Abstract
Background
Haematopoietic stem cell (HSC) infusion has demonstrated short-term improvement in liver functions in patients with chronic liver disease. The combination of HSC with mesenchymal stem cells (MSCs), which has an immunomodulatory effect, may augment the effects and enhance the duration of improvements on liver functions. The aim of the present study was to assess the safety of infusing the combination of autologous HSCs and MSCs in decompensated liver cirrhosis.
Methods
In phase I of the study, in vitro assessment was performed to observe the effect of coculturing MSCs with HSCs on their viability and cytokine profiles. Phase II of the study was to assess the safety of combination of stem cell infusions. Bone marrow (50 ml) was aspirated for MSC isolation and expansion using standard protocol. Patients received subcutaneous doses (n = 5) of granulocyte colony-stimulating factor (G-CSF) for stem cell mobilization followed by leukapheresis for harvesting HSCs using CliniMacs. HSCs and MSCs were infused through the hepatic artery under fluoroscopic guidance and were monitored for any adverse effects.
Results
In vitro studies revealed 94% viable HSCs in coculture similar to monoculture. HSCs released only interleukin (IL)-8, whereas MSCs secreted IL-8 and IL-6 in monocultures, and both IL-8 and IL-6 were secreted in coculture. G-CSF administration– and bone marrow aspiration–related complications were not observed. Infusion of the cells through the hepatic artery was safe, and no postprocedural complications were noted.
Conclusion
The combination of autologous HSC and MSC infusion is a safe procedure in patients with decompensated liver cirrhosis, and the outcomes needed to be assessed in larger studies.
Trial number
Keywords: stem cells, cirrhosis of liver, mesenchymal stem cells, combination of stem cells
Abbreviations: 7-AAD, 7-aminoactinomycin D; HSC, haematopoietic stem cell; MSC, mesenchymal stem cell; G-CSF, granulocyte colony-stimulating factor; CLD, chronic liver disease; MELD, Model for End-Stage Liver Disease; CBA, cytokine cytometric bead assay; IL, interleukin; MNC, mononuclear cell; TJLB, transjugular liver biopsy; SOP, standard operating procedure; DMEM-KO, Dulbecco's modified Eagle's Knock out medium; FBS, foetal bovine serum; cath-lab, cardiac catheterization laboratory; USG, ultrasonography; AFP, alpha-fetoprotein
Chronic liver disease (CLD), including hepatocellular carcinoma, accounts for 3.5% of total deaths worldwide.1 Currently, liver transplantation is the only curative treatment for these patients; it is possible in a small percentage of patients owing to lack of donor organs and financial implications. Every year, approximately 10% mortality occurs in patients awaiting liver transplantation.2 Therefore, alternative strategies, including cellular therapies, are under intense investigation in the treatment of CLD. Numerous studies such as randomized control trials using cell-based therapies have demonstrated improvement in the Model for End-Stage Liver Disease (MELD) score, ascites, renal function and quality of life in these patients.3, 4, 5, 6, 7 Recently, Moroni et al8 reported a safety profile of autologous macrophage therapy for liver cirrhosis.
Initial studies focused on bone marrow–derived haematopoietic stem cells (HSCs) for their regenerative capabilities, although it is still not clear whether they differentiate into hepatocytes or have a paracrine effect.9 Our experience with mobilized peripheral blood HSCs in patients with decompensated liver cirrhosis demonstrated beneficial effects as a bridge to liver transplantation.5 In recent years, the emphasis has shifted to mesenchymal stem cells (MSCs) for their advantages over other cell types. Both autologous and allogenic MSCs have been shown to improve liver functions without any adverse effect in the treatment of liver cirrhosis.10,11 A few randomized clinical trials with MSC infusions have reported improvement in the MELD score, quality of life and regression of fibrosis markers in animal models.6,11, 12, 13 These studies were neither able to track the location of the cells nor able to explain the effect on fibrosis. However, the multifaceted MSCs have immunomodulatory, anti-inflammatory and antifibrotic properties, making them superior to other cell types in the treatment of CLD. MSCs have also been shown to reduce the fibrogenic cytokine activity and promote hepatocellular proliferation through matrix metalloproteinase–induced collagen degradation and by reduction of hepatocyte apoptosis.14
With the aforementioned background, we hypothesized that regenerative capabilities of HSCs would be enhanced, if the microenvironment inside the liver is made suitable for regeneration by coinfusion with MSCs, which have a greater immunomodulatory effect.
In this proof-of-concept safety study, patients with decompensated cirrhosis of liver were infused a combination of autologous MSCs and HSCs and were monitored for safety assessment.
Study design
This is a prospective, single-centre, proof-of-concept safety study designed in two phases. Phase 1 involved in vitro studies to assess the effect of MSCs on HSC viability in a coculture system. Phase 2 involved infusion of MSCs and HSCs into the liver through the hepatic artery, followed by safety assessments in patients with CLD. The study was approved by the Institutional Committee for Stem Cell Research, Institutional Review Board and the Institutional Ethics Committee. Informed consent was obtained from all the patients. The study was registered at clinicaltrials.gov (NCT04243681).
Phase 1
The first phase included in vitro studies as per the following steps:
-
(i)
Isolation of clinical grade human bone marrow–derived MSCs and mobilized peripheral blood HSCs from three patients with cryptogenic cirrhosis of liver: The cells were compared with similar isolates from three patients without liver cirrhosis, who underwent bone marrow aspiration as part of evaluation for anaemia.
-
(ii)
Coculturing of MSCs with HSCs under in vitro conditions to assess the viability of HSCs and the inflammatory nature of MSCs in combination with HSCs: The cell count and viability were assessed at 72 h using the trypan blue exclusion method and by flow cytometry using 7-aminoactinomycin D (7-AAD). Inflammatory nature of MSCs was measured by estimating cytokine profiles using the human inflammatory cytokine cytometric bead assay kit (BD, BD FCAP array software) on a flow cytometer.
Phase II
The second phase of the study involved infusion of autologous MSCs and HSCs through the hepatic artery in patients with decompensated liver cirrhosis. An informed written consent was obtained from all the patients who agreed to be part of the study. All patients with decompensated liver cirrhosis were screened, and patients with diagnosis of cryptogenic cirrhosis of liver, aged between 20 and 70 years, with a Child-Pugh score of B or a MELD score higher than 10 but lower than 20 were included in the study. These patients were not willing for liver transplantation either owing to lack of donor tissue or owing to financial issues. A platelet count of higher than eighty thousand and an International Normalised Ratio (INR) of less than 1.6 along with a minimum life expectancy of more than 3 months as per the MELD score was required for inclusion in the study. Patients with history of liver tumours or any other cancer within the last 3 years, pregnant and lactating women and those with evidence of ongoing sepsis as per the surviving sepsis guideline were excluded. Further presence of hepatorenal syndrome and acute kidney injury not correctable with albumin or a combination of albumin and terlipressin infusion made patients ineligible for the study. In addition, patients with recent history of gastrointestinal bleeding or spontaneous bacterial peritonitis within the last 1 month and those with severe cardiorespiratory disease were excluded.
All patients underwent standard baseline investigations that included complete blood counts; peripheral CD34+ level analysis; liver function tests; renal function tests; viral screening for hepatitis B, hepatitis C and retroviral infection; analysis of fasting and postprandial blood sugar levels; analysis of serum alpha-fetoprotein (AFP) levels and Doppler ultrasonography (USG) of the abdomen or contrast-enhanced computed topography of the abdomen. Upper gastrointestinal endoscopy was performed to rule out varices. Any high-risk oesophageal varices found during endoscopy were banded before the stem cell procedure, and secondary beta-blocker prophylaxis was given as per standard practice.
Patients were admitted to the hospital for two times. The first admission was for transjugular liver biopsy (TJLB) and bone marrow aspiration for harvesting of MSCs, whereas the second admission was for HSCmobilization and infusion of the combination of MSCs and HSCs through the hepatic artery.
First hospital admission of patients in Phase II study
All patients underwent TJLB, and the hepatic venous pressure gradient was obtained by measuring wedge pressure and the free hepatic venous pressure measurement. The biopsy specimen was stained for both HSCs and MSCs using immunofluorescence staining procedures and enumerated as the percentage of cells per high-power field. On the subsequent day, under standard aseptic and antiseptic care, 40–50 ml of bone marrow was aspirated by the haematologist, and any postprocedural pain or complication was recorded. The bone marrow was immediately transported to the Good Medical Practice stem cell laboratory for processing as per the standard operating procedure for MSC isolation. The patient was discharged the next day from the hospital and advised readmission before stem cell infusion.
MSC isolation and expansion
MSCs were isolated using standard protocols. Mononuclear cell (MNC) fraction from the bone marrow was separated by density gradient centrifugation with HiSep™ and aspirated. MNCs were then suspended in Dulbecco's phosphate-buffered saline (DPBS) and washed twice and were cultured in Dulbecco's modified Eagle's Knock out medium supplemented with 10% foetal bovine serum and 2 mM glutamax in a humidified incubator at 37 °C and 5% CO2 in air. The cells were passaged 3–5 times until the required numbers of cells was obtained for transplantation after they reached 80% confluency.
Characterization and quality assessment of isolated and expanded MSCs
The isolated and expanded MSCs were characterized by phase-contrast microscopy (for spindle-shaped morphology), flow cytometry for MSC-specific surface markers (CD90, CD73 and CD105) and trilineage differentiation potential (osteocytes, adipocytes and chondrocytes). Quality of MSCs for transplantation was assured by sterility testing (microbial cultures), endotoxin measurement (using the commercially available endotoxin kit), mycoplasma testing (via real-time polymerase chain reaction), chromosomal stability (by karyotyping) and cell viability (by 7-AAD staining).
Preparation of MSCs for transplantation
On the day of transplantation, MSCs were harvested by trypsinization, and the viable cells were enumerated by trypan blue staining. Adequate cells were taken for transplantation (1 × 106 cells per kg of body weight) and washed twice with DPBS and finally once with normal saline. The cells were resuspended in 10 ml of normal saline, and 5 ml each was dispensed in two sterile vials. The tubes were sealed and transported to the cardiac catheterization laboratory (cath-lab) of the hospital along with the product release, characterization and quality testing reports.
Second hospital admission of patients in Phase II study
The patients were readmitted in the hospital for stem cell infusion. Daily subcutaneous injections of granulocyte colony-stimulating factor (G-CSF) (Neupogen; Roche Pharmaceutical) at a dose of 5 μg/kg of dry body weight of the patient were given for 5 days. This was given in two divided doses to enhance the number of circulating HSCs by mobilizing them from the bone marrow. CD34-positive stem cells, which is a marker of circulating HSCs, were enumerated before giving the first dose of G-CSF and subsequently on day 3 and day 5. Any injection-related side-effects were noted. On the fourth day of G-CSF injection, a 16-cm-length 12 French large bore multilumen Blue flex tip catheter (©Arrow International, USA) central line was inserted using local infiltration of 2% lignocaine. On the fifth day of receiving G-CSF, leukapheresis was initiated in the morning, and 100–200 ml of apheresis product was acquired as per the targeted cell numbers. Leukapheresis was started at a minimum flow rate higher than 60 ml/h. The vital signs of the patient were monitored continuously during the procedure. Any procedure-related complications were noted and handled as per standard practice protocols. After leukapheresis, the apheresis product was collected and shifted to the stem cell laboratory of the hospital for HSC isolation.
CD34+ HSC isolation
MNCs were isolated from the apheresis product using Hisep, and the CD34+ HSCs were isolated using CliniMACS using antihuman CD34 antibodies conjugated to superparamagnetic iron-dextran particles (Miltenyi Biotec, Germany). The cell pellet obtained after isolation was diluted in normal saline in 10-ml aliquots under sterile conditions maintaining room temperature under aseptic care.
Infusion of MSCs and HSCs through the hepatic artery
The patients were shifted to the cath-lab for stem cell infusion. Under fluoroscopic guidance, access to the aorta was made using the Seldinger technique after femoral artery access. The celiac axis and hepatic artery proper were catheterized using Simmon 1 catheter. The 5 French catheters were exchanged over a guide wire, and a 4 F slip catheter was placed beyond the origin of the gastroduodenal artery into either the right hepatic artery or left hepatic artery. Catheter position was confirmed with infusion of nonionic low osmolar contrast (visipaque) under fluroscopy. The MSCs, one million cells per kg of body weight (50–80 million cells) in 10 ml of normal saline, were infused slowly through the hepatic artery under fluoroscopic guidance. The vascular access was then sealed at the end of the procedure with use of a heparin plug to be used later for autologous CD34+ cell infusion. After 30 min, CD34+ stem cells were selectively infused as aliquots of 10 ml slowly over 1 min under fluoroscopic guidance. After the infusion, the catheter was flushed with 10 ml of normal saline slowly over 1 min. At the end of the infusion, the patency of the artery was confirmed by contrast injection. Adequate compression was applied at the puncture site, and the area was strapped with an adhesive plaster.
After the procedure, the patient was shifted to the high-dependency unit for overnight observation. During and after the procedure, all vital signs were monitored every hour. After the procedure, the puncture site was examined hourly to detect any haematoma or any procedural and postprocedural adverse effect including pain, hypotension and so on. If the pain score on the visual analogue scale was higher than 4, appropriate analgesics, preferably 50 mg of tramadol hydrochloride intramuscularly, were administered. On the subsequent day, colour Doppler USG of the abdomen was performed to observe any infusion-related complications such as hepatic artery thrombosis. If no complications were noted, the patient was discharged.
Follow-up of patients
The study patients were followed up every week for the first one month (with a compulsory physical visit at 4 weeks) and then every month for the next 3 months. At every monthly visit, the clinical findings and progress were noted in the pro forma. During each visit, physical examination included documentation of ascites, hepatic encephalopathy and amount of ascites based on USG of the abdomen. Their MELD score and Child-Pugh score were evaluated for improvement or deterioration. All complications of cirrhosis such as gastrointestinal bleeding, ascites, hepatic encephalopathy and so on, which may occur during the course of follow-up were managed as per standard of care following the updated American Association of Study of Liver Diseases guidelines.
Safety assessment
Any procedure-related adverse event was noted. Adverse event was defined as any untoward medical occurrence in a patient or clinical trial participant administered with a medicinal product and that does not necessarily have a causal relationship with this treatment. Any adverse event/adverse reaction that resulted in death or was life-threatening, required hospitalization or prolongation of existing hospitalization, or any event that resulted in persistent or significant disability or incapacity or resulted in a congenital anomaly or birth defect was considered as a serious adverse event. Serious side effects also included development of any of the following complications during the follow-up: acute renal failure, worsening hepatic decompensation, progressive elevation in serum AFP levels or development of liver mass on follow-up computed tomography scans.
The patients were closely monitored during leukapheresis and stem cell infusions for any procedure-related complications such as haemodynamic instability and dyselectrolytemia. After infusions, the patients were observed in the high-dependency unit. The patient's safety was evaluated at each visit, and clinical, laboratory and safety-related data were prospectively collected.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Phase I result
MSCs from patients with CLD are similar to MSCs from controls
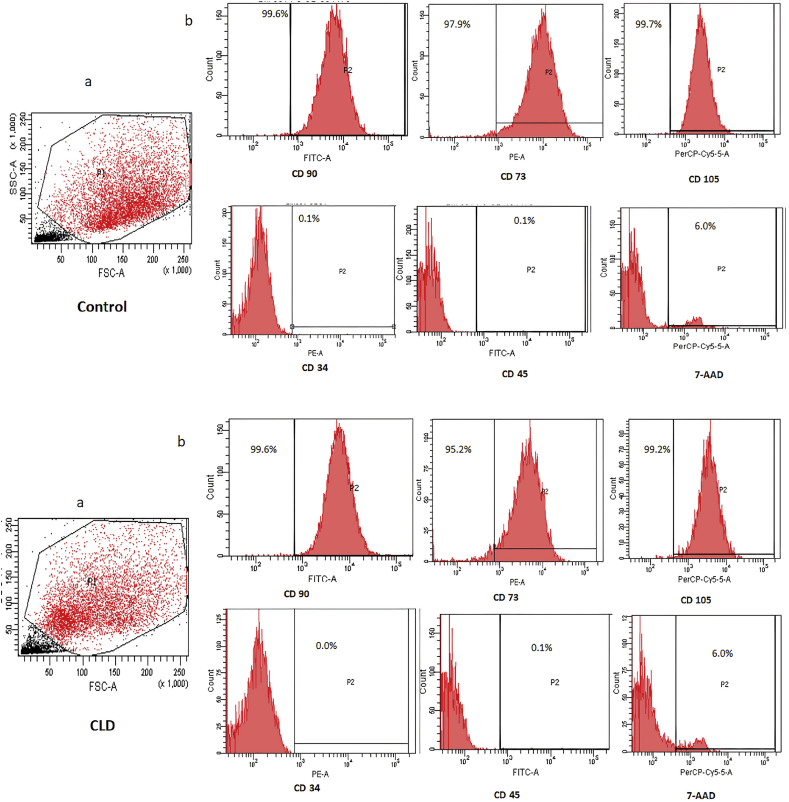
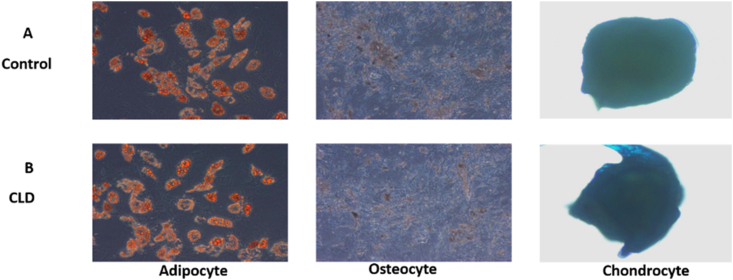
MSCs isolated from the bone marrow of patients with CLD were similar to the cells isolated from controls in terms of morphology, viability and trilineage differentiation. MSC yield was higher in patients with CLD than in controls (CLD: 0.2–0.3 million cells/ml of bone marrow; control: 0.07 million cells/ml of bone marrow). MSCs from controls and patients with CLD showed spindle-shaped fibroblast–like morphology and were positive for MSC markers CD90, CD73 and CD105 (95–99%) and negative for CD34 and CD45 on flow cytometry (Figure 1a, Figure 1b). The MSCs differentiated into osteoblasts, adipocytes and chondrocytes in both controls and patients with CLD (Figure 2). The cells showed more than 95% viability at all stages of culture by trypan blue and 7-AAD staining (94%). The cultures were negative for bacterial or fungal contamination as assessed by endotoxin and mycoplasma testing.
Figure 1.
Representative flow cytometry pictures of the healthy control (upper panel) and patient with CLD (lower panel). Panel a shows gating of MSCs at passage 3. Panel b shows MSCs positive for phenotypic marker staining for CD90, CD73 and CD105 and negative for CD34, CD45 and viability marker 7-AAD. 7-AAD, 7-aminoactinomycin D; MSC, mesenchymal stem cell; CLD, chronic liver disease.
Figure 2.
Trilineage differentiation of BM-derived MSCs in healthy controls and patients with CLD. BM-derived MSCs characterized for differentiation into adipocytes (formation of lipid droplets stained with Oil Red), osteocytes (demonstrated by calcium deposition stained with Alizarin Red) and chondrocytes (demonstrated by the deposition of extracellular matrix stained with toluidine blue) from healthy controls (A) and (B) patients with CLD. CLD, chronic liver disease; BM, bone marrow; MSC, mesenchymal stem cell.
MSC coculture does not alter cell count and viability of CD34+ cells
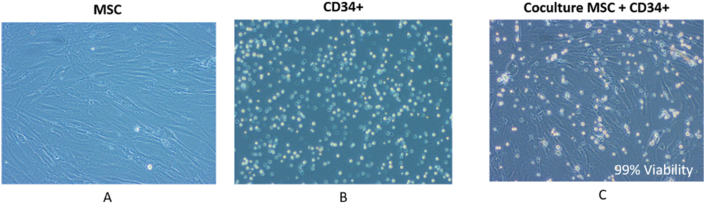
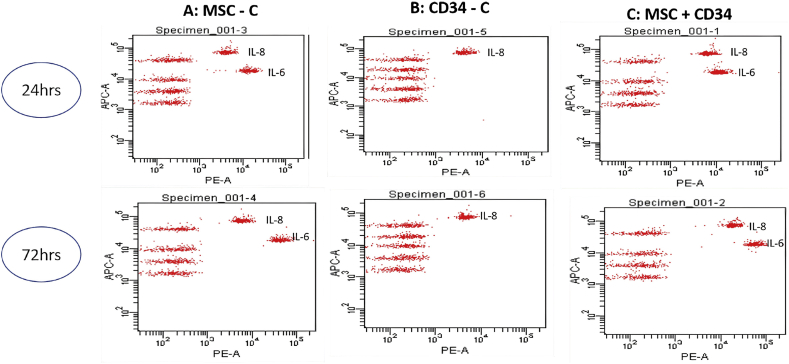
HSCviability and cell count in the coculture system were comparable with haematopoietic cell count and viability in monocultures of HSCs. The CD34+ cell count was higher in feeder coculture system, whereas CD34+ cell count and viability were significantly higher in the well insert coculture system (Figure 3). CD34+ cells secreted only interleukin (IL)-8, and MSCs secreted IL-8 and IL-6 in monocultures. Coculture of MSCs with HSCs secreted both IL-8 and IL-6. No other proinflammatory cytokines were secreted in cocultures (Figure 4) (Table 1).
Figure 3.
Cocultures of MSCs and CD34+ stem cells. Monoculture of MSCs (A) and HSCs (B) and coculture of MSCs and HSCs (C) at 72 h demonstrate 99% viable HSCs in cocultures. HSC, haematopoietic stem cell; MSC, mesenchymal stem cell.
Figure 4.
Cytokine analysis of culture supernatants of BM-derived stem cells from patients with CLD. Cytokine analysis from the BM-derived MSC culture supernatant shows IL-8 and IL-6 at 24 h and 72 h. The CD34+ culture supernatant secretes only IL-8. The coculture supernatants secrete IL-8 and IL-6. IL, interleukin; CLD, chronic liver disease; MSC, mesenchymal stem cell; BM, bone marrow.
Table 1.
Cell Counts and Cell Viability as per the Feeder and Well Insert Method at 72 h During Coculture of HSCs and MSCs when Compared with MonoCulture.
| Type of cell culture | Feeder method |
Well insert method |
||
|---|---|---|---|---|
| Cell count | Viability | Cell count | Viability | |
| Coculture CD34+ | 68,750 | 99.05 | 24,500 | 94.75 |
| Control CD34+ | 53,000 | 97.3 | 11,000 | 88.2 |
| Coculture MSC | 7000 | 100 | 23,000 | 100 |
| Control MSC | 38,500 | 98.7 | 10,500 | 100 |
HSC, haematopoietic stem cell; CD: cluster of differentiation; MSC: mesenchymal stem cell.
Phase II study results: safety data
Five patients with cryptogenic decompensated cirrhosis were included in the study. The baseline characteristics of the patients are shown in Table 2. Bone marrow aspiration performed in the sterile environment maintaining aseptic and antiseptic care was found to be a safe procedure. It was carried out under conscious sedation with propofol injection given by the anaesthetist. The mean number of bone marrow pricks to acquire 50 ml of bone marrow aspirate was 2. The pain score was lower than 4 out of 10 on the visual analogue scale after recovery of sedation in all the patients. No bone marrow site haematoma or infection was documented. All the patients were discharged on the subsequent day of bone marrow aspiration.
Table 2.
Baseline Characteristics of the Patients Included in the study.
| Parameter | Value |
|---|---|
| Age (mean ± SD), in years | 55.2 ± 3.70 |
| Male:female | 3:2 |
| Total bilirubin (mean ± SD), in mg/dl | 2.7 ± 1.41 |
| Serum albumin (mg/dl) | 3.2 ± 0.60 |
| Serum creatinine (mg/dl) | 0.93 ± 0.14 |
| MELD score | 14.2 ± 3.24 |
| Child-Pugh score (median, IQR) | |
| A | 1 |
| B | 8.5 (1.5) |
| Ascites | 100% |
| Hepatic encephalopathy | 0% |
| Gastrointestinal bleeding | 2/5 (40%) |
| History of resolved hepatorenal syndrome | 1/5 (20%) |
| Hepatic venous pressure gradient (median, IQR) | 12 (4) |
IQR: interquartile range; SD: standard deviation; MELD: Model for End-Stage Liver Disease.
During the second admission, all five patients received subcutaneous G-CSF injections, which did not result in any adverse effect. Increase in the total leucocyte count was an expected result of the injection. There was no symptomatic splenomegaly or increase in gastrointestinal bleeding owing to G-CSF injection. The central line insertion carried out by an anaesthetist under sterile conditions did not result in any adverse effect, except mild pain, with a score of lower than 4 out of 10 on the visual analogue scale, at the time of insertion not requiring any analgesic.
The process of leukapheresis was conducted under the guidance of a transfusion medicine specialist with continuous patient monitoring for any adverse effect. Blood pressure, pulse rate and oxygen saturation were documented every 15 min during the procedure and then every 30 min after the procedure for 6 h. Only one patient developed hypotension during the procedure, which reverted back by infusion of normal saline at a rate of 100 ml/h for 2 h and by slowing the rate of leukapheresis. No other adverse effects such as electrolyte imbalance were noted during the procedure.
During the process of hepatic artery catheterization and infusion of stem cells into the hepatic artery, technical success was 100%. The procedures were uneventful, and we did not observe bleeding or haematoma formation after the procedure. Follow-up Doppler USG on the subsequent day did not reveal any hepatic artery thrombosis or decrease in hepatic blood flow velocities when compared with preprocedure values. All patients were discharged within 24 h of the procedure.
All patients receiving the combination of stem cell infusions completed the follow-up for 3 months. Interim analysis at the end of 3 months showed a trend towards improvement of the MELD score from 14.2 ± 3.24 to 13.0 ± 1.84 (p = 0.131) and improvement in serum albumin levels from 3.2 ± 0.60 to 3.4 ± 0.82 (p = 0.67), with no worsening of renal parameters or clinical events such as hepatic encephalopathy or gastrointestinal bleeding.
Discussion
The present study aimed to assess the safety of the procedure of infusion of MSCs followed by HSCs into the liver. MSCs form <0.1% of adult bone marrow cells and have greater immunomodulatory property than HSCs.15 MSCs have the capacity to migrate towards the site of inflammation and modulate the secretion of cytokines and chemokines in response to the injury.16,17 Liu et al18 detected migration of MSCs towards areas of fibrosis in the liver. In addition, it has been shown that MSCs have a strong potential to differentiate into varied cell lineages and can reverse fibrosis in alcoholic cirrhosis, thereby improving liver function in patients with autoimmune hepatitis.6,19 Hepatocyte-like cells have also been derived from bone marrow MSCs.20 MSCs have been safely used in patients with liver cirrhosis including liver-transplanted subjects.21 However, concerns have been raised about the regenerative potential of MSCs when compared with HSCs, coupled with a small theoretical risk of promoting fibrogenesis.22
The concept of combining HSCs and MSCs in patients with cirrhosis arises from the hypothesis that the HSCs could possibly work better if the microenvironment of the liver is improved by the immunomodulatory effect of MSCs. Combination stem cell therapy has been used in graft versus host disease,23 and trials are ongoing in patients with severe limb ischaemia.(NCT00721006). Recently published data using the combination of MSCs and endothelial progenitor cells for reversal of lung injury after HSCtransplantation show an improvement in inflammation with lung tissue repair.24 Our study is a proof-of-concept safety study of use of combination stem cell therapy in patients with decompensated liver cirrhosis with an expectation that combination therapy would yield better results than monotherapy with HSC or MSCs. Furthermore, MSCs have been recently shown to promote the HSC niche and thereby help in increasing circulating HSCs to reach the areas of inflammation and fibrosis.25
Because the study involves the use of autologous HSCs and MSCs, phase I of the study was performed in vitro to evaluate the proinflammatory nature of MSCs derived from patients with cirrhosis in comparison with controls. Our study revealed that the cytokine profiles of MSCs from patients with CLD are comparable with the cytokine profiles of controls. This supports the fact that MSCs neither have antigen-presenting capability or express the major histocompatibility complex to render them hostile to the host environment26 nor promote fibrosis.
Another concern about the use of combination of MSCs and HSCs was regarding the behaviour of the cells in coculture. Therefore, a bench work with coculture of HSCs and MSCs was conducted in phase I of the study. It was found that there was no impact on the cell count, viability and cytokines released when compared with monoculture cells. Jing et al27 have already shown that in coculture, the mesenchymal stromal cell surface appears to be the predominant HSC proliferation site. Furthermore, coculture of HSCs and MSCs has shown that the MSC supports the proliferation and self-renewal capacity of HSCs.28 Our study addressed the concern regarding the conflicts between the two cell types in coculture. From the clinical standpoint, the concept of infusing MSCs 30 min before infusion of HSCs may not be sufficient, and in future, simultaneous infusion of the cells may be tried as coculturing of cells did not reveal any adverse effect of one cell type over another.
From a clinical viewpoint, safety of HSCinfusion in patients with liver cirrhosis has been demonstrated in multiple studies.29, 30, 31 Similarly, numerous studies have shown the safety of MSCs when used in patients with liver cirrhosis.32, 33, 34 By way of contrast, a recent study with G-CSF and use of autologous CD133+ cells has shown increased frequency of adverse effect when compared with the standard of care.35 This study however had a baseline heterogeneous population, and the dose and viability of the transfused HSC were much lower than in other studies.36 The cell isolation and infusion procedure has been time tested to be safe, and the present study did not find any adverse effect. The concerns in some studies of worsening of fibrosis with use of MSCs also need to be observed, and the paired biopsy results at 6 months would give more insights if any of the patients demonstrate worsening of fibrosis.
Although an interim analysis at 3 months showed a trend towards the improvement in the MELD score, this study was not designed and powered to comment on outcome data. This improvement in the MELD score was impacted mostly by the improvement in serum creatinine levels, which was similar to the findings of our initial published results with HSC use alone. There was no worsening of hepatic encephalopathy or gastrointestinal bleeding. Larger studies with a comparative population would be required to assess the benefit of this pilot study. The six-month analysis of the improvement in MSCs and HSCs in liver tissue would give further insights into the homing in of cells and increase in their numbers. The paired liver biopsy data, which are to be completed after 6 months, would give us more insight into the success of homing in of both cell types. However, to demonstrate that infusion of one cell line is better than infusing a combination of cell lines would need a head-to-head trial.
However, there are multiple unmet needs in this field of research as there is significant uncertainty about the best timing of infusion of cells (wherein it is better before decompensation?), the best quantity of cells to be used, the role of multiple infusion to tide over any waning effect over time and finally the theoretical risk of malignancy.
This study has proven the safety of infusion of the combination of MSCs and HSCs in patients with CLD. Future research in this field would be an attempt to find out the stage of liver cirrhosis at which stem cells need to be infused to obtain the best results. It should not be delayed to such an extent that the MSC fails to exert its immunomodulatory effect and the HSC's effort to regenerate proves futile. Tracking of the cells inside the body and the correct dose and time difference to be kept between HSC and MSC infusion needs to be an area of active research.
Credit authorship contribution statement
M.S., S.M., G.J. and P.N.R. contributed to the study concept and design; A.S. analysed the liver biopsy specimens. R.G., N.J., M.S., P.K., F.S. and A.K. involved in data collection. P.K., S.M. and S.J. performed stem cell isolation. S.D. was involved as an anaesthetist. J.R.S. performed stem cell infusion. Compilation and initial drafting were carried out by M.S., A.K. and P.K. Figures were contributed by S.M. and M.S. Final editing and critical revision was carried out by A.K., R.G., G.V.R. and D.N.R. All members approved the final draft.
Conflicts of interest
The authors have none to declare.
Acknowledgements
None.
Financial support
The authors have no financial grants to declare. The study was supported by Asian Institute of Gastroenterology, Hyderabad, India.
References
- 1.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019 Jan;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Fink M.A., Berry S.R., Gow P.J., et al. Risk factors for liver transplantation waiting list mortality. J Gastroenterol Hepatol. 2007 Jan;22:119–124. doi: 10.1111/j.1440-1746.2006.04422.x. [DOI] [PubMed] [Google Scholar]
- 3.Lin B.L., Chen J.F., Qiu W.H., et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: a randomized controlled trial. Hepatology. 2017 Jul;66:209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto T., Takami T., Sakaida I. Cell transplantation as a non-invasive strategy for treating liver fibrosis. Expet Rev Gastroenterol Hepatol. 2016;10:639–648. doi: 10.1586/17474124.2016.1134313. [DOI] [PubMed] [Google Scholar]
- 5.Sharma M., Rao P.N., Sasikala M., et al. Autologous mobilized peripheral blood CD34(+) cell infusion in non-viral decompensated liver cirrhosis. World J Gastroenterol. 2015 Jun 21;21:7264–7271. doi: 10.3748/wjg.v21.i23.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suk K.T., Yoon J.H., Kim M.Y., et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: phase 2 trial. Hepatology. 2016 Dec;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 7.Xue R., Meng Q., Dong J., et al. Clinical performance of stem cell therapy in patients with acute-on-chronic liver failure: a systematic review and meta-analysis. J Transl Med. 2018 May;16:126. doi: 10.1186/s12967-018-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moroni F., Dwyer B.J., Graham C., et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat Med. 2019 Oct;25:1560–1565. doi: 10.1038/s41591-019-0599-8. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.Y., Hong S.H. Hematopoietic stem cells and their roles in tissue regeneration. Int J Stem Cells. 2020;13:1–12. doi: 10.15283/ijsc19127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharaziha P., Hellström P.M., Noorinayer B., et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009 Oct;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 11.Mohamadnejad M., Alimoghaddam K., Bagheri M., et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013 Nov;33:1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 12.Khalifa Y.H., Mourad G.M., Stephanos W.M., Omar S.A., Mehanna R.A. Bone marrow-derived mesenchymal stem cell potential regression of dysplasia associating experimental liver fibrosis in albino rats. BioMed Res Int. 2019 Nov 6;2019:5376165. doi: 10.1155/2019/5376165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L., Gong Y., Wang B., et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014 Aug;29:1620–1628. doi: 10.1111/jgh.12653. [DOI] [PubMed] [Google Scholar]
- 14.Higashiyama R., Inagaki Y., Hong Y.Y., et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007 Jan;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 15.Wu J.Y., Scadden D.T., Kronenberg H.M. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24:759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uccelli A., Pistoia V., Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Le Blanc K., Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Liu W.H., Song F.Q., Ren L.N., et al. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 2015 Mar;19:511–520. doi: 10.1111/jcmm.12482. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang J., Zhang H., Zhao C., et al. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int J Rheum Dis. 2017 Sep;20:1219–1226. doi: 10.1111/1756-185X.13015. In press. [DOI] [PubMed] [Google Scholar]
- 20.Haga H., Yan I.K., Takahashi K., Matsuda A., Patel T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cells Transl Med. 2017 Apr;6:1262–1272. doi: 10.1002/sctm.16-0226. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detry O., Vandermeulen M., Delbouille M.H., et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I-II, open-label, clinical study. J Hepatol. 2017 Jul;67:47–55. doi: 10.1016/j.jhep.2017.03.001. In press. [DOI] [PubMed] [Google Scholar]
- 22.di Bonzo L.V., Ferrero I., Cravanzola C., et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008 Feb;57:223–231. doi: 10.1136/gut.2006.111617. In press. [DOI] [PubMed] [Google Scholar]
- 23.Lim J.Y., Park M.J., Im K.I., et al. Combination cell therapy using mesenchymal stem cells and regulatory T-cells provides a synergistic immunomodulatory effect associated with reciprocal regulation of TH1/TH2 and th17/treg cells in a murine acute graft-versus-host disease model. Cell Transplant. 2014 Apr;23:703–714. doi: 10.3727/096368913X664577. [DOI] [PubMed] [Google Scholar]
- 24.Zhang T., Chen Y.T., Shi J.R., et al. Effects of combined infusion of mesenchymal stem cells and endothelial progenitor cells on lung injury after hematopoietic stem cell transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020 Jun;28:1019–1024. doi: 10.19746/j.cnki.issn.1009-2137.2020.03.050. [DOI] [PubMed] [Google Scholar]
- 25.Frenette P.S., Pinho S., Lucas D., Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014 Nov;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 27.Jing D., Fonseca A.V., Alakel N., et al. Hematopoietic stem cells in co-culture with mesenchymal stromal cells--modeling the niche compartments in vitro. Haematologica. 2010 Apr;95:542–550. doi: 10.3324/haematol.2009.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walenda T., Bork S., Horn P. Co-culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J Cell Mol Med. 2010 Jan;14:337–350. doi: 10.1111/j.1582-4934.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan A.A., Parveen N., Mahaboob V.S., et al. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure: a preliminary study. Transplant Proc. 2008 May;40:1140–1144. doi: 10.1016/j.transproceed.2008.03.111. [DOI] [PubMed] [Google Scholar]
- 30.Mohamadnejad M., Namiri M., Bagheri M., et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007 Jun;13:3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai M., Zacharoulis D., Milicevic M.N., et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008 Aug;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 32.Amin M.A., Sabry D., Rashed L.A., et al. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27:607–612. doi: 10.1111/ctr.12179. [DOI] [PubMed] [Google Scholar]
- 33.Mohamadnejad M., Alimoghaddam K., Mohyeddin-Bonab M., et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007 Oct;10:459–466. [PubMed] [Google Scholar]
- 34.Zhang Z., Lin H., Shi M., et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012 Mar;27(suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 35.Newsome P.N., Fox R., King A.L., et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018 Jan;3:25–36. doi: 10.1016/S2468-1253(17)30326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma N., Singh A., Singh V. Haematopoietic stem cells in cirrhosis. Lancet Gastroenterol Hepatol. 2018;3:298. doi: 10.1016/S2468-1253(18)30041-4. [DOI] [PubMed] [Google Scholar]