Abstract
Achromatopsia (ACHM), also known as rod monochromatism or total color blindness, is an autosomal recessively inherited retinal disorder that affects the cones of the retina, the type of photoreceptors responsible for high-acuity daylight vision. ACHM is caused by pathogenic variants in one of six cone photoreceptor-expressed genes. These mutations result in a functional loss and a slow progressive degeneration of cone photoreceptors. The loss of cone photoreceptor function manifests at birth or early in childhood and results in decreased visual acuity, lack of color discrimination, abnormal intolerance to light (photophobia), and rapid involuntary eye movement (nystagmus). Up to 90% of patients with ACHM carry mutations in CNGA3 or CNGB3, which are the genes encoding the alpha and beta subunits of the cone cyclic nucleotide-gated (CNG) channel, respectively. No authorized therapy for ACHM exists, but research activities have intensified over the past decade and have led to several preclinical gene therapy studies that have shown functional and morphological improvements in animal models of ACHM. These encouraging preclinical data helped advance multiple gene therapy programs for CNGA3- and CNGB3-linked ACHM into the clinical phase. Here, we provide an overview of the genetic and molecular basis of ACHM, summarize the gene therapy-related research activities, and provide an outlook for their clinical application.
Key Points
| Achromatopsia (ACHM) is caused by mutations in one of six autosomal recessive genes and affects all aspects of daylight vision. |
| No therapy for ACHM has yet been approved, but several preclinical studies provided proof of concept for adeno-associated virus gene therapy. |
| Five clinical gene therapy trials are currently underway for CNGA3- and CNGB3-related ACHM. |
Introduction
Clinical Manifestation, Etiology, and Genetics of Achromatopsia
Achromatopsia (ACHM) is a rare genetic eye disease that is inherited in an autosomal recessive manner and affects approximately one in 30,000 people [1]. Unlike color blindness, in which mutations and rearrangements in the genes encoding the various cone photopigments affect only spectral sensitivity but not the main photoreceptor function [2], ACHM has grave consequences for all aspects of daylight vision mediated by the cone photoreceptors. Patients with ACHM have poor visual acuity, photophobia, and nystagmus and are not able to distinguish colors [3]. Nystagmus is often present at birth or manifests in early infancy. These symptoms are due to a primary functional defect of the cone photoreceptors that manifests in early infancy that is also reflected in a severely reduced or absent light-adapted electroretinogram (ERG) but a largely preserved scotopic ERG signal [4, 5]. In addition to this functional defect, many patients show varying degrees of morphologic changes in the cone-rich central (foveo-macular) part of the retina, ranging from loss of the outer segments of the cones to profound atrophy of the outer retina, including loss of the retinal pigment epithelium [3]. Currently, six genes are linked to ACHM (Table 1). Up to 90% of ACHM cases are due to mutations in CNGA3 (OMIM #216900) and CNGB3 (OMIM #262300) [6–8], which encode the alpha and beta subunits of the heterotetrameric cyclic nucleotide-gated (CNG) channel [9]. ACHM is genetically well-characterized, and there is a high percentage of solved cases [10]. ATF6 (OMIM #616517), GNAT2 (OMIM #613856), PDE6C (OMIM #613093), and PDE6H (OMIM #610024) are the other known ACHM genes [11]. With the exception of ATF6 [12], all other ACHM genes encode essential components of the signal transduction cascade known as phototransduction (or visual transduction) (Fig. 1), which is responsible for converting the light signal into voltage and calcium signals [13]. The absence or dysfunction of any of these phototransduction genes results in functional impairment of the phototransduction cascade. ATF6, on the other hand, encodes a transmembrane transcription factor that is localized in the endoplasmic reticulum (ER), can activate the unfolded protein response, and plays a role in ER homeostasis [14, 15]. Most cases are associated with mutations in the two cone CNG channel genes, and mutations in the other known ACHM genes are much rarer and together account for less than 6–8% of cases [6, 11]. ACHM genes that have not yet been identified or unidentified mutations may be responsible for the remaining 5% of unexplained cases [10].
Table 1.
Overview of achromatopsia genes, animal models and preclinical studies
| Gene | Chromosomal location | Phenotype/OMIM | Animal models | POC studies |
|---|---|---|---|---|
| ATF6 | 1q23.3 | ACHM7/605537 | KO mouse [12] | – |
| CNGA3 | 2q11.2 | ACHM2/600053 | KO mouse [66], cpfl5 mouse [91], ovine model [80] | [67, 80, 81, 83, 84, 96] |
| CNGB3 | 8q21.3 | ACHM3/605080 | KO mouse, mutant mouse [21], canine model [97], NHP model [51] | [75, 76, 89] |
| GNAT2 | 1p13.3 | ACHM4/139340 | Cpfl3 mouse [71] | [78] |
| PDE6C | 10q23.33 | Cone dystrophy 4/600827 | Cpfl1 mouse [72] | – |
| PDE6H | 12p12.3 | ACHM6/601190 | KO mouse [30] | – |
KO knockout, NHP non-human primate, OMIM Online Mendelian Inheritance in Man, POC proof of concept
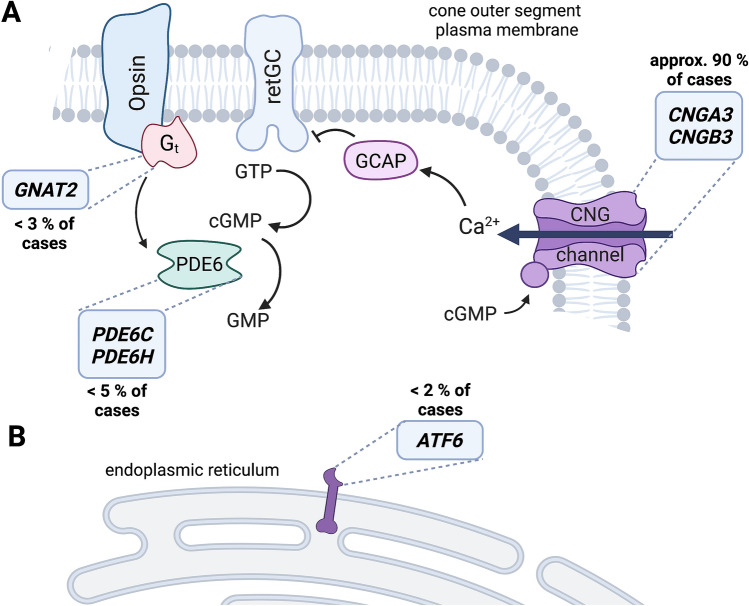
Fig. 1.
Known achromatopsia (ACHM) genes and their functions. A Most genes associated with ACHM encode proteins involved in phototransduction. The most common ACHM genes are CNGA3 and CNGB3, which encode the two subunits of the cyclic nucleotide-gated (CNG) channel, which is located in the plasma membrane of the outer segment. A less common disease gene is GNAT2, which encodes the cone transducin (Gt) that activates the phosphodiesterase (PDE6), which mediates the hydrolysis of the second messenger cyclic guanosine monophosphate (cGMP). The two genes encoding the cone PDE6 (PDE6C and PDE6H) have also been linked to ACHM. B The only known ACHM gene that does not encode a phototransduction cascade protein is ATF6. ATF6 is localized in the endoplasmic reticulum and is involved in endoplasmic reticulum stress and the unfolded protein response. The relative frequencies of ACHM mutations in Europe and Northern America are given next to the boxes with the gene names. Created with BioRender.com. GTP guanosine-triphosphate; retGC receptor guanylyl cyclase
The Cone CNG Channel and its Role in Phototransduction
The cone CNG channel is made up of three CNGA3 and one CNGB3 subunits [13, 16]. Each subunit comprises six transmembrane segments, a pore region, a C-linker region, and a cyclic nucleotide-binding domain. Within the retina, the cone CNG channel is located exclusively in the plasma membrane of the outer segment of cone photoreceptors [13]. Instead of CNGA3 and CNGB3, rod photoreceptors express the homologous genes CNGA1 and CNGB1, which encode the corresponding alpha and beta subunits of the rod CNG channel. In both types of photoreceptors, the CNG channel is an integral part of the phototransduction cascade (Fig. 1). While the phototransduction cascades of cones and rods are similar, they share only a few common proteins, and most key proteins are encoded by distinct homologous genes, which co-evolved during evolution [17].
In both cases, the activity of the CNG channel is controlled by the levels of cyclic guanosine monophosphate (cGMP), which is the central second messenger of the phototransduction cascade. cGMP is produced by the receptor guanylyl cyclase (retGC), and its levels are balanced by the guanylyl cyclase-activating protein (GCAP) and phosphodiesterase (PDE) (Fig. 1). In the dark, high levels of cGMP keep CNG channels open, which conduct a mixed Na+/Ca2+ current that maintains the cone cell in a depolarized state. This depolarization leads to continuous activation of synaptic voltage-gated calcium channels and release of glutamate from the cone synaptic terminal, transmitting the light signal to second-order neurons (bipolar and horizontal cells). The GCAP binds Ca2+ entering the outer segment through the CNG channel and inhibits retGC activity in a negative feedback mechanism, preventing excessive production of cGMP. Upon light exposure, photons hit the chromophores bound to the opsins (rhodopsin in rods and cone opsins in cones), triggering a conformational change and the release of the G-protein transducin, which activates the PDE to mediate hydrolysis of the second messenger cGMP [18]. The resulting drop in cGMP levels leads to closure of the CNG channel and hyperpolarization of the photoreceptor cell. This reduces the release of the neurotransmitter at the synaptic terminal, which is sensed by downstream glutamate receptors and triggers the transmission of the reverse signal to the brain.
Many inherited retinal disorders are caused by mutations in genes that encode proteins involved in the phototransduction cascades of rods and/or cones [19]. Although the phototransduction cascades of cones and rods are very similar in function and share some common proteins, many key proteins, although performing a similar function in the respective signaling cascade, are encoded by different genes and are expressed exclusively in the respective cell type (i.e., in only rods or only cones). Therefore, many inherited retinal diseases manifest in a primary functional defect of either the cone or the rod photoreceptors.
The Mutation Landscape in CNGA3- and CNGB3-Linked Achromatopsia
To date, more than 100 mutations in CNGA3 [20] and nearly 100 mutations in CNGB3 [6] have been found to cause inherited ACHM in humans. All known mutations are inherited in an autosomal recessive manner, and only homozygous or compound heterozygous patients show the typical symptoms of ACHM, whereas carriers have normal vision. Interestingly, a digenic and triallelic inheritance pattern with mutations in both genes has recently been identified in a subset of patients with ACHM [21]. The individual prevalence of CNGA3 or CNGB3 variants varies geographically. It is highest on Pingelap Atoll in Micronesia, where color-blind people make up almost 10% of the small island's indigenous population. This is because a typhoon in the eighteenth century decimated the atoll’s population, leaving behind a small group of survivors who passed on a particular mutation in the CNGB3 gene (p.S435F) [22] to their descendants [23]. Of the two genes, CNGB3 is the more common ACHM gene in Europe and the USA (estimated prevalence of ≥50%) [7, 8], whereas CNGA3 is most common in the Middle East and China. Among Israeli and Palestinian patients with ACHM, mutations in CNGB3 account for only 8% of ACHM cases, whereas mutations in CNGA3 account for more than 80% [19, 24]. A prevalence of approximately 80% for CNGA3-linked ACHM has also been reported for Chinese cohorts [25, 26]. Another difference between the two genes concerns the pattern of mutations. The majority of CNGB3 mutations are nonsense, frameshift, or splicing mutations that result in truncated or severely impaired channel proteins [27, 28]. In contrast, most CNGA3 mutations are missense mutations that affect only single amino acid residues of the protein [27, 28]. Folding, intracellular processing, and transport are thought to be impaired [29]. The effects of individual CNGA3 amino acid substitutions on CNG channel function have mainly been studied in vitro [21, 30–46]. Some insights have been gained, but the exact mechanisms linking CNGA3 amino acid substitutions to cone photoreceptor dysfunction and eventual degeneration are still not well understood.
Because cone photoreceptor function is strongly reduced or absent from early infancy in affected people, there is no true progression of clinical symptoms over time. However, animal studies and morphological data from patients with ACHM suggest progressive degeneration and loss of cones over time [47, 48]. It is believed that the principal development and morphology of cones in ACHM is initially similar to that of unaffected cones, and diseased cones begin to degenerate in young adulthood and are eventually lost through the induction of various cell death mechanisms [49–56]. Unfortunately, the disease mechanisms involved in cone degeneration are only partially understood, and there is a great need to better characterize the pathobiology in affected cone photoreceptors.
Animal Models of ACHM
There are several genetically modified and naturally occurring small and large animal models for ACHM [12, 21, 30, 51, 57–65] (Table 1). The first animal model developed was the Cnga3 knockout (KO) mouse, which helped to establish the genetic basis of CNGA3-linked ACHM [66]. Genetic inactivation of Cnga3 in mice results in nonfunctional cone CNG channels because CNGB3 cannot form functional CNG channels in the absence of CNGA3 [48, 66]. As a result, Cnga3 KO mice show selective loss of cone-mediated light responses [66], accompanied by progressive degeneration and cell death of cones [48]. An early hallmark of cone degeneration is the strong accumulation of the second messenger cGMP, suggesting its involvement in the degeneration process [50, 67, 68] (Fig. 1). Loss of cone photoreceptors in the Cnga3 KO retina progresses much more rapidly in the ventral and nasal (S cone-rich) portions than in the dorsal and temporal (M cone-rich) portions [48], suggesting that cone degeneration affects the M- and S-cones in different ways. This is in line with observations in other mouse models of inherited retinal disorders [69]. Several naturally occurring mouse, dog, and sheep models with mutations or deletions in the Cnga3 gene have since been identified that exhibit the typical features of ACHM and reduced cone function [58, 59, 62, 63].
There are also small and large animal models of CNGB3-associated ACHM [21, 57, 60, 61, 64, 65, 70]. Deletion of Cngb3 in mice or dogs results in severely reduced, but not absent, cone function and progressive degeneration of cone photoreceptors [60, 64]. The remaining cone function in Cngb3-deficient animal models can be attributed to residual irregular homomeric CNGA3 channels that are transported to the outer segment in the absence of CNGB3 protein. Animal models also exist for the other less frequent ACHM genes [12, 30, 57, 71, 72]. All these animal models are very valuable for studies on the pathobiology of ACHM and for testing the efficacy of potential (gene) therapies. This is especially true for large animal models that better mimic the morphological features of the human eye. An example of this is the observation of macular morphological changes in the recently discovered non-human primate model of PDE6C-linked ACHM, which also exhibits features observed in patients with ACHM, such as foveal thinning and subtle bull's eye maculopathy [57].
Development of Gene Therapy for ACHM
Clinical management of ACHM currently includes only specialized genetic counseling, vision aids, and tinted contact lenses or glasses to alleviate the symptoms of photophobia [73]. However, there is no curative treatment for ACHM. The availability of suitable animal models and the advent of recombinant adeno-associated virus (rAAV) vectors as efficient and safe retinal gene transfer vectors [74] have facilitated the preclinical development of novel gene therapies for ACHM (Table 1). As described, ACHM is caused by mutations that are inherited in an autosomal recessive manner. Therefore, it is a very attractive candidate disease for so-called gene supplementation (or augmentation) approaches, which aim to transfer a healthy copy of the disease-causing gene into affected cells (in this case, cone photoreceptors). Several groups have developed gene supplementation therapies and tested them in relevant animal models of ACHM. Some of these approaches have already moved to the translational phase and are currently being tested in phase I/II clinical trials. The following sections summarize the key findings of the preclinical studies and provide an overview of the current status of the clinical trials.
Gene Supplementation Therapy: Preclinical Studies
Several preclinical studies have tested rAAV vectors with known tropism for retinal photoreceptors for gene supplementation in animal models of CNGA3-, CNGB3-, and GNAT2-linked ACHM [62, 75–78]. The first study to provide evidence for efficient gene supplementation to cone photoreceptors used the naturally occurring Cpfl3 mouse model of GNAT2-linked ACHM [78]. These mice show little or no cone-driven light responses and normal rod-mediated ERG responses. Subretinal injection of an rAAV vector expressing mouse Gnat2 under the control of a human red opsin promoter resulted in increased light-adapted ERG responses and improved vision-guided behavior [78]. Despite the promising results, translation of this program is likely hampered by the low prevalence of GNAT2-linked ACHM, which accounts for less than 2% of ACHM [6].
More than a decade ago, rAAV-mediated Cnga3 gene supplementation showed promising results in rescuing the ACHM phenotype in the well-characterized Cnga3 KO mouse model, which lacks cone photoreceptor function from birth [77]. Cnga3 KO mice treated after eye opening were able to generate cone-driven light responses despite being born without cone-mediated visual processing. Thus, this study provided the first evidence of sufficient plasticity of the visual system such that new functions transferred postnatally to previously nonfunctional cones could be properly processed in the mouse visual cortex to elicit biologically meaningful visual behavior. The treatment also had a positive effect on retinal morphology, normalized cGMP levels in cones, delayed cone cell death, and reduced the inflammatory response of Müller glial cells typical of retinal degeneration [48]. The therapeutic effect was also observed when treatment was initiated at a more advanced stage of the disease at 1 or 3 months of age and was stable for at least 12 months after treatment [79]. Similar therapeutic effects with subretinal Cnga3 gene supplementation were reported from another study that used the naturally occurring cpfl5 mouse model of CNGA3-ACHM [62, 80]. The studies described so far used subretinal delivery of rAAV vectors for Cnga3 gene supplementation. Two other studies showed restoration of cone-mediated function in mouse models of CNGA3-ACHM after intravitreal delivery of engineered rAAV vectors that encoded Cnga3 under control of a cone-specific promoter [81, 82]. If translatable, such novel rAAV vectors could be used in future studies to target a larger fraction of cone photoreceptors without the need to detach the central (foveo-macular) part of the retina. Successful rAAV-based gene supplementation has also been described in the sheep model of CNGA3-ACHM [83]. Significant long-term improvement in cone function was demonstrated for at least 6 years after the one-time CNGA3 gene supplementation treatment [84]. These promising preclinical studies led to the initiation of three independent CNGA3 gene therapy programs (Table 2). Safety studies in sheep and non-human primate models revealed some evidence of inflammation after subretinal CNGA3 gene delivery but overall acceptable safety profiles for at least two different translatable CNGA3 gene therapy products [85–88]. Safety data have not yet been published for the third CNGA3 gene therapy product.
Table 2.
List of currently ongoing or recently completed achromatopsia gene therapy clinical trials
| Gene | Drug | Phase | Sponsor/company | NCT ID | Current status |
|---|---|---|---|---|---|
| CNGB3 | AAV2tYF-PR1.7-hCNGB3 | I/II | AGTC | 02599922 | Recruiting |
| CNGB3 | AAV8-hCAR-hCNGB3 (Entacingene turiparvovec) |
I/II LTFU |
MeiraGTx/Janssen |
03001310 03278873 |
Completed recruiting |
| CNGA3 | AAV8-hCAR-hCNGA3 |
I/II IIb |
RD-CURE | 02610582 | Completed recruiting |
| CNGA3 | AAV8-hG1.7-hCNGA3 | I/II | MeiraGTx/Janssen |
03758404 03278873 |
Completed recruiting |
| CNGA3 | AAV2tYF-PR1.7-hCNGA3 | I/II | AGTC | 02935517 | Recruiting |
LTFU long-term follow-up, NTC ID National Clinical Trial number of the ClinicalTrials.gov registry
Restoration of cone-mediated vision by rAAV-based gene supplementation therapy has also been described in mouse and dog models of CNGB3-related ACHM [75, 76]. Initial studies used subretinal delivery of rAAV (AAV5 or AAV8) vectors expressing human CNGB3 under control of cone-specific promoters and demonstrated long-term restoration of cone function and cone-mediated vision [75, 76]. Interestingly, the success rate of cone therapy in dogs was promoter and age dependent. Only the 2.1 kb human red opsin promoter and treatment at 28 weeks of age showed robust and sustained treatment effects [76]. Gene supplementation therapy also failed to restore normal visual acuity in older (6-month-old) Cngb1-deficient mice [75]. The exact reasons for the age dependence of treatment are not known but could be related to morphological changes observed in later stages of the disease. Interestingly, pretreatment with intravitreal ciliary neurotrophic factor (CNTF) allowed successful recovery of cone function and vision in 14- to 42-month-old Cngb3-mutant dogs [89]. The authors suggested that this was due to reversible CNTF-mediated shortening of outer segments and reduction of gene expression [89]. Based on these proof-of-concept studies, two independent translational programs for CNGB3-ACHM were initiated. Safety data obtained in mice, dogs, and cynomolgus monkeys were published from one of the programs [90–92]. The studies showed acceptable safety with vector- and dose-dependent inflammation and toxicity. Induction of neutralizing antibodies directed against the AAV capsid but not the CNGB3 transgene was demonstrated in the sera of treated animals from all species tested [90–92].
Gene Supplementation Therapy: Clinical Studies
All of the aforementioned translational programs for CNGA3- and CNGB3-linked ACHM have already reached the clinical phase [9] (Table 1). The first clinical trial evaluated the effect of three different doses (1 × 1010 to 1 × 1011 total vector genomes per eye) of AAV8.CNGA3 in nine patients with CNGA3-linked ACHM and has reported 1-year [93] and 3-year data [94]. Treatment involved a single subretinal injection into one eye, with the subretinal bleb covering the foveo-macular region. Despite this highly invasive delivery procedure, the treatment was well-tolerated and resulted in only mild and transient procedure- or drug-related adverse events [93, 94]. Transient subclinical induction of inflammatory markers has also been reported [87, 95]. Despite the congenital deprivation of cone-driven light signaling in patients with CNGA3-ACHM, treatment with AAV8.CNGA3 resulted in improvements in secondary endpoints related to cone function, including increases in visual acuity and contrast sensitivity against baseline in all nine treated patients [93], which persisted until at least 3 years after treatment [94]. A phase IIb clinical trial targeting treatment of the second eye of the first patients and treatment of children aged 6–12 years is ongoing (Table 1). Four other programs are currently in phase I/II of clinical trials, two on CNGA3-ACHM and two additional programs on CNGB3-ACHM (Table 1), but their data have not yet been published.
Conclusions and Outlook
In recent years, gene therapy has emerged as a viable treatment option for ACHM. Promising rAAV-based gene transfer technologies have been evaluated for safe and efficient gene delivery to the cone photoreceptors in multiple species. Several promising rAAV-based treatments for the most common forms of ACHM caused by mutations in CNGA3 or CNGB3 are currently being investigated in clinical trials. Although AAVs have natural limitations as a gene delivery system, vector engineering may help to develop improved rAAV variants that support transduction of a greater proportion of cone photoreceptors with higher efficiency and lower immunogenicity, ideally via less invasive administration routes.
Declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of Interest
SM is co-founder and shareholder of ViGeneron GmbH, a gene therapy company. MG, GR, SP, and CP have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
SM wrote the manuscript; all other authors contributed to the writing of the manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- 1.Francois J. Heredity in ophthalmology. Bull Soc Belge Ophtalmol. 1958;118(Pt 1):1–300. [PubMed] [Google Scholar]
- 2.Neitz J, Neitz M. The genetics of normal and defective color vision. Vis Res. 2011;51(7):633–651. doi: 10.1016/j.visres.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirji N, Aboshiha J, Georgiou M, Bainbridge J, Michaelides M. Achromatopsia: clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 2018;39(2):149–157. doi: 10.1080/13816810.2017.1418389. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti-Pierri R, Karali M, Melillo P, Di Iorio V, De Benedictis A, Iaccarino G, et al. Clinical and molecular characterization of achromatopsia patients: a longitudinal study. Int J Mol Sci. 2021;7(22):4. doi: 10.3390/ijms22041681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreasson S, Tornqvist K. Electroretinograms in patients with achromatopsia. Acta Ophthalmol (Copenh). 1991;69(6):711–716. doi: 10.1111/j.1755-3768.1991.tb02048.x. [DOI] [PubMed] [Google Scholar]
- 6.Felden J, Baumann B, Ali M, Audo I, Ayuso C, Bocquet B, et al. Mutation spectrum and clinical investigation of achromatopsia patients with mutations in the GNAT2 gene. Hum Mutat. 2019;40(8):1145–1155. doi: 10.1002/humu.23768. [DOI] [PubMed] [Google Scholar]
- 7.Kohl S, Varsanyi B, Antunes GA, Baumann B, Hoyng CB, Jagle H, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13(3):302–308. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- 8.Mayer AK, Van Cauwenbergh C, Rother C, Baumann B, Reuter P, De Baere E, et al. CNGB3 mutation spectrum including copy number variations in 552 achromatopsia patients. Hum Mutat. 2017;38(11):1579–1591. doi: 10.1002/humu.23311. [DOI] [PubMed] [Google Scholar]
- 9.Michalakis S, Schön C, Becirovic E, Biel M. Gene therapy for achromatopsia. J Gene Med. 2017;19:3. doi: 10.1002/jgm.2944. [DOI] [PubMed] [Google Scholar]
- 10.Weisschuh N, Obermaier CD, Battke F, Bernd A, Kuehlewein L, Nasser F, et al. Genetic architecture of inherited retinal degeneration in Germany: a large cohort study from a single diagnostic center over a 9-year period. Hum Mutat. 2020;41(9):1514–1527. doi: 10.1002/humu.24064. [DOI] [PubMed] [Google Scholar]
- 11.Georgiou M, Fujinami K, Michaelides M. Inherited retinal diseases: therapeutics, clinical trials and end points—a review. Clin Exp Ophthalmol. 2021;49(3):270–288. doi: 10.1111/ceo.13917. [DOI] [PubMed] [Google Scholar]
- 12.Kohl S, Zobor D, Chiang WC, Weisschuh N, Staller J, Gonzalez Menendez I, et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet. 2015;47(7):757–765. doi: 10.1038/ng.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michalakis S, Becirovic E, Biel M. Retinal cyclic nucleotide-gated channels: from pathophysiology to therapy. Int J Mol Sci. 2018;7(19):3. doi: 10.3390/ijms19030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10(11):3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420(6912):193–198. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb TD. Evolution of the genes mediating phototransduction in rod and cone photoreceptors. Prog Retin Eye Res. 2020;76:100823. doi: 10.1016/j.preteyeres.2019.100823. [DOI] [PubMed] [Google Scholar]
- 18.Luo DG, Xue T, Yau KW. How vision begins: an odyssey. Proc Natl Acad Sci USA. 2008;105(29):9855–9862. doi: 10.1073/pnas.0708405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanany M, Rivolta C, Sharon D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc Natl Acad Sci USA. 2020;117(5):2710–2716. doi: 10.1073/pnas.1913179117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remmer MH, Rastogi N, Ranka MP, Ceisler EJ. Achromatopsia: a review. Curr Opin Ophthalmol. 2015;26(5):333–340. doi: 10.1097/ICU.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 21.Burkard M, Kohl S, Kratzig T, Tanimoto N, Brennenstuhl C, Bausch AE, et al. Accessory heterozygous mutations in cone photoreceptor CNGA3 exacerbate CNG channel-associated retinopathy. J Clin Investig. 2018;128(12):5663–5675. doi: 10.1172/JCI96098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundin OH, Yang JM, Li Y, Zhu D, Hurd JN, Mitchell TN, et al. Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet. 2000;25(3):289–293. doi: 10.1038/77162. [DOI] [PubMed] [Google Scholar]
- 23.Sheffield VC. The vision of typhoon lengkieki. Nat Med. 2000;6(7):746–747. doi: 10.1038/77465. [DOI] [PubMed] [Google Scholar]
- 24.Zelinger L, Cideciyan AV, Kohl S, Schwartz SB, Rosenmann A, Eli D, et al. Genetics and disease expression in the CNGA3 form of achromatopsia: steps on the path to gene therapy. Ophthalmology. 2015;122(5):997–1007. doi: 10.1016/j.ophtha.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Liang X, Dong F, Li H, Li H, Yang L, Sui R. Novel CNGA3 mutations in Chinese patients with achromatopsia. Br J Ophthalmol. 2015;99(4):571–576. doi: 10.1136/bjophthalmol-2014-305432. [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Li S, Xiao X, Wang P, Zhang Q. Genotypes and phenotypes of genes associated with achromatopsia: a reference for clinical genetic testing. Mol Vis. 2020;26:588–602. [PMC free article] [PubMed] [Google Scholar]
- 27.Aboshiha J, Dubis AM, Carroll J, Hardcastle AJ, Michaelides M. The cone dysfunction syndromes. Br J Ophthalmol. 2016;100(1):115–121. doi: 10.1136/bjophthalmol-2014-306505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poloschek CM, Kohl S. Achromatopsia. Ophthalmologe. 2010;107(6):571–580. doi: 10.1007/s00347-010-2178-8. [DOI] [PubMed] [Google Scholar]
- 29.Tager J, Wissinger B, Kohl S, Reuter P. Identification of chemical and pharmacological chaperones for correction of trafficking-deficient mutant cyclic nucleotide-gated A3 channels. Mol Pharmacol. 2021;99(6):460–468. doi: 10.1124/molpharm.120.000180. [DOI] [PubMed] [Google Scholar]
- 30.Brennenstuhl C, Tanimoto N, Burkard M, Wagner R, Bolz S, Trifunovic D, et al. Targeted ablation of the Pde6h gene in mice reveals cross-species differences in cone and rod phototransduction protein isoform inventory. J Biol Chem. 2015;290(16):10242–10255. doi: 10.1074/jbc.M114.611921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buena-Atienza E, Ruther K, Baumann B, Bergholz R, Birch D, De Baere E, et al. De novo intrachromosomal gene conversion from OPN1MW to OPN1LW in the male germline results in Blue Cone Monochromacy. Sci Rep. 2016;24(6):28253. doi: 10.1038/srep28253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai G, Varnum MD. CNGA3 achromatopsia-associated mutation potentiates the phosphoinositide sensitivity of cone photoreceptor CNG channels by altering intersubunit interactions. Am J Physiol Cell Physiol. 2013;305(2):C147–C159. doi: 10.1152/ajpcell.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duricka DL, Brown RL, Varnum MD. Defective trafficking of cone photoreceptor CNG channels induces the unfolded protein response and ER-stress-associated cell death. Biochem J. 2012;441(2):685–696. doi: 10.1042/BJ20111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koeppen K, Reuter P, Kohl S, Baumann B, Ladewig T, Wissinger B. Functional analysis of human CNGA3 mutations associated with colour blindness suggests impaired surface expression of channel mutants A3(R427C) and A3(R563C) Eur J Neurosci. 2008;27(9):2391–2401. doi: 10.1111/j.1460-9568.2008.06195.x. [DOI] [PubMed] [Google Scholar]
- 35.Koeppen K, Reuter P, Ladewig T, Kohl S, Baumann B, Jacobson SG, et al. Dissecting the pathogenic mechanisms of mutations in the pore region of the human cone photoreceptor cyclic nucleotide-gated channel. Hum Mutat. 2010;31(7):830–839. doi: 10.1002/humu.21283. [DOI] [PubMed] [Google Scholar]
- 36.Kuniyoshi K, Muraki-Oda S, Ueyama H, Toyoda F, Sakuramoto H, Ogita H, et al. Novel mutations in the gene for alpha-subunit of retinal cone cyclic nucleotide-gated channels in a Japanese patient with congenital achromatopsia. Jpn J Ophthalmol. 2016;60(3):187–197. doi: 10.1007/s10384-016-0424-6. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Varnum MD. Functional consequences of progressive cone dystrophy-associated mutations in the human cone photoreceptor cyclic nucleotide-gated channel CNGA3 subunit. Am J Physiol Cell Physiol. 2005;289(1):C187–C198. doi: 10.1152/ajpcell.00490.2004. [DOI] [PubMed] [Google Scholar]
- 38.Matveev AV, Fitzgerald JB, Xu J, Malykhina AP, Rodgers KK, Ding XQ. The disease-causing mutations in the carboxyl terminus of the cone cyclic nucleotide-gated channel CNGA3 subunit alter the local secondary structure and interfere with the channel active conformational change. Biochemistry. 2010;49(8):1628–1639. doi: 10.1021/bi901960u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meighan PC, Peng C, Varnum MD. Inherited macular degeneration-associated mutations in CNGB3 increase the ligand sensitivity and spontaneous open probability of cone cyclic nucleotide-gated channels. Front Physiol. 2015;6:177. doi: 10.3389/fphys.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muraki-Oda S, Toyoda F, Okada A, Tanabe S, Yamade S, Ueyama H, et al. Functional analysis of rod monochromacy-associated missense mutations in the CNGA3 subunit of the cone photoreceptor cGMP-gated channel. Biochem Biophys Res Commun. 2007;362(1):88–93. doi: 10.1016/j.bbrc.2007.07.152. [DOI] [PubMed] [Google Scholar]
- 41.Patel KA, Bartoli KM, Fandino RA, Ngatchou AN, Woch G, Carey J, et al. Transmembrane S1 mutations in CNGA3 from achromatopsia 2 patients cause loss of function and impaired cellular trafficking of the cone CNG channel. Invest Ophthalmol Vis Sci. 2005;46(7):2282–2290. doi: 10.1167/iovs.05-0179. [DOI] [PubMed] [Google Scholar]
- 42.Reuter P, Koeppen K, Ladewig T, Kohl S, Baumann B, Wissinger B, et al. Mutations in CNGA3 impair trafficking or function of cone cyclic nucleotide-gated channels, resulting in achromatopsia. Hum Mutat. 2008;29(10):1228–1236. doi: 10.1002/humu.20790. [DOI] [PubMed] [Google Scholar]
- 43.Shaikh RS, Reuter P, Sisk RA, Kausar T, Shahzad M, Maqsood MI, et al. Homozygous missense variant in the human CNGA3 channel causes cone-rod dystrophy. Eur J Hum Genet. 2015;23(4):473–480. doi: 10.1038/ejhg.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Täger J, Kohl S, Birch DG, Wheaton DKH, Wissinger B, Reuter P. An early nonsense mutation facilitates the expression of a short isoform of CNGA3 by alternative translation initiation. Exp Eye Res. 2018;171:48–53. doi: 10.1016/j.exer.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Täger J, Wissinger B, Kohl S, Reuter P. Identification of chemical and pharmacological chaperones for correction of trafficking-deficient mutant cyclic nucleotide-gated A3 channels. Mol Pharmacol. 2021;99(6):460–468. doi: 10.1124/molpharm.120.000180. [DOI] [PubMed] [Google Scholar]
- 46.Tränkner D, Jägle H, Kohl S, Apfelstedt-Sylla E, Sharpe LT, Kaupp UB, et al. Molecular basis of an inherited form of incomplete achromatopsia. J Neurosci. 2004;24(1):138–147. doi: 10.1523/JNEUROSCI.3883-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sundaram V, Wilde C, Aboshiha J, Cowing J, Han C, Langlo CS, et al. Retinal structure and function in achromatopsia: implications for gene therapy. Ophthalmology. 2014;121(1):234–245. doi: 10.1016/j.ophtha.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalakis S, Geiger H, Haverkamp S, Hofmann F, Gerstner A, Biel M. Impaired opsin targeting and cone photoreceptor migration in the retina of mice lacking the cyclic nucleotide-gated channel CNGA3. Invest Ophthalmol Vis Sci. 2005;46(4):1516–1524. doi: 10.1167/iovs.04-1503. [DOI] [PubMed] [Google Scholar]
- 49.Arango-Gonzalez B, Trifunovic D, Sahaboglu A, Kranz K, Michalakis S, Farinelli P, et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS ONE. 2014;9(11):e112142. doi: 10.1371/journal.pone.0112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch M, Scheel C, Ma H, Yang F, Stadlmeier M, Gluck AF, et al. The cGMP-dependent protein kinase 2 contributes to cone photoreceptor degeneration in the Cnga3-deficient mouse model of achromatopsia. Int J Mol Sci. 2020;22:1. doi: 10.3390/ijms22010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Q, Lv JN, Wu KC, Zhang CJ, Liu Q, Jin ZB. Generation of nonhuman primate model of cone dysfunction through in situ AAV-mediated CNGB3 ablation. Mol Ther Methods Clin Dev. 2020;11(18):869–879. doi: 10.1016/j.omtm.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thapa A, Morris L, Xu J, Ma H, Michalakis S, Biel M, et al. Endoplasmic reticulum stress-associated cone photoreceptor degeneration in cyclic nucleotide-gated channel deficiency. J Biol Chem. 2012;287(22):18018–18029. doi: 10.1074/jbc.M112.342220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Morris L, Thapa A, Ma H, Michalakis S, Biel M, et al. cGMP accumulation causes photoreceptor degeneration in CNG channel deficiency: evidence of cGMP cytotoxicity independently of enhanced CNG channel function. J Neurosci. 2013;33(37):14939–14948. doi: 10.1523/JNEUROSCI.0909-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thomas MG, Kumar A, Kohl S, Proudlock FA, Gottlob I. High-resolution in vivo imaging in achromatopsia. Ophthalmology. 2011;118(5):882–887. doi: 10.1016/j.ophtha.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 55.Langlo CS, Patterson EJ, Higgins BP, Summerfelt P, Razeen MM, Erker LR, et al. Residual foveal cone structure in CNGB3-associated achromatopsia. Invest Ophthalmol Vis Sci. 2016;57(10):3984–3995. doi: 10.1167/iovs.16-19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubis AM, Cooper RF, Aboshiha J, Langlo CS, Sundaram V, Liu B, et al. Genotype-dependent variability in residual cone structure in achromatopsia: toward developing metrics for assessing cone health. Invest Ophthalmol Vis Sci. 2014;55(11):7303–7311. doi: 10.1167/iovs.14-14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moshiri A, Chen R, Kim S, Harris RA, Li Y, Raveendran M, et al. A nonhuman primate model of inherited retinal disease. J Clin Investig. 2019;129(2):863–874. doi: 10.1172/JCI123980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reicher S, Seroussi E, Gootwine E. A mutation in gene CNGA3 is associated with day blindness in sheep. Genomics. 2010;95(2):101–104. doi: 10.1016/j.ygeno.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka N, Dutrow EV, Miyadera K, Delemotte L, MacDermaid CM, Reinstein SL, et al. Canine CNGA3 gene mutations provide novel insights into human achromatopsia-associated channelopathies and treatment. PLoS ONE. 2015;10(9):e0138943. doi: 10.1371/journal.pone.0138943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sidjanin DJ, Lowe JK, McElwee JL, Milne BS, Phippen TM, Sargan DR, et al. Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet. 2002;11(16):1823–1833. doi: 10.1093/hmg/11.16.1823. [DOI] [PubMed] [Google Scholar]
- 61.Yeh CY, Goldstein O, Kukekova AV, Holley D, Knollinger AM, Huson HJ, et al. Genomic deletion of CNGB3 is identical by descent in multiple canine breeds and causes achromatopsia. BMC Genet. 2013;20(14):27. doi: 10.1186/1471-2156-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pang JJ, Deng WT, Dai X, Lei B, Everhart D, Umino Y, et al. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS ONE. 2012;7(4):e35250. doi: 10.1371/journal.pone.0035250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shamir MH, Ofri R, Bor A, Brenner O, Reicher S, Obolensky A, et al. A novel day blindness in sheep: epidemiological, behavioural, electrophysiological and histopathological studies. Vet J. 2010;185(2):130–137. doi: 10.1016/j.tvjl.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 64.Ding XQ, Harry CS, Umino Y, Matveev AV, Fliesler SJ, Barlow RB. Impaired cone function and cone degeneration resulting from CNGB3 deficiency: down-regulation of CNGA3 biosynthesis as a potential mechanism. Hum Mol Genet. 2009;18(24):4770–4780. doi: 10.1093/hmg/ddp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassall MM, Barnard AR, Orlans HO, McClements ME, Charbel Issa P, Aslam SA, et al. A novel achromatopsia mouse model resulting from a naturally occurring missense change in Cngb3. Invest Ophthalmol Vis Sci. 2018;59(15):6102–6110. doi: 10.1167/iovs.18-24328. [DOI] [PubMed] [Google Scholar]
- 66.Biel M, Seeliger M, Pfeifer A, Kohler K, Gerstner A, Ludwig A, et al. Selective loss of cone function in mice lacking the cyclic nucleotide-gated channel CNG3. Proc Natl Acad Sci USA. 1999;96(13):7553–7557. doi: 10.1073/pnas.96.13.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michalakis S, Mühlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, et al. Restoration of cone vision in the CNGA3-/- mouse model of congenital complete lack of cone photoreceptor function. Mol Therapy J Am Soc Gene Therapy. 2010;18(12):2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michalakis S, Xu J, Biel M, Ding XQ. Detection of cGMP in the degenerating retina. Methods Mol Biol. 2013;1020:235–245. doi: 10.1007/978-1-62703-459-3_16. [DOI] [PubMed] [Google Scholar]
- 69.Zhang T, Zhang N, Baehr W, Fu Y. Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci USA. 2011;108(21):8879–8884. doi: 10.1073/pnas.1017127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dixon CJ. Achromatopsia in three sibling Labrador Retrievers in the UK. Vet Ophthalmol. 2016;19(1):68–72. doi: 10.1111/vop.12265. [DOI] [PubMed] [Google Scholar]
- 71.Chang B, Dacey MS, Hawes NL, Hitchcock PF, Milam AH, Atmaca-Sonmez P, et al. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci. 2006;47(11):5017–5021. doi: 10.1167/iovs.05-1468. [DOI] [PubMed] [Google Scholar]
- 72.Chang B, Grau T, Dangel S, Hurd R, Jurklies B, Sener EC, et al. A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc Natl Acad Sci USA. 2009;106(46):19581–19586. doi: 10.1073/pnas.0907720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kohl S, Hamel C. Clinical utility gene card for: Achromatopsia—update 2013. Eur J Hum Genet. 2013;21:11. doi: 10.1038/ejhg.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Auricchio A, Smith AJ, Ali RR. The future looks brighter after 25 years of retinal gene therapy. Hum Gene Ther. 2017;28(11):982–987. doi: 10.1089/hum.2017.164. [DOI] [PubMed] [Google Scholar]
- 75.Carvalho LS, Xu J, Pearson RA, Smith AJ, Bainbridge JW, Morris LM, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet. 2011;20(16):3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19(13):2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michalakis S, Muhlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, et al. Restoration of cone vision in the CNGA3-/- mouse model of congenital complete lack of cone photoreceptor function. Mol Therapy J Am Soc Gene Therapy. 2010;18(12):2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13(6):685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mühlfriedel R, Tanimoto N, Schon C, Sothilingam V, Garcia Garrido M, Beck SC, et al. AAV-mediated gene supplementation therapy in achromatopsia type 2: preclinical data on therapeutic time window and long-term effects. Front Neurosci. 2017;11:292. doi: 10.3389/fnins.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai X, He Y, Zhang H, Zhang Y, Liu Y, Wang M, et al. Long-term retinal cone rescue using a capsid mutant AAV8 vector in a mouse model of CNGA3-achromatopsia. PLoS ONE. 2017;12(11):e0188032. doi: 10.1371/journal.pone.0188032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du W, Tao Y, Deng WT, Zhu P, Li J, Dai X, et al. Vitreal delivery of AAV vectored Cnga3 restores cone function in CNGA3-/-/Nrl-/- mice, an all-cone model of CNGA3 achromatopsia. Hum Mol Genet. 2015;24(13):3699–3707. doi: 10.1093/hmg/ddv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pavlou M, Schön C, Occelli LM, Rossi A, Meumann N, Boyd RF, et al. Novel AAV capsids for intravitreal gene therapy of photoreceptor disorders. EMBO Mol Med. 2021;2021:e13392. doi: 10.15252/emmm.202013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Banin E, Gootwine E, Obolensky A, Ezra-Elia R, Ejzenberg A, Zelinger L, et al. Gene augmentation therapy restores retinal function and visual behavior in a sheep model of CNGA3 achromatopsia. Mol Therapy J Am Soc Gene Therapy. 2015;23(9):1423–1433. doi: 10.1038/mt.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gootwine E, Ofri R, Banin E, Obolensky A, Averbukh E, Ezra-Elia R, et al. Safety and efficacy evaluation of rAAV2tYF-PR1.7-hCNGA3 vector delivered by subretinal injection in CNGA3 mutant achromatopsia sheep. Hum Gene Ther Clin Dev. 2017;28(2):96–107. doi: 10.1089/humc.2017.028. [DOI] [PubMed] [Google Scholar]
- 85.Ofri R, Averbukh E, Ezra-Elia R, Ross M, Honig H, Obolensky A, et al. Six years and counting: restoration of photopic retinal function and visual behavior following gene augmentation therapy in a sheep model of CNGA3 achromatopsia. Hum Gene Ther. 2018;29(12):1376–1386. doi: 10.1089/hum.2018.076. [DOI] [PubMed] [Google Scholar]
- 86.Tobias P, Philipp SI, Stylianos M, Martin B, Barbara W, Felix R, et al. Safety and toxicology of ocular gene therapy with recombinant AAV vector rAAVh.CNGA3 in nonhuman primates. Hum Gene Ther Clin Dev. 2019;30(2):50–56. doi: 10.1089/humc.2018.188. [DOI] [PubMed] [Google Scholar]
- 87.Reichel FF, Peters T, Wilhelm B, Biel M, Ueffing M, Wissinger B, et al. Humoral immune response after intravitreal but not after subretinal AAV8 in primates and patients. Invest Ophthalmol Vis Sci. 2018;59(5):1910–1915. doi: 10.1167/iovs.17-22494. [DOI] [PubMed] [Google Scholar]
- 88.Seitz IP, Michalakis S, Wilhelm B, Reichel FF, Ochakovski GA, Zrenner E, et al. Superior retinal gene transfer and biodistribution profile of subretinal versus intravitreal delivery of AAV8 in nonhuman primates. Invest Ophthalmol Vis Sci. 2017;58(13):5792–5801. doi: 10.1167/iovs.17-22473. [DOI] [PubMed] [Google Scholar]
- 89.Komaromy AM, Rowlan JS, Corr AT, Reinstein SL, Boye SL, Cooper AE, et al. Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia. Mol Therapy J Am Soc Gene Therapy. 2013;21(6):1131–1141. doi: 10.1038/mt.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ye GJ, Budzynski E, Sonnentag P, Nork TM, Miller PE, McPherson L, et al. Safety and biodistribution evaluation in CNGB3-deficient mice of rAAV2tYF-PR1.7-hCNGB3, a recombinant AAV vector for treatment of achromatopsia. Hum Gene Ther Clin Dev. 2016;27(1):27–36. doi: 10.1089/humc.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye GJ, Budzynski E, Sonnentag P, Nork TM, Miller PE, Sharma AK, et al. Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-PR1.7-hCNGB3, a recombinant AAV Vector for treatment of achromatopsia. Hum Gene Ther Clin Dev. 2016;27(1):37–48. doi: 10.1089/humc.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ye GJ, Komaromy AM, Zeiss C, Calcedo R, Harman CD, Koehl KL, et al. Safety and efficacy of AAV5 vectors expressing human or canine CNGB3 in CNGB3-mutant dogs. Hum Gene Ther Clin Dev. 2017;28(4):197–207. doi: 10.1089/humc.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fischer MD, Michalakis S, Wilhelm B, Zobor D, Muehlfriedel R, Kohl S, et al. Safety and vision outcomes of subretinal gene therapy targeting cone photoreceptors in achromatopsia: a nonrandomized controlled trial. JAMA Ophthalmol. 2020;138(6):643–651. doi: 10.1001/jamaophthalmol.2020.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reichel FF, Michalakis S, Wilhelm B, Zobor D, Muehlfriedel R, Kohl S, et al. Three-year results of phase I retinal gene therapy trial for CNGA3-mutated achromatopsia: results of a non randomised controlled tria. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2021-319067. [DOI] [PubMed] [Google Scholar]
- 95.Reichel FF, Dauletbekov DL, Klein R, Peters T, Ochakovski GA, Seitz IP, et al. AAV8 Can induce innate and adaptive immune response in the primate eye. Mol Therapy J Am Soc Gene Therapy. 2017;25(12):2648–2660. doi: 10.1016/j.ymthe.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mühlfriedel R, Tanimoto N, Schön C, Sothilingam V, Garcia Garrido M, Beck SC, et al. AAV-mediated gene supplementation therapy in achromatopsia type 2: preclinical data on therapeutic time window and long-term effects. Front Neurosci. 2017;11:292. doi: 10.3389/fnins.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia MM, Ying GS, Cocores CA, Tanaka JC, Komaromy AM. Evaluation of a behavioral method for objective vision testing and identification of achromatopsia in dogs. Am J Vet Res. 2010;71(1):97–102. doi: 10.2460/ajvr.71.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]