Abstract
Background
Saroglitazar—a unique dual peroxisome proliferator-activated receptor agonist was approved marketing authorization in India in 2013 for diabetic dyslipidemia. Postmarketing studies have additionally shown improvement in liver parameters in diabetic dyslipidemia patients with nonalcoholic fatty liver disease (NAFLD) who received saroglitazar.
Aim
The aim of this study was to evaluate the effect of saroglitazar on liver function test, liver fibrosis score by FibroScan, lipid profiles, HbA1c in NAFLD patients with diabetic dyslipidemia in southern India.
Methodology
A prospective, interventional, pilot study was performed to study the safety and efficacy of saroglitazar in NAFLD patients having type 2 diabetes mellitus. About 97 patients were screened, of which 85 patients were involved in the study based on the inclusion criteria. The clinical parameters and liver stiffness were measured at the baseline and also after 12 weeks of treatment with administration of saroglitazar 4 mg once daily. The change in the parameters at the baseline and after the end of the treatment was measured and was subjected to statistical analysis using SPSS software.
Results
The recruited patients received saroglitazar and were followed up for a period of 12 weeks. The clinical parameters such as fasting blood sugar, postprandial blood sugar, HbA1c, total cholesterol, triglycerides, SGPT, and liver stiffness showed significant difference after 12 weeks of treatment when compared with the baseline values. No adverse drug reaction was reported in patients receiving saroglitazar during the study.
Conclusion
Saroglitazar was found to show significant improvement in liver parameters in NAFLD patients with a significant reduction in liver fibrosis and triglycerides level.
Keywords: saroglitazar, diabetic dyslipidemia, non-alcoholic fatty liver disease, fibrosis level, ultrasound
Abbreviations: AACE, American Associaton of Clinical Endocrinologists; ADR, Adverse Drug Reaction; ALT, Alanine Transaminase; BMI, Body Mass Index; CDSCO, Central Drugs Standard Control Organisation; CT Scan, Computed Tomography Scan; DBP, Diastolic Blood Pressure; DCGI, Drug Controller General of India; FBS, Fasting Blood Sugar; GLP1Ra, Glucagon Like Peptide 1 Receptor agonist; HbA1C, Glycated Hemoglobin; HCV, Hepatitis - C Virus; HDL, High Density Lipoprotein; IHEC, Institutional Human Ethics Committee; LDL-C, Low Density Lipoprotein Cholesterol; LSM, Liver Stiffness Measurement; MRI, Magnetic Resonance Imaging; NAFLD, Nonalcoholic Fatty Liver Disease; NASH, Non-Alcoholic Steatohepatitis; Na2EDTA, Sodium Ethylenedinitrilotetraacetic acid; NPV, Negative Predictive Value; PPAR, Peroxisome Proliferator Activated Receptor; PPBS, Post Prandial Blood Sugar; SBP, Systolic Blood Pressure; SDB, Serum Direct Bilirubin; SGLT2i, Sodium Glucose Co-Transporter-2 Inhibitor; SGOT, Serum Glutamate Oxaloacetic Transaminase; SGPT, Serum Glutamate Pyruvic Transaminase; SPSS, Statistical Package for the Social Sciences; STB, Serum Total Bilirubin; TC, Total Cholesterol; TG, Triglycerides; TZD, Thiazolidinediones; T2DM, Type 2 Diabetes Mellitus; USG, Ultra Sonography; VLDL, Very Low Density Lipoprotein
Nonalcoholic fatty liver disease (NAFLD) is a genetic, environmental, metabolic, and stress-associated liver disease characterized by excess fat storage in liver parenchymal cells ranging from simple steatosis to steatohepatitis in the absence of excessive alcohol intake.1 It is a part of the metabolic syndrome, associated with many metabolic features, such as hypertension, obesity, diabetes, and hyperlipidemia.
Indians have higher insulin resistance and higher hepatic triglycerides in comparison with other races proposing that Indians are much susceptible to developing NAFLD.1 The prevalence of insulin resistance in Asian-Indians residing in India ranges from 7% to 55%.2,3 NAFLD is emerging as the most common liver abnormality in India. The management of patients with NAFLD consists of treatment of associated metabolic comorbidities such as hyperlipidemia, obesity, insulin resistance, and type 2 diabetes mellitus (T2DM). The higher prevalence of NAFLD in diabetic patients attributes to the use of antidiabetic drugs that exhibit beneficial effects in the management of NAFLD as well. Pioglitazone and empagliflozin are the few examples of antidiabetic drugs which also have potential effects on NAFLD.4,5 Simultaneous blood glucose reduction, weight reduction, increase in glucagon level, reduction in hepatic inflammatory cytokines, and lowering of lipid levels elicited by these drugs by various mechanisms facilitate improvement in the liver fibrosis condition which is reflected as reduction in the liver stiffness measurement (LSM).6,7 The pharmacotherapy of patients with NAFLD is still evolving. Current treatment for NAFLD mainly focuses on the simultaneous management of insulin resistance and dyslipidemia which are the key factors in the pathophysiology of NAFLD. Insulin sensitizers such as thiazolidinediones, SGLT2Is, which possess dual activity of maintaining blood glucose and lipid levels, are being used widely in the management of NAFLD. Alternatives such as therapies based on statins, cytoprotective and antioxidant agents, and incretin-based therapy are also being used for the NAFLD treatment.19 Saroglitazar is a glitazar class compound that has been approved by the central drug standard control organization of India for treating diabetes dyslipidemia with the excellent safety profile.8 Real-world evidence has showed that there was also a consistent improvement in liver parameters with reduction in ALT levels in NAFLD.9 Studies in northern India have shown improvement in liver parameters such as SGPT in diabetic dyslipidemia patients with NAFLD who received saroglitazar for 24 weeks.10, 11, 12 PPARα/γ are nuclear receptors which can control the metabolic process of lipid and glycemic parameters, respectively. PPAR-alpha and PPAR-gamma are the molecular targets of a number of marketed drugs. Saroglitazar is a dual PPARα (alpha) and PPARγ (gamma) agonist showing action predominantly on PPARα and moderately on PPARγ agonist.
There is a very high prevalence of NAFLD in individuals with T2DM. Studies have estimated that around one-third to two-thirds of diabetic patients have NAFLD.13, 14, 15 This results in adverse outcomes such as higher rates of mortality due to cirrhosis. The present study was designed to assess the effect of saroglitazar, a novel PPARα/γ agonist on NAFLD in patients with diabetic dyslipidemia.
Patients and methods
Study Design
A prospective, single-arm, open-label, interventional, pilot study was conducted from October 2017 to May 2018 in the Department of Medical Gastroenterology, SRM Medical College Hospital & Research Centre (SRMMCH&RC), Chennai, southern India. The Institutional Human Ethics Committee of SRMMCH&RC approved this study protocol (Approval No.1266/IEC/2017).
Study Population
Inclusion criteria: Subjects of both sex in the age group of 18–75 years with a body mass index (BMI) between 25 and 50 kg/msq with documented diagnosis of NAFLD established by imaging (ultrasound, CT scan, or MRI) within the 12 months preceding visit and reconfirmed by repeat USG abdomen during current visit. Patients with dyslipidemia (triglycerides > 150 mg/dl, total cholesterol > 200 mg/dl and LDL-C > 130 mg/dl) were included. Patients who are diagnosed with type 2 diabetes (HbA1c > 7%) and who are treated with oral medication, excluding TZD or saroglitazar drug users within the last 12 weeks were included.
Exclusion criteria: Use of drugs related with NAFLD for more than 12 sequential weeks in the one year before the first visit. Consumption of >3 units of alcohol per day (>21 units per week) if male and >2 units of alcohol per day (>14 units per week) if female for at least 3 consecutive months in the 5 years preceding visit 1 (note: 1 unit = 4 ounces of wine, 1 ounce of spirits/hard liquor or 12 ounces of beer). Use of tamoxifen, amiodarone, methotrexate, systemic glucocorticoids, tetracycline, anabolic steroids, estrogens in doses higher than used in vitamin A, oral contraceptives, l-asparaginase, sodium valproate, chloroquine, or antiretroviral drugs. Patients with serum positive for hepatitis B surface antigen and anti-HCV were excluded, as also, patients who had been taking SGLT2 inhibitors, pioglitazone, insulin, GLP1Ra drugs within the last 12 weeks. Lactating and pregnant women. Patients not willing to give consent and patients who were known to have cardiac and renal disorders after medical history interview were excluded. Being a pilot study, we randomly recruited 85 patients who were then subjected to intervention without blinding.
Procedure
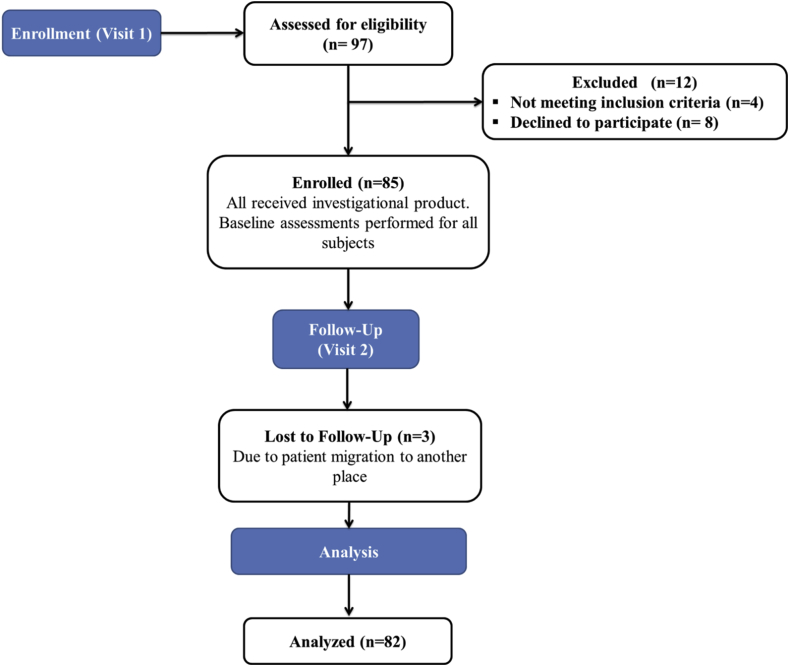
A total of 97 patients were considered for eligibility in which, 12 patients were excepted because of unwillingness and not meeting the inclusion criteria (Figure 1). Remaining 85 patients were included in the study and received 4 mg of saroglitazar once daily for 12 weeks. Fasting blood glucose, serum urea as renal function test, lipid profiles, liver function test, USG abdomen, FibroScan, and adverse effects were recorded at the starting point and after 12 weeks of the treatment.
Figure 1.
Study flowchart.
Biochemical Analysis
In the morning (7–9 am), 6 ml of blood samples were obtained by venous pinhole after overnight fasting, 2 ml of blood sample was shifted into Na2EDTA vacutainer then the plasma was separated by centrifugation for glucose estimation, remaining samples were transferred to plain tube and permitted to clot at room temperature for 45 min before centrifugation (centrifuge-5430R, Eppendorf) to separate the serum. The samples were then aliquoted into Eppendorf tubes and stored at −20 °C until analysis. Glucose, serum lipid profiles, and liver function tests were carried out by the fully automated clinical chemistry analyzer (EM 360, Transasia) using diagnostics kits (ERBA Diagnostics Mannheim GmbH).
Liver Stiffness Measurement
LSM was executed with the FibroScan (FibroScan 502 Touch, Echosens, Paris, France) under fasting conditions in accordance with the manufacture references by a specialized technician blinded to the patient's data. All the patients were fasted for at least 6 h before LSM. Dimensions were made with the M probe on the right lobe of the liver by the intercostal spaces with the patient lying in dorsal decubitus with the right arm in highest abduction. Ten successful attainments were performed on each patient. The median value characterized the liver elastic modulus. Only cases with 10 successful attainments were assessed. The liver stiffness was expressed in kiloPascal (kPa). Based on validation in an earlier cohort, we assumed an LSM cutoff of 7.9 kPa to exclude F3 fibrosis with 90% sensitivity and NPV. We also carried out sensitivity analysis using a range of other published cutoffs.
Statistical Analysis
The data were analyzed using SPSS (version 20; SPSS Inc., Chicago, IL, USA). Descriptive statistics are presented as a mean ± standard deviation. Statistical analysis was carried out by using Student's t-test. The minimum level of significance was fixed at P < 0.05. The Pearson's correlation coeffcients were estimated as well as tested for signifcance of the linear relationship between Fibrosis and triglycerides levels.
Results
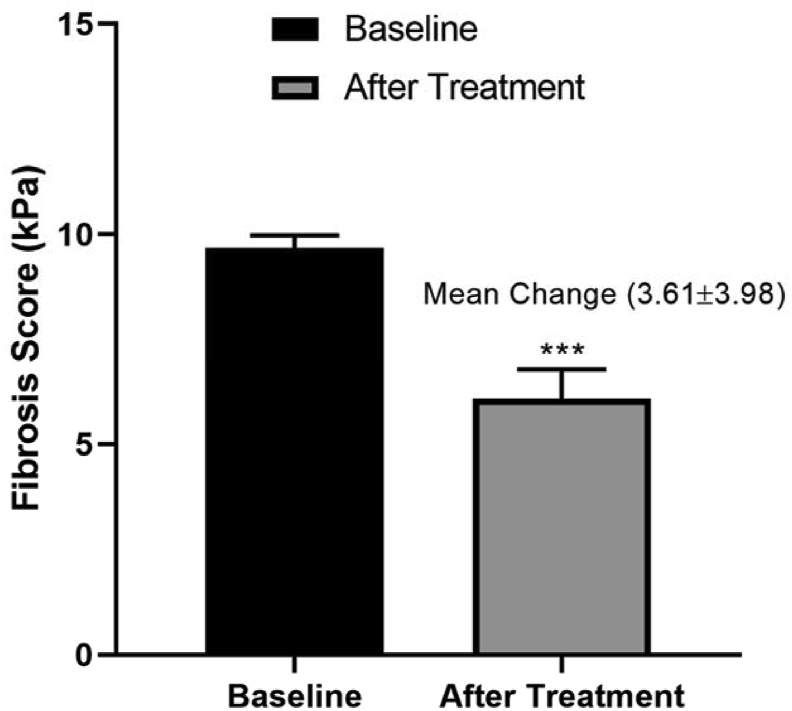
The mean age of the patients was 56.81 ± 4.06 years. All the 85 patients received saroglitazar treatment and were followed up for a period of 12 weeks. Of 85 patients, 44 (51.76%) were male and 41 (48.24%) were female. In this study, 72 patients (84.7%) were on statin therapy, that is, rosuvastatin 10 mg daily or atorvastatin 10 mg daily. Saroglitazar 4 mg was administered in addition to on-going antidiabetic monotherapy in 31 patients (36.8%) and to on-going antidiabetic dual therapy in 54 patients (63.2%). At the baseline, the antidiabetic medication reported includes metformin in 82 (96.47%) of the patients, followed by sulphonylureas in 61 (71.76%), gliptins in 15 (17.65%), alpha glucosidase inhibitors in 9 (10.59%). Patient's baseline characteristics are shown in Table 1. All clinical indices were examined after 12 weeks of treatment; there was no significant difference in SBP, DBP, LDL, HDL, VLDL, STB, SDB, SGOT, alkaline phosphate, total protein, serum albumin, and serum globulin from the baseline but FBS (P = 0.0001), PPBS (P = 0.002), HbA1C (P = 0.002), TC (P = 0.009), TG (P = 0.0001), SGPT (P = 0.02), blood urea (P = 0.04) (Table 2), and LSM value (P = 0.001) showed significant difference when compared with the baseline values (Figure 2).
Table 1.
Baseline Demographic Characteristics.
| Variable | Baseline value |
|---|---|
| Age (years) | 56.81 ± 4.06 |
| BMI | 25.94 ± 2.20 |
| Systolic blood pressure (mmHg) | 128.57 ± 5.46 |
| Diastolic blood pressure (mmHg) | 81.00 ± 7.45 |
| Fasting blood glucose (mg/dl) | 254.81 ± 2.74 |
| Postprandial blood glucose (mg/dl) | 264.10 ± 3.65 |
| HbA1C (%) | 10.29 ± 0.64 |
| Total cholesterol (mg/dl) | 230.30 ± 6.20 |
| Triglycerides (mg/dl) | 359.89 ± 5.46 |
| LDL (mg/dl) | 134.71 ± 4.23 |
| HDL (mg/dl) | 49.20 ± 3.08 |
| VLDL (mg/dl) | 19.65 ± 3.36 |
| Serum total bilirubin (mg/dl) | 0.80 ± 0.23 |
| Serum direct bilirubin (mg/dl) | 0.66 ± 0.29 |
| SGOT (u/l) | 64.28 ± 3.35 |
| SGPT (u/l) | 49.62 ± 3.31 |
| Alkaline phosphate (IU/L) | 103.86 ± 7.44 |
| Total protein (g/dl) | 7.02 ± 0.50 |
| Serum albumin (g/dl) | 4.44 ± 0.54 |
| Serum globulin (mg/dl) | 2.81 ± 0.26 |
| Blood urea (mmol/L) | 4.95 ± 0.28 |
| Fibrosis level (%) | 9.68 ± 0.30 |
BMI, body mass index; HbA1C, glycated hemoglobin; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein; SGOT, serum glutamate oxaloacetic transaminase; SGPT, serum glutamate pyruvic transaminase.
Table 2.
Diagnostics Parameters: Change From Baseline After the Saroglitazar Treatment.
| Parameter | Baseline | Follow-up (90th Day) | Mean change (95% CI) | P value |
|---|---|---|---|---|
| SBP (mmHg) | 128.57 ± 5.46 | 129.47 ± 7.36 | −2.88 ± 1.16 | 0.44 |
| DBP (mmHg) | 81.00 ± 7.450 | 82.00 ± 8.94 | −3.79 ± 1.79 | 0.47 |
| FBS (mg/dl) | 254.81 ± 2.74 | 248.56 ± 12.46 | 3.27 ± 9.24 | 0.0001∗∗∗ |
| PPS (mg/dl) | 264.10 ± 3.65 | 258.40 ± 14.04 | 2.44 ± 8.96 | 0.002∗∗ |
| HbA1C (%) | 10.29 ± 0.64 | 9.851 ± 0.946 | 0.24 ± 0.62 | 0.002∗∗ |
| TC (mg/dl) | 230.30 ± 6.20 | 226.61 ± 9.76 | 1.82 ± 5.55 | 0.009∗∗ |
| TG (mg/dl) | 359.89 ± 5.46 | 103.04 ± 16.52 | 252.39 ± 261.29 | 0.0001∗∗∗ |
| LDL (mg/dl) | 134.71 ± 4.23 | 130.71 ± 16.25 | 0.01 ± 7.99 | 0.06 |
| HDL (mg/dl) | 49.20 ± 3.082 | 49.000 ± 5.883 | 3.61 ± 3.98 | 0.80 |
| VLDL (mg/dl) | 19.65 ± 3.36 | 19.543 ± 5.321 | −1.37 ± 1.59 | 0.88 |
| STB (mg/dl) | 0.80 ± 0.23 | 0.7514 ± 0.2558 | −0.02 ± 0.13 | 0.17 |
| SDB (mg/dl) | 0.66 ± 0.29 | 0.5871 ± 0.3563 | 0.004 ± 0.14 | 0.17 |
| SGOT (u/L) | 64.28 ± 3.35 | 62.668 ± 4.742 | 0.77 ± 2.45 | 0.07 |
| SGPT (u/L) | 49.62 ± 3.31 | 48.457 ± 4.373 | 0.03 ± 2.31 | 0.02∗ |
| Alkaline phosphate (IU/L) | 103.86 ± 7.44 | 102.96 ± 19.74 | −4.00 ± 5.80 | 0.72 |
| Total protein (g/dl) | 7.02 ± 0.50 | 6.899 ± 0.838 | −0.09 ± 0.33 | 0.30 |
| Serum albumin (g/dl) | 4.4471 ± 0.5418 | 4.276 ± 0.889 | −0.05 ± 0.39 | 0.17 |
| Serum globulin (mg/dl) | 2.8114 ± 0.2673 | 2.8914 ± 0.6074 | −0.22 ± 0.06 | 0.31 |
| Blood urea (mmol/L) | 4.9586 ± 0.2824 | 4.8457 ± 0.3574 | 0.02 ± 0.19 | 0.04∗ |
| Fibrosis level (%) | 9.6857 ± 0.3047 | 6.0871 ± 0.7065 | 3.61 ± 3.98 | 0.0001∗∗∗ |
Values are expressed in Mean ± Standard deviation. Level of significance P > 0.05∗, P > 0.01∗∗, P > 0.001∗∗∗. SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; HbA1C, glycated hemoglobin; TC, total cholesterol; TG, triglycerides; LDL, low density lipoprotein; HDL, high density lipoprotein; VLDL, very low density lipoprotein; STB, serum total bilirubin; SDB, serum direct bilirubin; SGOT, serum glutamate oxaloacetic transaminase; SGPT, serum glutamate pyruvic transaminase.
Figure 2.
Effect of saroglitazar on fibrosis score in NAFLD with diabetic dyslipidemia patients after 12 weeks of treatment.
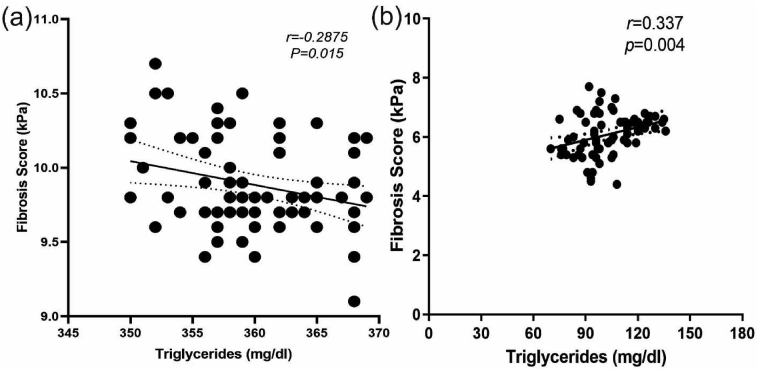
Liver fibrosis score decreased with the reduction of TG levels after the 12 weeks treatment of saroglitazar. Therefore, Pearson's correlation analysis revealed a positive relationship between LSM value and serum TG (P = 0.004, r = 0.337) (Figure 3). Comparison of severity of hepatic steatosis by ultrasonography before and after the intervention showed a significant difference in grading of fatty liver by the same radiologist blinded for previous values (P = 0.0413) (Table 3).
Figure 3.
Relationship between serum TG and fibrosis score before (a) and after the 12 weeks treatment of saroglitazar (b).
Table 3.
Liver Steatosis Grade Using Liver Ultrasound.
| Grade | Before treatment | After treatment | P value |
|---|---|---|---|
| Grade 0 n (%) | 0 (0) | 0 (0) | 0.0413∗ |
| Grade 1 n (%) | 19 (22.86) | 32 (45.71) | |
| Grade 2 n (%) | 46 (54.28) | 44 (54.29) | |
| Grade 3 n (%) | 20 (22.86) | 0 (0) |
Values are expressed in Mean ± Standard deviation. P > 0.05∗ significant.
Discussion
As per the International Diabetes Federation Report in 2011, prevalence of diabetes was estimated to be 371 million globally. By 2030, this prevalence may rise to 552 million. Diabetic populations are at an increased risk of developing NAFLD.16 The pathogenesis of NAFLD involves the pathophysiological changes in insulin production attributed to hyperinsulinemia which paves way for increased free fatty acid production. Lack of standard treatment for NAFLD in diabetic dyslipidemia patients necessitates a novel drug discovery.17 Saroglitazar, a novel dual PPAR agonist, was approved in 2013 by the Drug Controller General of India which has become the first drug to be given approval for the indication of NASH.22 Saroglitazar, one and only dual PPAR alpha/gamma agonist, has shown significant efficacy in improving both lipid as well as glycemic parameters with a good safety profile. A 24-week study conducted in Indian population was presented at the 25th Annual Scientific and Clinical Congress of the American Association of Clinical Endocrinologists which highlighted on saroglitazar that the drug could be a potential treatment option for the treatment of NAFLD associated with metabolic syndrome. In the study, a study population comprising 221 T2DM patients with dyslipidemia and NAFLD were administered with saroglitazar to assess its effect and safety in the management of NAFLD. The study results revealed reductions in triglycerides levels from 321 mg/do at the baseline to 129 mg/dl (P < 0.001) at week 24 and in HbA1C levels from 8.9% to 8.1% (P < 0.001). As per the results, 86 of 221 have shown improvement in fatty liver and 68 patients had normalization of liver enzymes.11
Saroglitazar showed impressive results in various clinical trials. In PRESS VI study, saroglitazar 4 mg tablets decreased the mean plasma triglyceride levels by −46.7 ± 3.02% (mean ± SE) causing a mean reduction of 139.5 ± 8.29 mg/dL in TG level from the baseline to the end of treatment. The reduction in TG level was statistically significant (P < 0.001) when compared with placebo and baseline. In the study, saroglitazar treatment was associated with a mean HbA1c reduction of 0.3%. Saroglitazar was found to be safe and well tolerated by the patients.20 This was a single center, prospective, open-label, interventional, single-arm, pilot study conducted to evaluate the safety and efficacy of saroglitazar in the treatment of NAFLD with diabetic dyslipidemia. Saroglitazar 4 mg was administered to 85 patients for a period of 12 weeks. In our study, significant improvement (P = 0.0001; N = 85) in the fibrosis score, as measured by FibroScan, was observed in all the recruited patients (grade-wise improvement) when compared with the baseline. This improvement can be attributed to the significant reduction in triglycerides (P = 0.0001), TC (P = 0.009), SGPT (P = 0.02), and glycemic parameters that is HbA1c (P = 0.002), FBS (P = 0.0001), and PPBS (P = 0.002). Several studies revealed that glucose control (HbA1C) may have a positive effect on the liver stiffness and may also act as an important tool for the management and prevention of liver conditions.23 Because dyslipidemia and hyperglycemia play a vital role in the pathogenesis of NAFLD, the reductions in lipid and glycemic parameters might have resulted in the improvement in NAFLD. The improvement in liver condition was reflected as the reduction in the liver fibrosis score and significant improvement (P = 0.0413) in the severity of hepatic steatosis was observed when compared with the baseline. At the end of treatment, no noticeable ADR were reported in any of the recruited patients.
In another trial, in comparison with pioglitazone, the efficacy analysis was performed after 24 weeks of follow-up (n = 39 in saroglitazar 4 mg; n = 33 in pioglitazone). Saroglitazar 4 mg significantly decreased (P < 0.001) plasma TG level from the baseline by 45% (−115.4 ± 68.11 mg/dL: absolute change ± SD), whereas the pioglitazone-treated group observed a reduction of −15.5% (−33.3 ± 162.41 mg/dL: absolute change ± SD) from the baseline at week 24. Saroglitazar 4 mg treatment also demonstrated marked decrease in lipid parameters such as VLDL (45.5%), LDL- 5%, total cholesterol (7.7%), and apolipoprotein-B (10.9%). Saroglitazar treatment was well tolerated and generally safe. Study findings revealed that no serious adverse events had occurred in the saroglitazar treatment arm and no persistent change in laboratory parameters in the patients were recorded. The study concluded that saroglitazar might be an effective and safe treatment option for improving hypertriglyceridemia in patients with T2DM.21 A recent trial, EVIDENCE-IV, which had studied the effect of different doses of saroglitazar on NAFLD, also has evidence resembling to that of our study, corroborating its potential in improving serum ALT, hepatic steatosis, and dyslipidemia.18
Limitations
No assessment was carried out to ensure the medication adherence and concomitant use of other therapeutic agents in patients receiving saroglitazar therapy. This study was conducted for a short period of 12 weeks with a small sample size. In this study, LSM was measured by the FibroScan which is inferior in the standard when compared with that of MRI scan. No biopsy was performed during this study for assessing the fibrosis score. Furthermore, no blinding was done to patients, investigators, and outcome assessors during the study. Baseline adjustment for the age should have been performed as the study population had a wide range in age which may affect LSM. The reductions in LSM should have been categorized and assessed as per BMI classification. The other limitations include absence of comparator group and being a single-centered study. Therefore, future studies with large sample size should be conducted for a longer duration among Indian Population to establish its potential use in the treatment of NAFLD in patients with diabetic dyslipidemia.
Saroglitazar was found to show significant improvement in the fatty liver condition which was evaluated by FibroScan and USG abdomen with significant reduction in the level of serum triglycerides. It has shown significant reduction in the liver fibrosis score. During the course of treatment, no adverse drug reaction was reported among the recruited patients.
CREDIT AUTHORSHIP CONTRIBUTION STATEMENT
Rajesh NA: conceived of the presented idea and Supervision, Drishya L: Methodology, Investigation and Writing – original draft, Ambati Murali Mohan Raju: Methodology, Investigation and Writing – original draft, Athi Narayanan L: Methodology, Investigation and Writing – original draft, Maria Alex: Methodology, Investigation and Writing – original draft, Kiran Kumar R: Data curation and Validation, Justin Jacob Abraham: Data curation and Validation, Vijayakumar TM: Writing- Reviewing and Editing. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Conflicts of interest
The authors have none to declare
Acknowledgments
The authors would like to thank Dean, SRM College of Pharmacy and Dean, SRM Medical College Hospital & Research Centre for their kind support and guidance.
Funding
This project was partially funded by SRMIST, Kattankulathur.
References
- 1.Misra A., Vikram N.K. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition. 2004;20:482–491. doi: 10.1016/j.nut.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Wasir J.S., Misra A. The metabolic syndrome in Asian-Indians: impact of nutritional and socio-economic transition in India. Metab Syndr Relat Disord. 2004;2:14–23. doi: 10.1089/met.2004.2.14. [DOI] [PubMed] [Google Scholar]
- 3.Misra A., Misra R. Asian Indians and insulin resistance syndrome: global perspective. Metab Syndr Relat Disord. 2003;1:277–283. doi: 10.1089/1540419031361390. [DOI] [PubMed] [Google Scholar]
- 4.Taheri H., Malek M., Ismail-Beigi F., et al. Effect of Empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Adv Ther. 2020;37:4697–4708. doi: 10.1007/s12325-020-01498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bril F., Kalavalapalli S., Clark V.C., et al. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs without type 2 diabetes. Clin Gastroenterol Hepatol. 2018 Apr;16:558–566. doi: 10.1016/j.cgh.2017.12.001. e2. Epub 2017 Dec 7. PMID: 29223443. [DOI] [PubMed] [Google Scholar]
- 6.Qiang S., Nakatsu Y., Seno Y., et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndrome. 2015;7:104. doi: 10.1186/s13098-015-0102-8. Published 2015 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xing B., Zhao Y., Dong B., Zhou Y., Lv W., Zhao W. Effects of sodium-glucose cotransporter 2 inhibitors on non-alcoholic fatty liver disease in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Investig. 2020 Sep;11:1238–1247. doi: 10.1111/jdi.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosale A., Saboo B., Sosale B. Saroglitazar for the treatment of hypertrig-lyceridemia in patients with type 2 diabetes: current evidence. Diabetes Metab Syndr Obes. 2015;8:189–196. doi: 10.2147/DMSO.S49592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul U., Parmar D., Manjunath K., et al. New dual peroxisome proliferator activated receptor agonist—saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence. Cardiovasc Diabetol. 2019;18:80. doi: 10.1186/s12933-019-0884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saboo B., Prajapati A., Joshi S., et al. The American Diabetes Association 75th Scientific Sessions Abstracts. 2015. To assess the effect of 4 mg Saroglitazar on patients of diabetes dyslipidemia with nonalcoholic fatty liver disease for 24 weeks at Diabetes Care Centre. [Google Scholar]
- 11.Joshi S., Ruby S., Saboo B., Chawla R., Bhandari S. The American Association of Clinical Endocrinologists Annual Meeting Abstracts. Late Breaking Abstracts. 2016. Saroglitazar in non-alcoholic fatty liver disease; p. 331. [Google Scholar]
- 12.Goyal O., Goyal P., Chinna R.S. Effect of saroglitazar on non-alcoholic fatty liver disease in patients with diabetic dyslipidemia: a prospective observational study. The 28th annual conference of asian pacific association for the study of the liver conference abstracts. Hepatol Int. 2019;13(suppl 1):S1–S266. Abstract#373–S204.) [Google Scholar]
- 13.Leite N.C., Salles G.F., Araujo A.L., Villela-Nogueira C.A., Cardoso C.R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. 26) [DOI] [PubMed] [Google Scholar]
- 14.Prashanth M., Ganesh H.K., Vima M.V., et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Phys India. 2009;57:205–210. 27. [PubMed] [Google Scholar]
- 15.Fan N., Zhang L., Xia Z., Peng L., Wang Y., Peng Y. Sex-specific association between serum uric acid and nonalcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Res. 2016;2016:3805372. doi: 10.1155/2016/3805372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia M.-F., Bian H., Gao X. NAFLD and diabetes: two sides of the same coin? Rationale for gene-based personalized NAFLD treatment. Front Pharmacol. 2019;10:877. doi: 10.3389/fphar.2019.00877. 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dharmalingam M., Yamasandhi P.G. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocrinol Metab. 2018;22:421–428. doi: 10.4103/ijem.IJEM_585_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedict M., Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. 2017;9:715–732. doi: 10.4254/wjh.v9.i16.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaton M.D. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Can J Gastroenterol. 2012;26:353–357. doi: 10.1155/2012/725468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jani R.H., Pai V., Jha P., et al. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI) Diabetes Technol Therapeut. 2014;16:63–71. doi: 10.1089/dia.2013.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai V., Paneerselvam A., Mukhopadhyay S., et al. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of saroglitazar 2 and 4 mg compared to pioglitazone 45 mg in diabetic dyslipidemia (PRESS V) J Diabetes Sci Technol. 2014;8:132–141. doi: 10.1177/1932296813518680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.“The World's First NASH Drug Approved in India: Zydus Cadila's Saroglitazar.” Trial Site News. 6 Mar. 2020. www.trialsitenews.com/the-worlds-first-nash-drug-approved-in-india-zydus-cadilas-saroglitazar/ [Google Scholar]
- 23.Watt Gordon P., Lee Miryoung, Pan Jen-Jung, Fallon Michael, Mccormick Joseph B., Fisher-Hoch Susan P. Increased hemoglobin A1c correlates with increased liver stiffness in a Mexican-American population. Diabetes Jul. 2018;67(suppl 1):2391. doi: 10.2337/db18-2391-PUB. [DOI] [Google Scholar]