Abstract
Chronic liver disease (CLD) is one of the leading causes of disability-adjusted life years in many countries. A recent understanding of nuclear bile acid receptor pathways has increased focus on the impact of crosstalk between the gut, bile acids, and liver on liver pathology. While conventionally used in cholestatic disorders and to dissolve gallstones, the discovery of bile acids’ influence on the gut microbiome and human metabolism offers a unique potential for their utility in early and advanced liver diseases because of diverse etiologies. Based on these findings, preclinical studies using bile acid-based molecules have shown encouraging results at addressing liver inflammation and fibrosis. Emerging data also suggest that bile acid profiles change distinctively across various causes of liver disease. We summarize the current knowledge and evidence related to bile acids in health and disease and discuss culminated and ongoing therapeutic trials of bile acid derivatives in CLD. In the near future, further evidence in this area might help clinicians better detect and manage liver diseases.
Keywords: cirrhosis, microbiome, bile acids, CLD, NAFLD
Abbreviations: ALP, Alkaline phosphatase; AD, Acute decompensation; AMACR, α-methylacyl-CoA racemase (AMACR); ASBT, Apical sodium dependent bile salt transporter; BA, Bile acid; BSEP, Bile salt export pump; BSH, Bile salt hydrolase; CA, Cholic acid; CLD, Chronic Liver Disease; CDCA, Chenodeoxycholic acid; CYP7A1, Cholesterol 7 α hydroxylase; CTP, Child-Turcotte-Pugh; DCA, Deoxycholic acid; DR5, Death receptor 5; ELF, Enhanced Liver Fibrosis; FXR, Farnesoid X receptor; FGF-19, Fibroblast growth factor-19; FGFR4, FGF receptor 4; GCA, Glycocholic acid; GDCA, Glycodeoxycholic acid; GLP-1, Glucagon-like peptide1; HVPG, Hepatic Venous Pressure Gradient; HBV, Hepatitis B virus; HCV, Hepatitis C virus; LCA, Lithocholic acid; LPS, Lipopolysaccharide; MELD, Model for End-Stage Liver Disease (MELD); MRI-PDFF, Magnetic resonance imaging derived proton density fat fraction; NAFLD, Non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, Nonalcoholic steatohepatitis; NTCP, Sodium taurocholate cotransporting polypeptide; OCA, Obeticholic acid; OST, Organic solute transporter; PBC, Primary biliary cirrhosis; PSC, Primary sclerosing cholangitis; PFIC, Progressive familial intrahepatic cholestasis; PXR, Pregnane X receptor; SHP, Small heterodimer partner; TBA, Total bile acids; TGR5, Takeda G-protein coupled receptor 5; TRAIL, TNF-related apoptosis-inducing ligand; UDCA, Ursodeoxycholic acid; UPLC-MS, Ultra-performance liquid chromatography with tandem mass spectrometry; VDR, Vitamin D receptor
Liver cirrhosis is the 8th most common cause of death in low-middle-income countries as per the World Health Organization's official report.1 The most common etiologies of cirrhosis are alcohol, chronic viral hepatitis, and the emerging menace of nonalcoholic fatty liver disease (NAFLD).2, 3, 4 Decompensated cirrhosis, in particular, has high mortality with a median survival of 2 years. In patients with cirrhosis, bacterial translocation further aggravates fluid shifts and increases mortality risk due to sepsis.5 Recent insights about the role of bile acid (BA) composition on insulin sensitivity and their dialog with the gut microbiome, apart from their well-known digestive functions, have drawn attention to their relevance in chronic liver disease (CLD). From numerous animal and human studies, we now know that through nuclear receptors, BAs play a role in regulating the qualitative and quantitative BA pool, gut microbiome, and glucose and lipid metabolism.6, 7, 8
An increase in studies characterizing the fecal and serum BA pool in cirrhosis has helped improve our understanding of the pathogenesis and enabled the development of diagnostic or prognostic markers of liver disease. As expected, many BA receptor–based therapeutics are currently in the pipeline. In this narrative review, we expand on the synthesis and metabolism of BAs, their effect on the gut microbiome, and the progression of CLD, in addition to discussing their emerging role in diagnostics and therapeutics.
Bile acid synthesis and metabolism in health
Primary BAs are synthesized from cholesterol in hepatocytes predominantly via the neutral/classic pathway and in small amounts by the acidic/alternate pathway. The rate-limiting and regulatory step in BA synthesis via classic pathway is enzymatically catalyzed by cholesterol 7 α hydroxylase (CYP7A1).9 This step is sensitive to negative feedback regulation via excess BAs. The primary BAs include cholic acid (CA) and chenodeoxycholic acid (CDCA) and are further conjugated to amino acids glycine and taurine (3:1) to increase their solubility. The BAs secreted by the hepatocytes are stored in the gallbladder and released postprandially into the proximal small bowel, where they carry out their well-known digestive functions such as emulsification and formation of micelles aiding in the absorption of fat- and fat-soluble vitamins. They are transported into the distal small bowel from where they are actively absorbed into the portal circulation and transported to the hepatocytes for resecretion via the enterohepatic cycle (Figure 1). The majority of the BAs are salvaged actively from the distal small bowel, and a small proportion escapes into the colon, where they are deconjugated by bacterial bile salt hydrolase and dehydroxylated to secondary BAs: deoxycholic acid and lithocholic acid. Ursodeoxycholic acid (UDCA) is a secondary BA formed from the bacterial epimerization of CDCA. These secondary BAs are also absorbed and form a minor fraction of the total BA pool in the body.
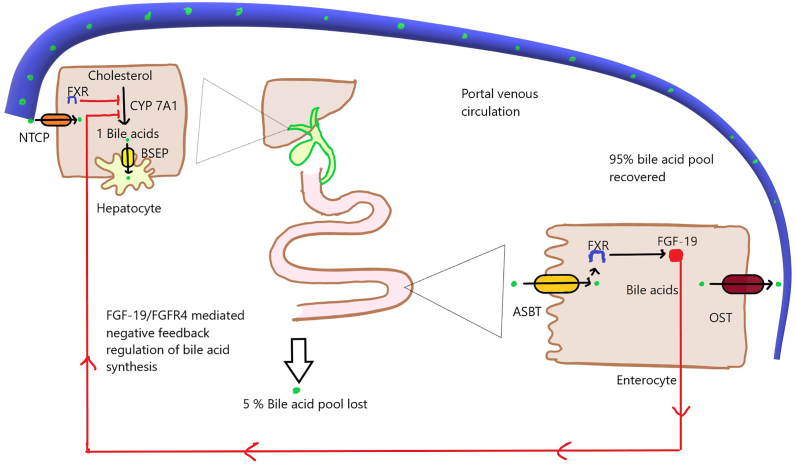
Figure 1.
Enterohepatic cycle: Primary bile acids are synthesized predominantly via the classic pathway (80%) and secreted via bile salt export pump (BSEP) following conjugation with glycine and taurine. Following storage, in the gallbladder, they are released postprandially into the proximal small bowel. The secreted bile acids are actively absorbed via luminal apical sodium-dependent bile salt transporter (ASBT) in the distal small bowel from where they are transported to the portal circulation via organic solute transporter (OST). The reabsorbed bile acids are taken up by hepatocyte sinusoidal membrane protein sodium taurocholate cotransporting polypeptide (NTCP) and resecreted. The efficient enterohepatic cycling enables reabsorption of up to 95% of the bile acids and loss of around 5% of the pool which is resynthesized. CYP7A1: cytochrome P450 family 7 subfamily A member 1; FGF-19:fibroblast growth factor 19; FGFR4: fibroblast growth factor receptor 4; FXR: farnesoid X receptor.
Apart from their well-known digestive functions in the gut, BAs act on a myriad of nuclear receptors such as farnesoid X receptor (FXR), vitamin D receptor and pregnane X receptor, and surface receptors such as Takeda G protein–coupled receptor 5 (TGR5) (Figure 2) to bring about their signaling effects. FXR receptors are expressed widely in hepatocytes as well as enterocytes. In the hepatocytes, BAs inhibit CYP7A1 via induction of small heterodimer partner, while in the enterocytes, they induce the production of fibroblast growth factor-19 (FGF-19), which acts via FGF receptor 4 (FGFR4) to inhibit CYP7A1 and BA synthesis. BAs also act on surface receptors such as TGR5 on enteroendocrine cells, causing the release of glucagon-like peptide-1, which acts as an incretin and plays a role in adipose tissue browning.9
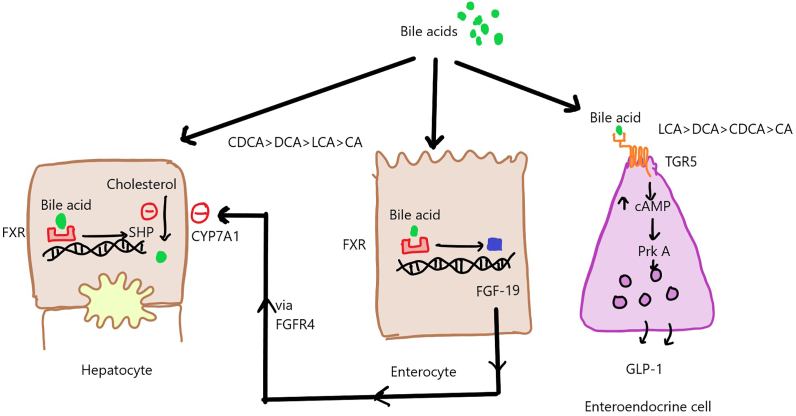
Figure 2.
Bile acid signaling via nuclear and surface receptors. CA: cholic acid; cAMP: cyclic adenosine monophosphate; CDCA: chenodeoxycholic acid; CYP7A1: cytochrome P450 family 7 subfamily A member 1; DCA: deoxycholic acid; FGF19: fibroblast growth factor 19; FGFR4: fibroblast growth factor receptor 4; FXR: farnesoid X receptor; GLP-1: glucagon-like peptide-1; LCA: lithocholic acid; PRK A: phosphoribulokinase A; SHP: small heterodimer partner; TGR-5: takeda-G-protein-receptor-5.
Dysregulated bile acid metabolism, cirrhosis, and dysbiosis: a multidirectional relationship
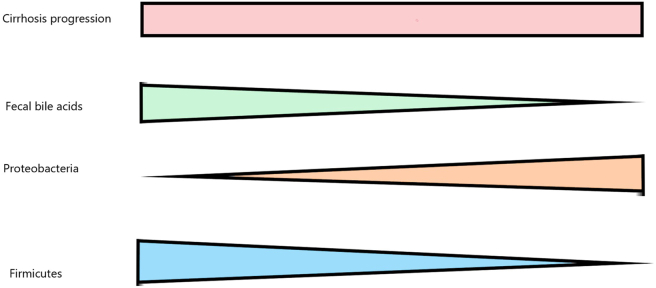
BA pool is depleted in patients with cirrhosis because of decreased synthesis and secretion of BAs from hepatocytes and disproportionate partitioning of BAs. Furthermore, the accumulation of BAs in the blood and within hepatocytes compounds the inhibition of CYP7A1, contributing to the BA pool depletion.10 The decreased levels of fecal BAs promote depletion of Firmicutes, particularly Blautia and Ruminococcus species, and expansion of proinflammatory pathogenic bacteria of the phylum Proteobacteria, particularly Enterobacteriaceae (Figure 3). This is because colonic microbial groups are responsible for deconjugation and 7-alpha dehydroxylation of BAs, and it is hypothesized that the presence of microbe toxic BAs (particularly deoxycholic acid [DCA]) in the intestine is one of the factors that keep undesirable microbial populations under control.11, 12, 13, 14, 15 The dysbiosis is linked to loss of membrane disrupting activity of secondary BAs directly as well as indirectly via loss of secretion of receptor-mediated antimicrobial peptides via BA signaling.11 The decrease in Firmicutes is possibly because BAs serve as primary fermentative electron acceptors for these bacteria.11 The resulting inflammation contributes to increased translocation of bacterial products such as lipopolysaccharide and compounds the progression of cirrhosis. Apart from depleted BA pool, reduced intestinal motility decreased gastric and pancreatobiliary secretions, and portal hypertensive enteropathy can disturb the normal intestinal microbial community in patients with cirrhosis.
Figure 3.
Effect of progression of cirrhosis on bile acids and microbiota.
Apart from these changes in patients with cirrhosis, the proinflammatory cytokines inhibit the classic pathway's key enzyme CYP7A1. Hence, the alternate pathway forms the major source of BA synthesis in cirrhosis.16 Patients with cirrhosis also have a depletion of 7α dehydroxylation bacteria, which leads to decreased secondary: primary BA ratio in cirrhosis.16 Only the emergence of new microbial groups does not foster a pathologic milieu. Even the normally prevalent microbial groups often shift toward a more toxin-producing metabolic pathway because of survival benefits in a dysbiotic environment, thereby disturbing homeostasis.17 Ridlon et al hypothesize that a decrease in DCA across different etiologies of cirrhosis could be beneficial as DCA could compound bacterial translocation and endotoxemia by irritating the gut mucosa, considering its membrane solubilizing potency.18
Gut microbiota composition and BA levels in liver disease
Primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC) patients have decreased alpha/intraindividual diversity of microbes with higher proinflammatory genera levels than healthy controls, with some groups responding after 6 months of UDCA therapy.19 Interestingly, Enterococcus was associated with an increase in TLCA (taurolithocholic acid) levels, a highly hydrophobic BA, in PSC patients.20, 21, 22, 23
In chronic hepatitis B virus (HBV) cirrhosis, there is a decrease in the conversion of primary to secondary BAs and increase in FGF-19 (feedback inhibition of de novo BA synthesis), with advancing fibrosis.24
In alcoholic cirrhosis, an increase in primary BAs correlated with Enterobacteriaceae populations,25 whereas in NAFLD, lower levels of Ruminococcaceae were associated with higher primary BAs in both obese and lean patients with NAFLD, whereas Veillonellaceae showed a positive association with primary BAs in lean patients only.26
Patients with autoimmune hepatitis (AIH) patients show lower alpha diversity compared with healthy controls with lower levels of commensal flora even after controlling for potential confounders, such as age, sex, and antibiotic use,27 but no correlation with BA levels has been done.28
BA-mediated toxicity and inflammation
BAs can induce cell necrosis by solubilizing the plasma membrane or by signaling programmed cell death/apoptosis. However, elevated BA concentrations, even in diseased states, rarely cause physical cellular damage.29 BAs as signaling molecules can trigger cellular death pathways or release chemokines to recruit inflammatory cells.30, 31, 32 In vitro studies in hepatocytes across species have shown that intracellular accumulation of BAs can cause oligomerization of the Fas receptor and activation of TNF-related apoptosis-inducing ligand receptor or death receptor 5.33,34 This leads to activation of death-inducing signaling complex, which leads to caspase 8 activation. Caspase 8 brings about further cleavage of antiapoptotic proteins and activation of proapoptotic proteins. The downstream signaling brings about mitochondrial permeability transition, the release of cytochrome C, and mitochondrial dysfunction.
Glycodeoxycholic acid (GCDCA) is primarily known to induce apoptosis of cholangiocytes and hepatocytes.13 In addition, reactive oxygen species generated due to excessive BAs (via phospholipase A2-induced membrane damage and interaction with nuclear receptors) overwhelm glutathione, which normally checks on repeated expected and stochastic cellular oxidative stressors. This increases the probability of cellular damage and necrosis. BAs can even increase Ca++ release from the endoplasmic reticulum (ER), triggering extracellular calcium entry into cells and activating caspases. A molecule called CHOP is involved in the ER stress pathway, and interestingly, CHOP knockout models have shown decreased liver fibrosis.33 The cytokines released from BA-induced cellular damage activate the hepatocyte stellate cells, repeated cycles of which can lead to irreversible fibrosis. However, intrahepatocellular accumulation of BAs is key to inducing hepatocyte damage.35 Exposure of hepatocytes to elevated BA concentrations as seen in obstructive cholestasis has shown to cause an increase in cytokines (interleukin [IL]-1β and IL-10), chemokines such as macrophage inflammatory protein cell adhesion molecules (ICAM-1 and VCAM-1), enzymes such as COX-2, and thereby influence immune cell levels and function.31
Evidence on the diagnostic and prognostic utility of BAs
BA derangements are common in the diseased liver and therefore may serve as markers of derangement and potential targets to help restore normal physiology. In healthy subjects, CA:CDCA typically ranges between 0.6 and 1, while in cirrhosis, it is reduced to 0.1 to 0.5 and further decreases as the severity of cirrhosis increases. The greatest decrease in CA occurs before overt symptoms appear, i.e., in early stages of cirrhosis.36 The standard assay to measure BAs in serum, urine, and stool is ultraperformance liquid chromatography with tandem mass spectrometry. Other separation-based assays include gas chromatography (GC)-MS, high-performance liquid chromatography-MS, supercritical fluid chromatography, capillary electrophoresis, enzyme-linked immunosorbent assay, thin-layer chromatography, and nuclear magnetic resonance (NMR) spectroscopy–based assays. However, the more commonly used 3-α-hydroxysteroid dehydrogenase–based colorimetric assays are better suited for total BA measurements than for individual BAs, and LC/GC-MS remains the gold standard.37
Kim et al found the fasting serum BA concentration to be a more sensitive test of disordered hepatobiliary function than conventional liver function tests. They observed that some of their patients with cirrhosis had higher serum BA concentrations despite normal transaminases.36
Across various etiologies of CLD, Alamoudi et al found that total urinary BAs were maximally increased in patients with PBC and only marginally in HBV infection. The increase in total urinary CA and CDCA was highest in PSC and lowest in HBV infection. The total urinary primary/secondary BA ratio was high in most cases of CLD and low only in PBC.38 In another study, including patients with alcoholic or nonalcoholic cirrhosis, serum conjugated primary BAs such as GCA, GCDCA, TCA, TCDCA, and TUDCA were higher in Child-Turcotte-Pugh (CTP) B and C than in CTP A, whereas secondary BAs showed a decreasing trend with advancing cirrhosis.39 Higher total (but not individual) BAs were also associated with increased 6-month mortality. Prediction accuracies of BAs were slightly lower than those of the Model for End-Stage Liver Disease score; yet, BAs can still serve as prognostic indicators for cirrhotic patients.
Alcoholic Liver Disease
In alcoholic cirrhosis, the total fecal BA pool shrinks with a reduction in both primary and secondary BAs.25,36 Taurine conjugates are higher than glycine conjugates. Although the absolute CDCA pool is significantly reduced in early alcoholic cirrhosis, it is largely unchanged in advanced stages. CA synthesis progressively decreases with cirrhosis; hence CA:CDCA ratio becomes a useful index of disease severity.36,40 Kakiyama et al found that alcohol intake increases stool BAs even in healthy subjects, with fecal total bile acid (TBA) levels being lowest in abstinent alcoholic cirrhotics. Second, alcoholic cirrhosis patients have higher serum BAs than nonalcoholic cirrhosis, and serum BAs were higher in both alcoholic/nonalcoholic cirrhosis in comparison to healthy subjects irrespective of alcohol intake.25
Nonalcoholic Fatty Liver Disease
NAFLD with liver fibrosis has higher total plasma BAs (with a disproportionate increase in primary BAs), which increase with advancing fibrosis. Glycine-conjugated CA and CDCA, 7-keto DCA, and GUDCA specifically show association with advancing fibrosis, whereas most secondary BAs show no significant difference.41
The fecal BA profile in NAFLD differs in obese and nonobese/lean patients (body mass index <25 kg/m2). In Asian lean NAFLD patients, total stool BAs were higher, with an increase in both unconjugated (CA and CDCA) and conjugated BAs (GUDCA and GCDCA) and fecal TBAs among those with advanced fibrosis.26 In obese NAFLD patients with liver fibrosis, fecal TBAs, total conjugated BAs, unconjugated BAs, and total fecal secondary BAs progressively decrease as fibrosis advances. The secondary to primary BA ratio and the unconjugated to conjugated BA ratios also show a decrease. There is no clinically relevant change in unconjugated primary BAs but conjugated primary BAs increase with advancing fibrosis.26
Viral Hepatitis
Serum TBAs are also significantly increased in cirrhosis because of HBV and hepatitis C virus (HCV) infection.42,43 Yan et al demonstrated an association of cirrhosis and serum TBAs to total cholesterol ratio in noncholestatic chronic HBV infection.44 Serum TBAs, particularly conjugated BAs, are elevated in HBV cirrhosis with a progressive increase as CTP grade advances.45 Fecal TBAs, unconjugated BAs, total fecal secondary BAs, and unconjugated secondary BAs decrease with advancing fibrosis as do the ratios of total secondary to primary BAs, DCA to CDCA, and unconjugated to conjugated BAs. In HCV cirrhosis, fasting serum TBAs are higher in severe cirrhosis than nonsevere cirrhosis, whereas biliary secondary BAs decrease as the cirrhosis advances.40 However, serum BAs show an increase in advanced cirrhosis, even with normal bilirubin.46
Hepatocellular Carcinoma
In early cirrhosis, the concentrations of serum TBAs and primary conjugated BAs are significantly higher in patients with underlying hepatocellular carcinoma (HCC) and can help suspect HCC before overt manifestations. However, TBAs do not show an association with mortality in this case.39
Primary Sclerosing Cholangitis and Primary Biliary Cholangitis
Compared with healthy controls, fecal glycine-conjugated primary BAs and serum total primary BAs are higher in PBC, whereas serum total secondary BAs are lower.47 Both serum and fecal secondary to primary BA ratios and hydrophobic to hydrophilic BA ratios are decreased in PBC, whereas serum and fecal conjugated to unconjugated BA ratios are increased. The data for serum BAs in PSC are confounded by UDCA therapy. But PSC patients have similar DCA levels, higher TBA levels, and decreased glycine:taurine BA ratios of CDCA and DCA, compared with controls. In a study by Mousa et al, serum TBAs were predominantly elevated only in patients on UDCA treatment. In the subset of patients who were not on UDCA, their concentration was higher than in healthy subjects (but within the normal range), increasing the conjugated fraction.48 An increase in serum levels of conjugated BAs (TCA and TCDCA) has been seen in experimental AIH models, but there is no evidence in humans yet.49
Acute Liver Failure
Horvatits et al investigated the role of fasting serum BAs as markers for acute decompensation (AD) of noncholestatic cirrhosis in hospitalized patients (excluding patients with PBC or PSC or those on UDCA). They found that in AD, TBAs, taurine, and glycine conjugates of primary BAs as well as UDCA, DCA, and unconjugated CDCA levels increase with an increase in CTP grade. Among the individual CTP components, BA levels correlated with total bilirubin and INR. In their sample of 39 AD patients, TCDCA had the strongest association with AD, whereas among the 11 ACLF patients, it was TCA and GCA. Interestingly, in a subsequent year-long follow-up in patients without AD/ACLF on initial admission, TBAs showed an independent association with new-onset ACLF and AD and increased CDCA and TBA levels more than ≥36.9 μmol/L predicted AD/ACLF with a 78% sensitivity.50 In this study, TBAs also positively correlated with the hepatic venous pressure gradient.
Pediatric Cholestatic Disorders
Serum BAs are elevated in childhood cholestatic disorders, such as Dubin-Johnson syndrome, progressive familial intrahepatic cholestasis (PFIC 1–3), and Alagille syndrome (AGS) with higher serum BA concentration in PFIC (PFIC 2 > PFIC 1) compared with AGS.51, 52, 53 There is no difference between total biliary bile salts between PFIC1 and PFIC2 (bile salt export pump deficiency). Pawlikowska et al found biliary bile salts and CDCA/CA ratio lower in PFIC, whereas the taurine:glycine (T:G) ratio and hydrophobicity index were not any different. Postsurgical diversion, AGS patients have a higher biliary CDCA/CA ratio compared with PFIC. A normalization of BAs postdiversion was associated with a longer duration of survival with native liver.54 Serum TBAs are typically low to normal in cholestasis because of BA synthesis disorders.55 Duodenal biliary TBA (dTBA), dTBA/sTBA ratio, and dTBA/serum gamma-glutamyl transferase (GGT) ratio were lower in infants with biliary atresia than healthy infants.56 BAs have not been shown to correlate with the severity of pruritis in childhood cholestatic disorders.57 Serum primary BAs (especially GCDCA and TCDCA) are higher in cholestasis due to biliary atresia than due to other causes and may help in noninvasively distinguishing etiology of childhood cholestasis.58
Bile acid therapeutics
Several therapies based on BAs and their signaling have been tested in various etiologies of CLD in various clinical trials and summarized in the following sections.
Ursodeoxycholic Acid
UDCA is a naturally occurring hydrophilic BA produced by epimerization of CDCA by colonic bacteria. On UDCA administration, there is a reduction in FXR-activating BAs in the pool, indirectly negating the FXR-mediated inhibition of BA synthesis. UDCA is approved as the first-line therapy for PBC. In therapeutic doses (>13 mg/kg), it improves liver transplant–free survival and is associated with fewer deaths per year in PBC with and without evidence of cirrhosis, with a more substantial effect in younger patients and those with higher baseline alkaline phosphatase (ALP).59
Studies have shown an improvement in Mayo risk score with UDCA in PSC, indicating improved survival.60, 61, 62 UDCA has also consistently been shown to improve liver biochemistry, but that is now considered to be a contentious endpoint in PSC.63, 64, 65, 66 A systematic review and a meta-analysis of randomized controlled trials evaluating UDCA in PSC showed no significant reduction in risk of death, liver transplant, ascites, encephalopathy, histological grade, and progression to cholangiocarcinoma.67,68 Despite questionable benefits, worsening symptoms and liver biochemistry were observed within 3 months of UDCA withdrawal in PSC patients.69 However, the American College of Gastroenterology (ACG) categorically recommends against doses more than 28 mg/kg because of higher incidence of adverse events.70,71
Multiple studies show biochemical and improvement in pruritis with UDCA therapy in cholestatic disorders, such as PFIC and AGS, with more significant benefit with doses >20 mg/kg.72, 73, 74 In PFIC, UDCA therapy even demonstrated reversal of liver fibrosis.72 In pediatric cholestasis secondary to long-term total parenteral nutrition, UDCA has shown improvement in liver biochemistry and resolution of splenomegaly, whereas in extrahepatic biliary atresia, it only offers symptomatic benefit.74, 75, 76, 77
norUDCA
norUDCA is a shortened derivative of UDCA relatively resistant to amidation. This helps in increasing its cholehepatic shunting. In 2017, a Phase II double-blind randomized controlled trial (DBRCT) of norUDCA showed a significant improvement in ALP levels in both sexes irrespective of pretreatment with UDCA within 12 weeks of therapy (NCT01755507). This was dose dependent, with the maximum dose of 1500 mg displaying a safety profile comparable to placebo. The 1000 mg and 1500 mg treatment groups also showed a decrease in spleen sizes. While pruritus, an expected adverse effect was not higher compared with placebo, the 1500 mg norUDCA group reported an increased number of headaches. However, this trial excluded patients with CTP B and C and those with serum bilirubin >3 mg/dL. Patients are currently being recruited for a 2-year long Phase 3 trial to evaluate 1500 mg norUDCA in PSC (NCT03872921).
Oral CA supplementation improves bile flow and liver function tests in inborn errors of BA synthetic pathway, such as 3β-hydroxysteroid dehydrogenase(3βHSDH) deficiency and α-methylacyl-CoA racemase (AMACR) deficiency. This is partly due to feedback inhibition of the BA synthetic pathway. In genetic defects of BA conjugation, conjugated primary BA supplementation may be potentially helpful.78
FXR-based therapeutics
Obeticholic acid (OCA) or 6-alpha ethyl CDCA is a potent and selective synthetic steroidal FXR agonist. It is known to promote bile flow or choleresis, which prevents the accumulation of hydrophobic BAs in the liver.79 FLINT trial–tested OCA in noncirrhotic nonalcoholic steatohepatitis (NASH) (NCT01265498). OCA in a dose of 25 mg showed significant improvement in NAFLD activity score (NAS), without worsening of fibrosis at the end of 72 weeks.80 There was also a significant improvement in fibrosis in the OCA group. However, the treatment group did not show resolution of NASH or reversal of diagnosis. Pruritus was seen in nearly 20%, with 2–3% developing very severe episodes of pruritus requiring withholding and/or discontinuation of the medication. In addition, OCA was associated with an increase in low-density lipoprotein (LDL) cholesterol levels. The 18-month interim analysis from the REGENERATE trial showed a significant improvement in fibrosis with no worsening of NASH in both 10 mg and 25 mg arms compared with placebo; however, there was no significant difference in NASH resolution without worsening of fibrosis.81 An extended follow-up of the same study showed significant improvement in NASH, with no worsening of fibrosis in the OCA group.82 Currently, the approval of OCA for noncirrhotic NASH hangs by a thin thread because of a significant proportion of patients experiencing pruritus (50%) and increased LDL cholesterol (17%) in the REGENERATE trial. The data on hypercholesterolemia are particularly concerning because most NASH patients have other risk factors for coronary artery disease. Currently, the REVERSE trial (Phase 3 NCT03439254) is ongoing to study the efficacy of 10 mg and 10–25 mg OCA in patients with compensated cirrhosis due to NASH.83 A recent review has discussed the role of various drugs, including FXR agonists, in patients with NAFLD.84
OCA has also demonstrated improvement in total bilirubin and ALP in PBC patients who showed poor response to UDCA and is already FDA approved for the indication at a lower dose.85,86 It is not recommended for patients with decompensated cirrhosis and is currently marketed for PBC with a black box warning for the same.
Cilofexor, a nonsteroidal non-BA FXR agonist, showed antifibrotic effects in a rat model of NASH (80). This was followed by a 12-week long Phase 2 DBRCT (NCT02943460). Total and primary serum BAs were lower in the 100 mg but not 30 mg cilofexor treatment group, albeit not significantly with secondary BAs showing a significant reduction. Liver enzymes decreased in a dose-dependent manner independent of UDCA use. There was no change in liver stiffness measured by FibroScan and enhanced liver fibrosis score, but serum TIMP-1, a fibrogenic cytokine, showed a decreasing trend in the 100 mg Cilofexor cohort.87 In 2020, a Phase 2 DBRCT of Cilofexor was done in patients with noncirrhotic NASH (NCT02854605). Both 100 mg and 30 mg treatment groups significantly reduced serum primary BAs, whereas only the 30 mg group had a relative and absolute reduction in total and secondary BAs. The 100 mg group showed a meaningful reduction in steatosis (measured using MRI-PDFF), but this must be interpreted with caution as the placebo group had significantly higher baseline levels of steatosis.88
Tropifexor (LJN542) is a synthetic nonsteroidal FXR modulator that was manufactured to address the limitations of the adverse effect profile of OCA. In preclinical studies, it was found to be 20 times more potent than OCA and has shown improvement in the quality of the gut microbiome and decreased hepatic steatosis in experimental models of cholestasis and NASH. Phase 1 studies recently concluded in humans have demonstrated its safety (NCT04408937).89
Nidufexor (LMB763) is another nonsteroidal FXR agonist with FXR-dependent gene modulatory activity in vivo.90 Nidufexor is currently being evaluated for NASH in Phase 2 clinical trials (NCT03804879).
FXR agonists are also being developed and evaluated in chronic hepatitis B infection, as they are hypothesized to interfere with viral replication (NCT03272009).
Aldafermin (NGM282), an FGF-19 analog (FXR downstream signaling molecule), has recently been tested in Phase 2 trials for PSC and NASH patients without cirrhosis, in doses of 0.3 mg, 1 mg, 3 mg, and 6 mg compared with placebo (NCT02443116).91, 92, 93, 94 In patients with NASH, at the end of 12 weeks, serum glycine-conjugated primary BAs significantly decreased from baseline. Greater reductions in GCA and GCDCA were observed with higher dose (1, 3, and 6 mg) groups. Aldafermin suppresses CYP7A1 and reduces the more hydrophobic glycine-conjugated BAs than hydrophilic taurine-conjugated BAs. In PSC, it showed a preferential reduction of secondary BAs.
TGR-5 receptor agonists have shown increased insulin sensitivity in mouse models of NAFLD.95,96
INT-767 is an FXR and TGR-5 agonist that improves high-fat diet–induced effects and promotes more insulin-sensitive adipocytes. In a rabbit model with high-fat diet–induced metabolic syndrome, INT-767 increased brown adipogenesis and prevented the development of NASH.97
All-trans retinoic acid (ATRA) is a permissive activator of FXR. Mice studies of ATRA have shown a marked reduction of fibrosis.98 In the clinical study of ATRA in combination with UDCA, only alanine aminotransferase (ALT) and BA intermediates showed a reduction.99
Apical sodium-dependent bile salt transporter and BA sequestrants have recently attracted attention owing to an experimental molecule, IMB17–15 demonstrating decreased hepatic fat content in hamsters fed a high-fat diet. Ge MX et al recently showed that IMB17-15 improves insulin sensitivity through activation of AMPK and PPAR-α pathway.100
A summary of studies evaluating BA-based therapeutics in CLD can be found in Table 1, Table 2, Table 3, Table 4.
Table 1.
Summary of Studies Evaluating Therapeutics in NAFLD and NASH.
| Author | Drug and dose | Inclusion criteria, stage of fibrosis | Type of study | Duration of therapy | Outcome |
|---|---|---|---|---|---|
| Ongoing | Elobixibat (IBAT inhibitor) 5 mg once daily | NAFLD, NASH | Randomized double-blind, placebo-controlled Phase 2 study | 16 weeks | Change in serum LDL cholesterol |
| Nadinskaia et al. WJG 2021101 | N = 174; 15 mg/kg/d UDCA 121 (69.5%) men and 53 (30.5%) women Men significantly younger than women |
Ultrasound-diagnosed NAFLD; FLI >60 | Open-label, multicenter, international uncontrolled trial | 6 months | Δ decrease in ALT, AST, and GGT during 0–3 m > 3–6 m. ↔ in NFS, FIB-4. Significant ↑ in HDL, ↓ in LDL-C, TC, TG Sex differences in response observed |
| Traussnigg et al, Wien Klin Wochenschr, 2021102 | 5 mg PX-104 (nonsteroidal FXR agonist) once daily | Nondiabetic NAFLD (n = 12) | Open-label Phase 2a study | 4 weeks | ↑ IS ↓ ALT and GGT ↔ ALP or serum lipids. ↔Hepatic steatosis: MRI-PDFF, 1H-MRS, and CAP ↔ Serum BAs Cardiac arrhythmia in two patients led to the termination of the study. |
| Newsome et al. Journal of hepatology 2020103 | N = 197 were randomized to receive Volixibat 5 mg (n = 49), Volixibat 10 mg (n = 50), Volixibat 20 mg (n = 49), or placebo (n = 49) once daily | Adults, ≥5% steatosis, and NASH without cirrhosis | Phase 2 randomized, double-blind, Phase II, placebo-controlled study | 48 weeks | Volixibat did not meet interim endpoints (24 weeks), i.e., ≥5% reduction in MRI-PDFF and ≥20% reduction in serum ALT. The study was terminated owing to a lack of efficacy |
| Pockros et al. Liver international, 2019104 | 5 mg, 10 mg, 25 mg OCA once daily | Biopsy-confirmed NASH without hepatic decompensation | Randomized, double-blind, placebo-controlled, Phase 2 study | 16 weeks | ↓ LDL-C with OCA + statin |
| Palmer et al. BMC pharmacology and toxicology 2018105 | 20, 40, or 80 mg Volixibat (n = 63); placebo (n = 21) once daily | Overweight and obese adults | Phase 1 study | 12 days | Volixibat (≥20 mg/day): maximal fecal BA excretion in obese and overweight adults |
| Harrison et al Hepatology 2020106 | Subcutaneous NGM282 1 mg (n = 24) NGM282 3 mg (n = 19) once daily | Paired biopsies, NASH as per NASH CRN criteria; F1-F3; liver fat ≥8%; ↑ ALT | Open-label, multicenter trial | 12 weeks | 50% and 68% in the 1 mg and 3 mg treatment arms, respectively, achieved significant histological improvement. 12% and 10% in the 1 mg and 3 mg groups, respectively, achieved NASH resolution without fibrosis worsening at 12 weeks |
| Younossi et al. Lancet 201981 | N = 931; placebo (n = 311) OCA 10 mg (n = 312); or OCA 25 mg (n = 308) | Stage F2-F3 fibrosis | Phase 3 multicenter, randomized, placebo-controlled trial | Interim analysis at 18 months (total study period = 4 years) | OCA 25 mg significantly improved fibrosis without worsening of NASH by 1.9 times (95% CI 1·4–2·8) c/w placebo. Greater proportion of patients receiving 25 mg OCA showed improvement in liver histology and in serum ALT and AST |
| Harrison et al. Lancet 201891 | Subcutaneous NGM282 (FGF-19 analog) 3 mg (n = 27), NGM282 6 mg (n = 28), or placebo (n = 27) once daily | Biopsy-confirmed NASH; Stage 1–3 fibrosis; liver fat ≥8%; ↑ ALT | Multicenter international randomized, double-blind, placebo-controlled, Phase 2 trial | 12 weeks | NGM282: ↓ ALT/AST, ↓ liver fat ↓ TGs seen only in 6 mg group and ↑ total and LDL cholesterol in both groups |
| Neuschwander-Tetri et al (FLINT trial) Lancet 201580NCT01265498 | OCA 25 mg (n = 141) or Placebo (n = 142) once daily | Histologically proven NASH or borderline NASH | Phase 3 Randomized double-blind, placebo-controlled trial | 72 weeks | OCA: improved liver histology (≥2-point NAS without worsening of fibrosis). Mean change in NAS: OCA > placebo. OCA: ↓ ALT and AST. ↑ ALP, ↓ GGT which reversed after stopping OCA. NASH resolution (OCA = placebo). OCA (ADE): ↑ LDL-C, Pruritis: 23% vs. 6% in placebo |
| Siddiqui et al Journal of Hepatology. 2020107 | Serum and biopsy samples of 196 patients who were enrolled in the FLINT trial; OCA group (n = 99), placebo group (n = 97) once daily | Histologically proven NASH or borderline NASH | 72 weeks | OCA: ↑ increase in lipoprotein levels, which improves after drug discontinuation | |
| Mueller et al. Journal of hepatology 2015108 | 20 mg/kg/day UDCA (n = 19) in two daily doses; Controls (n = 18) |
Morbidly obese (BMI >35 kg/m2) NAFLD scheduled for laparoscopic Roux-en-Y gastric bypass |
Randomized open-label study | 3 weeks | UDCA: ↓ AST, GGT, free FA, total and LDL-C, and ↑ TGs |
| Mudaliar et al. Gastroenterology 2013109 NCT00501592 |
Placebo (n = 23), 25 mg OCA (n = 20), or 50 mg OCA (n = 21) once daily | Patients with type 2 diabetes mellitus and NAFLD | Phase 2 Multicenter, randomized, double-blind, placebo-controlled study |
6 weeks | OCA: ↑ IS by 28% as c/w placebo OCA: ↓ GGT, ALT, and dose-related weight loss. ↑ LDL-C and FGF-19, a/w ↓ 7ɑ-hydroxy-4-cholesten-3-one and endogenous BAs |
| Ratziu et al J Hepatol. 2011110 | N = 126; high-dose UDCA (HD-UDCA; 28–35 mg/kg per day) | Biopsy-proven NASH and elevated ALT | Phase 2 randomized, double-blind, placebo-controlled multicenter trial | 12 months | HD-UDCA: ↓ ALT |
| Leuschner UF, Hepatology. 2010111 | N = 185; UDCA 23–28 mg/kg (n = 94) or placebo (n = 91) daily in three divided doses | NASH (per NAS and modified brunt score) | Randomized, double-blind, placebo-controlled study | 18 months | No difference in histology c/w placebo |
| Lindor et al. Hepatology 2004112 | N = 166; 13–15 mg/kg/day of oral UDCA (n = 80) or placebo (n = 86) four divided doses daily | Patients with biopsy-proven NASH | Prospective, randomized, double-blind, placebo-controlled trial | 24 months | UDCA (13–15 mg/kg/d): not effective in patients with NASH |
| Laurin et al. Hepatology 1996113 | 13–15 mg/kg/day of oral UDCA in divided doses with meals (n = 24); 2 g/day Clofibrate in two divided doses (n = 16 with NASH + hypertriglyceridemia) | Biopsy-proven NASH | Open-label study | 12 months | UDCA: ↓ ALP, ALT, GGT, and hepatic steatosis |
IS, insulin sensitivity; IBAT, ileal bile acid transporter; LDL-C, low-density lipoprotein cholesterol; OCA, obeticholic acid; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; LDL, low-density lipoprotein; FXR, Farnesoid X receptor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; ALP, alkaline phosphatase; MRI-PDFF, magnetic resonance imaging–proton density fat fraction; 1H-MRS, proton magnetic resonance spectroscopy; CAP, controlled attenuation Parameter; BA, bile acid; NASH CRN, nonalcoholic steatohepatitis clinical research network; FGF-19, fibroblast growth factor 19; PSC, primary sclerosing cholangitis; NAS, NAFLD activity score; TG, triglycerides; BMI, body mass index; UDCA, ursodeoxycholic acid; ADE, adverse drug events; FA, fatty acids; CI, confidence interval; NFS, NAFLD fibrosis score; FLI, Fatty Liver Index; FIB-4, Fibrosis-4 Index; TC, total cholesterol.
Table 2.
Summary of Studies in Primary Cholestatic Disorders in Adults.
| Author | Drug | Inclusion criteria, stage of fibrosis | Type of study, drug dose | Duration of therapy | Outcome |
|---|---|---|---|---|---|
| Kjærgaard, Kristoffer et al. Journal of Hepatology 2021114 | N = 8; daily oral OCA dose 5 mg for 1 month and 10 mg for 2 months or matching placebo | PBC | Single-center, double-blind placebo-controlled, crossover study | 3 months each with placebo or treatment | c/w placebo, OCA ↑ hepatic blood perfusion (11%). No significant difference in pruritis between the two treatments. |
| Kowdley et al. Journal of hepatology 2020115 AESOP (Assessment of Efficacy and Safety of OCA in PSC) |
N = 76; Placebo (n = 25), OCA 1.5–3.0 mg (n = 25), and OCA 5–10 mg (n = 26) once daily | PSC based on cholangiography Concomitant UDCA therapy in ≤50% |
Randomized, double-blind, placebo-controlled phase 2 trial | 24 weeks | Significant ↓ in serum ALP in the OCA 5–10 mg group compared with placebo. Pruritus was worse in the 5–10 mg OCA group. |
| Trauner et al. Hepatology 201987 | N = 52; Cilofexor 100 mg (n = 22), 30 mg (n = 20), or placebo (n = 10) orally once daily 46% patients on UDCA |
PSC patients without cirrhosis | Multicenter, randomized, double-blind, placebo-controlled, Phase 2 trial | 12 weeks | Significant ↓ in ALP, ALT, and AST, secondary BAs, HDL-C in 100 mg group, and ↓ GGT in both groups. Dose dependent ↓ in relative and absolute ALP concentration. Common ADEs: pruritus, abdominal pain, and nasopharyngitis. |
| Hegade et al. Lancet 2017116 | N = 22; IBAT inhibitor GSK2330672 or placebo (n = 11 each), 45 mg twice daily on days 1–3, followed by 90 mg twice daily on days 4–14 | PBC on UDCA for >8 weeks, serum ALP ≤10 times ULN | Phase 2a randomized, double-blinded, two-period crossover trial | 14 days | GSK2330672 reduced pruritus severity. Diarrhea, the most common adverse event |
| Fickert et al Journal of hepatology 2017117 | N = 159; norUDCA 500 mg (n = 39), 1000 mg (n = 41), 1500 mg (n = 39) or placebo (n = 40) once daily | PSC without concomitant UDCA therapy and with elevated serum ALP | Multicenter randomized, double-blind, placebo-controlled Phase 2 study | 12 weeks. Follow-up after 4 weeks after finishing study | ↓ in ALP (nearly to baseline), in all treatment arms. Notable ADEs: abdominal pain, fatigue, nasopharyngitis, headache, and pruritus. |
| Nevens et al. The New England journal of medicine 201683 (POISE trial) | N = 216; OCA: 10 mg (n = 73); 5 mg with adjustment to 10 mg if applicable (n = 70), or placebo (n = 73) | PBC with inadequate response to UDCA/intolerant to UDCA with 93% taking on UDCA | Randomized, double-blind, placebo-controlled parallel group Phase 3 trial | 12 months | Significant ↓ in ALP and total bilirubin in both treatment groups (irrespective of concomitant UDCA intake) Pruritus significantly higher in both treatment groups. |
| Trauner et al The lancet. Gastroenterology & hepatology 2019118 | N = 193; placebo (n = 48); OCA 5–10 mg (n = 55); OCA 10 mg (n = 53) | Open-label extension (OLE) of OCA in PBC patients included in POISE trial | 3-year interim analysis of 5-year open label extension of OCA in PBC patients included in POISE trial | 3 years | ALP significantly ↓ at 12, 24, 36, and 48 m (including 12 months of POISE trial) and total bilirubin ↓ at 12 m and 48 m only. Mean ↓ in liver enzymes (AST, ALT, GGT, and ALP) were persistent as in POISE trial and significant at each yearly time point. ALP, GGT, ALT, and AST showed significant ↓ starting as early as 3 months. CRP was significantly ↓ at 12 and 48 m. Similar results were observed across treatment groups ADEs: pruritus, fatigue, and nasopharyngitis Hepatic ADEs: esophageal varices and ascites. |
| Bowlus et al. Clinical Gastroenterology and Hepatology 2020119 | N = 17; OCA 5 mg or OCA 5–10 mg plus concomitant UDCA |
PBC patients with inadequate response to or intolerance to UDCA | Analysis of paired biopsies from the POISE study | Biopsy at baseline and at 3 years | Improvements or stabilization of ductular injury, fibrosis, and collagen morphometry. Significant ↓ in ALP, ALT, AST, GGT, CK-18, CRP, APRI, and ↑ FGF-19. |
| Harms et al. JHEP reports 2020120 | N = 187; Once daily OCA 5 mg, OCA 5–10 mg, or placebo At baseline: low risk (n = 47); moderate risk (n = 79); high risk (n = 89) based on GLOBE score and APRI. |
PBC patients with inadequate response to or intolerance to UDCA | Post hoc analysis of data from the POISE study | 12 months | Improvement or absence of worsening of baseline GLOBE score more likely in treatment groups. Significantly ↑ proportion of patients on OCA with ≥1 risk stage improvement irrespective of age-specific/nonspecific GLOBE scores used for defining risk stage. |
| Corpechot et al. Journal of Hepatology 2020121 | N = 780; UDCA 10–15 mg/kg/day (n = 190) orally in two divided doses started within 2 weeks of liver transplantation vs no preventive UDCA (n = 590) | PBC patients with liver transplant | Multicenter retrospective cohort study | 14 ± 7.4 yrs | Preventive UDCA therapy after liver transplant for recurrent PBC significantly a/w ↓ disease recurrence, graft failure, and 5-, 10-, 15-, 20-, and 25-year mortality. |
| Hirschfield et al. Gastroenterology 201585 | N = 165; OCA 10 mg (n = 38), 25 mg (n = 48), 50 mg (n = 41), placebo (n = 38) once daily N = 78 for OLE at 3–60 mg once daily (mean 20 mg) |
PBC patients on stable dose of UDCA for 6 months | Randomized double-blind placebo-controlled trial | 3 months | Daily doses of 10–50 mg OCA, significantly ↓ ALP, GGT, AST, and ALT. Significant ↓ in C4 and total endogenous BAs among all OCA groups and ↑ in FGF19 in 10 mg and 25 mg groups. Significant ↓ in total cholesterol and HDL-C across all dose groups and no significant change in LDL-C and TGs Significantly higher incidence of pruritus in OCA 25 mg and 50 mg groups. |
| Dilger K et al J Hepatol. 2012122 | N = 22; UDCA (15 mg/kg/day) once daily (n = 11); HC (n = 11) | Female patients, biopsy-proven PBC stage I, II, or II–III | Controlled trial | 3 weeks | PBC patients show higher rates of taurine conjugation in bile. ↓ after UDCA treatment |
| Lindor et al. Hepatology 200971 | High-dose UDCA (28–30 mg/kg/day in divided doses) vs placebo UDCA |
PSC not on UDCA treatment in 3 months prior | Randomized, double-blind placebo-controlled trial | 5 years | Death, liver transplant, minimal listing criteria for liver transplant, development of cirrhosis, esophageal and/or gastric varices, and cholangiocarcinoma more common in treatment group despite improved liver biochemistries. Study terminated after 6 years because of adverse effects. |
| Cullen et al. Journal of hepatology 2008123 | N = 31; UDCA 10 mg/kg (n = 11); 20 mg/kg (n = 11); 30 mg/kg (n = 9) | PSC; Most patients with UC also | Randomized trial | 24 months | Serum ALT and AST significantly ↓ with 20 mg/kg of UDCA, ALP, GGT with all three doses. Survival at all time points calculated (1–4 years) significantly ↑ only with 30 mg/kg/day. No significant histological improvement with any dose. No difference in side effect profile between the three doses of UDCA. |
| Olsson et al. Gastroenterology 2005124 | N = 198; 17–23 mg/kg/day of UDCA (n = 97) or placebo (n = 101) | PSC based on cholangiography with conventional radiological criteria not on UDCA treatment | Randomized placebo-controlled study | 5 years | No significant difference in liver enzymes and time to LT or death or percentage of patients with that endpoint in either group. ALP and ALT only tended to ↓ in UDCA-treated patients during the first 6 months. |
| Harnois et al. The American journal of gastroenterology 200160 | N = 128; high-dose UDCA (25–30 mg/kg/day, n = 23) in four divided doses, low-dose UDCA (13–15 mg/kg/day, n = 53), placebo (n = 52) | PSC without previous treatment with UDCA | Prospective study | 12 months | Significant improvement in serum ALP, AST, albumin, and bilirubin with both low- and high-dose UDCA. Improved Mayo risk score (therefore 4-year survival) only with high-dose UDCA. |
| Angulo et al. Journal of hepatology 1999125 | N = 137; low dose 5–7 mg/kg/day (n = 47) standard dose 13–15 mg/kg/day (n = 45) high dose 23–25 mg/kg/day (n = 45) in four divided doses | Clinical and histological evidence of PBC not previously treated with UDCA | Randomized, double-blind trial | 12 months | Improvement in ALP, AST, Mayo risk score: standard- and high-dose groups significantly > low-dose group; standard dose ≈ high-dose groups. |
| Lindor et al The New England journal of medicine 1997126 | N = 102; UDCA 13–15 mg/kg/day in four divided doses (n = 51), placebo (n = 51) | PSC for at least 6 months with biopsy within 3 months | Multicenter, randomized, double-blind, placebo-controlled trial | 24 months | Significant improvement in serum ALP, AST, bilirubin, and albumin levels at 1 and 2 years No difference in time to treatment failure, that is, death or histologic progression by two stages or decompensation or to liver transplantation irrespective of early histologic disease or presence of colitis. No significant changes in liver histology/symptoms/serum lipids after 2 years. |
| De Maria et al. Hepato-gastroenterology 1996127 | N = 59; UDCA 300 mg twice daily (n = 20) colchicine 0.6 mg orally twice daily (n = 19); and untreated control group (n = 20) | PSC based on clinical, biochemical, and radiology | Randomized controlled study | 24 months | No difference between groups in liver enzymes, liver function, liver size, and hepatic copper content. |
| van de Meeberg et al J Hepatol. 1996128 | N = 27; UDCA (10 mg/kg/day) in a single dose at bedtime (n = 13) or in three divided doses with meals (n = 14) | Early stage (Stage I-II) disease. PSC (n = 19), PBC (n = 8) | RCT | 3 months | Single- or multiple-dose UDCA have similar effects on liver biochemistry. |
UDCA, ursodeoxycholic acid; GGT, γ-glutamyltransferase; ALP, alkaline phosphatase; HC, healthy controls; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; IBD, inflammatory bowel disease; FGF19, fibroblast growth factor-19; HDL-C, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; OCA, obeticholic acid; IBAT, ileal bile acid transporter; BA, bile acid; ERC, endoscopic retrograde cholangiography; ELF, enhanced liver fibrosis; MRCP, magnetic resonance cholangiopancreatography; TIMP-1, tissue inhibitor of metalloproteinases-1; CRP, C-reactive protein; GLOBE, not an acronym; CK-18, cytokeratin 18; APRI, AST to platelet ratio index; ITT, intention-to-treat; GLDH, glutamate dehydrogenase; LT, liver transplantation; C4, 7 α-hydroxy-4-cholesten-3-one.
Table 3.
A Summary of Studies Evaluating Bile Acids in Pediatric Cholestatic Disorders.
| Author | Sample size and therapy | Inclusion criteria, stage of fibrosis | Type of study, drug dose | Duration of therapy | Outcome |
|---|---|---|---|---|---|
| Baumann et al, Clinics and research in hepatology and gastroenterology, 2021129 | N = 24; 10–200 μg/kg oral odevixibat daily | PFIC (n = 13), Alagille syndrome (n = 6), biliary atresia (n = 3), other causes of intrahepatic cholestasis (n = 2) | Open-label, multicenter Phase 2 study | 4 weeks | ↓ serum BA compared with baseline levels (reductions up to 98%). Improved pruritus (three scales) and sleep. No serious ADEs |
| van Wessel et al. Hepatology, 2021130 | N = 130, surgical biliary diversion | Compound heterozygous- or homozygous-predicted pathogenic ATP8B1 variants (PFIC) | Multicenter, combined retrospective, and prospective study | – | Postsurgical diversion serum BA concentrations <65 μmol/L show a trend of association (P = 0.05) with improved NLS |
| Deneau et al. The Journal of pediatrics 2019131 | N = 263; GGT normalization after 12 months (n = 122); non-norm (n = 141) | PSC with baseline serum GGT levels >50 IU/L | Retrospectively reviewed patient records | 12 months | ↓ in AST, ALT, ALP; 5-year survival with native liver better in the GGT normalization group |
| Black et al. Hepatology communications 2019132 | N = 22; Null (n = 7), ALT, and GGT persistently ≤29 IU/L; Flare (n = 8), ALT, and/or GGT >100 IU/L; indeterminant (n = 7), ALT, and/or GGT >29 IU/L and <100 IU/L; during UDCA dose reduction and withdrawal |
PSC | Treatment withdrawal and reintroduction study | 24 weeks; multiple phases | All flares responded to UDCA reinstitution |
| Shneider et al. Hepatology communications, 2018133 | N = 37; Maralixibat 70, 140, or 280 μg/kg/day (n = 25) or placebo (n = 12) once daily | Children with Alagille syndrome | Randomized double-blind, placebo-controlled Phase 2b trial | 13 weeks | Significant ↓ in ItchRO with 70 and 140 μg/kg/day but not 280 μg/kg/day (Maralixibat). A 1-point ↓ in pruritus in the drug-treated group. No serious ADEs |
| Dinler et al. The Turkish journal of pediatrics 1999134 | N = 24; Follow-up biopsy N = 17; UDCA 15–20 mg/kg/day |
Intrahepatic cholestasis (neonatal hepatitis 7, Byler disease 7, idiopathic intrahepatic cholestasis 10) | Uncontrolled trial | 12 months | Complete resolution of pruritus in 16.7% with some improvement in all. Significant ↓ in AST, ALT, ALP, bilirubin, GGT |
| Dinler et al. Pediatrics international 1999135 | Nine children aged between 1.5- and 9-years UDCA orally at doses of 15–20 mg/kg per day | PFIC1 (Byler disease) | Uncontrolled trial | 12 months | Pruritus resolved completely in 22.2%, ↓ in 22.2% and unchanged in 55.6%. Significant ↓ AST. ↔ GGT, cholesterol, and serum TBAs. Cholestatic changes on histology resolved in 22.2%, ↓ in 33.3%, and ↔ in 22.2%. No ADEs |
| Narkewicz et al. Journal of pediatric gastroenterology and nutrition 199877 | N = 13; CF (n = 6), Alagille syndrome (n = 4), PFIC (n = 2), and nonsyndromic intrahepatic bile duct paucity (n = 1). UDCA 15–20 mg/kg per day for 12 months, off for 6 months, and on again for 12 months |
Intrahepatic cholestatic liver disease for at least 6 months in a child >5 years old not previously on UDCA therapy | Open-label, crossover study | 2.5 years | Majority (75%) had biochemical or symptomatic relapse on UDCA discontinuation, requiring retreatment with UDCA. No sustained improvements in the biochemical indices at 24 months |
| Jacquemin et al. Hepatology, 199772 | N = 39; UDCA orally (20–30 mg/kg/day), Group 1 (n = 26) normal GGT, and group 2 (n = 13) high GGT. The dose of UDCA corresponded to a total daily dose of 28 ± 7 mg/kg of body weight in group 1 and 26 ± 7 mg/kg of body weight |
Children with PFIC | Uncontrolled trial | Group 1: 26 ± 16 months Group 2: 49 ± 11 months | ↓ in AST, ALT, serum TBAs in both groups and GGT only in group 2 Hepatomegaly and pruritus significantly ↓ in both groups. After therapy cessation: ↑ transaminases. No serious ADEs |
| Kardorff et al. Klinische Padiatrie, 199673 | N = 20; (biliary atresia n = 10, Alagille's syndrome n = 4, intrahepatic biliary hypoplasia n = 3, Byler disease/PFIC1 n = 3). UDCA 13 (7–26) mg/kg/d in two divided doses |
Inherited disorders of cholestasis treated for at least 6 months | Retrospective analysis | – | Significant ↓ GGT and GLDH, AST, and ALT ↔ Bilirubin and albumin. First 12 months: pruritus improved (20% only) |
BA, bile acid; ADE, adverse drug event; ItchRO, itch reported outcome; UDCA, ursodeoxycholic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; GLDH, glutamate dehydrogenase; PFIC, progressive familial intrahepatic cholestasis; NLS, native liver survival; TBA, total bile acids.
Table 4.
A Summary of Studies Evaluating Bile Acids in Alcoholic Liver Disease and Hepatocellular Carcinoma.
| Author | Study population | Parameter studied | Type of study | Duration of study | Outcome |
|---|---|---|---|---|---|
| Brandl et al. Journal of hepatology 2018136 | Alcoholic hepatitis (n = 132), alcohol use disorder (n = 9), and controls (n = 9) | Serum BAs | Multicenter prospective cohort study | – | Alcoholic hepatitis vs controls: Significant ↑ total and conjugated BAs, serum FGF19 ↓ De novo bile acid synthesis |
| Kakiyama et al Am J Physiol Gastrointest Liver Physiol. 201425 | N = 103; HC (n = 19), alcohol intake w/o liver disease (n = 6), cirrhotic patients (n = 78): currently drinking (n = 10), no history of alcohol consumption (n = 30), and alcoholic cirrhosis and abstinent for >6 m (n = 38) |
Fecal BAs | Prospective cohort study | – | MELD score significantly negatively correlated with total fecal BAs, total secondary BAs, and the secondary-to-primary BA ratio. Active alcohol intake influences BA composition independent of cirrhosis |
| Thomas et al. Cancers 2021137 | N = 32,535; incident HCC N = 216 among participants who provided a prediagnostic serum sample | Serum BAs | Prospective population-based cohort study Nested case–control study |
10 years | ↑ serum primary BAs and T:G ratio were strongly associated with HCC risk of ↑ 2°:1° primary BAs inversely associated with HCC risk |
| Petrick et al. International journal of cancer 2020138 | HBV N = 185; matched controls N = 61 from REVEAL-HBV cohort; HCV cases N = 96; matched controls N = 96 from REVEAL-HCV cohort |
15 serum BAs measured using LC-MS | Case–control study | Mean duration of follow-up HBV: 13 years HCV: 15 years |
↑ glycine- and taurine-conjugated primary BAs a/w 2–8 fold ↑ risk of HBV- and HCV-related HCC. ↑ DCA inversely a/w HBV-related HCC risk No ↑ risk of liver cancer with ↑ secondary BAs observed |
MELD, model for end-stage liver disease; BA, bile acid; FGF19, fibroblast growth factor 19; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, Hepatitis C virus; T:G, taurine:glycine.
In conclusion, Bile acid pathophysiology is a promising avenue for improving diagnosis and assessing the severity of CLD, especially secondary to NAFLD. Specific BA profile signatures can serve as early hints for certain types of CLD and help modulate metabolism to address the root cause of metabolic liver disease. However, the accuracy of BAs as noninvasive markers of advanced liver disease still needs more evaluation. We also need literature on BA profiles in autoimmune hepatitis. While approval of obeticholic acid came three decades after the experience with UDCA, the next decade should see a steep increase in BA-based drugs as can be estimated from the barrage of novel molecules currently being tested. Although BA-based drugs are now commonly used for cholestatic disorders and a fair number of studies are ongoing in the context of NAFLD and NASH, their therapeutic utility needs to be characterized in viral hepatitis. The cost of therapy is also an area that must be addressed. For example, OCA use in PBC currently costs a patient 1800–4500 per month. This becomes especially relevant in a country such as India, where the average monthly income of many would make it difficult to adhere to therapy for long.
Credit authorship contribution statement
N.F. contributed to writing the article, concept, and critical revision. A.E. contributed to critical revision and figures. S. contributed to concept and critical revision.
Conflicts of interest
The authors have none to declare.
Acknowledgements
The authors would like to thank Dr. Sabreena Sheikh for assistance in writing the article.
Funding
None.
References
- 1.The Top 10 Causes of Death. Accessed May 28, 2021. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.Wong M.C.S., Huang J.L.W., George J., et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16:57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 3.Wong R.J., Aguilar M., Cheung R., et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Pimpin L., Cortez-Pinto H., Negro F., et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718–735. doi: 10.1016/j.jhep.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj J.S., Heuman D.M., Hylemon P.B., et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu G., Pan L., Erickson S.K., et al. Removal of the bile acid pool upregulates cholesterol 7alpha-hydroxylase by deactivating FXR in rabbits. J Lipid Res. 2002;43:45–50. [PubMed] [Google Scholar]
- 7.Watanabe M., Houten S.M., Wang L., et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki T., Choi M., Moschetta A., et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabol. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Chiang J.Y.L., Ferrell J.M. Bile acid biology, pathophysiology, and therapeutics. Clinical Liver Disease. 2020;15:91–94. doi: 10.1002/cld.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlahcevic Z.R., Buhac I., Farrar J.T., Bell C.C., Swell L. Bile acid metabolism in patients with cirrhosis. Gastroenterology. 1971;60:491–498. doi: 10.1016/S0016-5085(71)80053-7. [DOI] [PubMed] [Google Scholar]
- 11.Ridlon J.M., Kang D.J., Hylemon P.B., Bajaj J.S. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakiyama G., Pandak W.M., Gillevet P.M., et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slocum M.M., Sittig K.M., Specian R.D., Deitch E.A. Absence of intestinal bile promotes bacterial translocation. Am Surg. 1992;58:305–310. [PubMed] [Google Scholar]
- 14.Ding J.W., Andersson R., Soltesz V., Willén R., Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 1993;25:11–19. doi: 10.1159/000129252. [DOI] [PubMed] [Google Scholar]
- 15.Fouts D.E., Torralba M., Nelson K.E., Brenner D.A., Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Lu L.-G. Therapeutic roles of bile acid signaling in chronic liver diseases. J Clin Transl Hepatol. 2018;6:425–430. doi: 10.14218/JCTH.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boursier J., Mueller O., Barret M., et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridlon J.M., Alves J.M., Hylemon P.B., Bajaj J.S. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microb. 2013;4:382–387. doi: 10.4161/gmic.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang R., Wei Y., Li Y., et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67:534–541. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 20.Kummen M., Hov J.R. The gut microbial influence on cholestatic liver disease. Liver Int. 2019;39:1186–1196. doi: 10.1111/liv.14153. [DOI] [PubMed] [Google Scholar]
- 21.Bajer L., Kverka M., Kostovcik M., et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23:4548–4558. doi: 10.3748/wjg.v23.i25.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little R., Wine E., Kamath B.M., Griffiths A.M., Ricciuto A. Gut microbiome in primary sclerosing cholangitis: a review. World J Gastroenterol. 2020;26:2768–2780. doi: 10.3748/wjg.v26.i21.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattner J. Impact of microbes on the pathogenesis of primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Chen L., Wang H., Cai W., Xie Q. Modulation of bile acid profile by gut microbiota in chronic hepatitis B. J Cell Mol Med. 2020;24:2573–2581. doi: 10.1111/jcmm.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakiyama G., Hylemon P.B., Zhou H., et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G929–G937. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G., You H.J., Bajaj J.S., et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020;11:4982. doi: 10.1038/s41467-020-18754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y., Li Y., Yan L., et al. Alterations of gut microbiome in autoimmune hepatitis. Gut. 2020;69:569–577. doi: 10.1136/gutjnl-2018-317836. [DOI] [PubMed] [Google Scholar]
- 28.Lou J., Jiang Y., Rao B., et al. Fecal microbiomes distinguish patients with autoimmune hepatitis from healthy individuals. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fickert P., Wagner M. Biliary bile acids in hepatobiliary injury - what is the link? J Hepatol. 2017;67:619–631. doi: 10.1016/j.jhep.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Perez M.-J., Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai S.-Y., Boyer J.L. The role of bile acids in cholestatic liver injury. Ann Transl Med. 2021;9 doi: 10.21037/atm-20-5110. 737-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolbright B.L., Dorko K., Antoine D.J., et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015;283:168–177. doi: 10.1016/j.taap.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen K., Jaeschke H., Copple B.L. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashby K., Navarro Almario E.E., Tong W., Borlak J., Mehta R., Chen M. Review article: therapeutic bile acids and the risks for hepatotoxicity. Aliment Pharmacol Ther. 2018;47:1623–1638. doi: 10.1111/apt.14678. [DOI] [PubMed] [Google Scholar]
- 35.Jones B.A., Rao Y.P., Stravitz R.T., Gores G.J. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1109–G1115. doi: 10.1152/ajpgi.1997.272.5.G1109. [DOI] [PubMed] [Google Scholar]
- 36.Kim M.J., Suh D.J. Profiles of serum bile acids in liver diseases. Korean J Intern Med. 1986;1:37–42. doi: 10.3904/kjim.1986.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter J.L., Fordtran J.S., Santa Ana C.A., et al. Accurate enzymatic measurement of fecal bile acids in patients with malabsorption. J Lab Clin Med. 2003;141:411–418. doi: 10.1016/S0022-2143(03)00040-4. [DOI] [PubMed] [Google Scholar]
- 38.Alamoudi J.A., Li W., Gautam N., et al. Bile acid indices as biomarkers for liver diseases I: diagnostic markers. World J Hepatol. 2021;13:433–455. doi: 10.4254/wjh.v13.i4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu N., Feng J., Lv Y., et al. Role of bile acids in the diagnosis and progression of liver cirrhosis: a prospective observational study. Exp Ther Med. 2019;18:4058–4066. doi: 10.3892/etm.2019.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick W.C., Bell C.C., Swell L., Vlahcevic Z.R. Cholic acid synthesis as an index of the severity of liver disease in man. Gut. 1973;14:895–902. doi: 10.1136/gut.14.11.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nimer N., Choucair I., Wang Z., et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism. 2021;116:154457. doi: 10.1016/j.metabol.2020.154457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neale G., Lewis B., Weaver V., Panveliwalla D. Serum bile acids in liver disease. Gut. 1971;12:145–152. doi: 10.1136/gut.12.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sang C., Wang X., Zhou K., et al. Bile acid profiles are distinct among patients with different etiologies of chronic liver disease. J Proteome Res. 2021;20:2340–2351. doi: 10.1021/acs.jproteome.0c00852. [DOI] [PubMed] [Google Scholar]
- 44.Yan L.-T., Wang L.-L., Yao J., et al. Total bile acid-to-cholesterol ratio as a novel noninvasive marker for significant liver fibrosis and cirrhosis in patients with non-cholestatic chronic hepatitis B virus infection. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000019248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X., Xie G., Zhao A., et al. Serum bile acids are associated with pathological progression of hepatitis B-induced cirrhosis. J Proteome Res. 2016;15:1126–1134. doi: 10.1021/acs.jproteome.5b00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shlomai A., Halfon P., Goldiner I., et al. Serum bile acid levels as a predictor for the severity of liver fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2013;20:95–102. doi: 10.1111/j.1365-2893.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen W., Wei Y., Xiong A., et al. Comprehensive analysis of serum and fecal bile acid profiles and interaction with gut microbiota in primary biliary cholangitis. Clin Rev Allergy Immunol. 2020;58:25–38. doi: 10.1007/s12016-019-08731-2. [DOI] [PubMed] [Google Scholar]
- 48.Mousa O.Y., Juran B.D., McCauley B.M., et al. Bile acid profiles in primary sclerosing cholangitis and their ability to predict hepatic decompensation. Hepatol Baltim Md. 2021 Jul;74:281–295. doi: 10.1002/hep.31652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou C., Jia H., Liu Y., et al. Metabolism of glycerophospholipid, bile acid and retinol is correlated with the early outcomes of autoimmune hepatitis. Mol Biosyst. 2016;12:1574–1585. doi: 10.1039/C6MB00092D. [DOI] [PubMed] [Google Scholar]
- 50.Horvatits T., Drolz A., Roedl K., et al. Serum bile acids as marker for acute decompensation and acute-on-chronic liver failure in patients with non-cholestatic cirrhosis. Liver Int. 2017;37:224–231. doi: 10.1111/liv.13201. [DOI] [PubMed] [Google Scholar]
- 51.Emerick K.M., Elias M.S., Melin-Aldana H., et al. Bile composition in Alagille syndrome and PFIC patients having partial external biliary diversion. BMC Gastroenterol. 2008;8:47. doi: 10.1186/1471-230X-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pawlikowska L., Strautnieks S., Jankowska I., et al. Differences in presentation and progression between severe FIC1 and BSEP deficiencies. J Hepatol. 2010;53:170–178. doi: 10.1016/j.jhep.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Togawa T., Mizuochi T., Sugiura T., et al. Clinical, pathologic, and genetic features of neonatal Dubin-Johnson syndrome: a multicenter study in Japan. J Pediatr. 2018;196:161–167. doi: 10.1016/j.jpeds.2017.12.058. e1. [DOI] [PubMed] [Google Scholar]
- 54.Schukfeh N., Metzelder M.L., Petersen C., et al. Normalization of serum bile acids after partial external biliary diversion indicates an excellent long-term outcome in children with progressive familial intrahepatic cholestasis. J Pediatr Surg. 2012;47:501–505. doi: 10.1016/j.jpedsurg.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Al-Hussaini A.A., Setchell K.D.R., AlSaleem B., et al. Bile acid synthesis disorders in Arabs: a 10-year screening study. J Pediatr Gastroenterol Nutr. 2017;65:613–620. doi: 10.1097/MPG.0000000000001734. [DOI] [PubMed] [Google Scholar]
- 56.Fukuoka T., Bessho K., Tachibana M., et al. Total bile acid concentration in duodenal fluid is a useful preoperative screening marker to rule out biliary atresia. J Pediatr Gastroenterol Nutr. 2018;67:383–387. doi: 10.1097/MPG.0000000000002037. [DOI] [PubMed] [Google Scholar]
- 57.Koofy N.E., Yassin N., Okasha S., William H., Elakel W., Elshiwy Y. Evaluation of the role of bile acids and serotonin as markers of pruritus in children with chronic cholestatic liver disease. Arab J Gastroenterol. June 2, 2021 doi: 10.1016/j.ajg.2021.04.001. Published online. S1687-1979(21)00020-4. [DOI] [PubMed] [Google Scholar]
- 58.Golden J., Zagory J.A., Fenlon M., et al. Liquid chromatography–mass spectroscopy in the diagnosis of biliary atresia in children with hyperbilirubinemia. J Surg Res. 2018;228:228–237. doi: 10.1016/j.jss.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Harms M.H., van Buuren H.R., Corpechot C., et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71:357–365. doi: 10.1016/j.jhep.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Harnois D.M., Angulo P., Jorgensen R.A., Larusso N.F., Lindor K.D. High-dose ursodeoxycholic acid as a therapy for patients with primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1558–1562. doi: 10.1111/j.1572-0241.2001.03777.x. [DOI] [PubMed] [Google Scholar]
- 61.O'Brien C.B., Senior J.R., Arora-Mirchandani R., Batta A.K., Salen G. Ursodeoxycholic acid for the treatment of primary sclerosing cholangitis: a 30-month pilot study. Hepatology. 1991;14:838–847. doi: 10.1002/hep.1840140516. [DOI] [PubMed] [Google Scholar]
- 62.Okolicsanyi L., Groppo M., Floreani A., et al. Treatment of primary sclerosing cholangitis with low-dose ursodeoxycholic acid: results of a retrospective Italian multicentre survey. Dig Liver Dis. 2003;35:325–331. doi: 10.1016/S1590-8658(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 63.Ponsioen C.Y., Lindor K.D., Mehta R., Dimick-Santos L. Design and endpoints for clinical trials in primary sclerosing cholangitis. Hepatology. 2018;68:1174–1188. doi: 10.1002/hep.29882. [DOI] [PubMed] [Google Scholar]
- 64.Stanich P.P., Björnsson E., Gossard A.A., Enders F., Jorgensen R., Lindor K.D. Alkaline phosphatase normalization is associated with better prognosis in primary sclerosing cholangitis. Dig Liver Dis. 2011;43:309–313. doi: 10.1016/j.dld.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al Mamari S., Djordjevic J., Halliday J.S., Chapman R.W. Improvement of serum alkaline phosphatase to <1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329–334. doi: 10.1016/j.jhep.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Trivedi P.J., Muir A.J., Levy C., et al. Inter- and intra-individual variation, and limited prognostic utility, of serum alkaline phosphatase in a trial of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2021;19:1248–1257. doi: 10.1016/j.cgh.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 67.Triantos C.K., Koukias N.M., Nikolopoulou V.N., Burroughs A.K. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2011;34:901–910. doi: 10.1111/j.1365-2036.2011.04822.x. [DOI] [PubMed] [Google Scholar]
- 68.Poropat G., Giljaca V., Stimac D., Gluud C. Bile acids for primary sclerosing cholangitis. Cochrane Database Syst Rev. 2011;1 doi: 10.1002/14651858.CD003626.pub2. CD003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wunsch E., Trottier J., Milkiewicz M., et al. Prospective evaluation of ursodeoxycholic acid withdrawal in patients with primary sclerosing cholangitis. Hepatology. 2014;60:931–940. doi: 10.1002/hep.27074. [DOI] [PubMed] [Google Scholar]
- 70.Lindor K.D., Kowdley K.V., Harrison M.E., American College of Gastroenterology ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol. 2015;110:646–659. doi: 10.1038/ajg.2015.112. quiz 660. [DOI] [PubMed] [Google Scholar]
- 71.Lindor K.D., Kowdley K.V., Luketic V.A.C., et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacquemin E., Hermans D., Myara A., et al. Ursodeoxycholic acid therapy in pediatric patients with progressive familial intrahepatic cholestasis. Hepatology. 1997;25:519–523. doi: 10.1002/hep.510250303. [DOI] [PubMed] [Google Scholar]
- 73.Kardorff R., Melter M., Rodeck B., Brodehl J. [Long-term ursodeoxycholic acid treatment of cholestatic liver diseases in childhood--clinical and biochemical effects] Klin Padiatr. 1996;208:118–122. doi: 10.1055/s-2008-1046459. [DOI] [PubMed] [Google Scholar]
- 74.Balistreri W.F. Bile acid therapy in pediatric hepatobiliary disease: the role of ursodeoxycholic acid. J Pediatr Gastroenterol Nutr. 1997;24:573–589. doi: 10.1097/00005176-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 75.Spagnuolo M., Iorio R., Vegnente A., Guarino A. Ursodeoxycholic acid for treatment of cholestasis in children on long- term total parenteral nutrition: a pilot study. Gastroenterology. 1996;111:716–719. doi: 10.1053/gast.1996.v111.pm8780577. [DOI] [PubMed] [Google Scholar]
- 76.Ullrich D., Rating D., Schröter W., Hanefeld F., Bircher J. Treatment with ursodeoxycholic acid renders children with biliary atresia suitable for liver transplantation. Lancet. 1987;2:1324. doi: 10.1016/s0140-6736(87)91208-6. [DOI] [PubMed] [Google Scholar]
- 77.Narkewicz M.R., Smith D., Gregory C., Lear J.L., Osberg I., Sokol R.J. Effect of ursodeoxycholic acid therapy on hepatic function in children with intrahepatic cholestatic liver disease. J Pediatr Gastroenterol Nutr. 1998;26:49–55. doi: 10.1097/00005176-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Sundaram S.S., Bove K.E., Lovell M.A., Sokol R.J. Mechanisms of Disease: inborn errors of bile acid synthesis. Nat Rev Gastroenterol Hepatol. 2008;5:456–468. doi: 10.1038/ncpgasthep1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellicciari R., Fiorucci S., Camaioni E., et al. 6α-Ethyl-Chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 80.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Younossi Z.M., Ratziu V., Loomba R., et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 82.Oral Abstracts (Abstracts 1–288) Hepatology. 2019;70:1–187. doi: 10.1002/hep.30940. [DOI] [Google Scholar]
- 83.Nevens F., Andreone P., Mazzella G., et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 84.Elhence A., Shalimar Treatment of non-alcoholic fatty liver disease - current perspectives. Indian J Gastroenterol. 2020;39:22–31. doi: 10.1007/s12664-020-01021-2. [DOI] [PubMed] [Google Scholar]
- 85.Hirschfield G.M., Mason A., Luketic V., et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–761. doi: 10.1053/j.gastro.2014.12.005. e8. [DOI] [PubMed] [Google Scholar]
- 86.Jhaveri M.A., Kowdley K.V. New developments in the treatment of primary biliary cholangitis - role of obeticholic acid. Ther Clin Risk Manag. 2017;13:1053–1060. doi: 10.2147/TCRM.S113052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trauner M., Gulamhusein A., Hameed B., et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology (Baltimore, Md) 2019;70:788–801. doi: 10.1002/hep.30509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel K., Harrison S.A., Elkhashab M., et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 89.Badman M.K., Chen J., Desai S., et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel non–bile acid FXR agonist tropifexor (LJN452) in healthy volunteers. Clin Pharmacol Drug Dev. 2020;9:395–410. doi: 10.1002/cpdd.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chianelli D., Rucker P.V., Roland J., et al. Nidufexor (LMB763), a novel FXR modulator for the treatment of nonalcoholic steatohepatitis. J Med Chem. 2020;63:3868–3880. doi: 10.1021/acs.jmedchem.9b01621. [DOI] [PubMed] [Google Scholar]
- 91.Harrison S.A., Rinella M.E., Abdelmalek M.F., et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 92.Harrison S.A., Neff G., Guy C.D., et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160:219–231. doi: 10.1053/j.gastro.2020.08.004. e1. [DOI] [PubMed] [Google Scholar]
- 93.Sanyal A.J., Ling L., Beuers U., et al. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep. 2021;3:100255. doi: 10.1016/j.jhepr.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirschfield G.M., Chazouillères O., Drenth J.P., et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70:483–493. doi: 10.1016/j.jhep.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 95.Kumar D.P., Asgharpour A., Mirshahi F., et al. Activation of transmembrane bile acid receptor TGR5 modulates pancreatic islet α cells to promote glucose homeostasis. J Biol Chem. 2016;291:6626–6640. doi: 10.1074/jbc.M115.699504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katsuma S., Hirasawa A., Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 97.Comeglio P., Cellai I., Mello T., et al. INT-767 prevents NASH and promotes visceral fat brown adipogenesis and mitochondrial function. J Endocrinol. 2018;238:107–127. doi: 10.1530/JOE-17-0557. [DOI] [PubMed] [Google Scholar]
- 98.He H., Mennone A., Boyer J.L., Cai S.-Y. Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology. 2011;53:548–557. doi: 10.1002/hep.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Assis D.N., Abdelghany O., Cai S.-Y., et al. Combination therapy of all-trans retinoic acid with ursodeoxycholic acid in patients with primary sclerosing cholangitis: a human pilot study. J Clin Gastroenterol. 2017;51:e11–e16. doi: 10.1097/MCG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ge M., Niu W., Ren J., et al. A novel ASBT inhibitor, IMB17-15, repressed nonalcoholic fatty liver disease development in high-fat diet-fed Syrian golden hamsters. Acta Pharmacol Sin. 2019;40:895–907. doi: 10.1038/s41401-018-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nadinskaia M., Maevskaya M., Ivashkin V., et al. Ursodeoxycholic acid as a means of preventing atherosclerosis, steatosis and liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2021;27:959–975. doi: 10.3748/wjg.v27.i10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Traussnigg S., Halilbasic E., Hofer H., et al. Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wien Klin Wochenschr. 2021;133:441–451. doi: 10.1007/s00508-020-01735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Newsome P.N., Palmer M., Freilich B., et al. Volixibat in adults with non-alcoholic steatohepatitis: 24-week interim analysis from a randomized, phase II study. J Hepatol. 2020;73:231–240. doi: 10.1016/j.jhep.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 104.Pockros P.J., Fuchs M., Freilich B., et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019;39:2082–2093. doi: 10.1111/liv.14209. [DOI] [PubMed] [Google Scholar]
- 105.Palmer M., Jennings L., Silberg D.G., Bliss C., Martin P. A randomised, double-blind, placebo-controlled phase 1 study of the safety, tolerability and pharmacodynamics of volixibat in overweight and obese but otherwise healthy adults: implications for treatment of non-alcoholic steatohepatitis. BMC Pharmacol Toxicol. 2018;19:10. doi: 10.1186/s40360-018-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harrison S.A., Rossi S.J., Paredes A.H., et al. NGM282 improves liver fibrosis and histology in 12 Weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1198–1212. doi: 10.1002/hep.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siddiqui M.S., Natta M.L.V., Connelly M.A., et al. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. 2020;72:25–33. doi: 10.1016/j.jhep.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mueller M., Thorell A., Claudel T., et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62:1398–1404. doi: 10.1016/j.jhep.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mudaliar S., Henry R.R., Sanyal A.J., et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. doi: 10.1053/j.gastro.2013.05.042. e1. [DOI] [PubMed] [Google Scholar]
- 110.Ratziu V., de Ledinghen V., Oberti F., et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 111.Leuschner U.F.H., Lindenthal B., Herrmann G., et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 112.Lindor K.D., Kowdley K.V., Heathcote E.J., et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 113.Laurin J., Lindor K.D., Crippin J.S., et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 114.Kjærgaard K., Frisch K., Sørensen M., et al. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J Hepatol. 2021;74:58–65. doi: 10.1016/j.jhep.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 115.Kowdley K.V., Vuppalanchi R., Levy C., et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hegade V.S., Kendrick S.F.W., Dobbins R.L., et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/S0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]
- 117.Fickert P., Hirschfield G.M., Denk G., et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 118.Trauner M., Nevens F., Shiffman M.L., et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4:445–453. doi: 10.1016/S2468-1253(19)30094-9. [DOI] [PubMed] [Google Scholar]
- 119.Bowlus C.L., Pockros P.J., Kremer A.E., et al. Long-term obeticholic acid therapy improves histological endpoints in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2020;18:1170–1178. doi: 10.1016/j.cgh.2019.09.050. e6. [DOI] [PubMed] [Google Scholar]
- 120.Harms M.H., Hirschfield G.M., Floreani A., et al. Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis. JHEPReport. 2021;3 doi: 10.1016/j.jhepr.2020.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Corpechot C., Chazouillères O., Belnou P., et al. Long-term impact of preventive UDCA therapy after transplantation for primary biliary cholangitis. J Hepatol. 2020;73:559–565. doi: 10.1016/j.jhep.2020.03.043. [DOI] [PubMed] [Google Scholar]
- 122.Dilger K., Hohenester S., Winkler-Budenhofer U., et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133–140. doi: 10.1016/j.jhep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 123.Cullen S.N., Rust C., Fleming K., Edwards C., Beuers U., Chapman R.W. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48:792–800. doi: 10.1016/j.jhep.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 124.Olsson R., Boberg K.M., de Muckadell O.S., et al. High-dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5-year multicenter, randomized, controlled study. Gastroenterology. 2005;129:1464–1472. doi: 10.1053/j.gastro.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 125.Angulo P., Dickson E.R., Therneau T.M., et al. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomized trial. J Hepatol. 1999;30:830–835. doi: 10.1016/s0168-8278(99)80136-6. [DOI] [PubMed] [Google Scholar]
- 126.Lindor K.D. Ursodiol for primary sclerosing cholangitis. N Engl J Med. 1997;336:691–695. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 127.De Maria N., Colantoni A., Rosenbloom E., Van Thiel D.H. Ursodeoxycholic acid does not improve the clinical course of primary sclerosing cholangitis over a 2-year period. Hepato-Gastroenterology. 1996;43:1472–1479. [PubMed] [Google Scholar]
- 128.van de Meeberg P.C., Wolfhagen F.H.J., Berge-Henegouwen G.P.V., et al. Single or multiple dose ursodeoxycholic acid for cholestatic liver disease: biliary enrichment and biochemical response. J Hepatol. 1996;25:887–894. doi: 10.1016/S0168-8278(96)80293-5. [DOI] [PubMed] [Google Scholar]
- 129.Baumann U., Sturm E., Lacaille F., et al. Effects of odevixibat on pruritus and bile acids in children with cholestatic liver disease: phase 2 study. Clin Res Hepatol Gastroenterol. 2021;45:101751. doi: 10.1016/j.clinre.2021.101751. [DOI] [PubMed] [Google Scholar]
- 130.van Wessel D.B.E., Thompson R.J., Gonzales E., et al. Impact of genotype, serum bile acids, and surgical biliary diversion on native liver survival in FIC1 deficiency. Hepatology. 2021 doi: 10.1002/hep.31787. n/a(n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deneau M., Perito E., Ricciuto A., et al. Ursodeoxycholic acid therapy in pediatric primary sclerosing cholangitis: predictors of gamma glutamyltransferase normalization and favorable clinical course. J Pediatr. 2019;209:92–96. doi: 10.1016/j.jpeds.2019.01.039. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Black D.D., Mack C., Kerkar N., et al. A prospective trial of withdrawal and reinstitution of ursodeoxycholic acid in pediatric primary sclerosing cholangitis. Hepatol Commun. 2019;3:1482–1495. doi: 10.1002/hep4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shneider B.L., Spino C., Kamath B.M., et al. Placebo-controlled randomized trial of an intestinal bile salt transport inhibitor for pruritus in Alagille syndrome. Hepatol Commun. 2018;2:1184–1198. doi: 10.1002/hep4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dinler G., Koçak N., Yüce A., Gürakan F., Ozen H. Ursodeoxycholic acid therapy in children with cholestatic liver disease. Turk J Pediatr. 1999;41:91–98. [PubMed] [Google Scholar]
- 135.Dinler G., Koçak N., Ozen H., Yüce A., Gürakan F. Ursodeoxycholic acid treatment in children with Byler disease. Pediatr Int. 1999;41:662–665. doi: 10.1046/j.1442-200x.1999.01143.x. [DOI] [PubMed] [Google Scholar]
- 136.Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis - PubMed. Accessed August 9, 2021. https://pubmed.ncbi.nlm.nih.gov/29654817/. [DOI] [PMC free article] [PubMed]
- 137.Thomas C.E., Luu H.N., Wang R., et al. Association between pre-diagnostic serum bile acids and hepatocellular carcinoma: the Singapore Chinese health study. Cancers. 2021;13:2648. doi: 10.3390/cancers13112648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Petrick J.L., Florio A.A., Koshiol J., et al. Prediagnostic concentrations of circulating bile acids and hepatocellular carcinoma risk: REVEAL-HBV and HCV studies. Int J Cancer. 2020;147:2743–2753. doi: 10.1002/ijc.33051. [DOI] [PMC free article] [PubMed] [Google Scholar]