Abstract
Eosinophilic myocarditis (EM) is an under-diagnosed inflammatory heart disease that often leads to severe left ventricular (LV) dysfunction. Meanwhile, severe secondary mitral regurgitation (MR) with valve disruption, possibly requiring mitral valve repair, is rarely concomitant with EM. We present the case of a 64-year-old female diagnosed with heart failure with severe LV dysfunction and localized asynergy. Echocardiography revealed severe secondary MR with mitral valve disruption. Cardiac magnetic resonance imaging (CMR) showed transmural late-gadolinium enhancement localized in the anterior wall and diffuse high-signal areas on T2-weighted images, suggesting non-ischemic and inflammatory heart disease. Although the peripheral eosinophil count was not elevated on admission, it gradually increased during hospitalization. These findings encouraged us to perform endomyocardial biopsy, which confirmed myocardial eosinophilic infiltration with mild fibrosis and necrosis, leading to the diagnosis of EM. Immunosuppressive treatment with oral corticosteroids improved LV dysfunction and completely resolved severe secondary MR. The current case highlighted that comprehensive assessment of laboratory, imaging, and pathological examinations including CMR is crucial to develop the appropriate therapeutic strategy for refractory heart failure. Immunosuppressive treatment should be considered as the first therapeutic option even in EM cases with severe secondary MR, possibly requiring mitral valve repair.
<Learning objective:The gradual increase in peripheral eosinophils during hospitalization, without significant peripheral eosinophilia on admission, is crucial for the diagnosis of eosinophilic myocarditis. Comprehensive assessment of laboratory, imaging, and pathological examinations including cardiac magnetic resonance imaging is mandatory when building an appropriate therapeutic strategy for refractory heart failure. Severe secondary mitral regurgitation with mitral valve disruption can be completely resolved via immunosuppressive treatment in cases of eosinophilic myocarditis.>
Keywords: Eosinophilic myocarditis, Mitral regurgitation, Cardiac magnetic resonance imaging
Introduction
Eosinophilic myocarditis (EM) is a rare inflammatory heart disease with various clinical presentations ranging from asymptomatic to life-threatening, and from rapid to gradual progression [1,2]. Moreover, peripheral eosinophilia, a key diagnostic feature of EM, is not always observed on admission [3]. Thus, these facts contribute to underdiagnosis or delayed diagnosis of EM, and subsequently, the appropriate timing of introduction of immunosuppressive therapy is sometimes missed.
EM is frequently accompanied by severe left ventricular (LV) dysfunction. A previous report demonstrated that the median value of baseline LV ejection fraction (LVEF) in EM was 35% [3]. Meanwhile, severe secondary mitral regurgitation (MR), possibly requiring mitral valve repair, is rarely concomitant with EM. In addition, a therapeutic strategy for such cases concomitant with severe secondary MR has not been established. Herein, we present a case of EM diagnosed as severe MR with mitral valve disruption, completely resolved by corticosteroids.
Case report
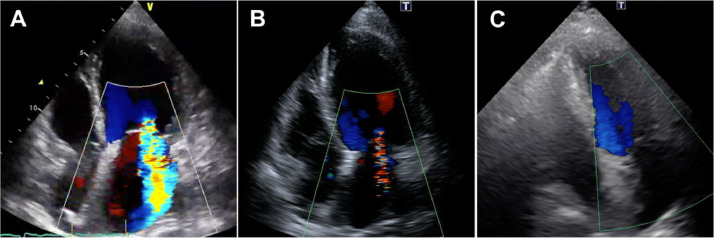
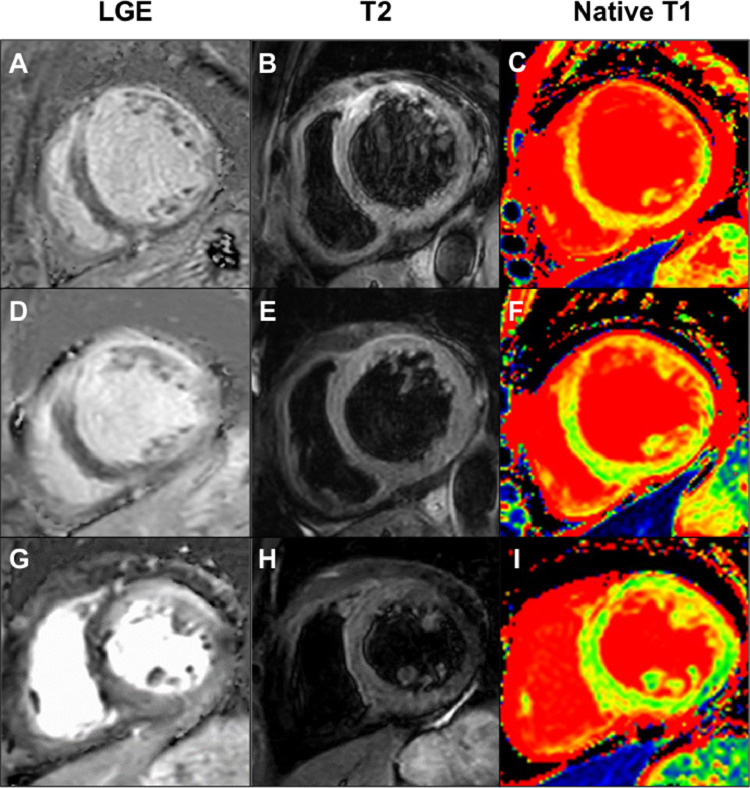
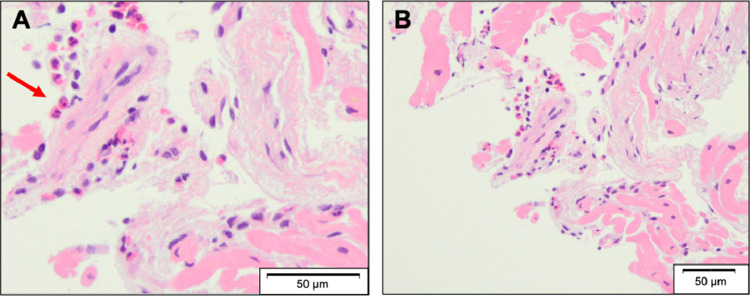
A 64-year-old female with a history of schizophrenia was admitted to a community hospital due to worsening dyspnea. Two months before this admission, she complained of palpitation without fever and chest pain, and then she felt dyspnea on exertion, which was gradually getting worse. Her schizophrenia was well-controlled with regular medications, including major tranquilizers. No new drugs or supplements had been started in the past several months. She was newly diagnosed with congestive heart failure due to non-ischemic cardiomyopathy based on the findings of transthoracic echocardiography (TTE), demonstrating severe LV dysfunction with severe MR, and coronary angiography with no significant stenosis of the epicardial coronary arteries. Since heart failure was not well-managed with medical treatment including inotropic agents, she was transferred to our hospital for a more detailed examination and intensive therapy that included mitral valve interventions. On admission, clinical examination revealed anasarca and pansystolic murmur at the apex on auscultation. Her blood pressure was 90/64 mmHg, and the heart rate was 121 bpm with regular rhythm under continuous dobutamine infusion (3 μg/kg/min). Electrocardiography showed sinus tachycardia with multiple premature atrial contractions, without a wide QRS wave and atrioventricular block. Chest radiography revealed bilateral pleural effusion and pulmonary congestion. TTE demonstrated severe MR with mitral valve disruption due to tethering and mitral annular expansion in addition to diffuse severe hypokinesis with localized asynergy. LV end-diastolic dimension (LVDd) was 60.6 mm, and LVEF was 25%. Tricuspid regurgitation pressure gradient (TRPG) was 55.6 mmHg, suggesting severe pulmonary hypertension (Fig. 1A; Online Movie 1). Blood tests revealed no significant elevation of eosinophils at 258/mm3 (<500/mm3) and a slight elevation of cardiac troponin I at 0.050 ng/mL (normal values less than 0.024 ng/mL). The plasma angiotensin-converting enzyme and soluble interleukin-2 receptor levels were within normal. Cardiac magnetic resonance imaging (CMR) revealed transmural late gadolinium enhancement (LGE) localized in the anterior wall and diffuse high-signal areas on T2-weighted images (Fig. 2A, B). In addition, the mean native T1, which can estimate the degree of myocardial fibrosis, was 1600 ± 296 ms (Fig. 2C). In the laboratory data on day 5, peripheral eosinophil count was elevated to 680/mm3. On day 10 of admission, she underwent endomyocardial biopsy (EMB) in addition to right heart catheterization, which showed an increase in mean pulmonary capillary wedge pressure (21 mmHg) and decreased cardiac output (1.68 L/min/m2). Although we tried to wean off the dobutamine, her heart failure deteriorated with remaining severe MR, considered as a medically refractory state. On day 17, the histopathological findings of the resected specimen on the EMB revealed myocardial eosinophilic infiltration with mild fibrosis and necrosis (Fig. 3), and she was diagnosed with EM. The etiology of EM remains unknown despite screening for infections, including parasites, malignancy, or autoimmune disease. We introduced immunosuppressive therapy based on these pathological findings. Oral prednisolone at 60 mg/day was initiated on day 21 for three days, and was then tapered to a dose of 30 mg/day. As she became hemodynamically stable after the introduction of PSL, dobutamine was gradually tapered. TTE performed on day 42 showed that MR was reduced from severe to mild. LVDd and LVEF were 54.4 mm and 32%, respectively. TRPG was improved to 16.2 mmHg (Fig. 1B; Online Movie 2). CMR revealed that the high-signal area on T2-weighted images decreased in size and the average native T1 was improved to 1385 ± 149 ms; however, focal transmural delayed enhancement at the anteroseptal wall remained unchanged (Fig. 2D–F). On day 45, the patient was discharged with a prednisolone of 30 mg/day. During the outpatient period, the prednisolone dose was tapered to a maintenance dose of 5 mg/day. Six months after discharge, the patient had no clinical adverse events. Blood tests showed that cardiac troponin I was normalized, TTE showed MR was completely resolved. LVDd and LVEF were normalized to 45.3 mm and 68%, respectively (Fig. 1C; Online Movie 3). CMR demonstrated that the high-intensity area on T2-weighted images disappeared and the average native T1 was further improved to 1336 ± 128 ms although the focal transmural LGE remained at the anteroseptal wall (Fig. 2G–I).
Fig. 1.
Serial change in transthoracic echocardiographic findings. (A) On admission. (B) Twenty-one days after the initiation of immunosuppressive therapy. (C) Six months after discharge.
Fig. 2.
Serial change in cardiac magnetic resonance imaging findings. (A–C) Before the initiation of immunosuppressive treatment. (D–F) Twenty-one days after the initiation of immunosuppressive treatment. (G–I) Six months after discharge. LGE, late gadolinium enhancement.
Fig. 3.
Microscopic findings in resected specimens (hematoxylin and eosin staining). Red arrow indicates the eosinophilic infiltration. (A) High-power field. (B) low-power field (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Discussion
EM is an under-diagnosed disease because its clinical presentations and examination findings vary. Peripheral eosinophilia is a key diagnostic feature, but it is not always observed on admission in patients with EM. A previous report based on a large series of published cases demonstrated that peripheral eosinophilia was absent on admission in approximately 25% of EM cases [3]. In the current case, peripheral eosinophilia was not observed on admission. The more important clue to reach a definitive diagnosis of EM is the gradual increase in peripheral eosinophils during hospitalization. A previous study demonstrated that upon monitoring the changes in peripheral eosinophil count, there was an increase in 3 of 4 patients with an initial eosinophil count of <500/mm3 to >500/mm3 7 to 12 days after admission, highlighting that the absence of eosinophilia on admission does not exclude the diagnosis of EM [4]. Therefore, in addition to the white blood cell count, the eosinophil count should be repeated after admission to decrease the probability of misdiagnosis.
Among the various imaging modalities, CMR is a first-line diagnostic tool for non-ischemic heart failure of unknown etiology. Subendocardial delayed enhancement patterns have been reported as characteristic of eosinophilic myocarditis [[5], [6], [7]], whereas other patterns, including diffuse patterns, have been reported in some papers [8]. In the current case, focal transmural delayed enhancement at the anteroseptal wall and diffuse myocardial hyperintensity in T2-weighted images were demonstrated, which might be attributable to extensive edema in the myocardium. These findings were not typical for EM but suggestive of ongoing inflammation. CMR is also helpful for the follow-up of the therapeutic effect with corticosteroids in inflammatory heart diseases. In the current case, the high-intensity area on T2-weighted image disappeared in the chronic phase, and the average native T1, one of the most useful prognostic markers to reflect the degree of diffuse fibrosis, was improved from 1600 ± 29 ms to 1385 ± 149 ms. These findings revealed adequate suppression of myocardial inflammation and would be helpful in choosing the appropriate maintenance dose of immunosuppressive therapy.
EMB is required for the definitive diagnosis of EM. However, its penetration is relatively low because of the concern of life-threatening complications, such as ventricular arrhythmias, especially in the critically ill state with continuous dobutamine infusion. Furthermore, it is not a very sensitive technique because inflammatory cell infiltrates are often localized in EM. A previous report demonstrated that the sensitivity of EMB based on autopsy specimens was estimated to be 54% [9]. This is likely to be lower in the beating heart owing to technical difficulties in the biopsy procedure. If inflammatory heart disease, such as EM, was strongly suspected based on other test results, EMB should be repeated in cases with negative results. In the current case, if the initial EMB test did not lead to a diagnosis, the new appearance of eosinophilia during hospitalization would have provoked the consideration of a repeated EMB.
In terms of the treatment strategy for secondary MR due to LV dysfunction, medical therapy should be initially optimized [10]. However, in some cases, heart failure cannot be controlled by medical therapy alone. More intensive therapy, including cardiac resynchronization therapy and mitral valve intervention, is required. Even though the guidelines do not strongly recommend mitral valve intervention in cases with low ejection fraction, it would have been considered if EMB had not confirmed EM or immunosuppressive therapy had not been effective. Basically, we should assess the etiology of LV dysfunction leading to secondary MR before proceeding with intensive treatment. However, an unstable hemodynamic state can contribute to rushing to perform early invasive strategies, including mitral valve intervention. It is still challenging to establish an appropriate therapeutic strategy for refractory heart failure with severe secondary MR as in the current case.
In conclusion, severe secondary MR with mitral valve disruption was completely resolved via immunosuppressive treatment in this patient with EM. Comprehensive assessment of laboratory, imaging, and pathological examinations is crucial to develop an appropriate therapeutic strategy for refractory heart failure.
Declaration of Competing Interest
The authors have nothing to disclose regarding the current study.
Acknowledgment
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jccase.2021.07.003.
Appendix. Supplementary materials
References
- 1.Rezaizadeh H., Sanches-Ross M., Kaluski E., Klapholz M., Haider B., Gerula C. Acute eosinophilic myocarditis: diagnosis and treatment. Acute Card Care. 2010;12:31–36. doi: 10.3109/17482940903578998. [DOI] [PubMed] [Google Scholar]
- 2.Thambidorai S.K., Korlakunta H.L., Arouni A.J., Hunter W.J., Holmberg MJ. Acute eosinophilic myocarditis mimicking myocardial infarction. Tex Heart Inst J. 2009;36:355–357. [PMC free article] [PubMed] [Google Scholar]
- 3.Brambatti M., Matassini M.V., Adler E.D., Klingel K., Camici P.G., Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J Am Coll Cardiol. 2017;70:2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto S., Kubo N., Hiramitsu S., Uemura A., Ohtsuki M., Kato S., Kato Y., Sugiura A., Miyagishima K., Mori N., Yoshida Y., Hishida H. Changes in the peripheral eosinophil count in patients with acute eosinophilic myocarditis. Heart Vessel. 2003;18:193–196. doi: 10.1007/s00380-003-0721-0. [DOI] [PubMed] [Google Scholar]
- 5.Chun W., Grist T.M., Kamp T.J., Warner T.F., Christian T.F. Images in cardiovascular medicine. Infiltrative eosinophilic myocarditis diagnosed and localized by cardiac magnetic resonance imaging. Circulation. 2004;110:e19. doi: 10.1161/01.CIR.0000135586.94417.3C. [DOI] [PubMed] [Google Scholar]
- 6.Petersen S.E., Kardos A., Neubauer S. Subendocardial and papillary muscle involvement in a patient with Churg–Strauss syndrome, detected by contrast enhanced cardiovascular magnetic resonance. Heart. 2005;91:e9. doi: 10.1136/hrt.2004.050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wassmuth R., Go¨bel U., Natusch A., Schneider W., Kettritz R., Dietz R., Luft F.C., Schulz-Menger J. Cardiovascular magnetic resonance imaging detects cardiac involvement in Churg–Strauss syndrome. J Card Fail. 2008;14:856–860. doi: 10.1016/j.cardfail.2008.07.227. [DOI] [PubMed] [Google Scholar]
- 8.Cummings K.W., Bhalla S., Javidan-Nejad C., Bierhals A.J., Gutierrez F.R., Woodard P.K. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29:89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 9.Burke A.P., Saenger J., Mullick F., Virmani R. Hypersensitivity myocarditis. Arch Pathol Lab Med. 1991;115:764–769. [PubMed] [Google Scholar]
- 10.O'Gara P.T., Mack M.J. Secondary mitral regurgitation. N Engl J Med. 2020;383:1458–1467. doi: 10.1056/NEJMcp1903331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.