved through alcohol cessation, particularly targeting the drinking habits of drinkers of higher strength spirits. The effects of such prevention measures would be manifold, as harmful alcohol use is problematic for a host of non-communicable and communicable diseases.
This online publication has been corrected. The corrected version first appeared at thelancet.com/lancetgh on December 22, 2021
Data sharing
Data are stored at the International Agency for Research on Cancer, where on-site access is welcomed via research collaborations in Lyon. Requests can be made to the corresponding author
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
ESCCAPE would like to acknowledge all participants for their time, and the support of study interviewers and endoscopy and laboratory staff at the Queen Elizabeth Central Hospital, Blantyre, Moi Teaching and Referral Hospital, Eldoret, and the Kilimanjaro Christian Medical Central, Moshi. The study was funded by the US National Cancer Institute (R21CA19165), Wereld Kanker Onderzoek Fonds (2018/1795), and the IARC Environment and Lifestyle Epidemiology Branch. In instances in which authors are identified as personnel of the IARC or WHO, the authors alone are responsible for the views expressed in this Article and they do not necessarily represent the decisions, policy, or views of these organisations.
Contributors
DRSM wrote the manuscript draft. DRSM did all analyses under the supervision of VM. DM, CD, and BTM led the fieldwork teams. All co-authors discussed the results, reviewed the text, interpreted the findings, and had access to all the study data. DRSM and VM accessed and verified the data for this analysis and were responsible for the decision to submit for publication.
ESCCAPE group
Nicolas Kigen, Margaret Oduor, Stephen Karuru Maina, Fatima Some, Caroline Kibosia, Amos Mwasamwaja, Alex Mremi, Ireen Kiwelu, Godfrey Mushi, Theresia Namwai, Remigi Swai, Godwin Kiwelu, Sophia Mustapha, Eliawawomy Mghase, Amana Mchome, Redfan Shao, Evarista Mallya, Kajiru Kilonzo, Anstead Kamkwantira, Mercy Kamdolozi, George Liomba, Mary Suwedi, Thandiwe Solomon, Rose Malamba, Steady Chasimpha, Behnoush Abedi-Ardekani, Christine Carreira, Clement Narh, Liacine Bouaoun.
Contributor Information
Valerie McCormack, Email: mccormackv@iarc.fr.
ESCCAPE:
Nicolas Kigen, Margaret Oduor, Stephen Karuru Maina, Fatima Some, Caroline Kibosia, Amos Mwasamwaja, Alex Mremi, Ireen Kiwelu, Remigi Swai, Godwin Kiwelu, Sophia Mustapha, Eliawawomy Mghase, Amana Mchome, Redfan Shao, Evarista Mallya, Kajiru Kilonzo, Anstead Kamkwantira, Mercy Kamdolozi, George Liomba, Steady Chasimpha, Clement Narh, Liacine Bouaoun, Behnoush Abedi-Ardekani, Godfrey Mushi, Theresia Namwai, Mary Suwedi, Thandiwe Solomon, Rose Malamba, and Christine Carreira
Supplementary Materials
References
- 1.Ferlay J, Ervik M, Lam F, et al. About cancer today. 2020. https://gco.iarc.fr/today/about
- 2.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer . World Health Organization; Lyon, France: 2012. Personal Habits and Indoor Combustions. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 4.Abnet CC, Arnold M, Wei W-Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asombang AW, Chishinga N, Nkhoma A, et al. Systematic review and meta-analysis of esophageal cancer in Africa: epidemiology, risk factors, management and outcomes. World J Gastroenterol. 2019;25:4512–4533. doi: 10.3748/wjg.v25.i31.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal I, Reinach SG, de Beer M. Factors associated with oesophageal cancer in Soweto, South Africa. Br J Cancer. 1988;58:681–686. doi: 10.1038/bjc.1988.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sammon AM. A case-control study of diet and social factors in cancer of the esophagus in Transkei. Cancer. 1992;69:860–865. doi: 10.1002/1097-0142(19920215)69:4<860::aid-cncr2820690404>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Vizcaino AP, Parkin DM, Skinner ME. Risk factors associated with oesophageal cancer in Bulawayo, Zimbabwe. Br J Cancer. 1995;72:769–773. doi: 10.1038/bjc.1995.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacella-Norman R, Urban MI, Sitas F, et al. Risk factors for oesophageal, lung, oral and laryngeal cancers in black South Africans. Br J Cancer. 2002;86:1751–1756. doi: 10.1038/sj.bjc.6600338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel K, Wakhisi J, Mining S, Mwangi A, Patel R. Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol. 2013;2013 doi: 10.1155/2013/503249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sewram V, Sitas F, O'Connell D, Myers J. Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa. Cancer Epidemiol. 2016;41:113–121. doi: 10.1016/j.canep.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw E, Schonland M. Oesophageal and lung cancers in Natal African males in relation to certain socio-economic factors. An analysis of 484 interviews. Br J Cancer. 1969;23:275–284. doi: 10.1038/bjc.1969.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menya D, Kigen N, Oduor M, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer. 2019;144:459–469. doi: 10.1002/ijc.31804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mmbaga EJ, Mushi BP, Deardorff K, et al. A case-control study to evaluate environmental and lifestyle risk factors for esophageal cancer in Tanzania. Cancer Epidemiol Biomarkers Prev. 2020;30:305–316. doi: 10.1158/1055-9965.EPI-20-0660. [DOI] [PubMed] [Google Scholar]
- 15.Bradshaw E, Schonland M. Smoking, drinking and oesophageal cancer in African males of Johannesburg, South Africa. Br J Cancer. 1974;30:157–163. doi: 10.1038/bjc.1974.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middleton DRS, Bouaoun L, Hanisch R, et al. Esophageal cancer male to female incidence ratios in Africa: a systematic review and meta-analysis of geographic, time and age trends. Cancer Epidemiol. 2018;53:119–128. doi: 10.1016/j.canep.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi R, Wilunda C, Magutah K, Mwaura-Tenambergen W, Wilunda B, Perngparn U. Correlates of alcohol consumption in rural western Kenya: a cross-sectional study. BMC Psychiatry. 2017;17:175. doi: 10.1186/s12888-017-1344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsunaga T, Larsen U. Prevalence of and risk factors associated with alcohol abuse in Moshi, northern Tanzania. J Biosoc Sci. 2008;40:379–399. doi: 10.1017/S0021932007002441. [DOI] [PubMed] [Google Scholar]
- 19.Francis JM, Weiss HA, Mshana G, Baisley K, Grosskurth H, Kapiga SH. The epidemiology of alcohol use and alcohol use disorders among young people in northern Tanzania. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoel E, Azalde G, Munthalic A, Eide AH, Natvig H, Braathen SH. Context and consequences of liquor sachets use among young people in Malawi. Afr J Drug Alcohol Stud. 2014;13:97–106. [Google Scholar]
- 21.Papas RK, Sidle JE, Wamalwa ES, et al. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS Behav. 2010;14:836–844. doi: 10.1007/s10461-008-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis JM, Grosskurth H, Kapiga SH, Weiss HA, Mwashiuya J, Changalucha J. Ethanol concentration of traditional alcoholic beverages in Northern Tanzania. J Stud Alcohol Drugs. 2017;78:476–477. doi: 10.15288/jsad.2017.78.476. [DOI] [PubMed] [Google Scholar]
- 23.Mawonike R, Chigunyeni B, Chipumuro M. Process improvement of opaque beer (chibuku) based on multivariate cumulative sum control chart. J Inst Brew. 2018;124:16–22. [Google Scholar]
- 24.Namondwe T, Ching'anda C, Gama AP, Matumba L. Consumption of illegal home-made alcohol in Malawi: a neglected public health threat. Alcohol. 2019;75:99–103. doi: 10.1016/j.alcohol.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Mosha D, Wangabo J, Mhinzi G. African traditional brews: how safe are they? Food Chem. 1996;57:205–209. [Google Scholar]
- 26.Menya D, Kigen N, Oduor M, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer. 2019;144:459–469. doi: 10.1002/ijc.31804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–872. [PubMed] [Google Scholar]
- 28.WHO Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Nov 14, 2013. https://www.who.int/publications/i/item/9789241506236
- 29.McCormack VA, Menya D, Munishi MO, et al. Informing etiologic research priorities for squamous cell esophageal cancer in Africa: a review of setting-specific exposures to known and putative risk factors. Int J Cancer. 2017;140:259–271. doi: 10.1002/ijc.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are stored at the International Agency for Research on Cancer, where on-site access is welcomed via research collaborations in Lyon. Requests can be made to the corresponding author