The epithelial sodium channel (ENaC) is a heterotrimeric ion channel that plays a key role in sodium and water homeostasis in tetrapod vertebrates. In the aldosterone-sensitive distal nephron, hormonally controlled ENaC expression matches dietary sodium intake to its excretion. Furthermore, ENaC mediates sodium absorption across the epithelia of the colon, sweat ducts, reproductive tract, and lung. ENaC is a constitutively active ion channel and its expression, membrane abundance, and open probability (PO) are controlled by multiple intracellular and extracellular mediators and mechanisms [9]. Aberrant ENaC regulation is associated with severe human diseases, including hypertension, cystic fibrosis, pulmonary edema, pseudohypoaldosteronism type 1, and nephrotic syndrome [9].
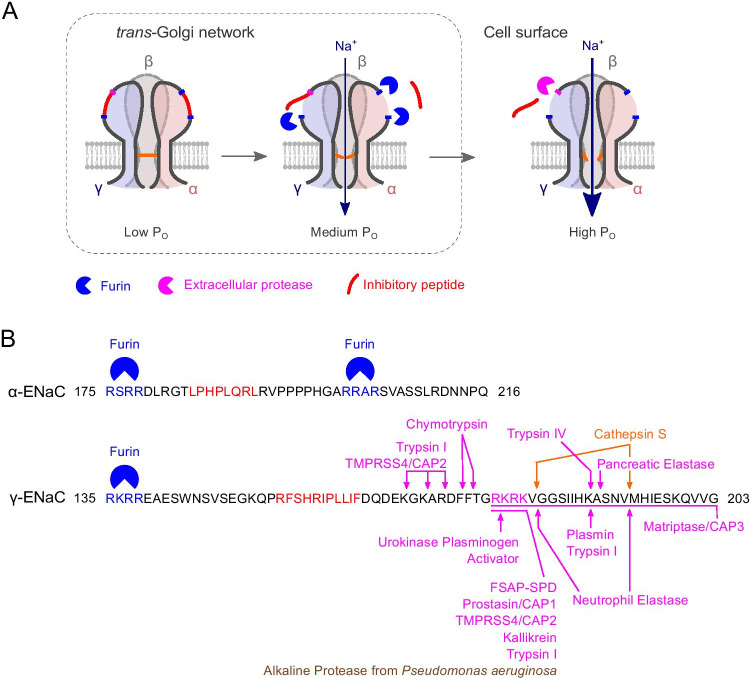
Canonical ENaC assembles in the αβγ-subunit combination. Its unique characteristic is the link between PO and cleavage of ENaC subunits by proteases [5] (Fig. 1a). The α- and γ-subunits contain inhibitory peptides embedded in their extracellular domains, which lock ENaC in a low-PO state. The α-subunit inhibitory peptide is flanked by cleavage sites for the endoprotease furin. Furin is present in the trans-Golgi network and cleaves the α-subunit twice, removing its inhibitory tract. The γ-subunit contains one furin cleavage site; therefore, the inhibitory tract remains attached to the γ-subunit until ENaC reaches the plasma membrane. Furin-processed ENaCs have an intermediate PO. The second cut releasing the γ-subunit inhibitory peptide is mediated by extracellular proteases (Fig. 1). Fully cleaved ENaCs display a high PO. The study by Artunc and colleagues adds factor VII activating protease (FSAP) to the list of ENaC-activating serine proteases [1]. Using Xenopus oocytes expressing human αβγ-ENaC, the authors demonstrate that a recombinant serine protease domain (SPD) of FSAP potently activates ENaC in a concentration- and time-dependent manner, whereas a metabolically inactive FSAP-SPD does not. Mutating a polybasic RKRK motif C-terminal to the ENaC γ-subunit inhibitory tract abolishes ENaC activation by FSAP-SPD.
Fig. 1.
ENaC activation by intra- and extracellular proteases. A Cartoon illustration of ENaC cleavage by furin within the trans-Golgi network and extracellular proteases at the cell surface. Please note that αβγ-ENaC assembles in a counter-clockwise subunit orientation [7]. PO, open probability. B Peptide sequences of the α- and γ-subunits of human ENaC showing the regions containing the inhibitory peptides within the extracellular loop. Furin consensus sites are shown in blue; the inhibitory peptides [7] are shown in red. The figure shows extracellular proteases cleaving the γ-subunit whose cleavage sites have been identified by mutagenesis studies [4–6]: Serine proteases are shown in magenta, cysteine proteases in orange, and metalloproteases in brown. CAP, channel-activating protease (alternative name for the indicated proteases); FSAP-SPD, factor VII activating protease—serine protease domain
Is proteolytic control of ENaC PO important in health and disease? In lung epithelia, the balance between membrane-bound proteases and soluble protease inhibitors has been suggested to adjust ENaC PO and, consequently, transepithelial sodium and water absorption, to the volume of liquid lining the airways and alveoli. Excess protease in the airway surface liquid contributes to airway dehydration in cystic fibrosis [10], whereas impaired ENaC cleavage promotes edema formation [8]. Physiological ENaC control by proteases in the kidney is less clear. While ENaC subunits are cleaved in human kidneys [11], it remains unclear whether ENaC cleavage is a specific regulatory mechanism adjusting channel PO to sodium re-absorption. The situation changes when proteases accidentally encounter ENaC. During acute nephrotic syndrome (NS), serine proteases or their zymogenic forms (e.g., plasminogen) are filtered into pre-urine. Active proteases can cleave ENaC in the distal nephron, thereby enhancing ENaC PO, resulting in sodium retention and edema, both hallmarks of NS. The identity of ENaC-activating proteases is not completely understood and the contribution of urinary plasmin to sodium retention is under debate [3]. The study by Artunc and colleagues demonstrates that active FSAP is present in urine of patients with NS and in mice with doxorubicin-induced NS [1]. While serine protease inhibitors protect mice from sodium retention in this model [2], FSAP-deficient mice do not show altered sodium retention or ENaC cleavage in comparison with wild-type mice [1]. These experiments suggest that FSAP alone does not cause ENaC-mediated sodium retention in this mouse model and imply a mechanism compensating for FSAP deficiency or an ENaC-activating protease cocktail in nephrotic urine. Identification of the main proteases causing ENaC activation in NS is an important task that might pave the way for the development of specific therapeutic strategies. The study by Artunc and colleagues provides an experimental blueprint aiming to complete this puzzle.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
The authors have no competing interests.
Footnotes
This article is a commentary to the original article 10.1007/s00424-021-02639-7
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Artunc F, Bohnert BN, Schneider JC, Staudner T, Sure F, Ilyaskin AV, Wörn M, Essigke D, Janess A, Nielsen NV, Birkendelf AL, Etscheid M, Haerteis S, Korbmacher C, Kanse SM (2021) Proteolytic activation of the epithelial sodium channel (ENaC) by factor VII activating protease (FSAP) and its relevance for sodium retention in nephrotic mice. Pflugers Arch Eur J Physiol In press [DOI] [PMC free article] [PubMed]
- 2.Bohnert BN, Essigke D, Janessa A, Schneider JC, Wörn M, Kalo MZ, Xiao M, Kong L, Omage K, Hennenlotter J, Amend B, Birkenfeld AL, Artunc F. Experimental nephrotic syndrome leads to proteolytic activation of the epithelial Na + channel in the mouse kidney. Am J Physiol Physiol. 2021;321:F480–F493. doi: 10.1152/ajprenal.00199.2021. [DOI] [PubMed] [Google Scholar]
- 3.Ehmke H (2020) Sodium retention by uPA in nephrotic syndrome? Acta Physiologica. 2020(228). 10.1111/apha.13393 [DOI] [PubMed]
- 4.Haerteis S, Krappitz A, Krappitz M, Murphy JE, Bertog M, Krueger B, Nacken R, Chung H, Hollenberg MD, Knecht W, Bunnett NW, Korbmacher C. Proteolytic activation of the human epithelial sodium channel by trypsin IV and trypsin I involves distinct cleavage sites. J Biol Chem. 2014;289:19067–19078. doi: 10.1074/jbc.M113.538470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleyman TR, Eaton DC. Regulating ENaC’s gate. Am J Physiol Physiol. 2020;318:C150–C162. doi: 10.1152/ajpcell.00418.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kota P, García-Caballero A, Dang H, Gentzsch M, Stutts MJ, Dokholyan NV. Energetic and structural basis for activation of the epithelial sodium channel by matriptase. Biochemistry. 2012;51:3460–3469. doi: 10.1021/bi2014773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noreng S, Bharadwaj A, Posert R, Yoshioka C, Baconguis I (2018) Structure of the human epithelial sodium channel by cryo-electron microscopy. Elife 7.10.7554/eLife.39340 [DOI] [PMC free article] [PubMed]
- 8.Planes C, Randrianarison NH, Charles RP, Frateschi S, Cluzeaud F, Vuagniaux G, Soler P, Clerici C, Rossier BC, Hummler E. ENaC-mediated alveolar fluid clearance and lung fluid balance depend on the channel- activating protease 1. EMBO Mol Med. 2010;2:26–37. doi: 10.1002/emmm.200900050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotin D, Staub O. Function and regulation of the epithelial Na+ channel ENaC. Compr Physiol. 2021;11:1–29. doi: 10.1002/cphy.c200012. [DOI] [PubMed] [Google Scholar]
- 10.Webster MJ, Reidel B, Tan CD, Ghosh A, Alexis NE, Donaldson SH, Kesimer M, Ribeiro CMP, Tarran R (2018) SPLUNC1 degradation by the cystic fibrosis mucosal environment drives airway surface liquid dehydration. Eur Respir J 52.10.1183/13993003.00668-2018 [DOI] [PMC free article] [PubMed]
- 11.Zachar R, Mikkelsen MK, Skjødt K, Marcussen N, Zamani R, Jensen BL, Svenningsen P. The epithelial Na+ channel α- and γ-subunits are cleaved at predicted furin-cleavage sites, glycosylated and membrane associated in human kidney. Pflugers Arch Eur J Physiol. 2019;471:1383–1396. doi: 10.1007/s00424-019-02321-z. [DOI] [PubMed] [Google Scholar]