Abstract
Background/Aims
Multiple definitions of sarcopenia exist and the acceptable criterion that best predicts outcome is lacking. We estimated the prevalence of sarcopenia based on four criteria and assessed their utility in predicting mortality in cirrhotics.
Methods
In a prospective observational study, consecutive Asian patients with cirrhosis underwent testing for handgrip strength (HGS) and estimation of skeletal muscle index (SMI) using computed tomography at the third lumbar vertebra. Sarcopenia was defined based on the Western cut-off (WC; SMI < 50 cm2/m2 for men and <39 cm2/m2 for women), Asian cut-off (AC; SMI < 36.5 cm2/m2 for men and 30.2 cm2/m2 for women), European Working Group on Sarcopenia in Older People—2nd meeting (EWGSOP2) definition incorporating low HGS (<27 kg for men and <16 kg for women) with low SMI (defined by the WC), and EWGSOP2 definition with low HGS and low SMI (defined by AC). Risk factors for mortality were assessed using multivariate Cox-proportional hazards.
Results
We included 219 patients with cirrhosis (168 men; mean age 42.6 years) with 50.2% patients having decompensation. Alcohol was the commonest aetiology (33.3%). The prevalence of sarcopenia was highest with the WC (men: 82.1%; women: 62.7%). There was a weak concordance among all criteria (Fleiss’ kappa 0.23, 95% confidence interval [CI] 0.10–0.37). Overall, 12-month survival was 86.1% (81.1–91.3%) over a median (interquartile range) follow-up of 12 (6–15) months. Ascites (hazards ratio [HR] 6.27 [95% CI 1.6–24.1]; P < 0.007) and SMI (HR 0.92 [0.85–0.98]; P = 0.021) were independent predictors of mortality. The 12-month mortality rate was higher in patients with sarcopenia, irrespective of criteria (log rank P < 0.05). Low HGS and low SMI (defined by AC) was the best for predicting mortality (HR 3.04 [1.43–6.43]; P = 0.004).
Conclusion
A weak concordance exists amongst various diagnostic definitions of sarcopenia. Sarcopenia diagnosed by a combination of low HGS and population-specific SMI cut-off (AC) best predicts mortality.
Keywords: sarcopenia, skeletal muscle index, EWGSOP2
Abbreviations: 6MWD, 6-min walk distance; AC, Asian cut-off; ACLF, acute on chronic liver failure; BMI, body mass index; CI, confidence interval; CT, computed tomography; CTP, Child-Turcotte-Pugh; EWGSOP2, European Working Group on Sarcopenia in Older People—2nd meeting; HCC, hepatocellular cancer; HE, hepatic encephalopathy; HGS, handgrip strength; HR, hazards ratio; HU, Hounsfield unit; IQR, interquartile range; LT, liver transplant; MELD, model for end-stage liver disease; SD, standard deviation; SMA, skeletal muscle area; SMI, skeletal muscle index; WC, Western cut-off
Introduction
Sarcopenia, or reduction in the skeletal muscle mass and/or strength,1 is a common complication of cirrhosis involving up to 30–70% of patients.2 Over the last two decades, its significance has been further recognized with an increased risk of poor outcomes in patients with cirrhosis, hepatocellular cancer (HCC) and liver transplant (LT) recipients.3, 4, 5, 6, 7, 8 There is, however, no consensus on the precise definition of sarcopenia, with the North American expert opinion statement recommending diagnosis based on skeletal muscle mass alone, and the European Working Group on Sarcopenia in Older People—2nd meeting (EWGSOP2) definition incorporating both low muscle strength and mass for a confirmed diagnosis.1,9 It is accepted that cross-sectional imaging modalities like computed tomography (CT) scans provide the most accurate estimates of skeletal muscle mass, and skeletal muscle index (SMI) is a consistent correlate of sarcopenia,10 enormous heterogeneity exists in the literature regarding the level of assessment (third or fourth lumbar vertebra), site of assessment (upper, mid or lower part of the lumbar vertebra), muscles to be included (psoas alone or total abdominal muscle area) and the patient population used for standardization (healthy controls, cirrhosis awaiting LT, HCC or solid organ cancers), resulting in multiple definitions for sarcopenia.8,11, 12, 13, 14, 15, 16, 17, 18 Defining sarcopenia is further complicated by differences in body composition of Asians and Caucasians, with Asians having a significantly higher amount of body fat for the same sex, age and body mass index (BMI), with lesser muscle mass.19, 20, 21 Therefore, there is a need for region-specific definitions of sarcopenia and their prospective validation in their specific cohorts.
A precise definition of sarcopenia is important for three major reasons: confirming the diagnosis, accurate prognostication and conducting future studies on different interventions targeting sarcopenia. In the absence of a universally accepted definition, the diagnostic criteria that best predict the mortality would have the most clinical relevance and, therefore, could be the definition of choice for defining sarcopenia. We evaluated the prevalence of sarcopenia in cirrhosis using different definitions available in the literature. In addition, we aimed to assess the concordance among these definitions of sarcopenia and their impact on mortality.
Materials and methods
Study Setting and Patient Management
This was a prospective observational study conducted at a single tertiary care centre in North India. Consecutive patients with cirrhosis between 18 and 60 years of age irrespective of aetiology, being prospectively followed up in the liver clinic from October 2018 to March 2020 were considered for inclusion. Cirrhosis was diagnosed based on a combination of clinical features (history and examination, history of decompensation of underlying chronic liver disease), biochemical and imaging findings (ultrasonography or CT abdomen).22 Patients with overt hepatic encephalopathy (HE), gastrointestinal bleed within the past 1 month, sepsis, acute on chronic liver failure (ACLF)23 and hospital admission in the preceding month before enrolment were excluded because of the high mortality in these patients. In addition, patients who had factors that could independently influence sarcopenia, including evidence of HCC at presentation, chronic obstructive pulmonary disease, congestive heart failure, chronic kidney disease, neuromuscular disorders and those unwilling for participation were also excluded.
All patients underwent blood investigations (complete blood counts, liver function tests and renal function tests), anthropometric measurements, nutritional assessment and estimation of SMI using multiphase CT (detailed below) at enrolment. They were followed up regularly at 3 monthly intervals, and also as and when required till March 2020. They also underwent laboratory testing at each visit and were evaluated for the development of complications such as new-onset or worsening ascites, HE, ACLF and gastrointestinal bleed. The underlying aetiology of cirrhosis and the ensuing complications were managed according to standard protocols. All patients underwent nutritional counselling at enrolment (after the assessment of sarcopenia) and subsequently at each visit. They were advised a caloric intake of about 30–35 kcal/kg/day and protein intake of 1.2–1.5 g/kg/day, as a part of routine patient care.24 The caloric requirement of patients with nonalcoholic fatty liver disease-related cirrhosis was adjusted according to their BMI.25 The follow-up duration was defined as the interval between the time of enrolment and the end of follow-up (March 2020), death or LT, whichever was earlier.
Nutritional Assessment and Anthropometric Measurements
Anthropometric measurements including weight, height and BMI were measured at enrolment. Dry weight was calculated to eliminate the added weight due to ascites (weight reduction of 5% for mild, 10% for moderate and 15% for gross ascites, 5% extra for pedal oedema),26 and was used to estimate BMI. Handgrip strength (HGS) was measured in the dominant arm using a digital hand dynamometer (DHD-1, Saehan Corporation, South Korea) according to standard protocols27 by trained personnel having experience of performing more than 100 procedures. A 6-min walk test was performed according to the recommendations of the American thoracic society to calculate the 6-min walk distance (6MWD).28
Estimation of Skeletal Muscle Area and SMI
All study participants underwent multiphase CT abdomen for ruling out HCC at inclusion. All CT images were acquired using a 256-slice dual-energy scanner (Somatom definition flash, Siemens). A transverse CT image in the venous phase at the mid-body of the third lumbar (L3) vertebral level was identified. The images were analysed using an image analyser software, 3D slicer software version 4.10.2 (http://www.slicer.org), which is an image computing platform used for quantitative imaging.29 Skeletal muscle was identified by a predefined CT density of −29 to +150 Hounsfield unit (HU). Skeletal muscle area (SMA) included the sum of areas of paraspinal, psoas, transverse abdominis, internal oblique, external oblique and rectus abdominis muscle at the L3 vertebral level. It was then normalized for the height and expressed as SMI in cm2/m2. L3-SMI was estimated because the different definitions compared in this study (detailed below) have estimated sarcopenia based on the L3-SMI cut-offs.
Diagnosis of Sarcopenia
Table 1 outlines the various definitions of sarcopenia and their respective cut-offs for SMI and HGS. Probable sarcopenia was defined according to the EWGSOP2 criteria using HGS.1 Sarcopenia was defined according to four diagnostic definitions. These included: a) North American expert opinion statement (henceforth referred to as the Western cut-off [WC]),9 b) SMI-based cut-off in a population of similar ethnicity (henceforth referred to as the Asian cut-off [AC]),16 c) confirmed sarcopenia based on the EWGSOP2 criteria using the SMI values of the WC (henceforth referred as HGS + WC) and d) confirmed sarcopenia based on the EWGSOP2 criteria using the SMI values of the AC (henceforth referred as HGS + AC).
Table 1.
Diagnostic Definitions of Sarcopenia.
| HGS |
Skeletal muscle index |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Probable sarcopenia1 | <27 kg | <16 kg | – | – |
| WC9 | – | – | <50.0 cm2/m2 | <39.0 cm2/m2 |
| AC18 | – | – | <36.5 cm2/m2 | <30.2 cm2/m2 |
| Confirmed sarcopenia (HGS + WC)1,9 | <27 kg | <16 kg | <50.0 cm2/m2 | <39.0 cm2/m2 |
| Confirmed sarcopenia (HGS + AC)1,18 | <27 kg | <16 kg | <36.5 cm2/m2 | <30.2 cm2/m2 |
AC, Asian cut-off; HGS, handgrip strength; WC, Western cut-off.
Ethical Clearance and Patient Consent- The study protocol was approved by the institutional ethics committee. Written informed consent was obtained from all the patients at enrolment and all procedures performed were as per the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Sample Size Estimation and Statistical Analysis
Sample size estimation was based on the AC definition of sarcopenia. Data on the prevalence of sarcopenia in a population of similar ethnicity, based on other diagnostic definitions, are lacking. The prevalence of sarcopenia in patients with cirrhosis, using the AC criteria, was 12.8%.18 Assuming an absolute precision of ±5% and a confidence interval (CI) of 95%, the sample size required for the estimation of the prevalence of sarcopenia was calculated to be 172 patients. Assuming 10% of patients getting lost to follow-up, a minimum of 190 patients were required in the study.
Categorical variables were represented as frequency (percentage) and continuous variables were represented as mean ± standard deviation (SD) or median (interquartile range [IQR]), as appropriate. Categorical variables were compared using the χ2 test, whereas continuous variables were compared using the independent sample t-test or Mann–Whitney U test, depending on the normality of distribution. A two-tailed P-value of less than 0.05 was considered to be statistically significant. Correlations between SMI, HGS and 6MWD were estimated using the Pearson correlation coefficient. Agreement between the diagnostic definitions of sarcopenia was assessed using the Cohen's Kappa coefficient (concordance rate between individual definitions) and Fleiss' Kappa coefficient (overall concordance among all definitions). Survival in patients with and without sarcopenia, defined based on the diagnostic definitions, was compared using the Kaplan–Meier analysis, and significance was estimated using the log-rank test. For the assessment of the predictors of survival in patients with cirrhosis, univariate and multivariable-adjusted Cox proportional hazards models were constructed. Results were expressed as hazards ratio (HR) with 95% CI and P-values for both univariate and multivariate analyses. The HR was also calculated for each diagnostic definition of sarcopenia after adjusting for the parameters significant on the multivariate analysis. All data were entered using Microsoft Excel 2011 and was analysed using R studio. In addition to the base package in R, survminer, survival, tidyverse, psych and irr packages were used.
Results
Baseline Characteristics
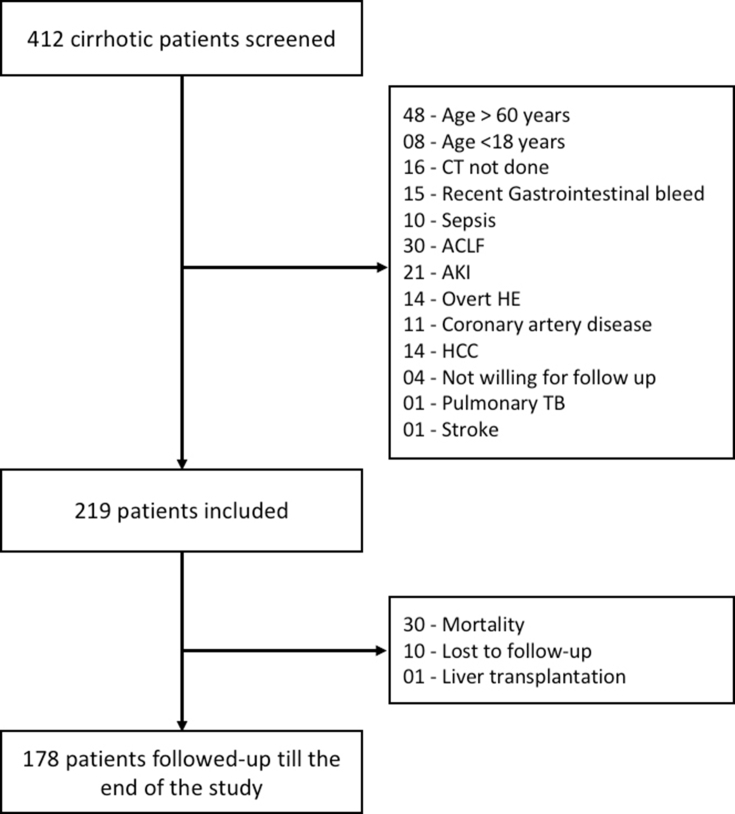
A total of 412 consecutive patients with cirrhosis attending the liver clinic were screened, and 219 patients (168 men; 76.7%) meeting the inclusion and exclusion criteria were recruited (Supplementary Figure 1). The baseline characteristics of the included cohort are summarized in Table 2. Mean age was 42.6 years (men: 42.4 ± 10.1 years and women: 43.5 ± 9.9 years; P = 0.49), with alcohol (33.3%) being the most common aetiology, followed by viral hepatitis (30.6%). Most patients had current or prior hepatic decompensation (Table 2). The median (IQR) model for end-stage liver disease (MELD) score was 12 (9–15) and Child-Turcotte-Pugh (CTP) score was 7 (5–9) (Child A, B and C: 49.3%, 39.7% and 11.0%, respectively). The patients were followed up for a median (IQR) duration of 12 (6–15) months, over which 30 patients succumbed to their illness (12-month survival 86.1% [81.1–91.3%]). Baseline characteristics were further analysed according to sex, and men were found to have more advanced liver disease as indicated by higher CTP scores (median CTP 7 [6–9] in men vs 6 [5–7] in women; P < 0.001) and MELD scores (median MELD 13 [10–16] in men vs 10 [8–12] in women; P < 0.001). They also had a higher SMA, SMI, HGS and 6MWD at baseline (Table 2).
Table 2.
Baseline Characteristics of the Entire Cohort (n = 219).
| Male (n = 168) | Female (n = 51) | P value | |
|---|---|---|---|
| Age (years) | 42.4 ± 10.1 | 43.5 ± 9.9 | 0.490 |
| Anthropometric parameters | |||
| • Height (cm) | 166.7 ± 6.2 | 153.1 ± 5.9 | <0.001 |
| • Weight (kg) | 61.8 ± 14.4 | 54.4 ± 13.7 | <0.001 |
| • BMI (kg/m2) | 22.1 ± 4.5 | 23.2 ± 5.5 | 0.170 |
| Symptom duration (months) | 16 (6–36) | 18 (5–60) | 0.366 |
| Aetiology of cirrhosis | |||
| • Alcohol | 72 (42.9%) | 1 (2.0%) | <0.001 |
| • Hepatitis B | 42 (25.0%) | 7 (13.7%) | |
| • Hepatitis C | 7 (4.2%) | 11 (21.6%) | |
| • Autoimmune hepatitis | 5 (3.0%) | 12 (23.5%) | |
| • Overlap syndrome | 0 (0.0%) | 2 (3.9%) | |
| • Nonalcoholic steatohepatitis | 5 (3.0%) | 7 (13.7%) | |
| • Cryptogenic | 26 (15.5%) | 9 (17.6%) | |
| • >1 aetiology a | 11 (6.5%) | 2 (3.9%) | |
| Decompensation | |||
| • Ascites (%) | 91/168 (54.2%) | 21/51 (41.2%) | 0.104 |
| • Hepatic encephalopathy (%) | 28/168 (16.7%) | 5/51 (9.8%) | 0.230 |
| • Gastrointestinal bleed (%) | 59/168 (35.1%) | 15/51 (29.4%) | 0.450 |
| Laboratory parameters | |||
| • Haemoglobin (g/dL) | 11.3 ± 2.4 | 10.4 ± 2.1 | 0.037 |
| • Platelet (× 109/L) | 91 (60–125) | 93 (60–124) | 0.844 |
| • Bilirubin (mg/dL) | 1.7 (0.9–2.8) | 1.1 (0.8–1.4) | <0.001 |
| • AST (U/L) | 51 (40–77) | 45 (35–72) | 0.288 |
| • ALT (U/L) | 34 (25–55) | 34 (23–58) | 0.889 |
| • ALP (U/L) | 286 (210–427) | 289 (195–438) | 0.815 |
| • Protein (g/dL) | 7.3 ± 0.7 | 7.4 ± 0.7 | 0.351 |
| • Albumin (g/dL) | 3.5 ± 0.7 | 3.7 ± 0.7 | 0.270 |
| • Calcium (mg/dL) | 8.6 ± 0.6 | 8.6 ± 0.7 | 0.808 |
| • Creatinine (mg/dL) | 0.8 (0.7–0.9) | 0.7 (0.6–0.8) | <0.001 |
| • INR | 1.5 ± 0.4 | 1.3 ± 0.2 | 0.002 |
| CTP | 7 (6–9) | 6 (5–7) | <0.001 |
| Child A/B/C | 75/69/24 | 33/18/0 | 0.004 |
| MELD | 13 (10–16) | 10 (8–12) | <0.001 |
| 6-MWD (m) | 347.1 ± 57.8 | 313.4 ± 48.4 | <0.001 |
| HGS (kg) | 26.6 (20.9–31.2) | 18.0 (15–20.4) | <0.001 |
| SMA (cm2) | 119.1 ± 22.9 | 85.9 ± 16.3 | <0.001 |
| SMI (cm2/m2) | 42.9 ± 8.0 | 36.7 ± 6.6 | <0.001 |
| Probable sarcopenia (%) | 87/168 (51.8%) | 18/51 (35.3%) | 0.039 |
| Sarcopenia criteria | |||
| • WC (%) | 138/168 (82.1%) | 32/51 (62.7%) | 0.004 |
| • AC (%) | 32/168 (19.0%) | 10/51 (19.6%) | 0.929 |
| • HGS + WC (%) | 73/168 (43.5%) | 11/51 (21.6%) | 0.005 |
| • HGS + AC (%) | 24/168 (14.3%) | 3/51 (5.9%) | 0.110 |
All values are expressed as mean ± SD or median (IQR) or percentage, as appropriate. Bold values indicate P value <0.05.
6-MWD, 6-min walk distance; AC, Asian cut-off; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CTP, Child-Turcotte-Pugh score; HGS, handgrip strength; INR, international normalized ratio; MAC, mid-arm circumference; MAMC, mid-arm muscle circumference; MELD, model for end-stage liver disease; SMA, skeletal muscle area; SMI, skeletal muscle index; TSFT, triceps skin-fold thickness; WC, Western cut-off.
Indicates the presence of more than one aetiology of cirrhosis such as viral hepatitis with co-existent alcoholic liver disease or non-alcoholic steatohepatitis.
Prevalence of Sarcopenia and Agreement Between Various Criteria
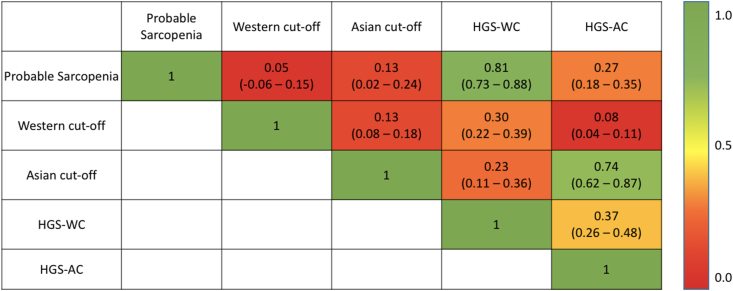
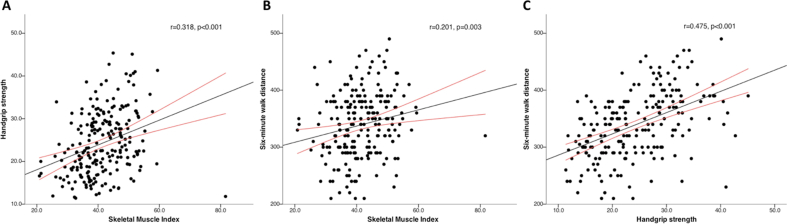
The prevalence of sarcopenia was 51.8%, 82.1%, 19.0%, 43.5% and 14.3% in men and 35.3%, 62.7%, 19.6%, 21.6% and 5.9% in women, based on the criteria for EWGSOP2 probable sarcopenia, WC, AC, HGS + WC and HGS + AC, respectively. The prevalence did not differ between men and women when estimated according to the AC (P = 0.929) and HGS + AC criteria (P = 0.110). Based on the EWGSOP2 criteria of probable sarcopenia, 114 patients had no sarcopenia. However, of these nonsarcopenic patients, the WC and AC criteria classified 86 of 114 (75.4%) and 15 of 114 (13.2%) patients as sarcopenic, respectively. Fig. 1 shows the agreement between various diagnostic criteria. We observed a high agreement between probable sarcopenia criteria and HGS-WC (Cohen's kappa coefficient 0.81, 95% CI 0.73–0.88) and a substantial agreement between HGS-AC and AC (Cohen's kappa coefficient 0.74, 95% CI 0.62–0.87). Overall, the diagnostic definitions were only fairly concordant (Fleiss' kappa coefficient 0.23, 95% CI 0.10–0.37). A weak positive correlation was identified between SMI and HGS (r = 0.318, P < 0.001) and SMI and 6MWD (r = 0.201, P = 0.003). A moderate positive correlation was seen between HGS and 6MWD (r = 0.475, P < 0.001) (Supplementary Figure 2).
Figure 1.
Heat map showing the concordance, as assessed by the Cohen's kappa coefficient, between various criteria for the diagnosis of sarcopenia. HGS, handgrip strength; AC, Asian cut-off; WC, Western cut-off.
Predictors of Mortality
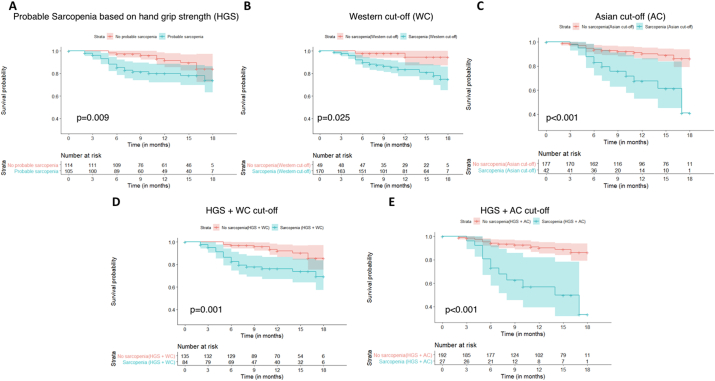
Over the study period, 30 of 219 patients (13.7%) expired and one patient underwent deceased donor LT. Mortality rates were significantly higher in patients with sarcopenia, irrespective of the diagnostic criteria used (Figure 2). The 1-year mortality rates, in patients with sarcopenia and without sarcopenia were 20% (95% CI: 11.4%–27.7%) and 8.5% (2.1%–14.4%) (log-rank P = 0.009) according to EWGSOP2 probable sarcopenia, 16.4% (10%–22.4%) and 5.5% (0%–12.8%) (log-rank P = 0.025) according to WC criteria, 32.5% (13.7%–47.3%) and 9.8% (4.8%–14.5%) (log-rank P < 0.001) according to AC criteria, 23.8% (13.4%–32.9%) and 8.0% (2.3%–13.4%) (log-rank P = 0.001) according to HGS + WC criteria and 43.1% (17.8%–60.6%) and 9.9% (5.1%–14.6%) (log rank P < 0.001) according to the HGS + AC criteria, respectively. On univariate Cox proportional hazard model, BMI, CTP and MELD scores, ascites, 6MWD, SMI and HGS were independent predictors of mortality (Table 3). Multivariate hazards were estimated using parameters that were independent predictors of mortality on the univariate analysis. Ascites was an independent predictor of mortality (HR 6.27, 95% CI 1.64–24.06, P = 0.007), whereas high SMI was protective (HR 0.92, 95% CI 0.85–0.98, P = 0.021).
Figure 2.
The Kaplan–Meier analysis of the survival probability in patients with and without sarcopenia, defined based on the diagnostic definitions of probable sarcopenia (based on handgrip strength) (A), Western cut-off (B), Asian cut-off (C), Handgrip strength and western cut-off (D), and handgrip strength and Asian cut-off (E). (Log-rank P < 0.05 for all categories.)
Table 3.
Univariate and Multivariate Cox-Proportional Hazards for the Predictors of Mortality.
| Univariate hazards |
Multivariate hazards |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 0.99 | 0.97–1.03 | 0.810 | |||
| Sex (Male) | 1.52 | 0.58–3.97 | 0.395 | |||
| BMI | 0.84 | 0.77–0.92 | <0.001 | 1.06 | 0.92–1.23 | 0.392 |
| Aetiology (Alcohol) | 1.94 | 0.95–3.98 | 0.070 | |||
| CTP score | 1.60 | 1.36–1.88 | <0.001 | 1.26 | 0.91–1.75 | 0.162 |
| MELD score | 1.17 | 1.09–1.25 | <0.001 | 1.04 | 0.92–1.12 | 0.510 |
| Ascites | 11.72 | 3.54–38.76 | <0.001 | 6.27 | 1.64–24.06 | 0.007 |
| 6MWD | 0.99 | 0.98–1.00 | 0.015 | 1.00 | 0.99–1.01 | 0.620 |
| SMI | 0.91 | 0.87–0.95 | <0.001 | 0.92 | 0.85–0.98 | 0.021 |
| HGS | 0.94 | 0.89–0.99 | 0.032 | 0.99 | 0.92–1.07 | 0.787 |
6MWD, 6-min walk distance; BMI, body mass index; CI, confidence interval; CTP, Child-Turcotte-Pugh; GI bleed, gastrointestinal bleed; HE, hepatic encephalopathy; HGS, handgrip strength; HR, hazards ratio; MELD, model for end-stage liver disease; SMI, skeletal muscle index. Bold values indicate P value <0.05.
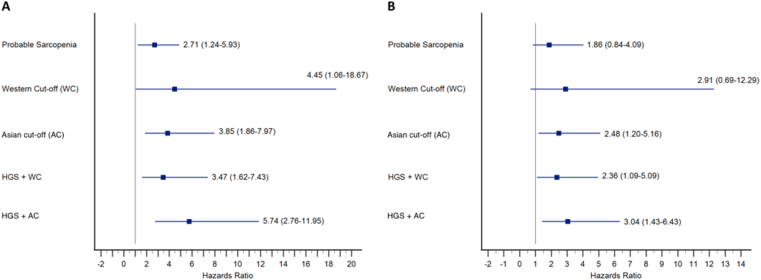
Sarcopenia, defined by all criteria, was also an independent predictor of mortality on the unadjusted univariate Cox proportional hazard models (Figure 3A). When adjusted for the presence of ascites, the HGS + AC criteria (HR 3.04, P = 0.004) had the highest HR for predicting mortality in comparison to the AC criteria (HR 2.48, P = 0.015), HGS + WC criteria (HR 2.36, P = 0.029), WC criteria (HR 2.91, P = 0.147) and probable sarcopenia criteria (HR 1.86, P = 0.124) (Figure 3B).
Figure 3.
Forest plots showing the results of the Cox-proportional hazards model for various diagnostic definitions of sarcopenia, their hazard ratios and 95% confidence intervals. The boxes represent the hazards ratio and the error bars represent the 95% confidence intervals. (A) Univariate hazards for various diagnostic definitions of sarcopenia as a predictor of mortality. (B) Multivariate hazards, adjusted for ascites, for various diagnostic definitions of sarcopenia as a predictor of mortality. HGS, handgrip strength; WC, Western cut-off; AC, Asian cut-off.
Discussion
In this study, we included 219 patients with cirrhosis and estimated the prevalence of sarcopenia using different diagnostic cut-offs. We observed significant heterogeneity among the diagnostic criteria, indicated by the wide variations in the prevalence of sarcopenia varying between 14.3% and 82.1% in men and 5.9% and 62.7% in women. The mortality rate in patients with sarcopenia was higher, irrespective of the definition used. Ascites and low SMI were independent predictors of mortality and patients found to be sarcopenic using the HGS + AC criteria had the highest risk of mortality.
The prevalence of sarcopenia in our cohort was found to range from 5.9% to 82.1%, depending on the definition used and the gender being studied. A major reason for this was the SMI cut-off, which was significantly lower for the AC criteria than that of similar Caucasian populations.8,11,12 In addition to the differences in genetics and body composition, one of the possible explanations for this could be an increased amount of abdominal and visceral fat in Asians as compared to Caucasians.30 Assuming a two-compartment model,31 the corresponding fat-free mass (composed mainly of muscle) would logically be lower in Asians for the same sex, weight and BMI. Therefore, the cut-off for muscle mass leading to sarcopenia is likely not a rigid construct and needs to be tailored to the ethnic group being studied.
A poor to fair agreement was observed between the diagnostic criteria for sarcopenia, except for the probable sarcopenia criteria and HGS-WC, and HGS-AC and AC criteria. Previous studies have also shown similar results in the geriatric population and patients with cirrhosis when comparing different diagnostic definitions.32, 33, 34 This lack of concordance between different definitions stems from the components of the definition. The EWGSOP2 probable sarcopenia, HGS + WC and HGS + AC criteria incorporate muscle function, whereas the WC and AC definition include muscle mass alone. Others have shown a poor to weak correlation between muscle mass and HGS,35 similar to the observations of our study. In a study by Tapper et al, the correlation between SMI and HGS in men and women were 0.168 and 0.283, respectively.36 One reason for this could be the overestimation of probable sarcopenia in cirrhotics due to a lower estimated HGS because of difficulties in the standardized assessment of muscle strength. It has been previously shown that HGS need not correlate with muscle mass in cirrhotics and may be influenced by other comorbidities such as clinical/subclinical HE37 and other acute decompensations, which are frequent issues in patients with cirrhosis. Moreover, HGS cut-offs are based on normative data from healthy individuals and evidence-based HGS cut-offs in cirrhotics is lacking. Defining sarcopenia based on HGS with SMI-based cut-off helps us select the patients who have both impaired muscle mass and function, and therefore, enables us to predict those who are at the highest risk of complications and mortality.
Although the prevalence of sarcopenia was highest when defined by the WC criteria, it did not translate into clinical utility as an independent predictor of mortality. The HR for predicting mortality was highest for HGS + AC criteria followed by the AC criteria, further suggesting the need for population-specific criteria. EWGSOP2 probable sarcopenia was an independent predictor of mortality on the univariate analysis, but not the multivariate analysis adjusted for ascites. This also suggests that sarcopenia, defined as low muscle strength and mass (as proposed by EWGSOP2), is probably a better predictor of mortality than the muscle mass (WC and AC criteria) alone.
Our data suggest that although low muscle strength (EWGSOP2 probable sarcopenia) was not independently associated with mortality, a combination of region-specific muscle mass and muscle strength (HGS + AC) identified the patients at the highest risk. This also suggests that decreased muscle strength may represent a significant clinical event in the natural course of cirrhosis, as it may be the forme fruste of sarcopenia and may translate into a subsequent risk of mortality. It is possible that the trend towards poor outcomes starts with decreased muscle strength, even though it starts independently contributing to mortality only when sarcopenia sets in. Identification of probable sarcopenia could therefore present a window of opportunity, and a different set of interventions may be useful in the future targeting this subgroup of patients.
Ascites was an independent predictor of mortality while high SMI was protective. MELD score, although a predictor of mortality on the univariate analysis, was not an independent predictor of mortality in our cohort on the multivariate analysis. This suggests that low SMI is a stronger predictor of mortality than the MELD score alone. Van Vugt et al showed that the median survival was lower in patients with sarcopenia, even in patients with an MELD score less than 15.38 Others also shown that the MELD-sarcopenia score is a better predictor of mortality in cirrhotics than the MELD score alone.39 Therefore, it is important to identify sarcopenia early and undertake steps to halt its progress.
The sample size was derived from the study involving compensated alcoholic cirrhotics. The study by Benjamin et al included both compensated and decompensated patients belonging to a group of only alcohol-related liver cirrhosis.18 Despite being an alcohol-related liver disease, the prevalence of sarcopenia was very low mainly because the cut-off of L3-SMI used was normative cut-offs (2 SD below mean of young healthy adults). This is a stringent cut-off and the overall prevalence was only 12.8% (compensated 6.4% and decompensated 15.8%). This method was chosen as the EWGSOP recommends the normative method of cut-off derivation. Also, if one chooses to use the cut-off at one SD below the mean of young healthy adults, the prevalence increases substantially. Although the prevalence is low, the patients with SMI of 2 SD below the mean of healthy adults (AC criteria) are truly having a very low muscle mass and carry a high mortality. The published WCs of CT–L3-SMI are mortality-based cut-offs, which are much higher than the normative ones, thereby, increasing the prevalence of sarcopenia. Hence, the need of the hour is to derive a mortality-based cut-off of SMI in patients with liver cirrhosis in the Indian scenario. Variations in the sarcopenia prevalence rates based depending on the study population and the methodology of sarcopenia assessment will remain the main reasons for the differences in various sarcopenia prevalence estimation studies.
Our study has a few limitations. Although the diseased cohort was not equally distributed for men and women, this is representative of the cirrhotic population in our region.40 The median follow-up was 1 year, and a longer follow-up with a sequential assessment of HGS and SMI could have further highlighted the role of rate of decline in HGS and SMI in predicting mortality. CT-based estimation of SMI is associated with the risks of radiation exposure and high cost of serial assessment, and it needs dedicated software for analysis, and hence defining sarcopenia based on SMI may lack wide acceptance from clinicians working outside research settings. Therefore, there is a need for simple bedside alternatives, which can estimate SMI and predict mortality in cirrhosis. Bioimpedance analysis, muscle ultrasound and dual-energy X-ray absorptiometry have been tested as alternatives, but have their limitations.41
The study highlights the benefits and limitations of using different diagnostic criteria for sarcopenia in routine clinical practice. In a large population of 219 patients, we compared five diagnostic criteria and showed immense heterogeneity in the prevalence of sarcopenia. Our study should pave way for future studies to determine appropriate population-specific HGS and SMI cut-offs in patients with cirrhosis.
To conclude, sarcopenia is highly prevalent in patients with cirrhosis and is associated with high mortality, irrespective of the definition used. Sarcopenia identified using the EWGSOP2 definition (including low HGS and population-specific SMI cut-off) best predicts mortality in patients with cirrhosis. There is a lack of concordance among the various diagnostic definitions of sarcopenia, and the definition being used should be tailored to the population being studied and study objectives.
Credit authorship contribution statement
The following authors contributed to this manuscript as listed:
Abhinav Anand: analysis and interpretation of data, statistical analysis, drafting of the manuscript, acquisition of data; Srikant Mohta: acquisition of data, drafting of the manuscript; Samagra Agarwal: analysis and interpretation of data; Sanchit Sharma: acquisition of data, drafting of the manuscript; Srikanth Gopi: acquisition of data; Deepak Gunjan: study supervision, drafting of the manuscript; Kumble S. Madhusudhan: acquisition of radiological data; Namrata Singh: study supervision, drafting of the manuscript; Anoop Saraya: study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content, study supervision.
Conflicts of interest
The authors have none to declare.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2021.03.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Figure 1.
Patient disposition in the entire cohort.
Supplementary Figure 2.
Scatter-plots demonstrating correlations between skeletal muscle index (SMI) (cm2/m2), handgrip strength (HGS) (kg), and 6-min walk distance (6MWD) (metres). (A) Scatter plots showing a weak positive correlation between SMI and HGS (r = 0.318, P < 0.001). (B) Scatter plots showing a weak positive correlation between SMI and 6MWD (r = 0.201, P = 0.003). (C) Scatter-plots showing a moderate positive correlation between HGS and 6MWD (r = 0.475, P < 0.001). The black line represents the trend line and the red lines indicate the 95% confidence interval.
References
- 1.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bojko M. Causes of sarcopenia in liver cirrhosis. Clin Liver Dis. 2019;14:167–170. doi: 10.1002/cld.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krell R.W., Kaul D.R., Martin A.R., et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transplant. 2013;19:1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montano-Loza A.J., Meza-Junco J., Prado C.M.M., et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. doi: 10.1016/j.cgh.2011.08.028. 173.e1. [DOI] [PubMed] [Google Scholar]
- 5.DiMartini A., Cruz R.J., Dew M.A., et al. Muscle mass predicts outcomes following liver transplantation. Liver Transplant. 2013;19:1172–1180. doi: 10.1002/lt.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamaguchi Y., Kaido T., Okumura S., et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transplant. 2014;20:1413–1419. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 7.Durand F., Buyse S., Francoz C., et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Carey E.J., Lai J.C., Wang C.W., et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017;23:625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey E.J., Lai J.C., Sonnenday C., et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70:1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K., Shin Y., Huh J., et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol. 2019;20:205–217. doi: 10.3348/kjr.2018.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prado C.M.M., Lieffers J.R., McCargar L.J., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 12.Tsien C., Garber A., Narayanan A., et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation: post-liver transplant sarcopenia. J Gastroenterol Hepatol. 2014;29:1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin L., Birdsell L., MacDonald N., et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara N., Nakagawa H., Kudo Y., et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Kalafateli M., Mantzoukis K., Choi Yau Y., et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121. doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamaguchi Y., Kaido T., Okumura S., et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32:1200–1205. doi: 10.1016/j.nut.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 17.van Vugt J.L.A., Buettner S., Alferink L.J.M., et al. Low skeletal muscle mass is associated with increased hospital costs in patients with cirrhosis listed for liver transplantation-a retrospective study. Transpl Int. 2018;31:165–174. doi: 10.1111/tri.13048. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin J., Shasthry V., Kaal C.R., et al. Characterization of body composition and definition of sarcopenia in patients with alcoholic cirrhosis: a computed tomography based study. Liver Int. 2017;37:1668–1674. doi: 10.1111/liv.13509. [DOI] [PubMed] [Google Scholar]
- 19.Stegenga H., Haines A., Jones K., Wilding J. On behalf of the Guideline Development Group. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ. 2014;349 doi: 10.1136/bmj.g6608. g6608–g6608. [DOI] [PubMed] [Google Scholar]
- 20.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Thornton J.C., Russell M., Burastero S., Heymsfield S., Pierson R.N. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60:23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 22.Tsochatzis E.A., Bosch J., Burroughs A.K. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 23.Moreau R., Jalan R., Gines P., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1-9. [DOI] [PubMed] [Google Scholar]
- 24.Merli M., Berzigotti A., Zelber-Sagi S., et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiavo L., Busetto L., Cesaretti M., Zelber-Sagi S., Deutsch L., Iannelli A. Nutritional issues in patients with obesity and cirrhosis. World J Gastroenterol. 2018;24:3330–3346. doi: 10.3748/wjg.v24.i30.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tandon P., Low G., Mourtzakis M., et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1473–1480.e3. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Sousa-Santos A.R., Amaral T.F. Differences in handgrip strength protocols to identify sarcopenia and frailty – a systematic review. BMC Geriatr. 2017;17:238. doi: 10.1186/s12877-017-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ATS Statement Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 29.Yip S.S.F., Parmar C., Blezek D., et al. Application of the 3D slicer chest imaging platform segmentation algorithm for large lung nodule delineation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raji A., Seely E.W., Arky R.A., Simonson D.C. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86:5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 31.Withers R.T., LaForgia J., Pillans R.K., et al. Comparisons of two-, three-, and four-compartment models of body composition analysis in men and women. J Appl Physiol. 1998;85:238–245. doi: 10.1152/jappl.1998.85.1.238. [DOI] [PubMed] [Google Scholar]
- 32.Shafiee G., Heshmat R., Ostovar A., et al. Comparison of EWGSOP-1and EWGSOP-2 diagnostic criteria on prevalence of and risk factors for sarcopenia among Iranian older people: the Bushehr Elderly Health (BEH) program. J Diabetes Metab Disord. 2020 doi: 10.1007/s40200-020-00553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traub J., Bergheim I., Eibisberger M., Stadlbauer V. Sarcopenia and liver cirrhosis-comparison of the European working group on sarcopenia criteria 2010 and 2019. Nutrients. 2020;12 doi: 10.3390/nu12020547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee W.-J., Liu L.-K., Peng L.-N., Lin M.-H., Chen L.-K. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-lan longitudinal aging study. J Am Med Dir Assoc. 2013;14:528.e1–528.e7. doi: 10.1016/j.jamda.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Wang C.W., Feng S., Covinsky K.E., et al. A comparison of muscle function, mass, and quality in liver transplant candidates: results from the functional assessment in liver transplantation study. Transplantation. 2016;100:1692–1698. doi: 10.1097/TP.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapper E.B., Derstine B., Baki J., Su G.L. Bedside measures of frailty and cognitive function correlate with sarcopenia in patients with cirrhosis. Dig Dis Sci. 2019;64:3652–3659. doi: 10.1007/s10620-019-05713-4. [DOI] [PubMed] [Google Scholar]
- 37.Maharshi S., Sharma B.C., Sachdeva S., Srivastava S., Sharma P. Efficacy of nutritional therapy for patients with cirrhosis and minimal hepatic encephalopathy in a randomized trial. Clin Gastroenterol Hepatol. 2016;14 doi: 10.1016/j.cgh.2015.09.028. 454–460.e3. [DOI] [PubMed] [Google Scholar]
- 38.van Vugt J.L.A., Alferink L.J.M., Buettner S., et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2018;68:707–714. doi: 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 39.Montano-Loza A.J., Duarte-Rojo A., Meza-Junco J., et al. Inclusion of sarcopenia within MELD (MELD-Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. doi: 10.1038/ctg.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sepanlou S.G., Safiri S., Bisignano C., et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinclair M. Controversies in diagnosing sarcopenia in cirrhosis-moving from research to clinical practice. Nutrients. 2019;11 doi: 10.3390/nu11102454. [DOI] [PMC free article] [PubMed] [Google Scholar]