Abstract
Introduction:
In classical EGFR-mutant non-small-cell lung cancer (NSCLC), EGFR tyrosine kinase inhibitor (TKI) therapy yields better outcomes than platinum-based chemotherapy. However, EGFR exon 20 insertion (ex20ins) NSCLC is relatively resistant to currently available EGFR TKIs. Though platinum-based chemotherapy is the frontline standard of care for EGFR ex20ins NSCLC, its efficacy is not fully described.
Study design:
A retrospective, single-center, case series
Methods:
Patients were identified through an electronic research database at a single institution and included if they had advanced EGFR ex20ins NSCLC, received platinum-based chemotherapy for metastatic disease, and had scans evaluable for response. Each patient’s demographics, tumor characteristics, and clinical course were recorded. Treatment response was evaluated using RECIST v1.1 criteria, and the PFS was calculated by the Kaplan-Meier method.
Results:
Among 27 patients identified with EGFR ex20ins NSCLC at our institution, 18 (67%) received platinum-based chemotherapy for metastatic disease and had scans evaluable for response. These patients received platinum-based chemotherapy in the first-line (N=17, 94%) and second-line settings (N=1, 6%). The objective response rate (ORR) to platinum-based chemotherapy was 39% (7 of 18 patients; 95% confidence interval [CI] 16-61). The median PFS with platinum-based chemotherapy was 7.1 months (95% CI, 6.3 – 13.7), and the median overall survival was 3.2 years (95% CI, 1.92 – NR).
Conclusions:
The efficacy of platinum-based chemotherapy in EGFR ex20ins NSCLC is similar to that expected for TKI sensitive EGFR-mutant NSCLC. Novel agents designed to specifically target ex20ins mutant EGFR should additionally improve outcomes.
Keywords: Epidermal growth factor receptor exon 20 insertion, tyrosine kinase inhibitor, non-small cell lung cancer, platinum-based chemotherapy
Microabstract
To better understand the efficacy of chemotherapy in treating EGFR exon 20 insertion lung cancer, we conducted a retrospective, single-institution case series (n = 29). We found that chemotherapy leads to an average seven months of progression-free survival for these patients. Our findings provide important context for novel therapies being developed for this rare subtype of lung cancer.
Introduction
In non-small-cell lung cancer (NSCLC), molecular profiling and the development of targeted therapies has led to significant improvements for patients with targetable mutations.1-4 Activating mutations in the epidermal growth factor receptor (EGFR) gene occur in 32% of patients with NSCLC.5 EGFR is a receptor tyrosine kinase that is heavily involved in cellular pathways promoting cell survival, proliferation, and migration. Increased activity of the EGFR protein drives cancer growth. Conformational changes in the classical EGFR L858R point mutation and exon 19 deletions destabilize the dormant form of EGFR molecules and shift the equilibrium towards the active form. In contrast, EGFR ex20ins mutations are thought to “lock” EGFR molecules in the active state.6
The classical EGFR L858R point mutation and exon 19 deletions comprise 85-90% of EGFR mutant NSCLC, and are frequently responsive to EGFR tyrosine kinase inhibitors (TKIs), with objective response rates (ORR) of up to 80%.1, 7, 8 This has led to the development of many successful EGFR targeted therapies in recent years, yielding progression-free survival (PFS) of greater than 10 months for first-generation EGFR TKIs and 19 months for third-generation EGFR TKIs (e.g., osimertinib).1, 9
Meanwhile, EGFR ex20ins NSCLC, which comprises ~4% of all EGFR mutant NSCLC, is associated with resistance to available EGFR TKIs and therefore confers poorer outcomes for patients.6, 10, 11 EGFR proteins with ex20ins mutations have binding pockets that are inaccessible to existing EGFR TKIs.12 Retrospective studies of EGFR ex20ins NSCLC treated with first-generation EGFR TKIs such as gefitinib and erlotinib reported ORR between 8 and 27% and a median PFS of less than 3 months.6, 13 Third-generation EGFR inhibitors have slightly better activity against EGFR ex20ins NSCLC.14, 15 Of note, a few EGFR ex20ins mutation variants, such as A763_Y764insFQEA insertion, are significantly more responsive to existing EGFR TKIs.16
Therefore, patients with EGFR ex20ins NSCLC are commonly treated with platinum-based chemotherapy as first-line systemic therapy. In clinical trials of EGFR TKIs versus platinum-based chemotherapy in classical EGFR mutant NSCLC, platinum-based chemotherapy yields an ORR of about 30% and median PFS of about 5-6 months.7, 8, 17 While presently chemotherapy is often given concurrently with immune checkpoint inhibitor immunotherapy in first-line NSCLC, the existing indications exclude EGFR mutant NSCLC and limited data suggest that immunotherapy monotherapy is ineffective against previously treated EGFR mutant NSCLC.18 Meanwhile, existing literature on the utility of anti-angiogenic therapy in EGFR mutant NSCLC is mixed.19
Although platinum-based chemotherapy, with or without anti-angiogenic therapy and/or immunotherapy, is a reasonable standard of care for EGFR ex20ins NSCLC, its efficacy in this subset of EGFR mutant NSCLC is not fully described in the literature. The purpose of this study is to describe the efficacy of platinum-based chemotherapy in EGFR ex20ins NSCLC. This will provide important real-world control in light of ongoing clinical trials of new targeted agents designed to target EGFR ex20ins insertion.
Methods
This retrospective study was conducted at the Stanford University Hospital & Clinics and Institutional Review Board approval was obtained from Stanford University. Eligible patients were those who were over 18 years of age at the time of diagnosis of EGFR ex20ins NSCLC. Using the STAnford medicine Research data Repository clinical database (STARR), all patients with EGFR ex20ins NSCLC who received platinum-based chemotherapy and had scans evaluable for treatment response were identified from December 1, 2013 to July 31, 2020.
Descriptive data (e.g., demographics, tumor characteristics, treatment courses) were collected for each patient. Of the 27 patients identified with EGFR ex20ins NSCLC, 18 received platinum-based chemotherapy and had scans that were evaluable for response. Molecular testing predominantly consisted of tissue testing with next-generation sequencing (NGS) through the Stanford Solid Tumor Actionable Mutation Panel (n = 14, 78%). A smaller subset had tissue PCR-based testing (n = 4, 22%) when diagnostics were performed outside our institution. Tumor measurements were made by clinical investigators MPS and JVA and subsequently reviewed by medical oncology investigator JWN. Tumor responses were determined using RECIST 1.1 criteria, and PFS was calculated using the Kaplan-Meier method. Statistical significance was defined at a p < 0.05.
Results
Patient demographics, characteristics, and treatments for the cohort of 18 patients are shown in table 1. The average age was 60 years, two-thirds were women, and less than a quarter had any smoking history. The histotype was adenocarcinoma in all cases, and all presented with stage IV disease at the time of systemic therapy. Fourteen (78%) patients underwent targeted NGS testing and the most commonly identified subtypes of EGFR ex20ins mutation included V769_D770insASV (28%) and A767_V769dup (28%). The remaining 4 insertion points were not characterized as these patients underwent PCR-based testing. Over half the patients had bone metastases, and fewer than half had metastases to liver, brain, or adrenal glands.
Table 1.
Patient Characteristics and Treatments
| Characteristic | n (%) |
|---|---|
| Total | 18 |
| Male/Female | 6 (33) / 12 (67) |
| Race/ethnicity | |
| Non-Hispanic white | 7 (39) |
| Hispanic | 4 (22) |
| Asian | 7 (39) |
| Mean age at diagnosis | 60y (range 32-81) |
| Positive smoking history | 4 (22) |
| Histological subtype | |
| Adenocarcinoma | 18 (100) |
| Ex20ins mutation subtype | |
| V769_D770insASV | 5 (28) |
| A767_V769dup | 5 (28) |
| S768_D770dup | 2 (11) |
| p.N771delinsGF | 1 (6) |
| M766_A767ins | 1 (6) |
| Unknown | 4 (22) |
| Co-mutations and other tumor markers | |
| TP53 | 7 (39) |
| PIK3CA | 1 (6) |
| APC | 1 (6) |
| ERBB2 | 1 (6) |
| PD-L1 (high expression, > 50%) | 1 (6) |
| CDK4 amplification | 1 (6) |
| Stage at systemic treatment initiation | |
| IV | 18 (100) |
| Location of metastases | |
| Brain | 3 (17) |
| Bone | 11 (61) |
| Liver | 5 (28) |
| Adrenals | 2 (11) |
| Prior local therapy | |
| Surgery | 1 (6) |
| Radiation | 14 (78) |
| Platinum-based chemotherapy regimen | |
| Carboplatin + pemetrexed | 10 (56) |
| Carboplatin + pemetrexed + bevacizumab | 5 (28) |
| Carboplatin + pemetrexed + pembrolizumab | 2 (11) |
| Carboplatin + paclitaxel + bevacizumab | 1 (6) |
| Line of therapy | |
| First-line | 17 (94) |
| Second-line | 1 (6) |
Most patients (17 of 18) received platinum-based chemotherapy as the first-line of therapy, whereas one patient received it as second-line therapy after progression on afatinib, which was given at an outside institution. Most patients (17 of 18) received carboplatin with pemetrexed, and one patient received carboplatin with paclitaxel. A third of the patients (6 of 18) had bevacizumab added to their chemotherapy regimen, and two patients had pembrolizumab added to their chemotherapy regimen.
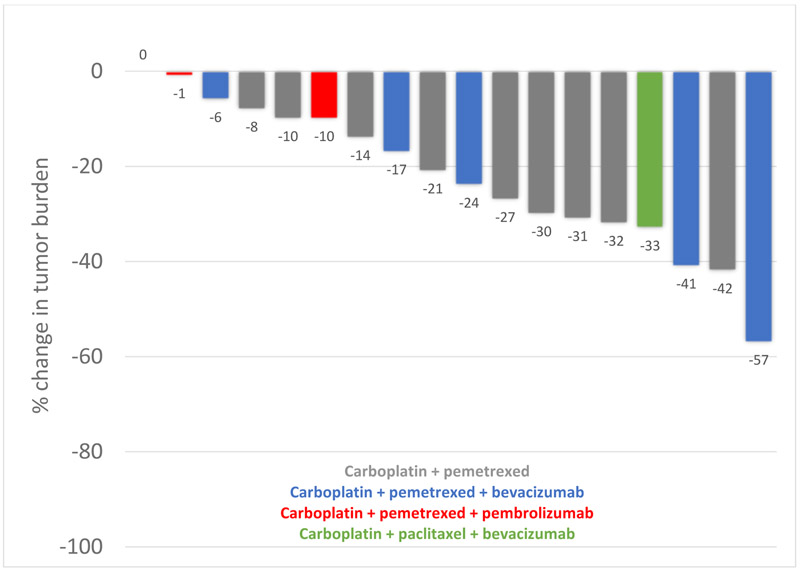
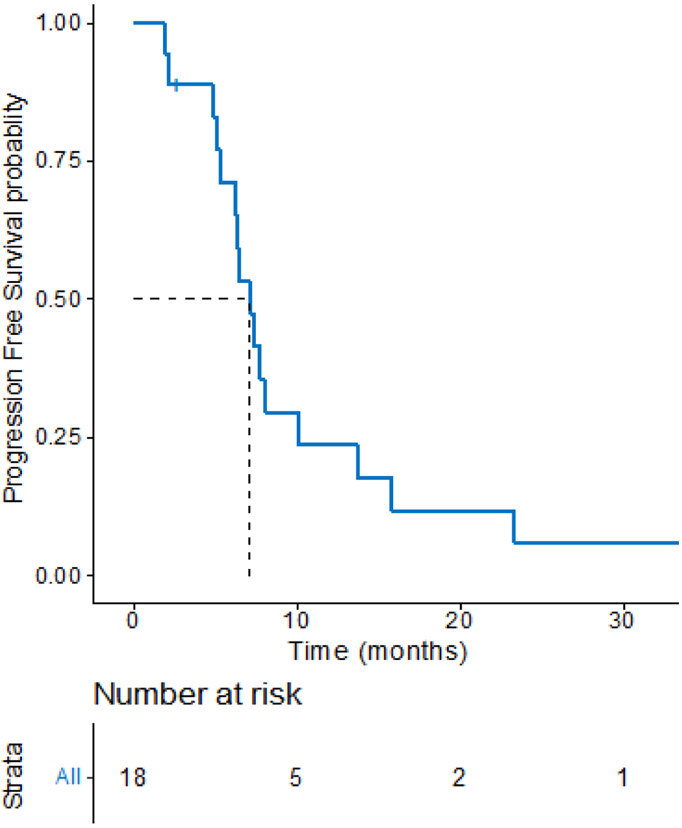
The objective response rate to platinum-based chemotherapy was 44% (8 partial response, 10 stable disease) with an average 22% reduction (s.d.15.6) in tumor burden, as shown in figure 1. The median PFS with platinum-based chemotherapy was 7.1 months (95% CI, 6.3 – 13.7), as shown in Figure 2. At the time of our analysis (data cutoff: July 31, 2020), 9 patients had died, and the median overall survival was 3.2 years (95% CI, 1.92 – NR). A sensitivity analysis excluding the two patients who received carboplatin/pemetrexed/pembrolizumab showed consistent results (data not shown).
Figure 1.
Best change in tumor burden for 18 patients with EGFR ex20ins NSCLC treated with platinum-based chemotherapy
Figure 2.
PFS Kaplan-Meier curve of 18 patients with EGFR ex20ins NSCLC treated with platinum-based chemotherapy
In the six patients who received bevacizumab with platinum-based chemotherapy, the mean tumor burden reduction was 30%, the ORR was 50%, and the median PFS was 6.2 months. In the two patients who received pembrolizumab with platinum-based chemotherapy, best response was stable disease, and the PFS were 2.3 months and 14.5 months.
In the five patients harboring the V769_D770insASV mutation, the mean tumor burden reduction was 21%, the ORR was 20%, and the median PFS was 5.2 months. In the five patients harboring the A767_V769dup mutation, the mean tumor burden reduction was 21%, the ORR was 40%, and the median PFS was 7.8 months.
Discussion
In this single-institution retrospective study, we describe the efficacy of platinum-based chemotherapy in 18 patients with EGFR ex20ins NSCLC. The ORR was 44%, median PFS was 7.1 months, and median OS was 3.2 years. These efficacy results are similar to those with platinum-based chemotherapy in EGFR TKI sensitive EGFR mutant NSCLC (i.e., L858R, ex19del).7, 8, 17 In the subgroup of 6 patients who received bevacizumab in combination with platinum-doublet therapy, the ORR and median PFS were not numerically different from the analysis of the overall population. In the two patients who received platinum-doublet chemotherapy in combination with pembrolizumab, both had stable disease, though notably one patient with high PD-L1 expression had a prolonged PFS of 14.5 months. In the subgroup of five patients harboring the A767_V769dup mutation, the ORR and median PFS were only slightly numerically greater than those in the subgroup of five patients harboring the V769_D770insASV mutation. Given the small sample size, we were unable to explore the association of clinical outcomes with therapy regimens, metastatic sites, specific ex20ins mutation, or presence of comutations (e.g. TP53).
Two recently published retrospective studies have described the clinical course of EGFR ex20ins NSCLC in Hispanic and Chinese patients. In the first study, 79 Hispanic patients with EGFR ex20ins NSCLC were treated with first-line EGFR TKI therapy or platinum-based chemotherapy with or without bevacizumab. Of the 50 patients who received first line platinum-based chemotherapy, the PFS was 6.9 months, consistent with the findings in our study. Unlike our study, 36% of tumors in this study had co-existing classical sensitizing EGFR mutations, which is much higher than previously described.20 In another study, 104 Chinese patients with EGFR ex20ins NSCLC who received first-line platinum-based chemotherapy demonstrated an ORR of 19.2% and a median PFS of 6.4 months, with the ORR being lower but the PFS being similar to our study.21
Limited data describe efficacy of single-agent immunotherapy in EGFR ex20ins NSCLC. A series of 36 patients with EGFR ex20ins NSCLC demonstrated an ORR of 25% and median PFS of 2.9 months. These values were numerically better than what was observed in 38 patients with classical EGFR sensitizing mutations, where the observed ORR was 0% and the median PFS was 1.9 months.22 In our study, only two patients received immunotherapy in combination with platinum-doublet chemotherapy and the value of this strategy in patients with EGFR ex20ins NSCLC needs further study.
Though existing EGFR TKIs have failed to improve the outcomes for patients with EGFR ex20ins NSCLC, novel TKIs designed to target the ex20ins EGFR protein are being tested against platinum-based chemotherapy in the frontline setting. These first line clinical trials will provide prospective data on the efficacy of platinum-based chemotherapy in EGFR ex20ins NSCLC. For these novel agents to be proven superior to first-line platinum chemotherapy, response rates over 40% and/or PFS over 8 months will likely be needed. Mobocertinib (TAK-788) is a promising novel TKI that covalently binds to EGFR molecules with selectivity towards ex20ins mutant EGFR over wild-type EGFR. Preliminary results from a phase II open-label, cohort expansion showed that mobocertinib demonstrated a high disease control rate of 86% (NCT02716116). Mobocertinib is now being studied through the global EXCLAIM extension study in 91 previously treated patients with advanced EGFR ex20ins NSCLC and is also being tested head-to-head against first-line carboplatin and pemetrexed in the EXCLAIM-2 study (NCT04129502).
Amivantamab, a novel bispecific antibody targeting EGFR and mesenchymal epithelial transition factor (MET) receptor, was recently approved for patients with locally advanced or metastatic EGFR ex20ins NSCLC after progression on or after platinum-based chemotherapy. This accelerated approval was based on results from the multicenter, multicohort, non-randomized, open label clinical trial CHRYSALIS. In the subset of 81 patients who had progressed on platinum-based chemotherapy, the ORR was 40% and the median duration of response was 11.1 months (NCT02609776). Based on these results, amivantamab in the second-line setting for EGFR ex20ins NSCLC yields an ORR similar to what we observe with platinum-based chemotherapy in the first-line setting. These results suggest that amivantamab may be efficacious against EGFR ex20ins as first-line therapy. A phase III study of combination amivantamab and carboplatin-pemetrexed therapy compared with carboplatin-pemetrexed in advanced EGFR ex20ins NSCLC is currently underway (NCT04538664).
Limitations of our study include a small cohort, retrospective approach, and conduct within a single institution. Due to not all tumors undergoing NGS testing, mutation subtypes were available for most but not all patients limiting possible comparison of platinum-based chemotherapy efficacy between groups.
Regarding our institutional practice patterns for EGFR Ex20ins NSCLC, our preferred platinum regimen includes carboplatin and pemetrexed, with or without bevacizumab. If immunotherapy is desired, then the four drug “IMpower 150” regimen consisting of atezolizumab, bevacizumab, carboplatin and paclitaxel could be considered; however, our institutional preference is generally to avoid PD-(L)1 inhibitors in patients with EGFR mutations due to uncertain effectiveness, except perhaps in patients with a smoking history or high PD-L1 expression.
Conclusion
EGFR ex20ins NSCLC has proven resistant to currently available EGFR TKIs, yielding significantly worse outcomes for patients when compared to those of classical EGFR mutant NSCLC. Our study adds real-world data to the literature of platinum-doublet efficacy in patients with EGFR ex20ins NSCLC. Until randomized studies of novel TKIs are complete, the current first-line standard of care for EGFR ex20ins NSCLC remains a platinum-based chemotherapy backbone, which has similar efficacy in this subtype as in other EGFR mutant NSCLC. Novel drugs to target EGFR ex20ins NSCLC will hopefully soon be approved and improve the outcomes for patients.
Clinical practice points
EGFR TKI therapies lead to better outcomes than platinum-based chemotherapy for patients with classical EGFR mutant NSCLC. However, in EGFR ex20ins NSCLC, currently available TKI therapies have proven ineffective, and chemotherapy is used instead. The efficacy of platinum-based chemotherapy in ex20ins NSCLC has not been fully described in the literature. In our study, we found that the objective response rate (ORR) to platinum based-chemotherapy was 39%, and the median progression-free survival was 7.1 months, suggesting that the efficacy of platinum-based chemotherapy in EGFR ex20ins NSCLC is similar to that expected for TKI sensitive EGFR-mutant NSCLC. Our study adds real-world data to the literature of platinum-doublet efficacy in patients with EGFR ex20ins NSCLC. Until randomized studies of novel TKIs are complete, the current first-line standard of care for EGFR ex20ins NSCLC remains a platinum-based chemotherapy backbone. Our preferred platinum regimen includes carboplatin and pemetrexed, with or without bevacizumab. We anticipate that novel therapies designed to specifically target ex20ins mutant EGFR will further improve outcomes.
Acknowledgements
STARR platform is developed and operated by Stanford Medicine Research IT team and is made possible by Stanford School of Medicine Research Office.
Footnotes
Disclosures: Unrelated to this work:
SKP reports personal fees from Pfizer, G1 Therapeutics, Blueprint Medicine, AstraZeneca, G1 Therapeutic; grants from Epicentrx, Bayer, Boehringer Ingelheim, 47 Inc.; all outside the submitted work.
KJR reports consulting with Varian and Group Well
HAW reports research support from ACEA Biosciences, Arrys Therapeutics, AstraZeneca/Medimmune, BMS, Celgene, Clovis Oncology, Exelixis, Genentech/Roche, Gilead, Merck, Novartis, Pharmacyclics, Seattle Genetics, Xcovery, Eli Lilly, and Pfizer; payment from Novartis and AstraZeneca; participation in a data safety or advisory board for AstraZeneca, Xcovery, Janssen, Daiichi Sankyo, Blueprint, Mirati, Helsinn, Merck, Takeda, Genentech/Roche, and Cellworks
JWN reports research support from Genentech/Roche, Merck, Novartis, Boehringer Ingelheim, Exelixis, Nektar Therapeutics, Takeda Pharmaceuticals, Adaptimmune, and GSK; serves an advisory role for AstraZeneca, Genentech/Roche, Exelixis, Jounce Therapeutics, Takeda Pharmaceuticals, Eli Lily and Company, Calithera Biosciences, Amgen, and Iovance Biotherapeutics; and reports honoraria from Research to Practice, MLI Peerview, Medscape, Biomedical Learning Institute, Prime Oncology, Rockpointe, CME Matters, and MJH CME; and reports royalties from UpToDate.
Declaration of Competing Interest
All remaining authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soria J-C, Ohe Y, Vansteenkiste J, et al. : Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378:113–125, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Solomon BJ, Mok T, Kim D-W, et al. : First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–2177, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Planchard D, Smit EF, Groen HJM, et al. : Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18:1307–1316, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Ou S-HI, Bang Y-J, et al. : Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371:1963–1971, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y-L, Yuan J-Q, Wang K-F, et al. : The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 7:78985–78993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyse S, Huang PH: Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 4:5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok TS, Wu Y-L, Thongprasert S, et al. : Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, et al. : Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu Y-L, Chen G, et al. : Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Arcila ME, Nafa K, Chaft JE, et al. : EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 12:220–229, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Mitsudomi T: Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci 107:1179–1186, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robichaux JP, Elamin YY, Tan Z, et al. : Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med 24:638–646, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naidoo J, Sima CS, Rodriguez K, et al. : Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: Clinical outcomes and response to erlotinib. Cancer 121:3212–3220, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piotrowska Z, Wang Y, Sequist LV, et al. : ECOG-ACRIN 5162: A phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions. JCO 38:9513–9513, 2020 [Google Scholar]
- 15.van Veggel B, Madeira R Santos JFV, Hashemi SMS, et al. : Osimertinib treatment for patients with EGFR exon 20 mutation positive non-small cell lung cancer. Lung Cancer 141:9–13, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Voon PJ, Tsui DWY, Rosenfeld N, et al. : EGFR exon 20 insertion A763-Y764insFQEA and response to erlotinib--Letter. Mol Cancer Ther 12:2614–2615, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Maemondo M, Inoue A, Kobayashi K, et al. : Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Cavanna L, Citterio C, Orlandi E: Immune checkpoint inhibitors in EGFR-mutation positive TKI-treated patients with advanced non-small-cell lung cancer network meta-analysis. Oncotarget 10:209–215, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander M, Halmos B: VEGF inhibitors in EGFR-mutated lung cancer: a never-ending story? Ann Transl Med 6:446, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardona AF, Rojas L, Zatarain-Barron ZL, et al. : EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP). Lung Cancer 125:265–272, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Li J, Xu H, et al. : EGFR exon 20 insertion mutations in Chinese advanced non-small cell lung cancer patients: Molecular heterogeneity and treatment outcome from nationwide real-world study. Lung Cancer 145:186–194, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Negrao MV, Reuben A, Robichaux JP, et al. : Association of EGFR and HER-2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non-small cell lung cancer. JCO 36:9052–9052, 2018 [Google Scholar]
- 23.Robichaux JP, Elamin YY, Vijayan RSK, et al. : Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell 36:444–457.e7, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le X, Goldman J, Clarke J, et al. : Abstract CT081: Poziotinib activity and durability of responses in previously treated EGFR exon 20 NSCLC patients - a Phase 2 study [Internet], in Tumor Biology. American Association for Cancer Research, 2020, pp CT081–CT081 [cited 2021 Feb 22] Available from: http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.AM2020-CT081 [Google Scholar]