Abstract
Objective:
To develop a Standardized Steroid dosing Regimen (SSR) by physicians treating childhood-onset systemic lupus erythematosus (cSLE) complicated by lupus nephritis (LN), using consensus formation methodology.
Methods:
Parameters influencing corticosteroid (CS) dosing were identified (Step-1). Data from children with proliferative LN were used to generate Patient Profiles (PP) (Step-2). Physicians rated change in activity of renal and extra-renal cSLE between two consecutive visits and proposed CS dosing (Step-3). Using PP ratings, the SSR was developed (Step-4) with refinements achieved in a physician focus group (Step-5). A second type of PP describing cSLE course for ≥4 months since kidney biopsy were rated to validate the SSR-recommended oral and intravenous CS-dosages (Step-6). PP adjudication was based on majority ratings for both renal and extra-renal disease courses, and consensus level was set at 80%.
Results:
Degree of proteinuria, estimated glomerular filtration rate, change in renal and extra-renal disease activity, and time since kidney biopsy influenced CS dosing (Steps-1/2). Considering these parameters in 5,056 PP-ratings from 103 raters, and renal and extra-renal course definitions, CS-dosing rules of the SSR were developed (Steps-3–5). Validation of the SSR for up to 6 months post kidney biopsy was achieved with 1,838 PP-ratings from 60 raters who achieved consensus for oral and intravenous CS dosage as per the SSR (Step-6).
Conclusion:
The SSR represents an international consensus on CS dosing for use in patients with cSLE and proliferative LN. The SSR is anticipated to be used for clinical care and standardize CS-dosage during clinical trials.
Keywords: Childhood-onset systemic lupus erythematosus, lupus nephritis, corticosteroids, treatment
INTRODUCTION
Corticosteroids (CS) remain one of the mainstays of therapy in childhood-onset systemic lupus erythematosus (cSLE), especially with major organ involvement such as lupus nephritis (LN). Due to lack of strong medical evidence, dosing of CS for cSLE treatment remains mainly provider dependent (1). Delphi surveys and expert opinion were previously employed to propose standards for CS use (2, 3), including for proliferative LN in children as part of the Consensus Treatment Plans for pediatric LN (CTPLN) (4). However, when tested in real life settings, providers followed the CS dosing recommended by the CTPLN in only 68% of patients by 3 months, and just 37% of patients by 6 months of induction therapy for LN (5).
Objectives for this study were to use consensus formation methods in conjunction with real-life patient data 1) to delineate determinants that influence CS dosage in cSLE with proliferative LN, and 2) develop as well as 3) initially validate the Standardized Steroid dosing Regimen (SSR).
PATIENTS AND METHODS
Overview.
Figure 1 sketches the experimental design (Steps 1–6). Building on the experience from the CTPLN (4), we focused on cSLE patients with biopsy proven, new-onset LN class III or IV with/without class V overlap, as per the International Society for Nephrology/Renal Pathology Society (ISN/RPS) (6). Consensus formation methodology was combined with statistical modeling of Patient Profiles (PP) ratings that were derived from a contemporary cSLE cohort. We invited an international group of physicians experienced in the care of pediatric LN to participate in this research.
Figure 1. Development of the Standardized Steroid dosing Regimen (SSR) for childhood-onset SLE.
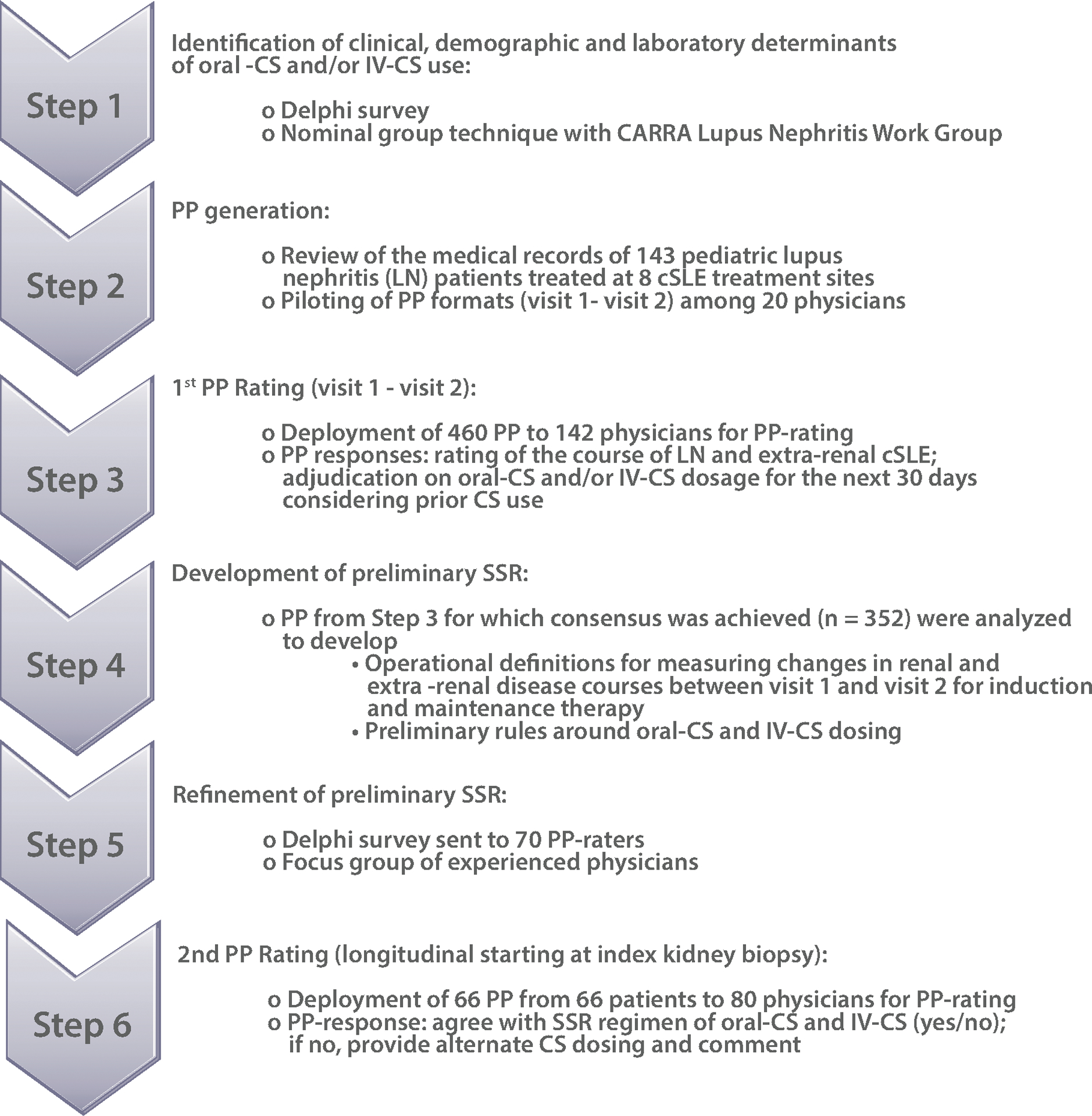
The experimental design used can be summarized in Step-1 to Step-6, and consists of various consensus formation methods, statistical analyses and the use of real-life data from pediatric patients with LN in Patient Profile ratings.
Abbreviations used are: Oral-CS: oral corticosteroids; IV-CS: intravenous corticosteroids; CARRA: Childhood Arthritis and Rheumatology Research Alliance; PP: patient profiles; LN: lupus nephritis; REDCap is a browser-based, metadata-driven electronic data capture software and workflow methodology for designing clinical and translational research databases; for additional details please see: https://projectredcap.org/software
Details of experimental design.
Step-1:
A review of the literature was conducted and revealed limited high-quality evidence regarding the dosing of CS in patients with cSLE (7). In April 2018, after literature review, followed by a Delphi survey, consensus was achieved around candidate determinants of oral-CS and IV-CS use (CS-determinants) at an in-person meeting held in Denver, CO, using modified nominal group technique (8). Demographic, laboratory and clinical parameters were identified as candidate CS-determinants. Consensus meeting participants were 51 members of the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Lupus Nephritis Work Group.
Step-2:
The medical records of 143 LN patients followed at eight pediatric tertiary care centers were retrospectively reviewed for up to 24 months, starting from the time of kidney biopsy that newly diagnosed the patient as having proliferative LN (6). Table 1 summarizes principal eligibility criteria of patients whose data were abstracted for PP generation.
Table 1.
Principal eligibility criteria for patients utilized for patient profile generation
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Patient could have had other renal biopsies indicating presence of other classes of LN
Lupus nephritis
International Society for Nephrology/ Renal Pathology Society
PP formats previously developed to judge the overall disease course of cSLE (9) were adapted and piloted among 20 physicians. PP included information about patient demographics (age, gender, race, ethnicity); organ involvement with SLE per the 1997 American College of Rheumatology (ACR) classification criteria (10); kidney biopsy results (LN class (6), activity and chronicity scores (11, 12)); vitals (body surface area, height, actual body weight, blood pressure); laboratory testing [complete blood count, erythrocyte sedimentation rate (ESR), C3, C4, anti-dsDNA antibodies], and the response variables for pediatric LN (LN-RVs) (4), namely proteinuria [spot urine protein/creatinine ratio (UPCR) or 24-hour timed proteinuria], renal function [estimated glomerular filtration rate (eGFR), serum creatinine], and urine sediment [white blood cells (WBC)/high powered field (HPF), red blood cells (RBC)/HPF and heme-granular or RBC casts]; scores of Systemic Lupus International Collaborating Clinics/ACR Damage Index (SDI) (13) and SLE Disease Activity Index (version SLEDAI-2K) (14); parent/patient global assessment of overall well-being; physician global assessment of overall disease activity; usage of oral-CS and IV-CS (dose, route, frequency), use of immunosuppressant (mycophenolate mofetil, cyclophosphamide with dose and frequency) and angiotensin system blockers (Yes/No). Each PP provided this information for two consecutive patient assessments [Visit-1, Visit-2] to describe the course of cSLE over a 4-week period.
Step-3:
Of 2,215 PP generated, 460 PP, with complete patients’ information, were selected for rating by 142 physicians (PP-raters) who were members of the CARRA Lupus Nephritis Work Group, the Pediatric Rheumatology European Society Lupus Working Party, or the Pediatric Nephrology Research Consortium. PP selection for the development dataset focused on capturing all permutations of combined renal and extra-renal disease courses between Visit-1 and Visit-2. PP-raters judged the renal and extra-renal disease courses as follows: active stable; active improved; active worsened; inactive; or not enough information. PP-raters were also asked to suggest oral-CS and IV-CS dosages for the 30 days following Visit-2, at stable, increasing, or tapering dosages. PP-raters were randomized to rate 66 PP each (also see supplemental figure S1).
Step-4:
Only PP for which consensus about the course of cSLE between visits was achieved were included in the dataset that was used to develop the SSR. As done in the past (9), adjudication of the renal and extra-renal course described in a given PP was based on majority vote among PP-raters, i.e. ≥50% of PP-raters agreed on one specific combination of renal plus extra-renal disease course between Visit-1 and Visit-2. SSR-recommended CS dosage was the median daily CS dose that achieved consensus by PP-raters.
Step-5:
Following statistical analysis (see below), an additional Delphi questionnaire was sent to a randomly selected subset of PP-raters (n=70). This questionnaire was aimed at clarifying maximum daily oral-CS dosages, use of divided daily oral-CS doses, IV-CS use upon initiation of induction therapy, and IV-CS and/or oral-CS dosages prescribed with flares (LN and/or extra-renal cSLE). This was followed by a focus group (KO, DML, SW, MKG) to clarify CS use for children <40 kg and the importance of the type of immunosuppressant prescribed (here: cyclophosphamide or mycophenolate mofetil) for the use of IV-CS. Step-5 information was utilized to refine the preliminary SSR (from Step-4) (4, 5).
Step-6:
Among available patients with ≥4 months of follow-up, 66 patients (or PP) were selected to serve as validation set. PP format used for Step-6 were similar to those of Step-3. However, in addition to renal and extra-renal disease course information, the SSR-recommended dosage of oral-CS and/or IV-CS for up to 6 months since kidney biopsy were shown (see supplemental figure S2). These 66 PP were sent to 80 PP-raters randomly selected from the pool of available PP-raters (Step-3); each PP-rater was asked to rate 33 PP. Specifically, PP-raters were asked whether the SSR-recommended CS dosage at each time point was acceptable for treating the vast majority (>80%) of patients with similar clinical presentations and comparable renal and extra-renal disease courses.
Data management & statistical analysis.
Following PP-ratings (Step-3), the frequencies and percent of agreement were calculated for each PP for the renal, extra-renal, and overall (renal plus extra-renal) disease courses. As the analysis unit, each PP was reviewed and rated by multiple pediatric rheumatologists and nephrologists, and their responses were summarized and analyzed to evaluate the level of consensus for oral-CS and IV-CS dosage as recommended by the SSR. Only PP with majority ratings (≥50%) for a given disease course were considered in these statistical analyses. Logistic regression analyses identified CS-determinants relevant to renal, extra-renal and overall disease courses (Step-3). Descriptive statistics (mean, standard deviation, median, Q1 and Q3) for the percent agreement among PP-raters for oral-CS and IV-CS dosing were computed, followed by distribution and probability plots (Step-4). CS dosing regimens for each disease course were summarized and synthesized to build the preliminary SSR (Step-4). Step-5 considered consensus among survey respondents. The validation of the SSR (Step-6) used statistics similar to Step-4. Research Electronic Data Capture (REDCap) software (https://projectredcap.org/software) was used for data capture, storage, surveys and PP-ratings. Data were analyzed using SAS, version 9.4.
The study was approved by the ethics committees / institutional review boards of the participating centers.
RESULTS
Proposed determinants that influence CS use in cSLE with LN.
For Step-1, 25 of 51 physicians of the CARRA Lupus Nephritis Work Group responded to the Delphi questionnaire (response rate: 49%) that aimed at confirming the LN-RVs and identifying additional CS-determinants. There was consensus (>80%) that complement levels (C3 and C4) and UPCR were important determinants of CS dosage. Structured discussions and voting as part of a nominal group exercise at a subsequent face-to-face meeting provided confirmation of these candidate CS-determinants. These included time since index kidney biopsy, kidney histological features, status and change of the LN-RVs since prior assessment, and extra-renal disease activity as measured by the scores from the respective domains of the SLEDAI-2K, ESR, physician global assessment of disease activity, and patient overall well-being. Case examples that were presented highlighted the extent of variation of CS use among group members (n=15) when treating proliferative LN, hence supporting the rationale for developing the SSR.
Determinants influencing PP-raters adjudication of status and changes in renal and extra-renal disease used in the SSR.
In Step-3, 103 physicians (response rate 103/142=73%) reviewed 460 PP producing 5,080 PP-ratings. Out of the 5,080 PP-ratings, 24 were excluded due to data quality issues resulting in 5,056 PP-ratings that were analyzed. These 460 PP represented 120 of the 143 LN patients with available data. Table 2 summarizes the baseline characteristics of these 120 cSLE patients represented by the PP. There were 352 PP (352/460=77%) that achieved majority ratings for the course of renal plus extra-renal disease between Visit-1 and Visit-2, hence qualified for inclusion in the subsequent steps of the SSR development.
Table 2:
Baseline characteristics of patients used for patient profile development
| Variables | Patients for Step-3 (N=120) | Patients for Step-6 (N=66) | |
|---|---|---|---|
| Mean +/− SD or (N) % | Mean +/− SD or (N)% | ||
| Age at LN onset (year) | 13.47 ± 3.06 | 13.50 ± 2.89 | |
| Female | (96) 80% | (55) 83% | |
| Race | |||
| White | (59) 49% | (35) 53% | |
| Black or African American | (45) 37.5% | (25) 38% | |
| Other | (13) 11% | (5) 8% | |
| Unknown | (3) 2.5% | (1) 1% | |
| Ethnicity | |||
| Hispanic | (35) 29% | (19) 29% | |
| Non-Hispanic | (84) 70% | (47) 71% | |
| Unknown | (1) 1% | (0) 0% | |
| Laboratory testing | |||
| UPCR (mg/mg)¥ | 3.04 ± 3.85 | 2.77 ± 2.46 | |
| Serum Creatinine (mg/dL) | 0.90 ± 0.70 | 0.98 ± 0.89 | |
| SDI Total Score at LN onset ¶ | 0.19 ± 0.58 | 0.15 ± 0.47 | |
|
SLEDAI-2K†; % with feature present |
|||
| Seizure | (2) 2% | (1) 2% | |
| Organic Brain Syndrome | (3) 3% | (1) 2% | |
| Visual Disturbances | (2) 2% | (0) 0% | |
| Cranial Nerve Disorder | (1) 1% | (1) 2% | |
| Lupus Headache | (1) 1% | (1) 2% | |
| CVA# | (1) 1% | (0) 0% | |
| Vasculitis | (10) 10% | (8) 14% | |
| Arthritis | (45) 43% | (28) 49% | |
| Myositis | (7) 7% | (5) 9% | |
| Urinary Casts | (36) 35% | (17) 30% | |
| Hematuria | (80) 77% | (47) 82 % | |
| Proteinuria | (92) 88% | (53) 93% | |
| Pyuria | (60) 58% | (36) 63% | |
| Rash | (58) 56% | (34) 60% | |
| Alopecia | (7) 7% | (4) 7% | |
| Mucosal Ulcers | (13) 12.5% | (8) 14% | |
| Pleurisy | (7) 7% | (1) 2% | |
| Pericarditis | (12) 12% | (5) 9% | |
| Low Complement | (96) 92 % | (53) 93% | |
| Increased DNA Binding | (93) 89% | (53) 93% | |
| Fever | (21) 20% | (12) 21% | |
| Thrombocytopenia | (15) 14% | (9) 16% | |
| Leukopenia | (19) 18% | (12) 21% | |
| SLEDAI-2K Total Score | 19.88 ± 7.43 | 20.86 ± 6.78 | |
| Oral Prednisone (equivalent‡) (mg/day) | |||
| <7.5 | (11) 9% | (6) 9% | |
| 7.5, 16 | (7) 6% | (1) 2% | |
| 16, 45 | (51) 42.5% | (28) 42% | |
| >45 | (51) 42.5% | (31) 47% | |
| Other medications at Visit-1; % present | |||
| IV Methylprednisolone | (3) 2.5% | (1) 2% | |
| Mycophenolate mofetil | (3) 2.5% | (1) 2% | |
| Cyclophosphamide | (33) 27.5% | (26) 39% | |
| Angiotensin system inhibitors | (51) 42.5% | (24) 36% | |
Urine protein creatinine ratio (UPCR)
Systemic Lupus International Collaborating Clinics/ACR Damage Index. For item definitions please see: Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis and rheumatism. 1996;39(3):363–9.
SLE disease activity index, version 2K. For item definitions please see: Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Cerebrovascular accident (CVA)
Other corticosteroid dosages were converted to prednisone equivalent doses
Among the proposed CS-determinants included in Step-3, the renal course was best predicted by the eGFR, UPCR, and urine RBCs. Pyuria was common (Table 2), however, was not associated with LN course (active stable; active improved; active worsened; inactive; odds-ratio (OR)=1.03, p-value=0.97). Course (active stable; active improved; active worsened; inactive) of extra-renal disease from PP-ratings was closely associated with change in extra-renal SLEDAI-2K score (OR=0.91, p-value=0.004). Thus, the following CS-determinants were considered in the preliminary SSR: patient actual body weight, time from index kidney biopsy, extra-renal SLEDAI-2K score, UPCR, urine RBCs, and eGFR. We used a conservative estimate for normal eGFR at ≥95 ml/min/1.73m2 (15, 16).
Summary and overarching principles of the SSR.
In the SSR, the oral-CS of choice is prednisone, and the IV-CS is methylprednisolone. It is understood that patients are treated with an additional immunosuppressant during induction and maintenance therapy for LN. Building on the CTPLN (4), the SSR considers time from the index kidney biopsy for the oral-CS and IV-CS use. The SSR assumes potential CS dose adjustments to occur every 4 weeks. More frequent adjustments may occur during the initial 4 weeks of induction therapy and with severe renal or extra-renal flares. In the following sections, we assumed patients’ body weights ≥40kg and a maximum daily oral-CS dosage at 60 mg; for cSLE patients with body weight <40kg the equivalent maximum dose is 1.5 mg/kg/day (Step-5). Lower oral-CS dosages (prednisone) for patients <40kg can be calculated as follows: daily SSR-dosage for ≥40kg patients × body weight × 0.025. We anticipate that the SSR can be used for the vast majority (>80%) of children as indicated by the PP-raters’ responses for oral-CS and IV-CS dosing (in Step 6).
Categorization of disease course between assessments.
The SSR categorizes changes in extra-renal activity (a–d) between patient assessments as follows: a) active- much worse; b) active-mild/moderate worse; c) active-stable or active-improved; and d) inactive. For induction therapy, changes in renal activity between assessments are categorized (A–D) as follows: A) active-worse; B) active-stable; C) active-improved or inactive prior to week 12; and D) inactive starting week 12. Changes in renal activity categories (i–iv) used during maintenance therapy are: i) LN flare after partial renal remission (PRR) or complete renal remission (CRR); ii) worse after PRR or CRR; iii) PRR stable; and iv) CRR or PRR improved. Table 3 summarizes the definitions of the changes in renal and extra-renal courses between visits that govern SSR-recommended CS dosages, with additional details pertaining to the interpretation of the changes of the LN-RVs provided in Figure 2 A–C. Logistic regression analysis identified variable thresholds that were used in the proposed definitions to classify renal courses (A-D) and extra-renal courses (a-d). Using these definitions allowed us to model renal and extra-renal courses (as per the PP-raters) with > 90% accuracy based on area under the curve from receiver operating characteristic curve analysis (see supplemental figure S3).
Table 3.
Categorization of renal and extra-renal disease courses between two consecutive assessments (Visit-1 and Visit-2) as used in the Standardized Steroid dosing Regimen (SSR)
| Renal courses | Extra-renal course$ | |
|---|---|---|
| Induction therapy (weeks 1–26) | Maintenance therapy (after week 27) | |
| LN* - Active worse#: | LN flare after PRR or CRR¶: | Active - Much worse: |
| Worsening of ≥ 1 LN-RV† | Worsening (persistent¥ & substantial) of ≥1 LN-RV | Δ SLEDAI score: ≥ +8 |
| LN - Active stable: | LN worse# after PRR or CRR: | Active - Mild-moderate worse |
| Neither Active Worse nor Active Improved | Worsening of ≥ 1 LN-RV | Δ SLEDAI score: +4 to +7 |
| LN - Active improved: | PRR stable: | Active - stable: |
| Improvement of ≥1 LN-RV with the remaining LN-RV(s) not being worse | PRR with changes of LN-RVs not qualifying for being worse or improved | Δ SLEDAI score: ± 3 |
| PRR‡: | PRR improved: | Active - Improved: |
| Clinically relevant improvement of ≥2 LN-RVs with the remaining LN-RV not being worse | PPR with improvement of ≥ 1 LN-RV with the remaining LN-RV(s) not being worse | Δ SLEDAI score: ≤- 4 |
| LN - Inactive: | CRR: | Inactive: |
| All LN-RVs are within normal range | All LN-RVs are within normal range | Absolute SLEDAI score: ≤ 2 |
Lupus Nephritis
All “worsening” is considered to be due to LN
Lupus nephritis response variables, i.e., Urine protein creatinine ratio (UPCR), hematuria, estimated glomerular filtration rate (eGFR). Normal values for the LN-RVs are always considered as “improved” for the purpose of the course definitions (for details please see Figure 2)
Partial renal remission assesses the change between baseline and week 26 and can only be measured starting week 26 of induction therapy
Complete renal remission
Worsening of 1 or more LN-RVs on >2 subsequent time points >1 week(s) apart as follows: Newly abnormal eGFR or abnormal eGFR that decreased by >10%; Persistent increase of UPCR to >0.5, after CRR; Persistent doubling of UPCR with values >1.0, after PRR; Newly active or worsening by 2 categories of urine RBCs.
Based on changes in the score of the SLE disease activity index, version 2K. For item definitions please see: Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Figure 2. Operational definitions for changes in the lupus nephritis response variables for use in the SSR.
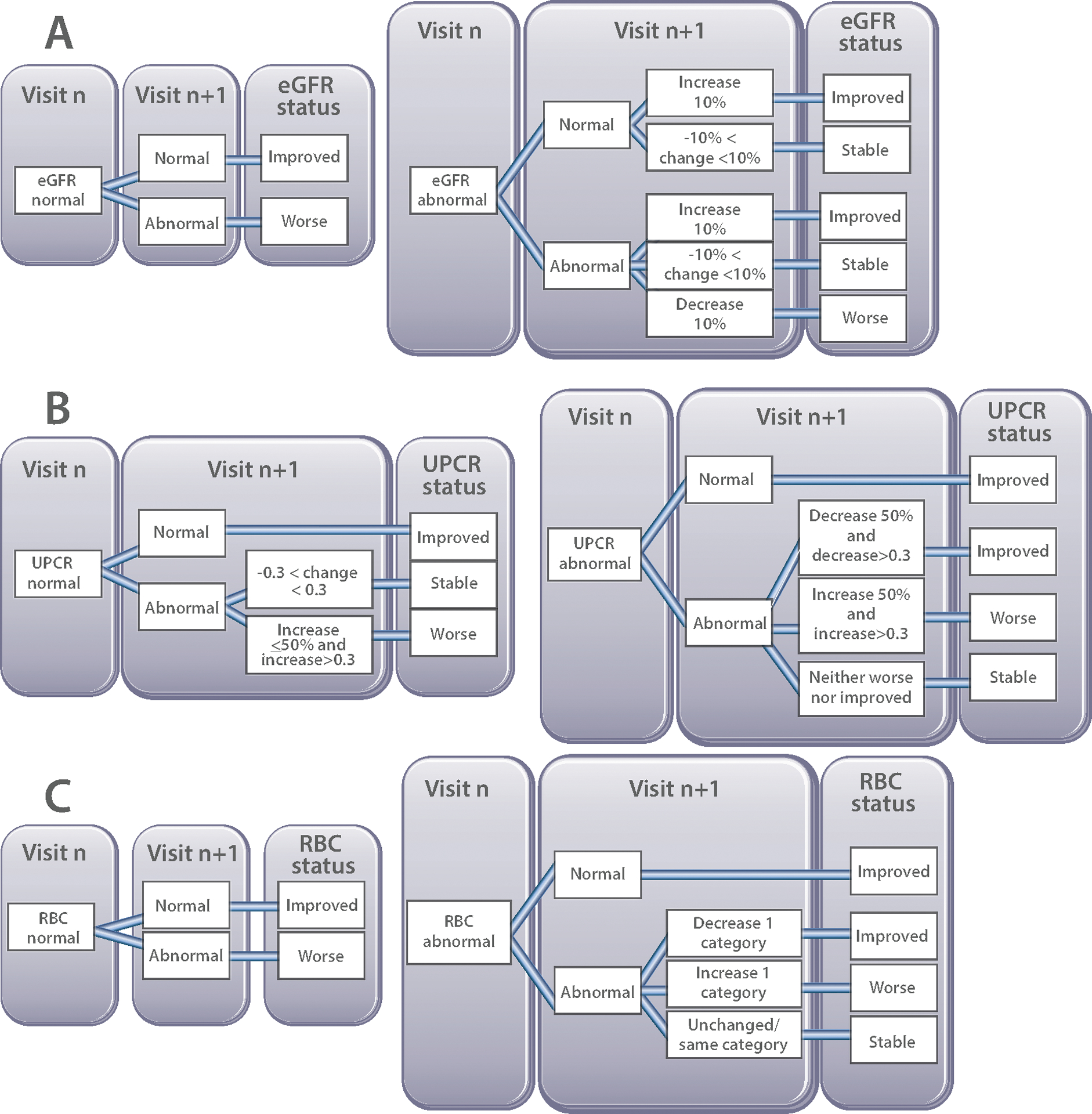
A: Estimated glomerular filtration rate (eGFR) of ≥95 ml/min/1.73 m2 was considered to be normal, and abnormal for eGFR values < 95 ml/min/1.73 m2, irrespective of patient age.
B: Values of the urine protein creatinine ratio (UPCR) from a random urine sample were considered normal for values ≤ 0.2 mg/mg and abnormal for values >0.2 mg/mg. Changes of the UPCR of ± 0.3 were deemed to represent stable proteinuria. Examples of changes of the UPCR between Visit 1 and Visit 2 and assessment of UPCR status are as follows: 0.5→0.25 (decrease by 50%, but decrease is <0.3, so UPCR is stable); 0.5→0.2 (UPCR normal); 0.5→0.8 (increase by >50% and increase by 0.3, so UPCR is worse); 0.5→0.75 (increase by 50%, but increase is <0.3, so UPCR is stable).
C: Only glomerular hematuria was considered when assessing urine microscopy, whereas pyuria and cellular casts were omitted. Five categories of glomerular hematuria measures in RBC/High Power Field (HPF) were defined as follows: normal: 0–5 RBC/HPF; mild: 6–10 RBC/HPF; moderate: 11–25 RBC/HPF; severe: 26–50 RBC/HPF; gross: >50 RBC/HPF.
SSR-recommended CS dosage during the initial 4 weeks post index kidney biopsy.
The SSR allows for up to 3 infusions of high dose methylprednisolone (30 mg/kg/dose; max 1 gram) upon diagnosis with proliferative LN. Maximum starting dosage of daily oral-CS is 60 mg, which may be given in divided doses. Depending on extra-renal disease activity and response of LN to therapy, oral-CS dosage may be decreased weekly, resulting in a minimum daily oral-CS dosage of 40 mg (maximum 60 mg) at week 4 of induction therapy.
SSR-recommended CS dosage during induction therapy (weeks 5–26).
The SSR is adjusted in at least monthly intervals during weeks 5–26 of induction therapy (minimum oral-CS dosage at week 26: 10 mg). Considering the pathology of LN, even if all LN-RVs have normalized, CS reduction is more conservative prior to week 12 than thereafter. Given the known toxicity of CS, small decreases of oral-CS occur even with stable renal and/or extra-renal activity. Figure 3A provides an example for SSR suggested adjustment of the CS dosage for a patient who experiences major worsening of extra-renal disease activity, in the setting of improving renal disease during the first 12 weeks post index kidney biopsy.
Figure 3. Examples of SSR suggested changes in prednisone (or equivalent dose of another corticosteroid).
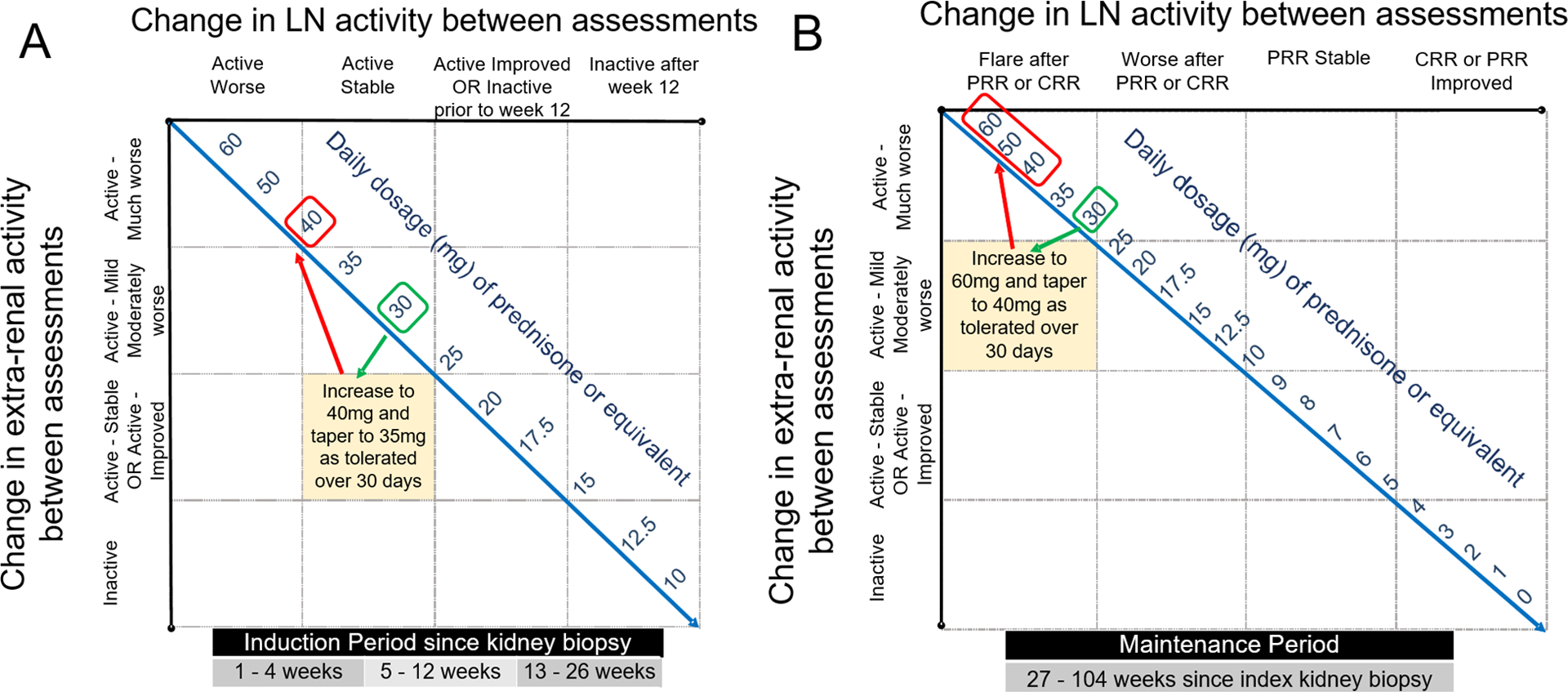
A: Suggested prednisone dose adjustment of a cSLE patient (≥ 40 kg) whose kidney biopsy showed proliferative lupus nephritis within the preceding 12 weeks. Upon re-assessment, the patient was taking prednisone 30 mg daily, his renal course was “Active-improved” and extra-renal disease course “Active-much worse”. The SSR recommends increasing daily prednisone dose to 40 mg. If tolerated, tapering oral-CS during the subsequent 4 weeks post assessment to 35 mg is proposed.
B: Suggested prednisone dose adjustment of a cSLE patient (≥ 40 kg) who completed induction therapy for LN, and achieved at least partial renal remission at week 26. Upon re-assessment, the patient was taking prednisone 30 mg daily and having a “LN flare after PRR”. The SSR recommends increasing the daily prednisone dose to 60 mg, irrespective of the extra-renal course. If renal response is improved with higher oral-CS doses, then oral-CS tapering can be initiated at day 10. The minimum allowable daily prednisone dose at day 30 following LN flare is 40 mg. If the patient has not improved by day 10, then intravenous pulse methylprednisolone (1–3 doses) should be considered.
SSR-recommended CS dosage during maintenance therapy (starting week 27).
PRR and CRR determination occurs upon completion of induction therapy at week 26. Based on focus group feedback, the SSR assumes that non-responders to induction therapy are likely to undergo repeat kidney biopsy, with CS-dosage then chosen according to (repeat) biopsy findings. For all other patients, CS dosage will depend on categories of both renal courses (i-iv) and extra-renal activity (a-d). As during induction therapy, provided there is well-controlled extra-renal disease, the SSR allows for CS tapering even in the setting of active stable renal disease. Likewise, with moderate worsening of extra-renal disease in the setting of improving renal disease, the CS dosage is kept stable. Patients who enter maintenance therapy in CRR and on oral-CS of 10 mg will taper oral-CS by 1–2 mg monthly, provided extra-renal disease is not worsening. An example of the SSR-recommended CS dosage for a patient receiving oral-CS at 30 mg at the time of a LN flare during maintenance therapy is shown in Figure 3B.
Major worsening of extra-renal disease.
Exploratory analyses suggested that daily oral-CS dosages at ≥40 mg are guided by the renal course, except in the setting of major extra-renal flares with potential organ damage. Major increase in extra-renal disease activity will prompt an increase of the oral-CS dosage in the SSR by 60–70% with stable renal courses, by 30–40% with renal improvement or with the use of oral-CS doses of ≥40 mg at the time of the extra-renal exacerbation. If extra-renal major deterioration fails to respond to increased oral-CS within 10–14 days as judged by the physician, then the SSR recommends the potential use of IV-CS.
SSR validation.
The complete SSR is shown in supplemental table S1. In Step 6, a total of 66 PP describing cSLE disease course for 4–6 months post index kidney biopsy were rated by 60 PP-raters which provided a total of 1,838 PP-ratings (response rate: 60 of 80 PP-raters invited=75%). Table 2 provides the baseline characteristics of the patients represented by the PP used in Step-6. PP-raters achieved agreement (95%) on all SSR-recommended oral-CS and/or IV-CS dosages. PP-raters stated that in >80% of their patients with similar cSLE features, both with regard to renal plus extra-renal disease courses, the SSR recommended oral-CS and/or IV-CS dosage was appropriate for clinical care.
DISCUSSION
In this study, an international group of pediatric rheumatologists and nephrologists collaborated to develop the SSR, a novel algorithm that standardizes oral-CS and IV-CS dosing decisions in cSLE patients with proliferative LN. As part of this research, determinants that influence medical decisions pertaining to CS-dosage were identified. This allowed us to model CS dosage, based on the renal and extra-renal disease courses with high accuracy.
Practice patterns are strongly variable among physicians (1, 3, 17, 18) and indicate that physician attributes may be more important than patient characteristics when prescribing CS (1, 3). While crucial in the treatment of cSLE, CS use is associated with damage accrual (19–21), and the severity of CS side effects make CS-dosage a constant focus for the treating physician. This emphasizes the importance of the developed SSR as an innovative tool to standardize CS dosage among physicians and centers treating cSLE patients.
A review of the literature has confirmed the lack of strong medical evidence to guide CS dosing in cSLE and LN (7). Our overall approach to this project was based on a stringent methodological framework using consensus formation techniques that were successfully used in previous pediatric rheumatology studies to develop the criteria for cSLE flare (9, 22), cSLE inactive disease and remission (23), the previous CTPLN (4), and classification criteria for Macrophage Activation Syndrome (24, 25). Our methodology was aligned with the recommendations of the Classification and Response Criteria Subcommittee of the ACR Committee on Quality Measures (26).
A strength of the SSR is that it has been derived from real-life patient data and was developed based on the consensus of a large number of experienced physicians who regularly treat cSLE and LN. The preliminary SSR developed in Steps 4/5 was further validated among a group of experts in the management of cSLE and LN using longitudinal data for the initial six months of induction therapy (Step 6). Our study validation achieved majority agreement (95%) among PP-raters for the use of the SSR-recommended oral-CS and IV-CS dosages which they considered acceptable in the vast majority (>80%) of their cSLE patients during induction therapy for LN. Although not specifically tested, we hypothesize that the SSR dosing for maintenance therapy can also be used for cSLE treatment that is not complicated by renal disease.
The SSR builds on the CTPLN which offers guidance for CS use with active proliferative LN, under the assumption of complete LN response at week 24 and CS use governed solely by LN (4). Unfortunately, such cSLE disease courses are rare based on the results of a pilot study testing the CTPLN (5). Different from the development of the CTPLN (4), the current study used real-life data from patients to deduce common CS use with cSLE. Disease courses considered in the SSR include variable courses of proliferative LN, extra-renal involvement with cSLE over time, multiorgan involvement with cSLE, renal and extrarenal flares, and inactive disease. Our validation exercise (Step-6) supports that the SSR is highly acceptable among physicians treating cSLE, supporting its future relevance for clinical care of and research in cSLE.
By design the SSR is expected to support CS dosing decisions in most patients with cSLE. However, the limitations of this study are related to the known marked phenotypic variation with cSLE which may prohibit SSR use in patients with extreme phenotypes, such as children with life threatening acute manifestations of cSLE. Notably, cSLE patients requiring renal replacement therapy were not reflected in our PP. Thus, additional validation is needed to evaluate the usefulness of the SSR in such situations.
Chronic, especially high-dose use of CS is a major risk factor for infections, a leading cause of death in SLE (7). Due to their CS-sparing properties, concurrent use of immunosuppressive medications is recommended during induction therapy for LN, and was considered in the development of the SSR. In exploratory analysis, use of mycophenolate mofetil as opposed to cyclophosphamide did not influence CS dosing by the PP-raters.
A short-coming of the SSR assumes that patients are adherent to their medications, including CS. Non-adherence to CS would be associated with lack of cSLE and LN improvement, or even disease flares. Such uncontrolled cSLE courses would be reflected in a lack of tapering of CS as per the SSR and likely prompt clinicians to consider patient’s difficulties with adherence to a treatment plan.
Personalized dosing of CS that take into account a cSLE patient’s pharmacogenetic and pharmacodynamic make-up are a highly active area of research (27, 28). We consider the SSR a long-needed tool to advance such research in the association between CS use, CS pharmacology, and control of inflammation or development of damage with cSLE.
We are cognizant that the development of the SSR is a dynamic process especially with new progress in biomarker studies. This would require, in our opinion, validation in large longitudinal datasets that capture the differential accrual of disease damage with specific CS uses. Indeed, additional insights in the biological factors that modulate response to and damage from CS should be used in future research to enhance the SSR. In this context, the SSR provides a new template to which other CS dosing regimens can be compared. Potentially, the use of biologic agents with marked steroid sparing effects may support more rapid CS tapering. Such a steroid sparing effect could be quantified using the SSR and, subsequently, may necessitate updates to the SSR for patients treated with such medications.
In clinical trials, variability of CS dosing introduces bias and threatens the validity of study results (17). By standardizing CS use, the SSR can counterbalance such bias, and possibly increase the willingness of physicians to contribute patients to studies. The SSR may also support CS use in clinical care, especially by health care providers with less exposure to the treatment of cSLE and pediatric LN. We would like to note that, to enhance the widespread use of the SSR, a web-based calculator is in development.
Supplementary Material
ACKNOWLEDGEMENT & FUNDING:
This project was supported by Pediatric musculOskeletal & RheumaTology Innovation COre center (PORTICO), a National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Core Center of Excellence in Clinical Research focused on Pediatric Musculoskeletal Diseases, under award number P30AR076316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was supported by NIAMS under the award number R34 AR071651. Further, this publication was supported by an Institutional Clinical and Translational Science Award, NIH/NCATS grant number 8UL1TR000077, and REDCap is supported in part by the National Institutes of Health (NIH/NCATS UL1 TR000445). Dr. Nathalie Chalhoub’s contribution to this study was funded in part by grant 2UL1TR001425 from the National Center for Advancing Translational Sciences, and support from the Internal Medicine Scholars Training for Academic Research (IM STAR) program funded by the Department of Internal Medicine at the University of Cincinnati College of Medicine. Dr. Sonia Savani’s contribution to this study was funded in part by a T32 Training Grant (AR050958). Dr. Jianghong Deng was supported from Beijing Municipal Bureau of Foreign Affairs Training’s Award. Dr. Coziana Ciurtin is supported by a Centre of Excellence (Centre for Adolescent Rheumatology Versus Arthritis) grant (21593), as well as a grant from NIHR UCLH BRC (BRC 525 III/CC). Dr. Clovis A. Silva was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 303422/2015-7), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2015/03756-4) and by Núcleo de Apoio à Pesquisa “Saúde da Criança e do Adolescente” da USP (NAP-CriAd). Dr. Prasad Devarajan is supported by NIDDK (P50DK096418). The authors wish to acknowledge CARRA and the ongoing Arthritis Foundation financial support of CARRA. We are grateful for the contributions of the Pediatric Nephrology Research Consortium (PNRC), and to Drs. von Scheven and Cooper for providing us access to background information about their unpublished research on corticosteroid dosing with pediatric lupus nephritis. We thank the Coordinating Center of the Pediatric Rheumatology Collaborative Study Group (PRCSG) in assisting with this project.
Contributor Information
Nathalie E. Chalhoub, Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA;.
Scott E. Wenderfer, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA;.
Deborah M. Levy, Department of Pediatrics, The Hospital for Sick Children and The University of Toronto, Toronto, Ontario, Canada;.
Kelly Rouster-Stevens, Department of Pediatrics, Emory University, Children’s Healthcare of Atlanta, Atlanta, Georgia, USA;.
Amita Aggarwal, Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India;.
Sonia I Savani, Department of Pediatrics; Medical University of South Carolina, Charleston, South Carolina, USA:.
Natasha M Ruth, Department of Pediatrics, Medical University of South Carolina, Charleston, South Carolina, USA;.
Thaschawee Arkachaisri, Department of Pediatric Subspecialties, KK Women’s and Children’s Hospital, Singapore;.
Karen Onel, Division of Pediatric Rheumatology, Hospital for Special Surgery, New York, NY, USA;.
Beatrice Goilav, The Children’s Hospital at Montefiore, Albert Einstein College of Medicine, Bronx, NY, USA;.
Raju P Khubchandani, Section of Pediatric Rheumatology, SRCC Children’s Hospital, Mumbai, India;.
Jianghong Deng, Department of Rheumatology, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, China;.
Adriana R Fonseca, Pediatric Rheumatology Unit, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil;.
Stacy P. Ardoin, Nationwide Children’s Hospital, Columbus, Ohio, USA,.
Coziana Ciurtin, Centre for Adolescent Rheumatology, University College London, London, UK,.
Ozgur Kasapcopur, Department of Pediatrics, Cerrahpasa Medical School, Istanbul University-Cerrahpasa, Istanbul, Turkey;.
Marija Jelusic, Department of Pediatrics, University of Zagreb School of Medicine, Zagreb, Croatia;.
Adam M. Huber, Department of Pediatrics, IWK Health Centre and Dalhousie University, Halifax, Nova Scotia, Canada;.
Seza Ozen, Department of Pediatrics, Hacettepe University, Ankara, Turkey;.
Marisa S Klein-Gitelman, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann & Robert H Lurie Children’s Hospital of Chicago, Chicago, IL, USA;.
Simone Appenzeller, Department of Orthopedics, Rheumatology and Traumatology, School of Medical Science, University of Campinas, Campinas, Brazil;.
André Cavalcanti, Hospital das Clínicas da Universidade Federal de Pernambuco, Recife, Pernambuco, Brazil;.
Lampros Fotis, Department of Pediatrics, ATTIKON General Hospital, National and Kapodistian University of Athens, Athens, Greece;.
Sern Chin Lim, Department of Pediatrics, University Teknologi MARA, Sg Buloh, Selangor, Malaysia;.
Rodrigo M Silva, Department of Pediatrics, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil;.
Julia Ramírez- Miramontes, Department of Pediatrics, Instituto Mexicano del Seguro Social, Monterrey, N.L México;.
Natalie L Rosenwasser, Department of Pediatrics, University of Washington, Seattle Children’s Hospital, Seattle, Washington, USA;.
Claudia Saad-Magalhaes, Department of Pediatrics, Sao Paulo State University (UNESP), Botucatu, Brazil;.
Dieneke Schonenberg-Meinema, Department of Pediatrics, Emma Children’s Hospital, Amsterdam University Medical Centers, Amsterdam, The Netherlands;.
Christiaan Scott, Department of Paediatrics and Child Health, Red Cross War Memorial Children’s Hospital and University of Cape Town, Cape Town, South Africa;.
Clovis A. Silva, Department of Pediatrics, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brazil;.
Sandra Enciso, Department Pediatric Rheumatology, Hospital de la Beneficencia Española, Mexico City, Mexico;.
Maria T Terreri, Department of Pediatrics, Universidade Federal de Sao Paulo, SP, Brazil;.
Alfonso-Ragnar Torres-Jimenez, Department of Pediatric Rheumatology; National Medical Center La Raza IMSS, Mexico City, Mexico;.
Maria Trachana, Pediatric Immunology and Rheumatology Referral Center,1st Pediatric clinic, Aristotle University of Thessaloniki, Greece;.
Sulaiman M Al-Mayouf, Department of Pediatrics, King Faisal Specialist Hospital and Research Center, Alfaisal University, Riyadh, Saudi Arabia;.
REFERENCES
- 1.Brunner HI, Klein-Gitelman MS, Ying J, Tucker LB, Silverman ED. Corticosteroid use in childhood-onset systemic lupus erythematosus-practice patterns at four pediatric rheumatology centers. Clin Exp Rheumatol. 2009;27(1):155–62. [PubMed] [Google Scholar]
- 2.Ilowite NT, Sandborg CI, Feldman BM, Grom A, Schanberg LE, Giannini EH, et al. Algorithm development for corticosteroid management in systemic juvenile idiopathic arthritis trial using consensus methodology. Pediatric Rheumatology. 2012;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criteria for steroid-sparing ability of interventions in systemic lupus erythematosus: report of a consensus meeting. Arthritis and rheumatism. 2004;50(11):3427–31. [DOI] [PubMed] [Google Scholar]
- 4.Mina R, von Scheven E, Ardoin SP, Eberhard BA, Punaro M, Ilowite N, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis care & research. 2012;64(3):375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper JC, Rouster-Stevens K, Wright TB, Hsu JJ, Klein-Gitelman MS, Ardoin SP, et al. Pilot study comparing the childhood arthritis and rheumatology research alliance consensus treatment plans for induction therapy of juvenile proliferative lupus nephritis. Pediatr Rheumatol Online J. 2018;16(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Journal of the American Society of Nephrology : JASN. 2004;15(2):241–50. [DOI] [PubMed] [Google Scholar]
- 7.Deng J, Chalhoub NE, Sherwin CM, Li C, Brunner HI. Glucocorticoids pharmacology and their application in the treatment of childhood-onset systemic lupus erythematosus. Seminars in arthritis and rheumatism. 2019;49(2):251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delbecq AL, Van de Ven AH, Gustafson DH. Group techniques for program planning : a guide to nominal group and Delphi processes. Glenview, Ill.: Scott, Foresman; 1975. [Google Scholar]
- 9.Brunner HI, Mina R, Pilkington C, Beresford MW, Reiff A, Levy DM, et al. Preliminary criteria for global flares in childhood-onset systemic lupus erythematosus. Arthritis care & research. 2011;63(9):1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 11.Austin HA 3rd, Boumpas DT, Vaughan EM, Balow JE. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int. 1994;45(2):544–50. [DOI] [PubMed] [Google Scholar]
- 12.Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25(4):689–95. [DOI] [PubMed] [Google Scholar]
- 13.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis and rheumatism. 1996;39(3):363–9. [DOI] [PubMed] [Google Scholar]
- 14.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91. [PubMed] [Google Scholar]
- 15.Pierrat A, Gravier E, Saunders C, Caira M-V, Aït-Djafer Z, Legras B, et al. Predicting GFR in children and adults: a comparison of the Cockcroft-Gault, Schwartz, and modification of diet in renal disease formulas. Kidney international. 2003;64(4):1425–36. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology : JASN. 2009;20(3):629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corzillius M, Bae SC. Methodological issues of corticosteroid use in SLE clinical trials. Lupus. 1999;8(8):692–7. [DOI] [PubMed] [Google Scholar]
- 18.Walsh M, Jayne D, Moist L, Tonelli M, Pannu N, Manns B. Practice pattern variation in oral glucocorticoid therapy after the induction of response in proliferative lupus nephritis. Lupus. 2010;19(5):628–33. [DOI] [PubMed] [Google Scholar]
- 19.Brunner HI, Silverman ED, To T, Bombardier C, Feldman BM. Risk factors for damage in childhood-onset systemic lupus erythematosus: cumulative disease activity and medication use predict disease damage. Arthritis Rheum. 2002;46(2):436–44. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez-Suarez R, Ruperto N, Gastaldi R, Pistorio A, Felici E, Burgos-Vargas R, et al. A proposal for a pediatric version of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index based on the analysis of 1,015 patients with juvenile-onset systemic lupus erythematosus. Arthritis and rheumatism. 2006;54(9):2989–96. [DOI] [PubMed] [Google Scholar]
- 21.Heshin-Bekenstein M, Trupin L, Yelin E, von Scheven E, Yazdany J, Lawson EF. Longitudinal disease- and steroid-related damage among adults with childhood-onset systemic lupus erythematosus. Semin Arthritis Rheum. 2019;49(2):267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunner HI, Holland M, Beresford MW, Ardoin SP, Appenzeller S, Silva CA, et al. American College of Rheumatology Provisional Criteria for Global Flares in Childhood-Onset Systemic Lupus Erythematosus. Arthritis care & research. 2018;70(6):813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mina R, Klein-Gitelman MS, Ravelli A, Beresford MW, Avcin T, Espada G, et al. Inactive disease and remission in childhood-onset systemic lupus erythematosus. Arthritis care & research. 2012;64(5):683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis & rheumatology (Hoboken, NJ). 2016;68(3):566–76. [DOI] [PubMed] [Google Scholar]
- 25.Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. Expert consensus on dynamics of laboratory tests for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. RMD open. 2016;2(1):e000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh JA, Solomon DH, Dougados M, Felson D, Hawker G, Katz P, et al. Development of classification and response criteria for rheumatic diseases. Arthritis and rheumatism. 2006;55(3):348–52. [DOI] [PubMed] [Google Scholar]
- 27.Ngo D, Beaulieu E, Gu R, Leaney A, Santos L, Fan H, et al. Divergent effects of endogenous and exogenous glucocorticoid-induced leucine zipper in animal models of inflammation and arthritis. Arthritis Rheum. 2013;65(5):1203–12. [DOI] [PubMed] [Google Scholar]
- 28.Northcott M, Gearing LJ, Nim HT, Nataraja C, Hertzog P, Jones SA, et al. Glucocorticoid gene signatures in systemic lupus erythematosus and the effects of type I interferon: a cross-sectional and in-vitro study. The Lancet Rheumatology. 2021;3(5):e357–e70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


