Abstract
The construction of axially chiral N-heterobiaryls is of great interest as a result of their occurrence in organocatalysts, chiral ligands, natural products, and biologically active molecules. Despite remarkable achievements in this area, strategies for the preparation of new classes of axially chiral N-heterobiaryls remain to be further explored. Herein, we report the enantioselective synthesis of axially chiral arylquinolizones through an intramolecular atroposelective cycloisomerization. The reaction proceeds via the Brønsted acid-enhanced dearomatization of pyridine by a copper catalyst that allows for the formation of the desired products in excellent yields and enantioselectivities. The utility of this methodology is illustrated by a synthesis on gram scale production and transformation of the products into chiral thiourea catalysts. Mechanistic studies demonstrate that Brønsted acid plays a significant role in promoting the reactivity of the reaction, while both the steric and electronic effects of aryl substituents in substrate play a role in controlling the stereoselectivity.
Subject terms: Synthetic chemistry methodology, Asymmetric catalysis
Methods to assemble axially chiral biaryl molecules with nitrogen-containing heterocycles can have implications in ligand development for homogeneous catalysis. Here the authors report enantioselective syntheses of axially chiral arylquinolizones, via an intramolecular atroposelective cycloisomerization.
Introduction
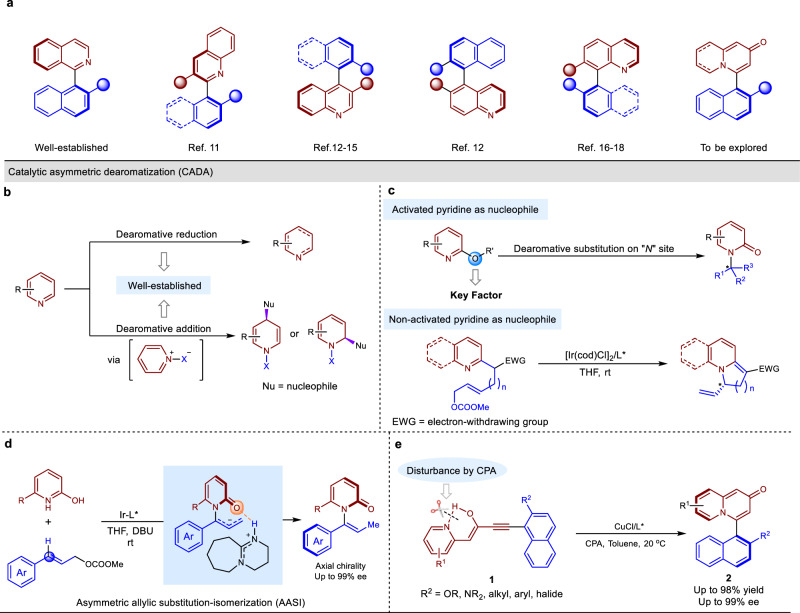
In recent years, substantial effort has been devoted to the efficient and modular catalytic synthesis of axially chiral N-heterobiaryls because of their broad applicability to pharmaceuticals, functional materials and catalysis (Fig. 1a)1–5. 1-(Isoquinolin-1-yl)naphthalene-2-ol (QUINOL), a representative example of an axially chiral N-heterobiaryl, has been studied extensively as a precursor to accessing a variety of ligands or catalysts, including QUINAP, IAN, and QUINOX6–10. Thus, there has been increased interest in the development of methods for the facile construction of axially chiral arylquinolines and arylisoquinolines11–18. Various strategies for the synthesis of these compounds, including dynamic kinetic resolution, enantioselective functionalization of prochiral N-heterobiaryls and enantioselective cross-coupling, have been developed1–5. However, to the best of our knowledge, there was no catalytic approach for accessing axially chiral arylquinolizidine skeletons so far, despite the fact that quinolizidines are widespread components of pharmaceuticals19–21.
Fig. 1. Background and concept design.
a Representative examples of axially chiral axially chiral N-heterobiaryls. b State of the art of CADA using pyridine as an electrophile. c State of the art of CADA using pyridine as a nucleophile. d AASI for the synthesis of axially chiral enamides. e Atroposelective cycloisomerization to axially chiral arylquinolizones.
On the basis of the previous reports on the preparation of quinolizidine analogs, we considered the catalytic asymmetric dearomatization (CADA) reaction of pyridines as a challenging but potentially efficient strategy to address this synthetic problem22–28. Enantioselective hydrogenation represents a direct and well-established approach for the CADA of pyridines (Fig. 1b)29–31. More recently, the enantioselective nucleophilic addition to pyridinium salts that are preactivated at the nitrogen site has been reported as well (Fig. 1b)32–41. However, in spite of this catalytic system, a significant drawback of this strategy is the need to remove the protecting group from the nitrogen at a later stage. Nevertheless, pyridine analogs in these cases are used as electrophiles. A brief survey of the literature reveals that the direct CADA of pyridine derivatives that behave as nucleophiles is much less explored. In this context, activated 2-hydroxy- and 2-alkoxypyridines have been employed (Fig. 1c)42–46. A unique example of the enantioselective dearomatization of unactivated nucleophilic pyridines was reported by the You group in 2014 (Fig. 1c)47,48. In their study, the iridium-catalyzed enantioselective allylic substitution was applied to generate the centrally chiral products using pyridine nitrogen as the nucleophilic site. The only report on the efficient CADA of nucleophilic pyridine derivatives to give axially chiral compounds was disclosed by our group, but the substrates were still limited to 2-hydroxypyridine analogs (Fig. 1d)49.
As part of our continuing efforts in axially chiral compounds26,49–51, we envisioned that CADA of nucleophilic pyridines might also be used for the atroposelective cycloisomerization to axially chiral arylquinolizones. If successful, it would represent the first example of their facile construction from readily prepared alkyne starting materials. At the outset of our studies, we anticipated that several challenges that may arise during our efforts to develop this cycloisomerization process: (1) The sp2 nitrogen lone pair of the pyridine directed outward the ring skeleton which is well directed to have overlap with vacant metal orbital in producing an σ bonding interaction. This may cause pyridine to be a good ligand, resulting in poisoning and deactivation of the metal catalyst. (2) The lack of assistance by -OR or -NHR group and bulky ortho-substituents in arene 1 may lead to the low reactivity of π-activation by TM-catalysis. (3) The ratio of enol and keto isomers of starting materials may cause difficulty in balancing the enantioselectivity and reactivity.
In this work, we report a Brønsted acid-enhanced copper-catalyzed atroposelective cycloisomerization to access axially chiral arylquinolizones via dearomatization of pyridines 1 (Fig. 1e).
Results
Reaction optimizations
To commence our study, we evaluated the reaction conditions by using 1a as the model substrate for the synthesis of axially chiral arylquinolizones. At first, the use of AgOTf26 with L1 gave product 2a in low 29% ee, albeit with a good yield of 70% (Table 1, entry 1). The low ee of 2a can be attributed to the strong background reaction since the reaction could proceed rapidly in the presence of silver catalyst without the ligand26. Next, we examined the copper catalyst for the reaction. While the use of CuBF4 afforded the product in 50% yield with 36% ee (entry 2), changing the catalyst from CuBF4 to CuCl resulted in a substantial improvement in the ee of 2a (73%) (entry 3). For both copper sources, the reaction rate was slower compared to AgOTf, and the background reaction was less significant. The solvent was also evaluated, and toluene was found to give a small improvement of enantioselectivity to 76% ee, though the yield was still moderate (entries 4–6). The ee of 2a was decreased to 20–69% when L2–L5 were used as ligands for the catalyst (entries 7–10).
Table 1.
Optimized conditions for the reactiona.
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | Ligand | Solvent | Additives | t (h) | Yield (%)b | Ee (%)c |
| 1 | AgOTf | L1 | DCE | – | 0.5 | 70 | 29 |
| 2 | CuBF4 | L1 | DCE | – | 72 | 50 | 36 |
| 3 | CuCl | L1 | DCE | – | 72 | 51 | 73 |
| 4 | CuCl | L1 | THF | – | 72 | 38 | 69 |
| 5 | CuCl | L1 | MeCN | – | 72 | 34 | 56 |
| 6 | CuCl | L1 | Toluene | – | 72 | 45 | 76 |
| 7 | CuCl | L2 | Toluene | – | 72 | 30 | 20 |
| 8 | CuCl | L3 | Toluene | – | 72 | 35 | 46 |
| 9 | CuCl | L4 | Toluene | – | 72 | 56 | 69 |
| 10 | CuCl | L5 | Toluene | – | 72 | 30 | 35 |
| 11d | CuCl | L1 | Toluene | – | 72 | 51 | 90 |
| 12d | CuCl | L1 | Toluene (2.0 mL) | – | 72 | 70 | 90 |
| 13d | CuCl | L1 | Toluene (3.0 mL) | – | 72 | 58 | 90 |
| 14d | CuCl | L1 | Toluene | (R)-CPA | 36 | 88 | 92 |
| 15d | CuCl | L1 | Toluene | TsOH | 36 | 67 | 90 |
| 16d | CuCl | L1 | Toluene | PhCOOH | 36 | 57 | 90 |
| 17d | CuCl | L1 | Toluene | (R)-CPA (0.5 eq) | 36 | 83 | 91 |
| 18d | CuCl | L1 | Toluene | (R)-CPA (0.2 eq) | 36 | 76 | 90 |
| 19d | CuCl | L1 | Toluene | NaBArF | 72 | 26 | 13 |
| 20d | CuCl | L1 | Toluene | DBU | 72 | N.D.e | N.D.e |
 | |||||||
aReaction conditions: 1a (0.1 mmol), catalyst (10 mol%), ligand (15 mol%), solvent (1.0 mL), 20 °C.
bYields of isolated products.
cEe values were determined by chiral HPLC.
d1b was used as the substrate.
eN.D. = Not detected. Compound 1b was decomposed.
To further optimize the reaction conditions, we reasoned that a bulkier group on the naphthalene ring may be useful for improving the level of enantiocontrol. As expected, the use of 1b bearing an -OEt instead of -OMe at the 2-position of the naphthalene ring significantly improves the enantioselectivity of 2b (90% ee), although the yield was still moderate (51%) (Table 1, entry 11). Conducting the reaction at lower concentrations afforded improved levels of reactivity (entries 12 and 13). Given the existence of enol and pyridine groups in substrate 1, we hypothesized chiral phosphoric acid (CPA) may be beneficial for not only the reactivity but enantioselectivity because of their hydrogen-bonding interactions52–56. With inexpensive BINOL phosphoric acid as the CPA, the reaction reached completion within 36 h, providing 2b in higher yield (88%) and ee (92%) (entry 14). Examination of other acid additives showed that (R)-CPA was superior in this system (entries 14–18). Lower yield and a slight decrease in ee were obtained by decreasing the amount of (R)-CPA (entries 19 and 20). On the other hand, the use of additives NaBArF and DBU resulted in detrimental effects on the reaction (entries 19 and 20). It should be noted that π-activation by a low-cost copper catalyst for the enantioselective construction of atropisomers is rare and the example was only reported very recently57.
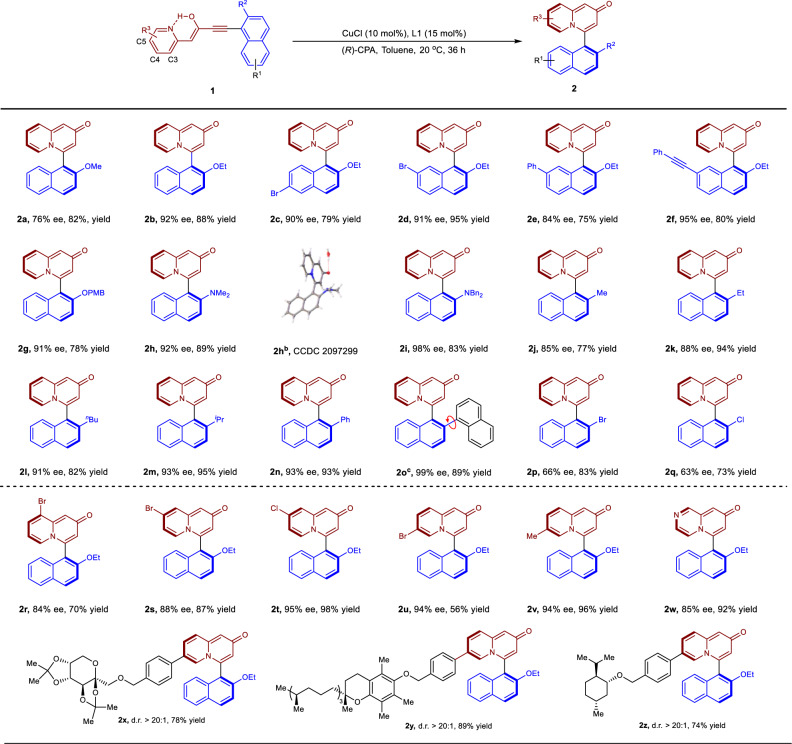
Substrate scope
Having identified optimized conditions for the reaction, we next explored the substrate scope. In general, the substituent R1 on naphthol core such as bromo-, phenyl-, and alkyne- group has little effect on the yields and enantioselectivities, and the products could be isolated in 75–95% yield with 84–95% ee (Fig. 2, compounds 2c–2f). Variation of R2 group in 1 proved to have dramatic effects on the enantioselectivity of the reaction (2a, 2g–2q). Replacement of -OEt with -OPMB provided the product in 91% ee and 78% yield (compound 2g). Notably, protected amines were also compatible at this position, leading to the desired 2h and 2i in excellent yields with high enantioselectivities of 92 and 98% ee, respectively. The absolute configuration of 2h was determined by single-crystal X-ray analysis, and others were assigned by analogy to 2h. It should be noted that, unlike previous enantioselective addition of alkynes to axially chiral heterobiayls11,57–63, substituent R2 is not limited to -OH, ether or amine. In fact, it was found to be amenable to both alkyl and aryl groups, providing the products in 85–99% ee (compounds 2j–2o). However, alteration of -OEt to a halogen group such as -Br and -Cl resulted in moderate ee of 66 and 63%, respectively (compounds 2p and 2q). We speculated that the low ee of 2p and 2q may be attributed to their smaller size compared to other substituents.
Fig. 2. Substrate scope of the reaction.
Reaction conditions: 1 (0.1 mmol), CuCl (10 mol%), L1 (15 mol%), (R)-CPA (0.1 mmol), toluene (2.0 mL), 20 °C for 36 h. The yield of isolated products. Ee values were determined by chiral HPLC. OPMB = p-methoxybenzylb. The single-crystal X-ray of 2h contains one molecule of H2Oc. There are conformers that existed around the red arrow.
To further demonstrate the generality of this protocol, the cyclization of a variety of substituted pyridines was explored. Substrates 1 bearing substituents at C3, C4, or C5 positions of pyridine were well tolerated, affording products in 84–94% ee with 56–96% yield (Fig. 2, compounds 2r–2v). Modification of the central core of pyridine to pyrazine afforded the product 2w in 85% ee and 92% yield. Since quinolizone derivatives are indispensable frameworks of numerous natural products and pharmaceuticals, the decoration of the substrate with complex bioactive molecules was also investigated. For instance, diacetonefructose derivative 1x could undergo atroposelective cyclization to generate product 2x in 78% yield with >20: 1 dr. The reaction also tolerated tocopherol and (L)-menthol derivatives, which delivered 2y and 2z in excellent yield (89 and 74% yield) and dr value (>20:1).
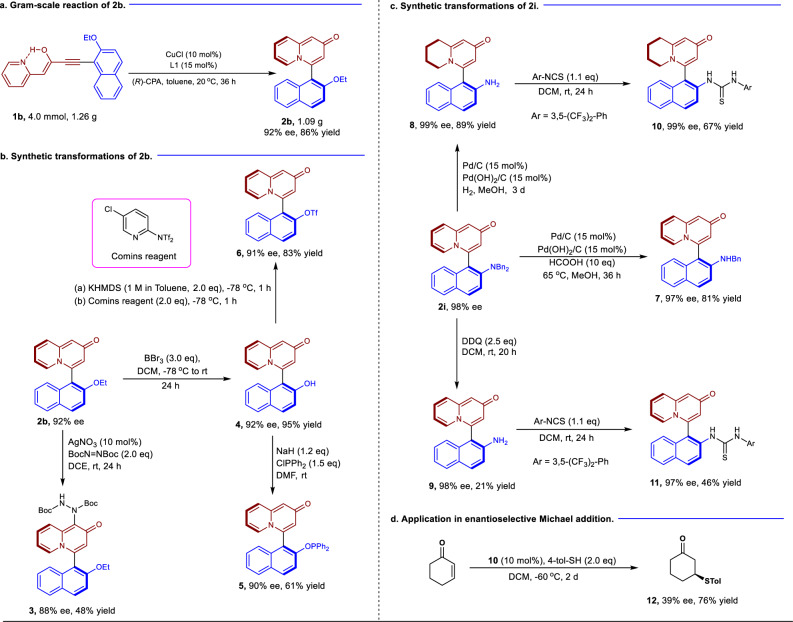
Transformations and applications of the products
Having established these robust and general reaction conditions, we next explored the scale-up of the procedure to gram-scale. The synthesis of 2b was conducted on a 4.0 mmol scale, and the product was obtained in 92% ee with 86% yield (1.09 g) (Fig. 3a). Subsequently, a series of synthetic transformations were performed based on products 2b and 2i. As can be seen from Fig. 3b, direct amination by silver catalysis led to product 3 in 88% ee with 48% yield. Next, deprotection of 2b by BBr3 delivered compound 4 in 92% ee and 95% yield. Using 4 as a substrate, the axially chiral phosphinite 5 could be readily generated in 90% ee. Importantly, the coupling precursor 6 could be easily obtained in 83% yield while maintaining the enantioselectivity in the presence of Comins reagent.
Fig. 3. Large-scale experiments and synthetic applications.
a Gram-scale reaction of 2b. b Synthetic transformations of 2b. c Synthetic transformations of 2i. d Enantioselective Michael addition using compound 10 as the catalyst.
The selective reductions of 2i in the different reductive systems were then carried out (Fig. 3c). Interestingly, the use of HCOOH as a reductant under palladium catalysis afforded compound 7 in 97% ee with 81% yield, whereas using H2 as a reductant, compound 8 was generated using the same catalyst. The direct deprotection of the dibenzyl group of 2i was finally achieved through oxidative conditions without any loss in enantioselectivity, albeit with a yield of 21%. Nevertheless, the axially chiral thiourea catalysts 10 and 11 which derived from 8 and 9 respectively were generated in both high enantioselectivities. To probe the reactivity of the catalyst, the enantioselective Michael addition of thiophenol with cyclohexenone was chosen as the model reaction. The 1,4-addition product 12 was obtained in a good yield of 76% with 39% ee. These results demonstrate the potential utility of this approach for the synthesis of new organocatalysts for asymmetric catalysis. (Fig. 3d).
Mechanistic studies
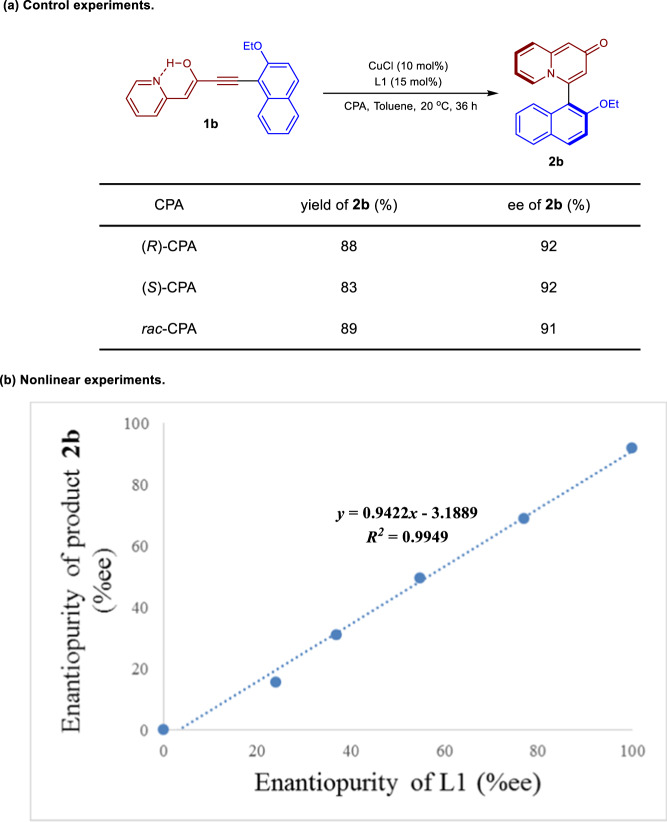
To probe the reaction mechanism, a series of control experiments were conducted. First, the effect of the additive was investigated by comparing the two enantiomers and racemate of CPA. As can be seen from Fig. 4a, there is no obvious change in either yield or enantioselectivity when the (R) isomer of the CPA was replaced with the enantiomer of the racemic mixture. Similar results were also obtained by using other representative substrates for the reaction (see Supplementary Table 3 for details). In addition, the reaction displays a clear linear relationship between the enantiopurity of phosphoramidite L1 and that of product 2b (Fig. 4b). These observations indicate that only one molecule of L1 is likely to be ligated to the copper catalyst during the cycloisomerization process, whereas CPA is either not involved in the enantiodetermining transition state or far from the site of reactivity and not responsible for the enantiocontrol of the reaction.
Fig. 4. Mechanistic studies of enantioselectivities of reaction.
a Control experiments. b Nonlinear experiments.
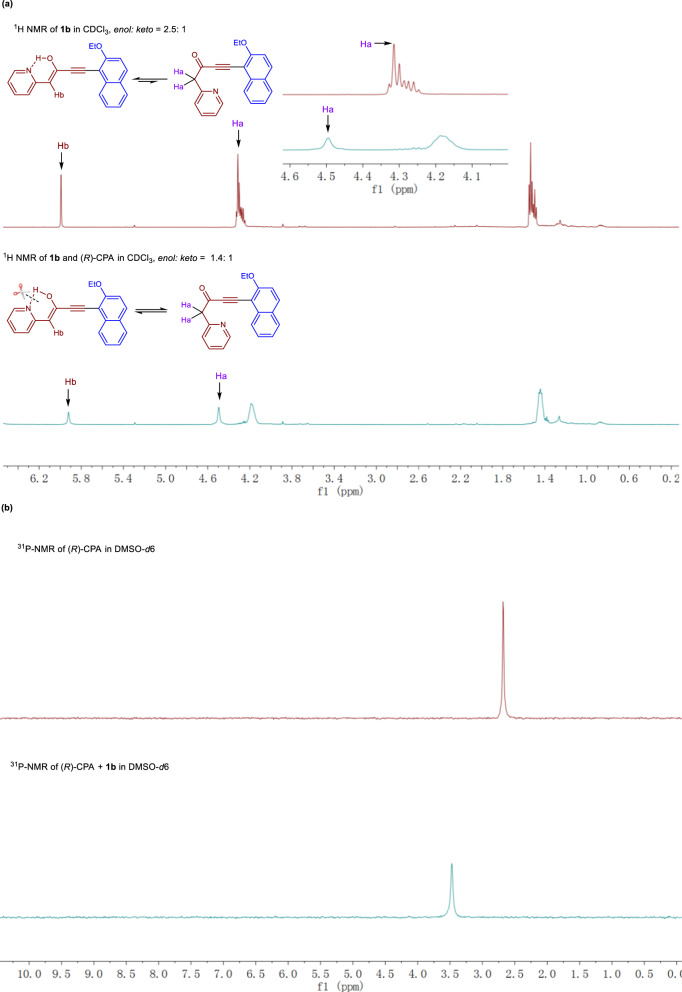
Next, we investigated how the CPA affects the reactivity of cycloisomerization. Considering the co-existence of the enol and keto equivalent of 1b, we investigated the interactions of (R)-CPA and 1b by 1H-NMR and 31P-NMR. As can be seen from Fig. 5a, the original ratio of enol/keto was calculated as 2.5:1 by 1H-NMR spectroscopy, and the Ha and Hb signals were recorded as δ 4.31 and δ 5.99. In the case of the addition of (R)-CPA, the ratio of enol/keto was decreased to 1.4/1. Moreover, the mixture of (R)-CPA (1.0 equiv) and 1b (1.0 equiv) exhibited, two signals (δ 4.50 and δ 5.92) for Ha and Hb, respectively, at a significantly different chemical shift compared to 1b in the absence of the CPA additive. The interactions were further confirmed by 31P-NMR studies in DMSO-d6 (Fig. 5b). It is worth mentioning that mixture of CPA and 1b in CDCl3 was homogeneous, while the CPA itself forms a suspension at the same concentration. These observations all point to the formation of a hydrogen-bond complex between the CPA and the starting material 1b, which disrupts the intramolecular hydrogen-bonding in the enol form of 1b, leading to greater amounts of the keto form.
Fig. 5. Mechanistic studies of the reactivity of the cycloisomerization.
a 1H NMR studies of a mixture of 1b and (R)-CPA. b 31P NMR studies of mixture of 1b and (R)-CPA.
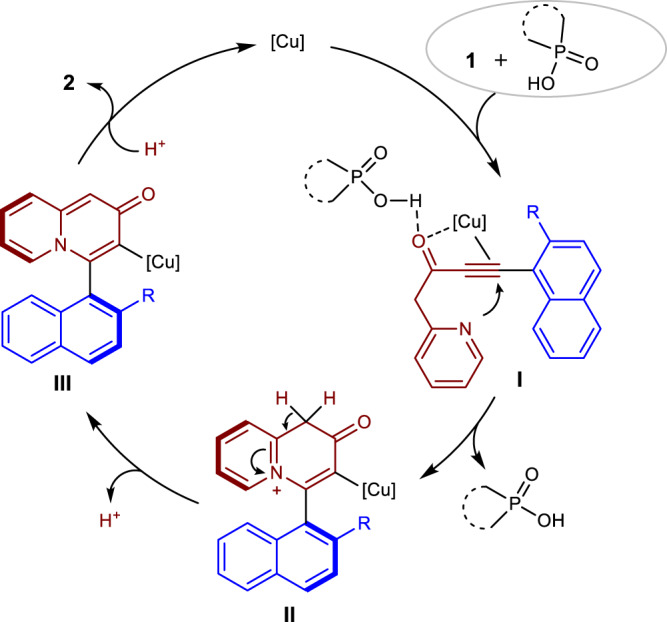
On the basis of the results above, a proposed mechanism is outlined in Fig. 6. First, substrate 1 coordinating with CPA increases the ratio of keto/enol because of the hydrogen-bonding interactions. Meanwhile, the π-activation by copper catalyst with the direction of carbonyl occurred (see Supplementary Fig. 6 for details). The CPA hydrogen-bonded to the carbonyl in I making the alkyne more electrophilic which facilitated the intramolecular nucleophilic addition to the alkyne to form chiral intermediate II. The following isomerization and protonation of III would then occur to yield product 2 and regenerate the copper catalyst.
Fig. 6. Proposed catalytic cycle of the reaction.
Copper catalyst plays a role in controlling the stereoselectivity, while CPA plays a significant role in promoting the reactivity of the reaction.
Finally, linear free energy relationships (LFERs) were studied to probe the trends in enantioinduction by substrates64–70. Firstly, Charton values71–73, which are related to the van der Waals radius of the functional group, was used to relate the enantioselectivities. Unfortunately, no correlation was found when selected products were taken into consideration (Table 2). In addition, a non-linear correlation was also observed when Sterimol parameter65,70 was exploited (see Supplementary Tables 4 and 5 for details). However, when the electronic effect was divided into an electron-donating group (Table 2, entries 1–3), alkyl group (entries 4–7), and electron-withdrawing group (entries 8 and 9), good correlations were obtained (Fig. 7)74–77. Therefore, it seems that (a) the steric effect may play a role in enantioselectivity albeit that Charton values can not describe this influence accurately; (b) other factors such as electronic effect may also participate in the control of stereoselectivity.
Table 2.
Examination of steric effect on enantioselectivitya.
 | ||||
|---|---|---|---|---|
| Entry | R | er | Charton value | △△G≠ (kcal K−1 mol−1)b |
| 1 | OMe | 88.06:11.94 | 0.36 | 1.16 |
| 2 | OEt | 96.0:4.0 | 0.48 | 1.85 |
| 3 | Ph | 96.52:3.48 | 0.57 | 1.93 |
| 4 | Me | 92.42:7.58 | 0.52 | 1.46 |
| 5 | Et | 94.02:5.98 | 0.56 | 1.60 |
| 6 | nBu | 95.45:4.55 | 0.68 | 1.77 |
| 7 | iPr | 96.66:3.34 | 0.76 | 1.96 |
| 8 | Cl | 18.32:81.68 | 0.55 | 0.87 |
| 9 | Br | 16.95:83.05 | 0.65 | 0.93 |
aReaction conditions: 1 (0.1 mmol), CuCl (10 mol%), L1 (15 mol%), (R)-CPA (0.1 mmol), toluene (2.0 mL), 20 °C for 36 h. Yields of isolated products, er values were determined by chiral HPLC.
bΔΔG≠ = RTln(er), R = 0.001986 kcal K−1 mol−1, T = 293.15 K.
Fig. 7. Correlation of the representative substrate parameters to enantioselectivities.
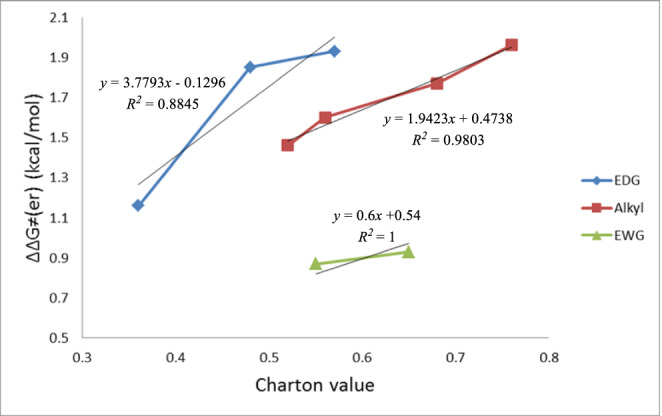
EDG electron-donating group, EWG electron-withdrawing group.
Based on the mechanistic studies and absolute configuration of product 2, we proposed two possible stereoinduction models. As shown in Fig. 8, the transition-state model A accounts for the observed stereochemistry of axial chirality. It is obvious that model B is disfavored relative to A since the steric repulsion between the R group and the backbone of the ligand. Thus, the bulky substituents of the R group are crucial to suppress model B which contributes to the obvious improvement in the enantioselectivity. This result is also consistent with our substrate scope investigations illustrated in Fig. 2.
Fig. 8. Proposed stereoinduction modes.
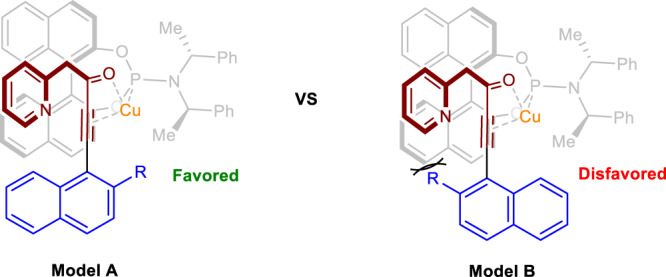
Cl− is omitted for simplicity.
Discussion
In conclusion, we have developed a strategy for the synthesis of axially chiral arylquinolizones. This transformation was achieved by a copper-catalyzed enantioselective dearomatization of nucleophilic pyridines. Control experiments indicated that this transformation was enhanced by Brønsted acid by changing the enol/keto ratio of the substrate. The synthetic utility of the strategy was demonstrated by obtaining a series of axially chiral arylquinolizone analogues. Moreover, the axially chiral quinolizone-type thiourea could be used as a new catalyst for enantioselective Michael addition, albeit with moderate ee. LFERs analysis indicated that both a steric hindrance and electronic effect participate in the control of stereoselectivity. Further studies on the reaction mechanism are currently in progress in our laboratory.
Methods
General procedure for the synthesis of enantioenriched compounds 2
To a vial was added CuCl (1.0 mg, 10 mol%), L1 (8.5 mg, 15 mol%), toluene (2.0 mL), and stirring bar. The vial was wrapped with Teflon tape and fitted with a corresponding cap. The vial was stirred at room temperature for 30 min. Then compound 1 (0.1 mmol, 1.0 eq) and phosphoric acid (34.8 mg, 0.1 mmol, 1.0 eq) were added into the reaction mixture. The reaction was stirred at 20 °C for 36 h. The reaction was then diluted with EA and washed with water three times. The extracts were dried and the product was purified by column chromatography over silica gel. Full experimental details and characterization of new compounds can be found in the Supplementary Information.
Supplementary information
Acknowledgements
Y.H. thank the financial support from the Natural Science Foundation of Jiangsu Province (BK20180447) and the Fundamental Research Funds for the Central Universities (30918011313 and 30919011273). W.Y. thank the financial support of the National Natural Science Foundation of China (21776138 and 22078161). We especially thank Prof. Yi-Ming Wang (University of Pittsburgh) for proofreading of the paper.
Author contributions
Y.H. lead the contact of the paper. Y.H. and W.Y. jointly supervised the research. X.-L.M. and X.-L.Z. performed the experiments and prepared the Supplementary Information. Y.H. and X.-L.M. checked the experimental data and co-wrote the paper with contributions from the other authors.
Peer review
Peer review information
Nature Communications thanks Xin-Yuan Liu and the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2097299 (2h). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
X.-L.M., X.-L.Z., W.Y., and Y.H. are listed as inventors on a Chinese patent application describing the synthesis of compound 10 (application No. 2021108066134). All authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenbin Yi, Email: yiwb@njust.edu.cn.
Ying He, Email: yhe@njust.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-27989-3.
References
- 1.Cheng JK, Xiang S-H, Li S, Ye L, Tan B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 2021;121:4805–4902. doi: 10.1021/acs.chemrev.0c01306. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y-B, Tan B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 2018;51:534–547. doi: 10.1021/acs.accounts.7b00602. [DOI] [PubMed] [Google Scholar]
- 3.Carmona JA, Rodríguez-Franco C, Fernández R, Hornillos V, Lassaletta JM. Atroposelective transformation of axially chiral (hetero)biaryls. From desymmetrization to modern resolution strategies. Chem. Soc. Rev. 2021;50:2968–2983. doi: 10.1039/d0cs00870b. [DOI] [PubMed] [Google Scholar]
- 4.Cheng D-J, Shao Y-D. Advances in the catalytic asymmetric synthesis of atropisomeric hexatomic N-heterobiaryls. Adv. Synth. Catal. 2020;362:3081–3099. [Google Scholar]
- 5.Kumarasamy E, Raghunathan R, Sibi MP, Sivaguru J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 2015;115:11239–11300. doi: 10.1021/acs.chemrev.5b00136. [DOI] [PubMed] [Google Scholar]
- 6.Jiang P-Y, Fan K-F, Li S, Xiang S-H, Tan B. Metal-free oxidative cross-coupling enabled practical synthesis of atropisomeric QUINOL and its derivatives. Nat. Commun. 2021;12:2384. doi: 10.1038/s41467-021-22621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramírez-López P, et al. A dynamic kinetic C–P cross–coupling for the asymmetric synthesis of axially chiral P, N ligands. ACS Catal. 2016;6:3955–3964. [Google Scholar]
- 8.Bhat V, Wang S, Stoltz BM, Virgil SC. Asymmetric synthesis of QUINAP via dynamic kinetic resolution. J. Am. Chem. Soc. 2013;135:16829–16832. doi: 10.1021/ja409383f. [DOI] [PubMed] [Google Scholar]
- 9.Cortright SB, Johnston JN. IAN-amines: direct entry to a chiral C2-symmetric zirconium(IV) β-diketimine complex. Angew. Chem. Int. Ed. 2002;41:345–348. doi: 10.1002/1521-3773(20020118)41:2<345::aid-anie345>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Malkov AV, Dufková L, Farrugia L, Kočovský P. Quinox, a quinoline-type N-oxide, as organocatalyst in the asymmetric allylation of aromatic aldehydes with allyltrichlorosilanes: the role of arene–arene interactions. Angew. Chem. Int. Ed. 2003;42:3674–3677. doi: 10.1002/anie.200351737. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, et al. Design and atroposelective construction of IAN analogues by organocatalytic asymmetric heteroannulation of alkynes. Angew. Chem. Int. Ed. 2020;59:23077–23082. doi: 10.1002/anie.202010598. [DOI] [PubMed] [Google Scholar]
- 12.Shen D, Xu Y, Shi S-L. A bulky chiral N-heterocyclic carbene palladium catalyst enables highly enantioselective Suzuki–Miyaura cross-coupling reactions for the synthesis of biaryl atropisomers. J. Am. Chem. Soc. 2019;141:14938–14945. doi: 10.1021/jacs.9b08578. [DOI] [PubMed] [Google Scholar]
- 13.Shao Y-D, et al. Organocatalytic atroposelective Friedländer quinoline heteroannulation. Org. Lett. 2019;21:4831–4836. doi: 10.1021/acs.orglett.9b01731. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Xu Y, Wang Z, Zhong R, Wang Y. Construction of axial chirality by rhodium-catalyzed asymmetric dehydrogenative Heck coupling of biaryl compounds with alkenes. J. Org. Chem. 2021;86:4262–4273. doi: 10.1002/anie.201408805. [DOI] [PubMed] [Google Scholar]
- 15.Shao Y-D, Han D-D, Dong M-M, Yang X-R, Cheng D-J. A one-pot stepwise approach to axially chiral quinoline-3-carbaldehydes enabled by iminium–allenamine cascade catalysis. Org. Chem. Front. 2021;8:605–612. [Google Scholar]
- 16.Luo J, et al. Enantioselective synthesis of biaryl atropisomers by Pd-catalyzed C-H olefination using chiral spiro phosphoric acid ligands. Angew. Chem. Int. Ed. 2019;58:6708–6712. doi: 10.1002/anie.201902126. [DOI] [PubMed] [Google Scholar]
- 17.Da B-C, Xiang S-H, Li S, Zhou Y-G. Chiral phosphoric acid catalyzed asymmetric synthesis of axially chiral compounds. Chin. J. Chem. 2021;39:1787–1796. [Google Scholar]
- 18.Miyaji R, Asano K, Matsubara S. Induction of axial chirality in 8-arylquinolines through halogenation reactions using bifunctional organocatalysts. Chem. Eur. J. 2017;23:9996–10000. doi: 10.1002/chem.201701707. [DOI] [PubMed] [Google Scholar]
- 19.Weinreb SM. Studies on total synthesis of the cylindricine/fasicularin/lepadiformine family of tricyclic marine alkaloids. Chem. Rev. 2006;106:2531–2549. doi: 10.1021/cr050069v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael JP. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2008;25:139–165. doi: 10.1039/b612166g. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RD, MacCoss M, Lawson ADG. Rings in drugs. J. Med. Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 22.Enamorado MF, Connelly CM, Deiters A, Comins DL. A concise synthesis of the Lycopodium alkaloid cermizine D. Tetrahedron Lett. 2015;56:3683–3685. [Google Scholar]
- 23.James MJ, Grant ND, O’Brien P, Taylor RJK, Unsworth WP. Catalytic dearomatization approach to quinolizidine alkaloids: five step total synthesis of (±)-Lasubine II. Org. Lett. 2016;18:6256–6259. doi: 10.1021/acs.orglett.6b03017. [DOI] [PubMed] [Google Scholar]
- 24.Shinde PS, Shaikh AC, Patil NT. Efficient access to alkynylated quinalizinones via the gold(I)-catalyzed aminoalkynylation of alkynes. Chem. Commun. 2016;52:8152–8155. doi: 10.1039/c6cc03414d. [DOI] [PubMed] [Google Scholar]
- 25.Fan X, He Y, Zhang X, Wang J. Sustainable and selective synthesis of 3,4-dihydroquinolizin-2-one and quinolizin-2-one derivatives via the reactions of penta-3,4-dien-2-ones. Green Chem. 2014;16:1393–1398. [Google Scholar]
- 26.Min X-L, Sun C, He Y. Synthesis of 1‑amino‑2H‑quinolizin-2-one scaffolds by tandem silver catalysis. Org. Lett. 2019;21:724–728. doi: 10.1021/acs.orglett.8b03935. [DOI] [PubMed] [Google Scholar]
- 27.Wiesenfeldt MP, Moock D, Paul D, Glorius F. Enantioselective hydrogenation of annulated arenes: controlled formation of multiple stereocenters in adjacent rings. Chem. Sci. 2021;12:5611–5615. doi: 10.1039/d0sc07099h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang WW, et al. Controllable synthesis of 3-iodo-2H-quinolizin-2-ones and 1,3-diiodo-2H-quinolizin-2-ones via electrophilic cyclization of azacyclic ynones. Chem. Commun. 2019;55:12607–12610. doi: 10.1039/c9cc06250e. [DOI] [PubMed] [Google Scholar]
- 29.Wagener T, Luckemeier L, Daniliuc CG, Glorius F. Interrupted pyridine hydrogenation: asymmetric synthesis of δ-Lactams. Angew. Chem. Int. Ed. 2021;60:6425–6429. doi: 10.1002/anie.202016771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gribble MW, Guo S, Buchwald SL. Asymmetric Cu-catalyzed 1,4-dearomatization of pyridines and pyridazines without preactivation of the heterocycle or nucleophile. J. Am. Chem. Soc. 2018;140:5057–5060. doi: 10.1021/jacs.8b02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gribble MW, Liu RY, Buchwald SL. Evidence for simultaneous dearomatization of two aromatic rings under mild conditions in Cu(I)-catalyzed direct asymmetric dearomatization of pyridine. J. Am. Chem. Soc. 2020;142:11252–11269. doi: 10.1021/jacs.0c04486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancheño OG, Asmus S, Zurro M, Fisher T. Highly enantioselective nucleophilic dearomatization of pyridines by anion-binding catalysis. Angew. Chem. Int. Ed. 2015;54:8823–8827. doi: 10.1002/anie.201502708. [DOI] [PubMed] [Google Scholar]
- 33.Di Carmine G, et al. Enantioselective dearomatization of alkylpyridiniums by N-heterocyclic carbene-catalyzed nucleophilic acylation. J. Org. Chem. 2018;83:2050–2057. doi: 10.1021/acs.joc.7b02996. [DOI] [PubMed] [Google Scholar]
- 34.Flanigan DM, Rovis T. Enantioselective N-heterocyclic carbene-catalyzed nucleophilic dearomatization of alkyl pyridiniums. Chem. Sci. 2017;8:6566–6569. doi: 10.1039/c7sc02648j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertuzzi G, et al. Nucleophilic dearomatization of pyridines under enamine catalysis: regio-, diastereo-, and enantioselective addition of aldehydes to activated N-alkylpyridinium salts. Org. Lett. 2017;19:834–837. doi: 10.1021/acs.orglett.6b03824. [DOI] [PubMed] [Google Scholar]
- 36.Bertuzzi G, et al. Catalytic enantioselective addition of indoles to activated N-benzylpyridinium salts: nucleophilic dearomatization of pyridines with unusual C-4 regioselectivity. ACS Catal. 2016;6:6473–6477. [Google Scholar]
- 37.Bertuzzi G, Bernardi L, Fochi M. Nucleophilic dearomatization of activated pyridines. Catalysts. 2018;8:632. [Google Scholar]
- 38.Robinson DJ, Spurlin SP, Gorden JD, Karimov RR. Enantioselective synthesis of dihydropyridines containing quaternary stereocenters through dearomatization of pyridinium salts. ACS Catal. 2020;10:51–55. [Google Scholar]
- 39.Yao K, et al. Pd-catalyzed asymmetric allylic substitution cascade using α-(pyridin-1-yl)-acetamides formed in situ as nucleophiles. Chem. Sci. 2019;10:1767–1772. doi: 10.1039/c8sc04626c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lutz JP, Chau ST, Doyle AG. Nickel-catalyzed enantioselective arylation of pyridine. Chem. Sci. 2016;7:4105–4109. doi: 10.1039/c6sc00702c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chau ST, Lutz JP, Wu K, Doyle AG. Nickel-catalyzed enantioselective arylation of pyridinium ions: Harnessing an iminium ion activation mode. Angew. Chem. Int. Ed. 2013;52:9153–9156. doi: 10.1002/anie.201303994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu G, et al. Access to N-substituted 2-pyridones by catalytic intermolecular dearomatization and 1,4-acyl transfer. Angew. Chem. Int. Ed. 2019;58:1980–1984. doi: 10.1002/anie.201812937. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Yang Z-P, Huang L, You S-L. Highly regio- and enantioselective synthesis of N-substituted 2-pyridones: iridium-catalyzed intermolecular asymmetric allylic amination. Angew. Chem. Int. Ed. 2015;54:1873–1876. doi: 10.1002/anie.201409976. [DOI] [PubMed] [Google Scholar]
- 44.Cheng L-J, Brown APN, Cordier CJ. Enantioselective propargylic [1,3]-rearrangements: copper-catalyzed O-to-N migrations toward C–N bond formation. Chem. Sci. 2017;8:4299–4305. doi: 10.1039/c7sc01042g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Kähny M, Breit B. Rhodium-catalyzed chemo-, regio-, and enantioselective addition of 2-pyridones to terminal allenes. Angew. Chem. Int. Ed. 2014;53:13780–13784. doi: 10.1002/anie.201407935. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues A, Lee EE, Batey RA. Enantioselective palladium(II)-catalyzed formal [3,3]-sigmatropic rearrangement of 2-allyloxypyridines and related heterocycles. Org. Lett. 2010;12:260–263. doi: 10.1021/ol9025759. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z-P, Wu Q-F, You S-L. Direct asymmetric dearomatization of pyridines and pyrazines by iridium-catalyzed allylic amination reactions. Angew. Chem. Int. Ed. 2014;53:6986–6989. doi: 10.1002/anie.201404286. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z-P, Wu Q-F, Shao W, You S-L. Iridium-catalyzed intramolecular asymmetric allylic dearomatization reaction of pyridines, pyrazines, quinolines, and isoquinolines. J. Am. Chem. Soc. 2015;137:15899–15906. doi: 10.1021/jacs.5b10440. [DOI] [PubMed] [Google Scholar]
- 49.Sun C, et al. Asymmetric allylic substitution-isomerization to axially chiral enamides via hydrogen-bonding assisted central-to-axial chirality transfer. Chem. Sci. 2020;11:10119–10126. doi: 10.1039/d0sc02828b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, et al. Tandem iridium catalysis as a general strategy for atroposelective construction of axially chiral styrenes. J. Am. Chem. Soc. 2021;143:10686–10694. doi: 10.1021/jacs.1c04400. [DOI] [PubMed] [Google Scholar]
- 51.Min X-L, Xu X-R, He Y. Axial-to-central chirality transfer for construction of quaternary stereocenters via dearomatization of BINOLs. Org. Lett. 2019;21:9188–9193. doi: 10.1021/acs.orglett.9b03558. [DOI] [PubMed] [Google Scholar]
- 52.Lin J-S, et al. A dual-catalytic strategy to direct asymmetric radical aminotrifluoromethylation of alkenes. J. Am. Chem. Soc. 2016;138:9357–9360. doi: 10.1021/jacs.6b04077. [DOI] [PubMed] [Google Scholar]
- 53.Lin J-S, et al. Cu/chiral phosphoric acid-catalyzed asymmetric three-component radical-initiated 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 2019;141:1074–1083. doi: 10.1021/jacs.8b11736. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y-F, et al. Catalytic enantioselective desymmetrizing functionalization of alkyl radicals via Cu(I)/CPA cooperative catalysis. Nat. Catal. 2020;3:401–410. [Google Scholar]
- 55.Li Z-L, Fang G-C, Gu Q-S, Liu X-Y. Recent advances in copper-catalysed radical-involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020;49:32–48. doi: 10.1039/c9cs00681h. [DOI] [PubMed] [Google Scholar]
- 56.Gu Q-S, Li Z-L, Liu X-Y. Copper(I)-catalyzed asymmetric reactions involving radicals. Acc. Chem. Res. 2020;53:170–181. doi: 10.1021/acs.accounts.9b00381. [DOI] [PubMed] [Google Scholar]
- 57.Xu M-M, et al. Enantioselective synthesis of axially chiral biaryls by Diels–Alder/Retro-Diels–Alder reaction of 2-pyrones with alkynes. J. Am. Chem. Soc. 2021;143:8993–9001. doi: 10.1021/jacs.1c04759. [DOI] [PubMed] [Google Scholar]
- 58.Imase H, et al. Highly enantioselective construction of axial chirality by palladium-catalyzed cycloisomerization of N-alkenyl arylethynylamides. Org. Lett. 2009;11:1805–1808. doi: 10.1021/ol900373z. [DOI] [PubMed] [Google Scholar]
- 59.Shibuya T, Shibata Y, Noguchi K, Tanaka K. Palladium-catalyzed enantioselective intramolecular hydroarylation of alkynes to form axially chiral 4-aryl 2-quinolinones. Angew. Chem. Int. Ed. 2011;50:3963–3967. doi: 10.1002/anie.201100152. [DOI] [PubMed] [Google Scholar]
- 60.Liang Y, et al. Enantioselective construction of axially chiral amino sulfide vinyl arenes by chiral sulfide-catalyzed electrophilic carbothiolation of alkynes. Angew. Chem. Int. Ed. 2020;59:4959–4964. doi: 10.1002/anie.201915470. [DOI] [PubMed] [Google Scholar]
- 61.Zhang J, Simon M, Golz C, Alcarazo M. Gold-catalyzed atroposelective synthesis of 1,1’-binaphthalene-2,3’-diols. Angew. Chem. Int. Ed. 2020;59:5647–5650. doi: 10.1002/anie.201915456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, et al. Organocatalytic atroposelective intramolecular [4+2] cycloaddition: Synthesis of axially chiral heterobiaryls. Angew. Chem. Int. Ed. 2018;57:6491–6495. doi: 10.1002/anie.201801824. [DOI] [PubMed] [Google Scholar]
- 63.Peng L, et al. Organocatalytic asymmetric annulation of ortho-alkynylanilines: Synthesis of axially chiral naphthyl-C2-indoles. Angew. Chem. Int. Ed. 2019;58:17199–17204. doi: 10.1002/anie.201908961. [DOI] [PubMed] [Google Scholar]
- 64.In linear free energy relationships (LFERs) in asymmetric catalysis. Asymmetric Synthesis II: More Methods and Applications. (eds Christmann, M., Bräse, S.) (Wiley-VCH, Weinheim, Germany, 2012).
- 65.Santiago CB, Guo J-Y, Sigman MS. Predictive and mechanistic multivariate linear regression models for reaction development. Chem. Sci. 2018;9:2398–2412. doi: 10.1039/c7sc04679k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harper KC, Sigman MS. Using physical organic parameters to correlate asymmetric catalyst performance. J. Org. Chem. 2013;78:2813–2818. doi: 10.1021/jo4002239. [DOI] [PubMed] [Google Scholar]
- 67.Sigman MS, Harper KC, Bess EN, Milo A. The development of multidimensional analysis tools for asymmetric catalysis and beyond. Acc. Chem. Res. 2016;49:1292–1301. doi: 10.1021/acs.accounts.6b00194. [DOI] [PubMed] [Google Scholar]
- 68.Miller JJ, Sigman MS. Quantitatively correlating the effect of ligand-substituent size in asymmetric catalysis using linear free energy relationships. Angew. Chem. Int. Ed. 2008;47:771–774. doi: 10.1002/anie.200704257. [DOI] [PubMed] [Google Scholar]
- 69.Li S-L, et al. Atroposelective catalytic asymmetric allylic alkylation reaction for axially chiral anilides with achiral Morita–Baylis–Hillman carbonates. J. Am. Chem. Soc. 2018;140:12836–12843. doi: 10.1021/jacs.8b06014. [DOI] [PubMed] [Google Scholar]
- 70.Harper KC, Bess EN, Sigman MS. Multidimensional steric parameters in the analysis of asymmetric catalytic reactions. Nat. Chem. 2012;4:366–374. doi: 10.1038/nchem.1297. [DOI] [PubMed] [Google Scholar]
- 71.Charton M. Steric effects. I. Esterification and acid-catalyzed hydrolysis of esters. J. Am. Chem. Soc. 1975;97:1552–1556. [Google Scholar]
- 72.Charton M. Steric effects. II. Base-catalyzed ester hydrolysis. J. Am. Chem. Soc. 1975;97:3691–3693. [Google Scholar]
- 73.Charton M. Steric effects. 7. Additional V constants. J. Org. Chem. 1976;41:2217–2220. [Google Scholar]
- 74.Belot V, et al. Steric scale of common substituents from rotational barriers of N-(o-substituted aryl)thiazoline-2-thione atropisomers. J. Org. Chem. 2017;82:10188–10200. doi: 10.1021/acs.joc.7b01698. [DOI] [PubMed] [Google Scholar]
- 75.Bott G, Field LD, Sternhell S. Steric effects. A study of a rationally designed system. J. Am. Chem. Soc. 1980;102:5618–5626. [Google Scholar]
- 76.Lunazzi L, et al. Rotational barriers of biphenyls having heavy heteroatoms as ortho-substituents: experimental and theoretical determination of steric effects. Org. Biomol. Chem. 2012;10:1847–1855. doi: 10.1039/c1ob06688a. [DOI] [PubMed] [Google Scholar]
- 77.Ruzziconi R, Spizzichino S, Mazzanti A, Lunazzi L, Schlosser M. The biphenyl-monitored effective size of unsaturated functional or fluorinated ortho substituents. Org. Biomol. Chem. 2010;8:4463–4471. doi: 10.1039/c0ob00136h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and Supplementary Information file, or from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2097299 (2h). The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.