Abstract
Background
Portal cavernoma cholangiopathy (PCC) refers to abnormalities of the extra- and intrahepatic bile ducts in patients with portal cavernoma. The literature on PCC in children is very scarce. This study aimed at characterizing PCC in children with extrahepatic portal venous obstruction (EHPVO) using endoscopic ultrasound (EUS) and magnetic resonance cholangiography/portovenography (MRC/MRPV).
Methods
A total of 53 consecutive children diagnosed with EHPVO were prospectively evaluated for PCC using MRC/MRPV and EUS. Chandra classification was used for type of involvement and Llop classification for grading of severity.
Results
All 53 children (100%) had PCC changes on MRC/EUS, but none were symptomatic. Extrahepatic ducts (EHDs) and intrahepatic ducts were involved in majority (85%), and 58.5% had severe changes. Periductal thickening/irregularity (71%) was the commonest change in intrahepatic ducts, whereas irregular contour of the duct with scalloping (68%); common bile duct (CBD) angulation (62.3%) were the frequent changes in the EHDs. Increased CBD angulation predisposed to CBD strictures (P = 0.004). Both left and right branches of portal vein were replaced by collaterals in all children. Among the EUS biliary changes, para-pericholedochal, intrapancreatic, and intramural gall bladder collaterals had significant association with severity, with higher frequency of occurrence in children with the most severe Llop Grade.
Conclusions
PCC develops early in the disease course of EHPVO, in children, but is asymptomatic despite severe changes. EUS biliary changes are more likely to be observed with increasing severity of PCC.
Keywords: magnetic resonance cholangiography, portal vein, children, endoscopic ultrasonography
Abbreviations: CBD, Common bile duct; EHPVO, Extrahepatic portal venous obstruction; EUS, Endoscopic ultrasound; GB, Gall bladder; MRC/MRPV, Magnetic resonance cholangiography/portovenography; PCC, Portal cavernoma cholangiopathy; PHT, Portal hypertension
Extrahepatic portal venous obstruction (EHPVO) is the commonest cause of portal hypertension (PHT) in children in developing countries.1,2 Despite a long list of possible etiologic factors (umbilical sepsis, umbilical vein catheterization, dehydration, congenital abnormalities, surgery, intra-abdominal sepsis, prothrombotic states), the etiology remains elusive in the large majority of children.2 Apart from variceal bleeding, other manifestations of EHPVO include anemia, abdominal distension and postbleed ascites. Endoscopic eradication of varices is the standard treatment, although surgery is advocated in certain circumstances.1,2
Complications and long-standing sequelae include growth retardation, hypersplenism, occult blood loss due to gastropathy, ectopic varices, and portal cavernoma cholangiopathy (PCC). The latter refers to abnormalities of the extrahepatic and intrahepatic bile ducts due to compression by collaterals. Adults with EHPVO, harbor features of PCC in 80–100% of patients, but most of them are asymptomatic.3, 4, 5, 6 Severe PCC can sometimes cause symptoms due to cholelithiasis, choledocholithiasis, cholangitis, and even secondary biliary cirrhosis, in the long term.7 The possibility of such complications has led some to consider shunt surgery as a better option. Although endoscopic retrograde cholangiopancreatography (ERCP) has been used by a few investigators to explore the occurrence of PCC, the literature on PCC in children, remains very scarce.4,8,9 An evaluation for the presence of PCC in childhood will yield a better understanding of its natural history and may aid in determining the prognosis in a given patient. It would also allow clinicians to tailor the treatment, giving an option of surgical shunting, as the primary modality of treatment in severe cases, to avoid complications of PCC. Accordingly, this study was conceptualised, to evaluate PCC in children using magnetic resonance cholangiography/magnetic resonance portovenography (MRC/MRPV) and endoscopic ultrasound (EUS).
Methods
Study subjects
Consecutive children with diagnosed EHPVO, undergoing endoscopic therapy were prospectively screened for enrolment. EHPVO was diagnosed on the basis of splenomegaly and portal cavernoma on ultrasonography. Relevant history obtained from the parents and information from medical case records were recorded in a predesigned proforma, which included the demographic information like age, gender, and other details such as age at diagnosis, clinical presentation, anthropometry, details of bleeding episodes, clinical examination, and investigation details (hemogram, liver function tests, coagulogram, upper gastrointestinal endoscopy [UGIE] findings). All enrolled children underwent UGIE for conformation of eradication of esophageal varices. Children with other causes of PHT like cirrhosis and NCPF, those who have undergone shunt surgery, those with contraindication to MRI like history of allergy to MR contrast and those with deranged renal function tests were excluded. After enrolment, MRC/MRPV and EUS were done within a weeks’ time of one another. (The cost of the EUS/MRCP was highly subsidized as per public sector norms and moreover was not borne by the patients.) For sedation during the procedures, intravenous midazolam (0.1–0.2 mg/kg) and/or ketamine (1–2 mg/kg) was used. The study protocol was approved by Institutional Ethics Committee (IEC NK/4772/DM/188).
Study procedures
Magnetic resonance cholangiography/magnetic resonance portovenography
MR evaluation was performed on a 3 Tesla Philips Ingenia machine. The imaging protocol included MRC and intravenous contrast (0.2 ml/kg), enhanced MRPV sequences in the axial and coronal planes. The sequences acquired included T1WI and T2WI, BTFE (balanced turbo-field echo), thick and thin slab MRC, and postcontrast dynamic DIXON. The MR study was exported to the work station and evaluated for changes in both extrahepatic ducts (EHDs) and intrahepatic ducts (IHDs). IHDs were evaluated for any dilation, strictures/narrowing with or without upstream dilation and periductal thickening (T2 hyperintense, relative to liver parenchyma).10 The changes in EHDs were documented using the descriptions proposed earlier by the working party of the Indian National Association for the Study of Liver (INASL)11 that included extrinsic impressions (smooth impressions on the bile duct, with a nodular contour, more than one-quarter of ductal diameter); irregular ductal contour (fine-wavy, irregular contour of the bile duct walls due to contiguous shallow indentations less than one-quarter of the ductal diameter) and strictures (with or without accompanying upstream dilation). Bile duct angulation, degree of angulation of the common bile duct (CBD), usually seen at the superior border of the pancreas was measured using electronic callipers. Coronal thick-slab HASTE (half-Fourier single-shot turbo spin echo) sequences were used for the purpose. Lines were placed along the long axis of the duct centrally across the lumen above and below the site of angulation and the angle measured. An angle of <145 degrees was regarded as significant.12 The gall bladder (GB), IHD, and EHD were carefully examined for the presence of intraluminal signal voids suggestive of calculi/sludge.
Classifications
Classification of extent of involvement was done using the Chandra's classification:13 type 1: only EHDs, type 2: only IHDs, type 3a: EHDs and unilateral IHD, type 3b: EHDs and bilateral IHDs. Grading of severity was done using Llop's classification14: grade 1: irregularities/angulations, grade 2: strictures without upstream dilation, grade 3: strictures with upstream dilation. Since the adult diameters of bile ductular dilation could not be applied to children, grade 3 of Llop et al. classification was modified according to the available pediatric data. Dilatation was defined as ductal diameter of >4.1 mm for EHD and >2 mm for IHDs.15, 16, 17
Endoscopic ultrasound
EUS examination was performed by a single experienced gastroenterologist using a radial endoscope (Pentax EG-3670 URK radial echoendoscope, Pentax Corp., Japan or GF-UE 160 radial echoendoscope, Olympus Corp., Japan at 7.5 MHz) with the patient in a left-sided recumbent position under conscious sedation, if required. On EUS, presence of bile duct abnormalities including strictures/stones, dilatation, mass, duct wall thickening, and loss of wall stratification, if present were noted. The classification described earlier, was used to define the collaterals as epicholedochal, intracholedochal, paracholedochal and pericholedochal.18 The GB was also evaluated for presence of sludge/stones, wall thickening, and collaterals. Intrapancreatic collaterals and their location were also assessed.
Statistical analysis
It was performed using SPSS (Statistical Package for Social Sciences) software version 20.0 (SPSS Inc, Chicago, IL). Normality of the data was tested using Shapiro–Wilk test. Frequencies of various findings of PCC like bile duct irregularities and strictures, were calculated as percentages. Categorical data between groups were compared using Fisher exact tests. Continuous variables between groups were compared using Mann–Whitney U test for two groups and Kruskal–Wallis test for >2 groups. Multinomial logistic regression was used for predicting severity of PCC grades. All tests were two-tailed and a P value of <0.05 was considered statistically significant.
Results
Demographic characteristics
Of the 57 children enrolled in the study, 4 children (one of which had symptomatic PCC and grade 3 PCC on MRC) were excluded due to difficulty in negotiating the echoendoscope into the stomach. Thus, 53 children who successfully completed the study were analysed. Median age at diagnosis was 7.5 years (range: 1.8–13 years) and at the time of enrolment into the study was 10.8 years (range: 6.1–17 years). All children had cavernous transformation of portal vein (PV) identified on ultrasound Doppler at presentation. Majority (81%) of children had upper gastrointestinal bleed as a symptom at presentation, followed by abdominal distension (13.2%) and abdominal pain (5.8%). All the children were on prophylaxis with propranolol (1 mg/kg/day). Esophageal variceal eradication was achieved in all prior to enrolment into the study. No predisposing factors were identified and thus the etiology of EHPVO in our cohort was predominantly idiopathic. None had jaundice at the time of study, and no one had significant symptoms of PCC. However, if we consider those children excluded due to difficulty in performing EUS (n = 4), symptomatic PCC in our cohort would be 1.7%. The characteristics of the study population are tabulated in Table 1.
Table 1.
Characteristics of the Study Population (n = 53).
| Characteristics | At enrolment |
|---|---|
| Age (years) (Median, range) | 10.8 (6.1–17) |
| Sex (n, %) | Boys: 37 (70) |
| Anthropometry (Median, range) | |
| 1. Weight Z score | −1.51 (−7.2 to 0.98) |
| 2. Height Z score | −0.64 (−6.5 to 2.46) |
| 3. Underweight | 4 (7.5%) |
| 4. Stunting | 6 (11.3%) |
| Lab parameters (Median, range) | |
| 1. Total bilirubin (mg/dl) | 0.8 (0.1–1.05) |
| 2. Conjugated bilirubin (mg/dl) | 0.2 (0.09–0.8) |
| 3. AST (U/L) | 36 (14–61) |
| 4. ALT (U/L) | 27 (9–68) |
| 5. ALP (U/L) | 199 (67–487) |
| 6. GGT (U/L) | 12 (7–46) |
| 7. Serum total protein (g/dl) | 7.2 (4.3–8.5) |
| 8. Serum albumin (g/dl) | 4.2 (2.8–4.8) |
| 9. Hemoglobin (g/dl) | 10.2 (4.1–13.5) |
| 10. Total leucocyte count (x109/L) | 4.7 (1.4–11.7) |
| 11. Platelet count (x109/L) | 89 (11–371) |
| 12. INR | 1.1 (0.9–1.9) |
| MRC changes | |
| 1. Extrahepatic ducts (n = 53) | 1. Extrinsic impressions†: 10 (19%) |
| 2. Irregular contour/scalloping‡: 36 (68%) | |
| 3. Angulation¶: 33 (62.3%) | |
| 4. Mean CBD angle: 139.5 ± 11.6 degrees | |
| 5. Pericholecystic collaterals: 46 (86.8%) | |
| 6. Gallbladder calculi/sludge: 9 (17%) | |
| 7. Strictures: 16 (30.2%) | |
| 2. Intrahepatic ducts (n = 53) | 1. No abnormality: 8 (15%) |
| 2. Smooth dilation§: 7 (13.3%) | |
| 3. Periductal thickening£: 38 (71.7%) | |
| 4. Strictures: 26 (49%) | |
| 3. Ductal diameters (Mean ± SD) | 1. Common bile duct: 3.96 ± 1.42 mm |
| 2. Common hepatic duct: 3.74 ± 1.76 mm | |
| 3. Left hepatic duct: 3.75 ± 1.71 mm | |
| 4. Right hepatic duct: 2.69 ± 1.21 mm | |
| EUS changes n (%) | 1. Para-pericholedochal collaterals: 49 (92.5%) |
| 2. Intracholedochal collaterals: 5 (9.4%) | |
| 3. Intramural GB collaterals: 39 (73.6%) | |
| 4. GB wall thickening: 34 (64.2%) | |
| 5. Intrapancreatic collaterals: 40 (75.3%) | |
| 6. GB calculi/sludge: 5 (9.4%) | |
Underweight and stunting: weight for age and height for age less than 2 standard deviations below the median of WHO growth standards, respectively; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; INR, international normalised ratio; NA, not applicable; MRC, magnetic resonance cholangiography; CBD, common bile duct; GB, gall bladder; EUS, endoscopic ultrasound.
Extrinsic impressions: smooth impressions on the bile duct >one-fourth ductal diameter.
Irregular ductal contour/scalloping: fine-wavy impressions due to contiguous shallow indentations <one-fourth ductal diameter.
Smooth dilatation: no evidence of wall thickening/irregularity/focal narrowing.
Angulation: degree of angulation of the CBD seen at the superior border of the pancreas.
Periductal thickening: presence of T2 hyperintense thickening around the intrahepatic ducts.
Magnetic resonance cholangiography changes
All 53 (100%) children had PCC changes on MRC, but none were symptomatic. Anatomical involvement, according to the Chandra classification (Figure 1), was type 3b in 45 (85%) and type 1 in 8 (15%). Severity of PCC was grade 1 in 4 (7.5%), grade 2 in 18 (34%), and grade 3 in 31 (58.5%) children. The various abnormalities of biliary tree on MRC are tabulated in Table 1, and representative images are shown in Figure 2 (A-D).
Figure 1.
(A–B): Coronal magnetic resonance cholangiography images showing the anatomical types of involvement of biliary tree in children with portal cavernoma cholangiopathy. A: Chandra type 1 (involvement of common bile duct [CBD] with normal intrahepatic ducts); B: Chandra type 3b (involvement of CBD and both the intrahepatic ducts).
Figure 2.
(A–D): Magnetic resonance cholangiography (MRC) images showing biliary abnormalities in children with portal cavernoma cholangiopathy. A: Axial T2 HASTE image showing peri-ductal thickening (white arrow) with dilated left hepatic duct (red arrow); coronal MRC images showing:- B: narrowing/stricture of the left hepatic duct (white arrow) with upstream dilation; C: shallow impressions (red arrows) and extrinsic indentation (white arrow) on extrahepatic ducts; D: shallow impressions (red arrows), increased angulation of the common bile duct (white arrow), scalloping of the cystic duct (yellow arrow).
Changes in intrahepatic ducts
Involvement of the IHD of any kind was seen in 45 children (85%). Among these children, periductal thickening/irregularity was the most common (84.4%) followed by narrowing/stricture of either hepatic duct (57.7%). Narrowing/stricture was seen in 26 children with left hepatic duct (LHD) being the site of the stricture in majority (81%). The left and common hepatic ducts showed a significant larger mean diameter than right hepatic duct (P < 0.001).
Changes in extrahepatic ducts
CBD changes were seen in all the 53 (100%) children either alone (15%) or in combination (85%) with changes in IHD. Strictures/narrowing were seen in 16 (30.2%) children of which half (8) had accompanying upstream dilation. Middle one-third of CBD was the most common site of stricture/narrowing (56.2%) followed by lower one-third (25%) and upper one-third (18.8%). Significant correlation was observed between increased CBD angulation and occurrence of CBD strictures (spearman's ρ = −0.39, P = 0.004). No significant association was found between modified Llop MRC grading and age at enrolment (P = 0.7) or duration of disease (P = 0.9). Among the 46 children who had pericholecystic collaterals, 9 (19.6%) had GB calculi/sludge. No significant associations were observed between the presence of GB calculi/sludge with increased CBD angulation or CBD strictures or presence of pericholecystic collaterals.
Magnetic resonance portovenography findings
On MRPV, the different categories of block observed were a) PV block at the hilum (9.4%), b) entire PV replaced by collaterals (36%) with patent splenic vein (SV) and superior mesenteric vein (SMV), c) extension of block into SV with patent SMV (PV+SV: 15%), d) extension of block into SMV with patent SV (PV+SMV: 5.6%), e) complete block of PV, SV, and SMV (PV+SV+SMV: 34%). In all the children, both the left and right branches of PV were blocked and replaced by collaterals. Among the children with a PV + SV block with a patent SMV (8), modified MRC Llop grade 1, 2, and 3 changes were present in 0% (0), 37.5% (3), 62.5% (5), respectively. Similarly, among those with a PV + SMV block with a patent SV (3), modified MRC Llop grade 1, 2, and 3 changes were present in 0% (0), 33.3% (1), and 66.7% (2), respectively. Of the 18 children who had complete block (PV+SV+SMV), grade 1 changes were seen in 1 (5.6%), grade 2 in 6 (33.3%), and grade 3 in 11 (61.1%). No significant association was seen between the different types of block and MRC Llop severity grades.
Endoscopic ultrasound changes
The distribution of EUS biliary changes are summarized in Table 1 and representative images in Figure 3. Presence of intracholedochal collaterals had significant association with type of block (P = 0.002), with higher frequency of these collaterals in children having PV block extending either into SV, SMV, or both. Similarly presence of intrapancreatic collaterals were seen more frequently in children having accompanying SMV block (P = 0.04). Intramural GB collaterals on EUS and pericholecystic collaterals on MRC were seen in a similar number of patients (39 & 46 respectively; P = 0.36). On further analysing patients with intracholedochal collaterals (n = 5), intramural GB collaterals (n = 39), and intrapancreatic collaterals (n = 40), no significant associations were seen with the MRC changes like extrinsic compressions, cholelithiasis, CBD strictures/narrowing, or intrahepatic narrowing/strictures. We found that para-pericholedochal collaterals, wall thickening of GB, intramural GB collaterals, and intrapancreatic collaterals had significantly higher frequency of occurrence in children having Llop grade 3 MRC changes (P = 0.004, 0.009, 0.03, and 0.03, respectively). The clinical, biochemical, radiologic, and EUS biliary findings in different modified MRC Llop severity grades is shown in Table 2.
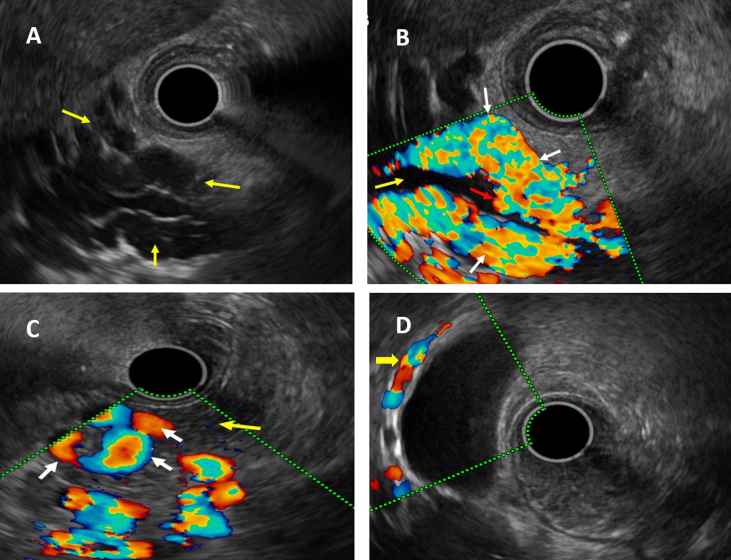
Figure 3.
(A–D): Endoscopic ultrasound (EUS) images showing various collaterals in children with portal cavernoma cholangiopathy. A: EUS image showing multiple anechoic, tubular structures at porta (yellow arrows); B: colour Doppler EUS image of the same patient showing para-pericholedochal collaterals (white arrows) around the common bile duct (yellow arrow) and intracholedochal collateral (red arrow); EUS colour Doppler images showing C: intrapancreatic collaterals (white arrows) in the pancreatic parenchyma (yellow arrow); D: intramural gall bladder collaterals (yellow arrows).
Table 2.
Comparison of Characteristics among Modified Llop Severity Grades.
| Severity grade (Llop) Feature |
Grade 1 (n = 4) |
Grade 2 (n = 18) |
Grade 3 (n = 31) |
P value |
|---|---|---|---|---|
| Age in years (Median, IQR) | 11.9 (8.3,14.6) | 10.9 (8.3,13) | 10.2 (7.7,12.8) | 0.74 |
| Gender (Boys, n [%]) | 4 (100) | 13 (72) | 20 (65) | 0.33 |
| Duration of disease in years (Median, IQR) | 2.5 (0.3, 7.5) | 3.2 (0.3, 5.7) | 2.3 (0.5, 5.8) | 0.98 |
| Lab parameters (Median, IQR) | ||||
|
1.05 (0.3, 1.4) | 0.8 (0.6,1.1) | 0.8 (0.5,1) | 0.63 |
|
0.25 (0.1,0.3) | 0.2 (0.1,0.2) | 0.2 (0.1,0.4) | 0.28 |
|
30.5 (23.2,40) | 38.5 (26.5,42) | 34 (27,44) | 0.57 |
|
20 (18,27) | 27.5 (19,35) | 28 (20,38) | 0.41 |
|
174 (92,264) | 211 (154,261) | 199 (139,280) | 0.63 |
|
10.5 (7.7,11.7) | 11.5 (10.7,16) | 12 (10,18) | 0.24 |
|
6.5 (4.8,6.9) | 7.2 (7,7.6) | 7.2 (6.9,7.6) | 0.04 |
|
3.7 (2.9,4.1) | 4.2 (4,4.6) | 4.2 (3.9,4.3) | 0.07 |
|
9.6 (8.3,11.6) | 11 (9.6,12) | 10 (8.9,11.7) | 0.27 |
|
4.7 (3.8,6.5) | 5.4 (4.3,6.8) | 4.3 (2.6,6.1) | 0.08 |
|
86 (60,97) | 107 (77,151) | 78 (54,102) | 0.06 |
|
1.1 (1, 1.2) | 1.1 (1, 1.2) | 1.1 (1, 1.2) | 0.98 |
| Type of block n (%) | 0.84 | |||
|
3 (75%) | 8 (44.5%) | 13 (42%) | |
|
1 (25%) | 10 (55.5%) | 18 (58%) | |
| CBD angle (Mean ± SD) | 157.7 ± 10.6° | 141.4 ± 5.9° | 136.1 ± 12° | <0.01 |
| EUS changes n (%) | ||||
|
2 (50%) | 17 (94.4%) | 30 (96.7%) | <0.01 |
|
0 (0) | 2 (11.1%) | 3 (9.7%) | 0.78 |
|
1 (25%) | 8 (44.4%) | 25 (80.6%) | <0.01 |
|
1 (25%) | 12 (66.7%) | 26 (84%) | <0.01 |
|
1 (25%) | 13 (72.2%) | 26 (84%) | <0.01 |
|
0 (0) | 2 (11.1%) | 3 (9.7%) | 0.78 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; INR, international normalised ratio; PV, portal vein; SV, splenic vein; SMV, superior mesenteric vein; EUS, endoscopic ultrasound; CBD, common bile duct; GB, gall bladder.
Discussion
Although the data on natural history of PCC is scarce, there is some evidence that PCC exists even in children with EHPVO. Reported incidence among the few studies varies from 20 to 92% with symptomatic PCC in children ranging from 0 to 7%.4,19,20 As reported in few previous studies,14 there were no clinical or laboratory parameters to predict the presence of PCC on MRC in our study. Biliary tract imaging is therefore essential to confirm the presence of PCC.
MRC has replaced conventional ERCP for evaluation of PCC due to its many advantages, and ERCP is used only for therapeutic purposes. Reported involvement of EHD ranges from 70% to 100%3,4,6,9,21,22 and that of IHD from 40% to 100%4,9,22, 23, 24, 25 with predominance of changes in LHD owing to the abundance of collateral vessels where the umbilical vein joins the left branch of PV.25 Our results are comparable with those of previous studies.3,4,10,20,22 Increased angulation of the CBD is attributed to the dilatation of anterior and superior pancreatico-duodenal veins displacing the CBD.26 As the angulation increases and approaches toward more acuteness, strictures and choledocholithiasis can develop there by leading to symptomatic PCC. In our study, mean CBD angle differed significantly between those who had CBD narrowing/strictures (n = 16) and those who did not (n = 37) ([132.7 ± 10.3° vs. 142.5 ± 10.9°, P = 0.005]). It also differed significantly among different Llop severity grades with increasing acuteness of the CBD angle as the grade progresses (P = 0.002).
Cholelithiasis in children in the setting of PCC has been reported in few studies.20,27,28 Chronic bile stasis because of obstruction in the biliary outflow and reduction in bile duct/GB contractility due to fibrosis or ischemia may lead to de novo stone formation in biliary tract/GB.29 In a study by Yamada et al., GB wall thickening was seen in 62% and cholelithiasis in 14.2%.28 Our study showed comparable results with GB wall thickening in 64.2% and cholelithiasis/sludge in 17% on MRC. EUS however, showed stones/sludge only in 9.4%; this would be considered the more accurate estimation. Other possible causes of cholelithiasis were excluded by appropriate investigations.
The role of EUS in evaluation of PCC is evolving. EUS with Doppler needs to be done in patients of EHPVO presenting with features of biliary obstruction, to identify the cause of obstruction viz., bile duct collaterals, strictures, calculi, or a tumorous growth.29 Bleeding from the intracholedochal collaterals during ERCP in patients with PCC leading to disastrous consequences have been reported.30,31 Although there are few adult studies reporting PCC changes on EUS, literature regarding EUS evaluation of PCC in children is very scarce. Our study showed the presence of para-pericholedochal collaterals in 92.5% of patients. There was also a significant association of EUS biliary collaterals with severity according to modified MRC Llop grades, with higher frequency of these collaterals in patients having grade 3 changes (P < 0.05). We observed a significant association with type of block and presence of these collaterals. Those with extension of block into SV/SMV had higher frequency of intracholedochal collaterals (P < 0.05). Thus, children with complete (PV+SV+SMV) block may be at higher risk of bleeding during ERCP and cannulation.
We saw a higher proportion of blocks with extension into SV or SMV in 21% and complete block (PV+SV+SMV) in 34% of patients as reported previously by few researchers.14,20 A study among adolescents showed that SMV block had a significant association with increasing MRC grades with higher occurrence of grade 2 and 3 changes compared with grade 1 among children with SMV block.20 However, findings regarding PV+SV block with patent SMV, PV+SMV block with patent SV and complete block of PV+SV+SMV and the whole group comparisons were not described. Overall in a scenario, where very few patients have grade 1 changes, identifying any single type of block and their association with MRC severity would be irrelevant. In the study by Llop et al., additional block of SV±SMV was seen in 65.7%, and no significant association was observed between the type of block and severity of MRC changes.14 In our study, though there was high frequency of grade 3 changes compared to grade 1 in those having additional block of SV±SMV, the results were not significant. We observed that irrespective of the type of block, majority of patients (nearly two-thirds for each type of block) had grade 3 changes with grade 1 changes in a few. Thus, the extent of block does not determine the severity of PCC.
We also observed that along with PV cavernoma, the left and right branches of PV were replaced by collaterals in all the children in our cohort. Thus, the Meso-Rex shunt surgery, recommended by the Baveno VI consensus,32 would not have been possible in any of these children. Similar findings were also observed by Sen Sarma et al.,20 and it can be said that meticulous evaluation for vascular anatomy is essential prior to recommending shunt surgeries in children with EHPVO to identify the subset with blocked left branch of PV. We observed a high frequency of spontaneous splenorenal shunts (54.7%) compared with older literature.33,34 This could be attributed to the presence of complete block leading to extensive collateralization. It is more likely, however, that the advancement in MRI technology (the earlier reports on natural shunts being before the MRI era) led us to pick up more of these shunts. This shunting, however, did not attenuate the severity of PCC, possibly indicating an early occurrence of the fixed component of PCC, prior to the development of natural shunts.
The natural history of PCC following PV thrombosis (PVT) and PV cavernoma formation is not well studied. In one adult study,14 73% of acute PVT patients had PCC detectable on MRC performed within a median of 33 months and of those who had had their first MRC performed within a year, 60% had PCC after an episode of acute PVT. It has been observed that patients with symptomatic PCC are older suggesting that it is a progressive condition and long-term PHT/obstruction are required to produce symptoms.11,13,35 In a recent prospective study in adults, 15.6% had symptomatic PCC, and the median time from index bleed to symptomatic PCC was 12 years.36 A study in adolescents, which had 5 patients with symptomatic PCC, showed a higher duration of disease in these children compared with those with asymptomatic PCC. However, the authors did not describe how the duration of PHT was calculated.20 In our study, median duration of disease (calculated from the time of first symptom onset) was 2.5 years (range: 3 months to 10.5 years). We did see one 13-year-old child with symptomatic PCC, during the study period; however, EUS could not be done, as he came in severe cholangitis and succumbed despite an emergent biliary drainage. We observed that there was a universal occurrence of PCC even in young children with EHPVO. All 13 children aged less than 8 years had evidence of PCC. All children with duration of disease less than 6 months (n = 16) also had evidence of PCC. This reiterates the fact that PCC develops very early in the course of EHPVO, as reported in the previous studies.14 However, it can also be argued that the duration of PHT in children with EHPVO cannot be determined exactly. Usually, the duration of PHT is calculated from the time of first appearance of symptoms. The time period for which the cavernoma and PHT have existed prior to the presenting symptom, cannot be ascertained, as it is largely an idiopathic disorder. In a given patient, we cannot pinpoint the time point at which the cavernoma formed and the PHT started developing. Thus, these children could be harboring the portal cavernoma and the changes consequent to it, much before the symptom onset.
The limitation of our study was that we were not able to study younger children less than 6 years old with EUS. They were not included keeping in mind a possible difficulty in negotiating the larger diameter echoendoscope in young children. It is probable that these younger children might also be harboring PCC changes, and this information would have given us a better idea about the impact of age and duration of PHT on the occurrence of PCC. Although EUS findings do not provide additional information as a roadmap for shunt surgery, it is useful prior to intervention in symptomatic biliary obstruction due to PCC.
To conclude, ours is the first pediatric study to date to evaluate PCC in children with EHPVO aged 6 years and older using both MRC/MRPV and EUS. As there are no definite clinical and biochemical parameters to determine PCC, we recommend the use of these modalities for evaluating EHPVO children with PCC. Despite the common occurrence of severe PCC in children, it remains asymptomatic in this age group and need for interventions is rare prior to adulthood. A more acute CBD angle predisposes to stricture/narrowing and thereby more severe PCC grade. Those with complete venous block need a meticulous evaluation for intracholedochal varices by EUS prior to any drainage procedure for biliary obstruction. The exact clinical significance of occurrence of severe PCC early in childhood is still unclear. Our understanding of the natural history of PCC is incomplete. Will it progress to symptomatic PCC and secondary biliary cirrhosis in a significant proportion? Should we prefer surgical shunt as a primary treatment modality instead of endoscopic obliteration of varices in an effort to reduce PCC-related morbidity in adulthood? This can be answered by further studies including large cohort of adults with EHPVO, who have lived with the disease since childhood.
Credit authorship contribution statement
VV- data curation, investigation, formal analysis, writing-original draft and editing; SSR-resources, investigation, supervision and review; AB- resources, investigation, supervision and review; SBL- concept, design, methodology, supervision, writing- review and editing and final approval.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Yachha S.K., Khanduri A., Sharma B.C., Kumar M. Gastrointestinal bleeding in children. J Gastroenterol Hepatol. 1996;11:903–907. [PubMed] [Google Scholar]
- 2.Yachha S.K. Portal hypertension in children: an Indian perspective. J Gastroenterol Hepatol. 2002;17:S228–S231. doi: 10.1046/j.1440-1746.17.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 3.Dilawari J.B., Chawla Y.K. Pseudosclerosing cholangitis in extrahepatic portal venous obstruction. Gut. 1992;33:272–276. doi: 10.1136/gut.33.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khuroo M.S., Yattoo G.N., Zargar S.A., et al. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–813. [PubMed] [Google Scholar]
- 5.Bayraktar Y., Balkanci F., Ozenc A., et al. The “pseudo-cholangiocarcinoma sign” in patients with cavernous transformation of the portal vein and its effect on the serum alkaline phosphatase and bilirubin levels. Am J Gastroenterol. 1995;90:2015–2019. [PubMed] [Google Scholar]
- 6.Nagi B., Kochhar R., Bhasin D., Singh K. Cholangiopathy in extrahepatic portal venous obstruction. Radiological appearances. Acta Radiol. 2000;41:612–615. doi: 10.1080/028418500127345992. [DOI] [PubMed] [Google Scholar]
- 7.Khuroo M.S., Rather A.A., Khuroo N.S., Khuroo M.S. Portal biliopathy. World J Gastroenterol. 2016;22:7973–7982. doi: 10.3748/wjg.v22.i35.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poddar U., Thapa B.R., Bhasin D.K., Prasad A., Nagi B., Singh K. Endoscopic retrograde cholangiopancreatography in the management of pancreaticobiliary disorders in children. J Gastroenterol Hepatol. 2001;16:927–931. doi: 10.1046/j.1440-1746.2001.02545.x. [DOI] [PubMed] [Google Scholar]
- 9.Malkan G.H., Bhatia S.J., Bashir K., et al. Cholangiopathy associated with portal hypertension: diagnostic evaluation and clinical implications. Gastrointest Endosc. 1999;49:344–348. doi: 10.1016/s0016-5107(99)70011-8. [DOI] [PubMed] [Google Scholar]
- 10.Jabeen S., Robbani I., Choh N.A., et al. Spectrum of biliary abnormalities in portal cavernoma cholangiopathy (PCC) secondary to idiopathic extrahepatic portal vein obstruction (EHPVO)—a prospective magnetic resonance cholangiopancreaticography (MRCP) based study. Br J Radiol. 2016;89:20160636. doi: 10.1259/bjr.20160636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhiman R.K., Saraswat V.A., Valla D.C., et al. Portal cavernoma cholangiopathy: consensus statement of a working party of the Indian national association for study of the liver. J Clin Exp Hepatol. 2014;4:S2–S14. doi: 10.1016/j.jceh.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keizman D., Shalom M.I., Konikoff F.M. An angulated common bile duct predisposes to recurrent symptomatic bile duct stones after endoscopic stone extraction. Surg Endosc Interv Tech. 2006;20:1594–1599. doi: 10.1007/s00464-005-0656-x. [DOI] [PubMed] [Google Scholar]
- 13.Chandra R., Kapoor D., Tharakan A., Chaudhary A., Sarin S.K. Portal biliopathy. J Gastroenterol Hepatol. 2001;16:1086–1092. doi: 10.1046/j.1440-1746.2001.02562.x. [DOI] [PubMed] [Google Scholar]
- 14.Llop E., de Juan C., Seijo S., et al. Portal cholangiopathy: radiological classification and natural history. Gut. 2011;60:853–860. doi: 10.1136/gut.2010.230201. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Wang X.-L., Li S.-X., et al. Ultrasonographic dimensions of the common bile duct in Chinese children: results of 343 cases. J Pediatr Surg. 2013;48:1892–1896. doi: 10.1016/j.jpedsurg.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Khalili K., Wilson S.R. Elsevier Mosby; 2005. The Biliary Tree and Gall Bladder. Diagnostic Ultrasound; pp. 171–212. [Google Scholar]
- 17.Ashkar L., Maheshwari S., Pressaco L., Cholangiopancreatography M.R. Saunders Elsevier; 2006. Clinical Magnetic Resonance Imaging; pp. 483–2540. [Google Scholar]
- 18.Sharma M., Rameshbabu C.S. Portal cavernoma cholangiopathy: an endoscopic ultrasound based imaging approach. J Clin Exp Hepatol. 2014;4:S53–S61. doi: 10.1016/j.jceh.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier-Villars M., Franchi S., Gauthier F., Fabre M., Pariente D., Bernard O. Cholestasis in children with portal vein obstruction. J Pediatr. 2005;146:568–573. doi: 10.1016/j.jpeds.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Sen Sarma M., Yachha S.K., Rai P., Neyaz Z., Srivastava A., Poddar U. Cholangiopathy in children with extrahepatic portal venous obstruction. J Hepato-Biliary-Pancreat Sci. 2018;25:440–447. doi: 10.1002/jhbp.582. [DOI] [PubMed] [Google Scholar]
- 21.Özkavukcu E., Erden A., Erden I. Imaging features of portal biliopathy: frequency of involvement patterns with emphasis on MRCP. Eur J Radiol. 2009;71:129–134. doi: 10.1016/j.ejrad.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Chevallier P., Denys A., Novellas S., Schmidt S., Schnyder P., Bruneton J.N. Magnetic resonance cholangiography features of biliary abnormalities due to cavernous transformation of the portal vein. Clin Imag. 2006;30:190–194. doi: 10.1016/j.clinimag.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Sezgin O., Oğuz D., Altintaş E., Ü Saritaş, Şahin B. Endoscopic management of biliary obstruction caused by cavernous transformation of the portal vein. Gastrointest Endosc. 2003;58:602–608. doi: 10.1067/s0016-5107(03)01975-8. [DOI] [PubMed] [Google Scholar]
- 24.Perlemuter G., Béjanin H., Fritsch J., et al. Biliary obstruction caused by portal cavernoma: a study of 8 cases. J Hepatol. 1996;25:58–63. doi: 10.1016/s0168-8278(96)80328-x. [DOI] [PubMed] [Google Scholar]
- 25.Dhiman R.K., Behera A., Chawla Y.K., Dilawari J.B., Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walser E.M., Runyan B.R., Heckman M.G., et al. Extrahepatic portal biliopathy: proposed etiology on the basis of anatomic and clinical features. Radiology. 2011;258:146–153. doi: 10.1148/radiol.10090923. [DOI] [PubMed] [Google Scholar]
- 27.Chiu B., Superina R. Extrahepatic portal vein thrombosis is associated with an increased incidence of cholelithiasis. J Pediatr Surg. 2004;39:1059–1061. doi: 10.1016/j.jpedsurg.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Yamada R.M., Hessel G. Ultrasonographic assessment of the gallbladder in 21 children with portal vein thrombosis. Pediatr Radiol. 2005;35:290–294. doi: 10.1007/s00247-004-1343-0. [DOI] [PubMed] [Google Scholar]
- 29.Harmanci O., Bayraktar Y. How can portal vein cavernous transformation cause chronic incomplete biliary obstruction? World J Gastroenterol. 2012;18:3375–3378. doi: 10.3748/wjg.v18.i26.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tighe M., Jacobson I. Bleeding from bile duct varices: an unexpected hazard during therapeutic ERCP. Gastrointest Endosc. 1996;43:250–252. doi: 10.1016/s0016-5107(96)70327-9. [DOI] [PubMed] [Google Scholar]
- 31.Mutignani M., Shah S.K., Bruni A., Perri V., Costamagna G. Endoscopic treatment of extrahepatic bile duct strictures in patients with portal biliopathy carries a high risk of haemobilia: report of 3 cases. Dig Liver Dis. 2002;34:587–591. doi: 10.1016/s1590-8658(02)80093-7. [DOI] [PubMed] [Google Scholar]
- 32.Shneider B.L., de Ville de Goyet J., Leung D.H., et al. Primary prophylaxis of variceal bleeding in children and the role of MesoRex bypass: summary of the Baveno VI pediatric satellite symposium. Hepatology. 2016;63:1368–1380. doi: 10.1002/hep.28153. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez F., Bernard O., Brunelle F., Hadchouel P., Odièvre M., Alagille D. Portal obstruction in children. I. Clinical investigation and hemorrhage risk. J Pediatr. 1983;103:696–702. doi: 10.1016/s0022-3476(83)80460-0. [DOI] [PubMed] [Google Scholar]
- 34.Dilawari J.B., Chawla Y.K. Spontaneous (natural) splenoadrenorenal shunts in extrahepatic portal venous obstruction: a series of 20 cases. Gut. 1987;28:1198–1200. doi: 10.1136/gut.28.10.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khare R., Sikora S.S., Srikanth G., et al. Extrahepatic portal venous obstruction and obstructive jaundice: approach to management. J Gastroenterol Hepatol. 2005;20:56–61. doi: 10.1111/j.1440-1746.2004.03528.x. [DOI] [PubMed] [Google Scholar]
- 36.Shukla A., Gupte A., Karvir V., Dhore P., Bhatia S. Long term outcomes of patients with significant biliary obstruction due to portal cavernoma cholangiopathy and extra-hepatic portal vein obstruction (EHPVO) with No shuntable veins. J Clin Exp Hepatol. 2017;7:328–333. doi: 10.1016/j.jceh.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]